Abstract
Objective
The aim of this study was to compare the cytotoxic effects of endodontic cements on human tooth germ stem cells (hTGSCs). MTA Fillapex, a mineral trioxide aggregate (MTA)-based, salicylate resin containing root canal sealer, was compared with iRoot SP, a bioceramic sealer, and AH Plus Jet, an epoxy resin-based root canal sealer.
Material and Methods
To evaluate cytotoxicity, all materials were packed into Teflon rings (4 mmµ3 mm) and co-cultured with hTGSCs with the aid of 24-well Transwell permeable supports, which had a pore size of 0.4 µm. Coverslips were coated with MTA Fillapex, iRoot SP and AH Plus Jet and each coverslip was placed onto the bottom of one well of a six-well plate for scanning electron microscopy (SEM) analysis. Before the cytotoxicity and SEM analysis, all samples were stored at 37ºC and at 95% humidity and 5% CO2 for 24 hours to set. The cellular viability was analyzed using MTS test (3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxy-methoxy-phenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium). The cytotoxic effects and SEM visualization of the tested materials were analyzed at 24-hour, 72-hour, one-week and two-week periods.
Results
On the 1st day, only MTA Fillapex caused cytotoxicity compared to negative control (NC) group (p<0.008). No significant difference was observed between the other tested materials at this period (p>0.05). After 14 days of incubation with the test materials, MTA Fillapex exhibited significantly higher cytotoxicity compared with iRoot SP, AH Plus Jet and the NC group (P<0.008). In the SEM analysis, the highest levels of cell attachment were observed for iRoot SP and the control group. After 24 hours, MTA Fillapex reduced the number of cells attached to the surface.
Conclusions
Within the limitations of this study, sealers exerted different cytotoxic effects on hTGSCs. Although all materials have exerted cellular toxicity, iRoot SP and AH Plus Jet may promote better attachment to hTGSCs.
Keywords: Stem cells, Cytotoxicity, Scanning electron microscopy, Calcium silicate, Endodontics
INTRODUCTION
Dental materials used for the root canal filling process can cause some unexpected effects when exposed to periapical tissues. Filling materials or their byproducts contact the surrounding tissues via dentinal tubules and apical openings4. Therefore, an ideal root canal filling material should be biocompatible and antimicrobial. In addition, it should provide an effective seal and be dimensionally stable3. Moreover, it should have the ability to induce biological cell responses that contribute to regeneration. Therefore, researchers have sought to develop a new generation of endodontic sealers for successful root canal treatment.
In most cases, cell culture techniques are utilized to test the efficiency of sealers in cytocompatibility and induction capacity of hard tissue deposition using multiple well-established cell lines25. Furthermore, recent studies based on material-cell interactions have revealed that dental materials might have the capacity to induce repair/regeneration by stimulating periodontal ligament (PDL) cells in the apical region10. Besides, cell-based therapies demonstrated dentin/pulp-like tissue regeneration when dental pulp stem cells (DPSCs) were transplanted into immunocompromised mice18. Therefore, since human tooth germ stem cells (hTGSCs) originate from both the periodontium and the dental pulp25, determination of material-cell interactions using hTGSCs might help enlighten conditions that are likely to arise during clinical usage of these materials.
For many years, various materials based on zinc oxide eugenol, polyketone, epoxy resin, calcium hydroxide, silicone, methacrylate resin, glass ionomer, and resin modified glass ionomer have been used to fill root canals4. Recently, new types of filling materials containing mineral trioxide aggregate (MTA) and calcium silicate have been developed. MTA is a biocompatible material for cells14. The biocompatibility, pulp healing, dentine bridge formation and reparative effects of MTA have been demonstrated by in vivo studies in which healthy human/murine pulp cell responses were observed14,19. Aside from its biocompatibility, MTA is a reliable material in clinical use14.
In addition to MTA, calcium-silicate-based MTA-like materials, such as bioceramics, have also been introduced to the market for the same purposes. iRoot SP (Innovative BioCreamix Inc., Vancouver, Canada) is a new premixed, injectable bioceramic root canal sealer26. Given the similar chemical compositions of iRoot SP and MTA, iRoot SP can also induce cellular reparative processes29.
Recently, MTA Fillapex (Angelus Indústria de Produtos Odontológicos S/A, Londrina, PR, Brazil), a new MTA-based root canal material, has been developed. The chemical composition of MTA Fillapex consists of two main components: MTA and salicylate resin. Due to its MTA content, it has also been introduced as a biologic material by the manufacturer.
