Abstract
The side effects of chemotherapy on the lips may cause esthetic and functional impact and increase the risk of infection. HPA Lanolin® is an option for supportive therapy because it has anti-inflammatory, antimicrobial and moisturizing properties.
Objective:
To evaluate the efficacy of this product in the prevention of lip alterations in a population of patients undergoing chemotherapy.
Material and Methods:
Patients undergoing chemotherapy (n=57) were examined and distributed into two groups: study (used HPA Lanolin®) and control (without supportive therapy on the lips). We evaluated the patients two weeks after chemotherapy, registering oral alterations, symptoms of pain, discomfort, limitation of mouth opening and dehydration, classified according to a visual analogue scale.
Results:
Patients who used HPA Lanolin® had lower dehydration and experienced improvement of lip dryness (p<0.001). The main symptoms were dehydration, discomfort, limitation of mouth opening, pain. The main clinical signs were dry lips, mucositis, cheilitis, hematoma, swelling and cracking. We found no difference concerning the variables of pain, discomfort, and limitation of mouth opening between the study and control group.
Conclusions:
We suggest that HPA Lanolin® is effective in reducing the symptoms of dehydration and the signs of lip dryness resulting from toxicity of chemotherapy, proving to be an interesting alternative supportive therapy for cancer patients.
Keywords: Chemotherapy, Lanolin, Lip, Patient care
INTRODUCTION
Lip alterations related to cancer treatment represent 32.5% to 46% of the oral manifestations that occur due to the toxicity of chemotherapy4-6. The chemotherapeutic agents themselves can cause a rupture of the lips, bleeding, ulceration, and this has an impact on the quality of life of cancer patients. It increases the risk of systemic dissemination of bacteria from the mouth, which infiltrates through the ulcers and causes secondary infections9-11,16.
The main chemotherapy agents whose toxicity is related to the onset of oral lesions are the following: Actinomycin D, Amsacrine, Bleomycin, Cytarabine, Daunorrubicin, Doxatel, Doxorubicin, Etoposide, Floxuridine, 5-Fluoracil, Methotrexate, Mitoxantrone, Primacine, Tioguanine, Vinblastine, Vindesine and Melphalan9. These agents deserve special attention for the doctor of dental surgery (DDS) for the diagnosis and monitoring of oral lesions.
For proper oral care during chemotherapy, meticulous attention should be paid to the lips because they are an external contact area that can be a port for bacteria. One of the options for supportive therapy for the lips of patients undergoing chemotherapy is topical lanolin ointment, which can be applied to the lips as often as necessary to keep them moist, and it has a low risk of side effects. The efficacy of lanolin-based products applied during the anticancer treatment has been cited as an option to prevent and to treat lip lesions12-14, but there are no studies in the literature about the use of lip products for cancer patients.
Lanolin is a natural wax obtained from sheep wool grease, and it has different degrees of purity. Its composition is of high structural complexity, mixing high molecular weight esters. Lanolin is a unique product without substitutes, and it has a low allergenic capacity. This composition helps it to absorb water, making it an excellent moisturizer, as well as having excellent adherence10. There is great concern in dermatology about contact dermatitis and allergic conditions in patients using lanolin for skin and lips3,17. The HPA Lanolin® (high purified anhydrous) is a 100% natural substance, and it is considered to be the purest of lanolin. It has anti-inflammatory, antimicrobial, moisturizing, and protective effects on the epithelium. The purification process removes impurities and allergens, eliminating odor, flavor, preservatives, and bleaches. The absence of these impurities results in fewer reports of allergic reactions1,15.
There are very few prospective studies about the involvement of the lips in chemotherapy side effects. Although some articles quote a statement about lanolin lip moisturizer as an option as well, currently there is no prospective study in the literature evaluating the efficacy of this product when applied to the lips of cancer chemotherapy patients. This article aims to evaluate the efficacy of HPA Lanolin® in the prevention of lip alterations in a population of patients undergoing chemotherapy.
