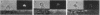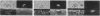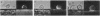Abstract
Mouse B lymphocytes that were specifically bound to dinitrophenylated bovine serum albumin on nylon fibers exhibited continuous morphological changes, whereas bound T lymphocytes remained more or less spherical. Cinematomicrographic studies showed that the shape changes were associated with local and global movements, although the attached cells did not translocate along the fiber. Cap formation induced by anti-immunoglobulin was always found to be opposite to the point of attachment. The movements and the shape changes were prevented by cytochalasin B and colchicine. Treatment with these agents did not prevent cap formation but led to randomization of the position of the caps with respect to the fiber. Exposure to concanavalin A or attachment of cells to concanavalin A fibers prevented both movement and patch and cap formation, suggesting that cellular structures regulating the mobility of various receptors are altered by binding to concanavalin A fibers. These observations also indicate that interactions of local areas of the lymphocyte surface with certain ligands and substrates can strongly affect the movement and morphology of the entire cell.
Keywords: cell shape, cell mobility, cap formation, concanavalin A
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhisey A. N., Freed J. J. Ameboid movement induced in cultured macrophages by colchicine or vinblastine. Exp Cell Res. 1971 Feb;64(2):419–429. doi: 10.1016/0014-4827(71)90096-6. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. S., Wallach D. F., Tsai S. Temperature-induced variations in the surface topology of cultured lymphocytes are revealed by scanning electron microscopy. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2492–2496. doi: 10.1073/pnas.70.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polliack A., Lampen N., Clarkson B. D., De Harven E., Bentwich Z., Siegal F. P., Kunkel H. G. Identification of human B and T lymphocytes by scanning electron microscopy. J Exp Med. 1973 Sep 1;138(3):607–624. doi: 10.1084/jem.138.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., D'Eustachio P., Edelman G. M. Immunological functions of lymphocytes fractionated with antigen-derivatized fibers. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3894–3898. doi: 10.1073/pnas.70.12.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Edelman G. M. Binding of thymus- and bone marrow-derived lymphoid cells to antigen-derivatized fibers. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3774–3778. doi: 10.1073/pnas.69.12.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. The pattern of binding of fluorescein-labeled concanavalin A to the motile lymphocyte. J Reticuloendothel Soc. 1970 Nov;8(5):458–464. [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor redistribution by concanavalin A, anti-mitotic agents and alterations of pH. Nature. 1973 Nov 16;246(5429):152–155. doi: 10.1038/246152a0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. The effects of concanavalin A on the mobility of lymphocyte surface receptors. Exp Cell Res. 1973 Sep;81(1):143–155. doi: 10.1016/0014-4827(73)90121-3. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]