According to the manufacturers, both iRoot SP and MTA Fillapex require the moisture that originates from dentinal tubules or periapical tissues in order to set and harden. Calcium silicate, which is the main ingredient of iRoot SP, generates calcium silicate hydrates in the presence of water, similarly to MTA26. Torabinejad, et al.20 (1995) stated that MTA is mainly composed of tricalcium silicate, tricalcium aluminate, tricalcium oxide, and silicate oxide, and it is used to prevent leakage between the root canal system and its surrounding tissue. Due to the hard tissue deposition capacity of MTA and the fact that it is associated with fewer inflammatory reactions, MTA has been used as a pulp capping agent1,14. MTA is also used for root perforation repair and retrograde filling11,21. Recently, it has been recommended for regenerative endodontic procedures5. Although fewer studies have been published compared with MTA, the biocompatibility of iRoot SP has been documented29,30. MTA Fillapex has not been evaluated as extensively12,16,17. In addition, no study has compared the cytotoxic effects of iRoot SP and MTA Fillapex or examined the interactions between the sealers and hTGSCs.
AH Plus Jet is an epoxy resin-based root canal sealer that is currently used as a root canal filling material. Cytotoxic evaluations of this sealer demonstrated that its cytotoxicity decreased at longer setting times8.
The purpose of this study was to investigate the potential cytotoxic effects of iRoot SP and MTA Fillapex on hTGSCs relative to AH Plus Jet, which has proven to be a non-toxic dental material.
MATERIAL AND METHODS
Material
The test materials were iRoot SP® (Innovative BioCreamix Inc., Vancouver, Canada. Lot No:10002SP), MTA Fillapex® (Angelus Ind. Prod. Odontológicos S/A, Londrına, PR, Brazil. Lot No: 15824) and an epoxy-resin based root canal sealer (AH Plus Jet®; Dentsply DeTrey GmbH, 78467 Konstanz, Germany. Lot No: 1005001642). All the materials used are injectable, which means that they are self-mixing or premixed; therefore, the mixing process was out of the contributors' control.
Cell culture
Fully characterized and cryopreserved Passage 2 hTGSCs25 were defrosted and cultured in growth medium containing Dulbecco's modified Essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 1% PSF (penicillin, streptomycin, and fungizone) solution. The cells were incubated at 37ºC in a humidified atmosphere with 5% CO2.
Cell viability assay
To evaluate cell viability, the sealers were prepared according to the manufacturers' instructions and embedded into Teflon rings with a diameter of 4 mm and a height of 3 mm, followed by being covered with Mylar sheets to form cylindrical specimens of each sealer under aseptic conditions. To obtain standardization in terms of setting times of iRoot SP (4 hours), MTA Fillapex (2 hours) and AH Plus Jet (8 hours), the rings were stored in an incubator with a humidified 5% CO2, 95% air atmosphere for 24 hours at 37ºC. For each material, six rings (n=6) were prepared for each time point (day 1, day 3, day 7, and day 14). After 24 hours, the set sealer disks were removed from the teflon rings, placed on transwell inserts (cat no. 3472, Corning, NY 14831, USA and Canada) and co-cultured with hTGSCs (4000 cells/well) in 24-well plates. All sealers were submerged in media with the addition of 1.5 mL of medium to each well. Because the 24-well transwell inserts had a pore size of 0.4 µm, the sealers and cells could be incubated in the same medium without direct contact. The cells and sealers were incubated for 14 days, with the media being changed every other day.
Cell viability was measured on days 1, 3, 7, and 14 using MTS assays (CellTiter96 Aqueous One Solution, Promega, Southampton, UK) according to the manufacturer's instructions. MTS (3-(4, 5-dimethyl-thiazol-2-yl)-5-(3-carboxy-methoxy-phenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium) is a tetrazolium-salt-based colorimetric assay used for detecting the activity of enzymes, mostly present in the mitochondria, that reduce MTS to formazan, yielding a purple color. Before the addition of 300 µl of MTS solution on top of the cells, the sealers, inserts, and medium in each well of the 24-well plates, were removed. The plates were incubated with MTS for 2 hours, then 100 µl of MTS solution was transferred from one well of the 24-well plate to one well of a 96-well plate in order to read the absorbance at 450 nm in an ELISA plate reader (Biotek, USA).