MATERIAL AND METHODS
After receiving approval from the Amaral Carvalho Foundation Research Ethics Committee, we examined 57 patients undergoing chemotherapy with drugs that carry a high risk of developing oral mucositis. We excluded patients with a history of allergies to HPA Lanolin®. All patients signed the free and clarified consent. After the training and calibration of four dental examiners, the 57 participants were distributed into two patient groups, study (SG) and control (CG). Patients included in the SG (n=25) applied HPA Lanolin® to their lips 6 times/day from the beginning of chemotherapy until two weeks after the end of chemotherapy. Patients included in the CG (n=32) did not use HPA Lanolin®. When necessary, patients received an oral care regimen to treat oral mucositis with low laser level therapy and rinse solutions with an enzymatic system (Biotène®).
We evaluated the patients on the first day of chemotherapy and two weeks after the end of chemotherapy, according to the period of HPA Lanolin® use, examining the mouth, classifying existing lesions according to the presence or absence of clinical aspects of dry lips, classifying oral mucositis according to the criteria of the World Health Organization (WHO)19, and registering symptoms of pain, discomfort, sense of limitation of mouth opening, and dehydration, classified according to a visual analogue scale. The results were analyzed with the SPSS statistical software package, applying the Shapiro-Wilkes and Mann-Whitney tests.
RESULTS
We included 57 patients, 27 men (47.7%) and 30 women (52.6%), with a median age of 58 (17-38). The SG group, with 25 patients, applied HPA Lanolin® to the lips, and the CG group, with 32 patients, did not apply the product. There were no missing data.
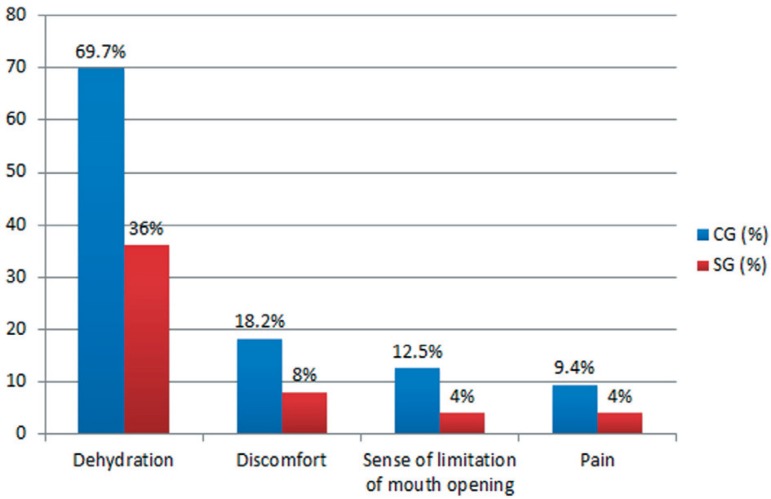
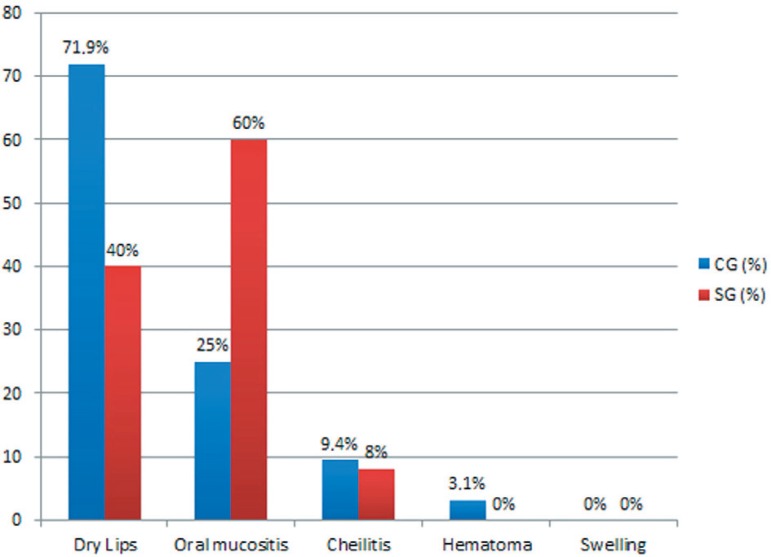
Several results of the study are presented here in graphic format. They include the symptoms reported by patients two weeks after the end of chemotherapy (Figure 1), when we might see dehydration, discomfort, sense of limitation of mouth opening and pain less frequency in patients that used the product. The clinical signs of alterations caused by chemotherapy (Figure 2) were dry lips, mucositis, cheilitis, hematoma, swelling and cracking.
Figure 1.
Symptoms of 57 patients undergoing chemotherapy. SG, Study Group; CG, Control Group
Figure 2.
Stomatologic alterations in 57 patients undergoing chemotherapy. SG, Study Group; CG, Control Group
We examined the efficacy of HPA Lanolin® in the reduction of lip symptoms resulting from chemotherapy with the Mann-Whitney test, and we found a difference between the CG and SG groups only in the variable of dehydration (p<0.001). Regarding mucositis, even with the use of lanolin, 60% of patients from the SG presented signs of oral mucositis, probably due to the history of previous ulcers caused by chemotherapy. The oral mucositis scoring by the WHO scale showed in the study group (SG) grade 0 (n=8/25), grade 1 and 2 (n=10/25), grade 3 and 4 (n=7/25), and in the control group (CG) grade 0 (n=23/32), grade 1 and 2 (n=8/32), and grade 3 and 4 (n=1/32).
We found no difference in the variables of pain, discomfort, and limitation of mouth opening between the two groups of patients. All these variables were found statistically not significant (p>0.05). Although there was no significant difference in lip dryness, we observed that patients in the SG (40%) group had less dry mouth than those in the CG (71.9%) (Figure 2).
DISCUSSION
The aim of this study was to evaluate the efficacy of HPA Lanolin® in the prevention of lip alterations in a population of patients undergoing chemotherapy. We showed that lip dryness and dehydration due to chemotherapy can be alleviated with the use of HPA Lanolin®. The maintenance of a protective film on the lips offered comfort to the patients, and lubrication and protection of the lips. Therefore, we can also deduce that lip dryness was reduced, preventing other conditions such as erosions and ulcers, which could lead the patient to a more severe clinical status, such as poor nutrition and more severe infections18.
There are no studies related to the use of HPA Lanolin® as a treatment for the oral side effects of chemotherapy, or for other oral alterations not related to chemotherapy, so there are no protocols in place for the topical lip product. However, HPA Lanolin® has proven effective in preserving the integrity of the nipples and areolas in mothers who have maintained a constant film of the product while breastfeeding, showing lower rates of trauma and pain1. It has also been found effective in the treatment of anal fissures in children by applying lanolin cream in the perianal area twice a day2.
HPA Lanolin® was also important in reducing lip dryness and discomfort. The moisturizing property of the product, due to emulsification in the presence of moisture, may explain the attenuation of lip dryness, because the emulsification facilitates the transport of water to the deeper layers of the epithelium1,7. This product can be used as supportive therapy for cancer patients, reducing some of the painful side effects of chemotherapy, since there are no contraindications for its use. This product is easily applied, well tolerated and contributes to the comfort of the patient during the critical period of chemotherapy. The elimination of the impurities and allergenic components of the HPA Lanolin® enhances its safety for this use over other products with similar properties1,15 such as Vaseline® (petroleum jelly) and mineral oil. While these other products do form a film that helps to alleviate the dryness, they should be avoided because they are associated with the aspiration of particles of lipids and pneumonia. Moreover Vaseline® is highly flammable and its use should be avoided in children receiving oxygen therapy because on the risk of burns of face8.
Based on the results of our study, we suggest that HPA Lanolin® is effective in reducing the symptoms of dehydration and the signs of lip dryness resulting from toxicity of chemotherapy without side effects. Further studies are necessary to conclusively prove the efficacy of HPA Lanolin® in the prevention of lip alterations of patients undergoing chemotherapy.
ACKNOWLEDGMENTS
We declare that we do not have a conflict of interest or external funding for this research.