SEM analysis
SEM analysis was performed to observe the surface interactions between hTGSCs and the root canal sealing materials in vitro. The sealers were prepared according to the manufacturers' instructions, and 0.8 g of each was applied to the surface of sterile coverslips (20 mmµ20 mm) (Isolab, Laborgerate Gmbh), forming 400 mm2 plugs with a thickness of 2 mm. Coverslips with no material were used as a control group. After coating the coverslips, they were stored in an incubator with a humidified 5% CO2, 95% air atmosphere for 24 hours at 37ºC. For each material, one coverslip was prepared for each time point (day 1, day 3, day 7, and day 14). The coverslips coated with the sealing materials were sterilized under ultraviolet light for 30 minutes, then each coverslip was placed into one well of a 6-well plate. Next, the cells were seeded onto the coverslips at a concentration of 25,000 cells/well in 2 mL of growth media. The cells and materials were incubated in an incubator with a humidified 5% CO2, 95% air atmosphere for 14 days at 37ºC, and the medium was changed every other day. Next, the medium was removed and the cells in the wells were fixed via incubation with 2% paraformaldehyde at 4ºC for 30 minutes. The coverslips were air-dried at room temperature for 1 hour. Visualization of the cells on the coverslips was performed using a Carl Zeiss EVO 40 model SEM instrument (Dresden, Germany). The coverslips were coated with a gold layer (5 nm thick) with a sputter coater (Model BAL-TEC SCD 005 Sputter Coater, Balzers, Liechtenstein) to impart electrical conductivity. The accelerating voltage was 5 kV for all experiments. SEM images were obtained from specific areas of interest at various magnifications (200× and 500×).
Statistical analyses
Statistical analysis was performed using the SPSS 15.0 (Statistical package for social sciences) program for Windows. The intergroup comparisons of parameters were analysed by Kruskal-Wallis test whereas Mann-Whitney U Test with Bonferroni adjustment was used for determining the group, causing the difference. Statistical significance level was set at p<0.05. In the Mann-Whitney U Test on which Bonferroni adjustment was applied, significance level was accepted as 0.008 (0.05/6).
RESULTS
MTS assay
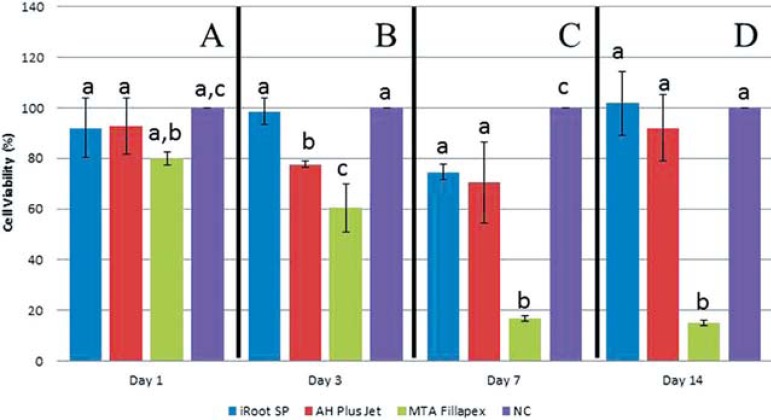
The results of the MTS analysis revealed that MTA Fillapex showed a significant toxic effect compared with the negative control group (P<0.008), starting on day 1. The non-significant reduction in cell viability observed with MTA Fillapex relative to the other groups (P>0.05) was observed on day 1. On days 3, 7 and 14, MTA Fillapex showed significant toxic effects compared with iRoot SP (P<0.008). On the other hand, on day 3, iRoot SP and AH Plus Jet exerted significantly different results on hTGSCs (p<0.008). Only on day 7, we observed a significant reduction in cell viability with iRoot SP compared with the negative control group (p<0.008) (Figure 1).
Figure 1.