REFERENCES
- 1.Abou-Dakn M, Fluhr JW, Gensch M, Wockel A. Positive effect of HPA Lanolin versus expressed breastmilk on painful and damaged nipples during lactation. Skin Pharmacol Physiol. 2010;24:27–35. doi: 10.1159/000318228. [DOI] [PubMed] [Google Scholar]
- 2.Büyükyavuz B, Savas Ç, Duman L. Efficacy of lanolin and bovine type I collagen in the treatment of childhood anal fissures: a prospective, randomized, controlled clinical trial. Surg Today. 2010;40:752–756. doi: 10.1007/s00595-009-4141-3. [DOI] [PubMed] [Google Scholar]
- 3.Castanedo-Tardan MP, Zug KA. Patterns of cosmetic contact allergy. Dermatol Clin. 2009;27:265–280. doi: 10.1016/j.det.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Wang R, Cheng S, Chang Y. Assessment of chemotherapy-induced oral complications in children with cancer. J Pediat Oncol Nurs. 2004;21:33–39. doi: 10.1177/1043454203259947. [DOI] [PubMed] [Google Scholar]
- 5.Chen HM. Patient's experiences and perceptions of chemotherapyinduced oral mucositis in a day unit. Care Nursing. 2008;31:363–369. doi: 10.1097/01.NCC.0000305762.89109.29. [DOI] [PubMed] [Google Scholar]
- 6.Cowen D, Tarideu C, Schubert M, Peterson D, Resbeut M, Faucher C, et al. Low energy Helium-Neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys. 1997;38:697–703. doi: 10.1016/s0360-3016(97)00076-x. [DOI] [PubMed] [Google Scholar]
- 7.Hagen RL. Lanolin for sore nipples. Arch Pediatr Adolesc Med. 1999;153(9):658. [PubMed] [Google Scholar]
- 8.Kennedy L, Diamond J. Assessment and management of chemotherapy-induced mucositis in children. J Pediat Oncol Nurs. 1997;14:164–174. doi: 10.1016/s1043-4542(97)90052-7. [DOI] [PubMed] [Google Scholar]
- 9.Köstler Wj, Hejna M, Wenzel C, Zielinski CC. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51:290–315. doi: 10.3322/canjclin.51.5.290. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval RL, Koga DH, Buloto LS, Suzuki R, Dib LI. Management of chemo- and radiotherapy induced oral mucositis with low-energy laser: initial results of A.C. Camargo Hospital. J Appl Oral Sci. 2003;11(4):337–341. doi: 10.1590/s1678-77572003000400012. [DOI] [PubMed] [Google Scholar]
- 11.Santos PSS, Coracin FL, Barros JCA, Dulley FL, Nunes FD, Magalhães MG. Impact of oral care prior to HSCT on the severity and clinical outcomes of oral mucositis. Clin Transplan. 2011;25:325–328. doi: 10.1111/j.1399-0012.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 12.Santos PSS, Mesaggi AC, Montesso A, Magalhães MHCG. Oral mucositis: recent perspectives on prevention and treatment. Rev Gauch Odontol. 2009;57:339–344. [Google Scholar]
- 13.Schubert MM, Peterson DE, Lloid ME. Oral complications. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic cell transplantation. 2nd ed. Malden, MA: Blackwell Science; 2005. pp. 751–763. p. [Google Scholar]
- 14.Semba SE, Mealy BL, Hallmon WW. Dentistry and the cancer patient: Part 2 - oral health management of the chemotherapy patient. Compend. 1994;15:1378–1387. [PubMed] [Google Scholar]
- 15.Tanchev S, Vulkova S, Georgieva V, Gesheva I, Tsvetkov M. Lansinoh in the treatment of sore nipples in breastfeeding women. Akush Ginekol. 2004;43(Suppl 3):27–30. [PubMed] [Google Scholar]
- 16.Tolentino ES, Centurion BS, Ferreira LHC, Souza AP, Damante JH, Rubira-Bullen IRF. Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. J Appl Oral Sci. 2011;19:448–454. doi: 10.1590/S1678-77572011000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakelin SH, Smith H, White IR, Rycroft RJ, McFadden JP. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28–31. doi: 10.1046/j.1365-2133.2001.04277.x. [DOI] [PubMed] [Google Scholar]
- 18.Watters AL, Epstein JB, Agulnik M. Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol. 2011;47:441–448. doi: 10.1016/j.oraloncology.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Handbook for reporting results of cancer treatment. Geneva: The Organization; 1979. [Google Scholar]