MTS-reeducing activity relative to the negative control group of human tooth of human tooth germ stem cells after 1, 3, 7, and 14 days of incubation with the test materials. Data are shown as the mean±standard deviation (n=6). Differing letters indicate statically significant diferences. MTA Fillapex was more toxic than iRoot SP and AH Plus Jet NC: Negative Control
SEM analysis
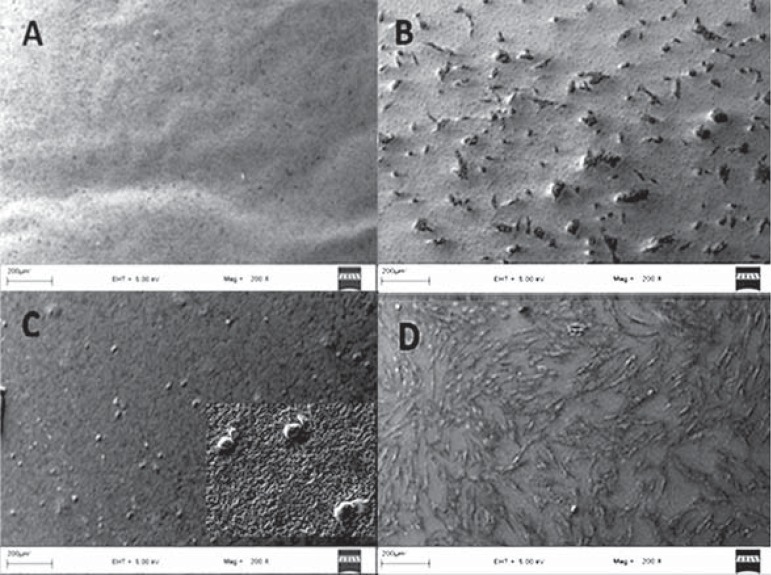
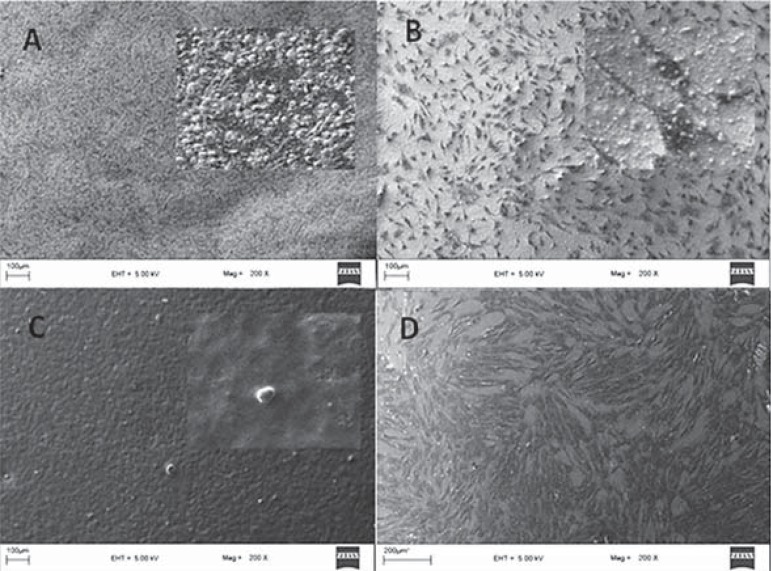
To visualize interactions between the endodontic sealer surfaces and hTGSCs, cells were seeded onto material-coated coverslips, which were placed in six-well plates. SEM analysis was performed on days 1, 3, 7, and 14. The highest levels of cell attachment were observed for iRoot SP and the control group over the entire period. On day 1, cells on the coverslips coated with MTA Fillapex and AH Plus Jet displayed a rounded shape, rather than adopting a spreading configuration (Figure 2). After 24 hours, MTA Fillapex exerted its cytotoxic effects, reducing the number of cells attached to the surface (Figure 3). Cells were literally embedded in the AH Plus Jet material and showed significant morphological changes on day 7.
Figure 2.
Scanning electron microscopy (SEM) analysis of human tooth germ stem cells cultured on various sealer surfaces on day 1 - A. AH Plus Jet, B. iRoot SP, C. MTA Fillapex, D. Control
Figure 3.
Scanning electron microscopy (SEM) analysis of human tooth germ stem cells cultured on various sealer surfaces on day 14 - A. AH Plus Jet, B. iRoot SP, C. MTA Fillapex, D. Control
DISCUSSION
This study used hTGSCs that were isolated from the third molars of young adults and were composed of cells derived from both dental follicles and dental pulp tissue25. These cells contain both osteogenic and odontogenic cellular compounds24, so they have the ability to differentiate into both osteogenic and odontogenic cells. In fact, the filling materials are mostly in contact with odontogenic and osteogenic cellular remnants, on which they exert some effects, such as cytotoxicity, inflammation, or proliferation. Further investigation of material-cell interactions in vitro using hTGSCs might provide valuable data for estimating the possible biological effects of dental materials in vivo. In this study, hTGSCs were used as cellular material, and cell viability was measured with the commonly used MTS reaction7. Studies have shown that the dental follicle includes precursors of cementoblasts, osteoblasts, and periodontal ligament cells13. Zhang, et al.29 (2010) demonstrated the mineralization ability and biocompatibility of iRoot SP with human osteoblast-like cells. Consistent with Zhang, et al.29 (2010), the results of the MTS analysis reported here demonstrated a non-cytotoxic response to iRoot SP, which was also confirmed by SEM analysis at the end of the two week evaluation period in this study. The strong attachment of hTGSCs to the material surface suggests the biocompatibility of iRoot SP sealer. The molecules released from materials and the material surface structures observed during/after setting periods, are the critical factors that affect the material-cell interactions. In this study, material-cell interactions were observed using SEM analysis. Borges, et al.2 (2012) demonstrated the solubility of calcium-silicate containing endodontic cements by the aid of scanning electron microscopy and energy-dispersive spectroscopy (SEM/EDX) methods. The external surface analysis of iRoot SP with SEM method demonstrated a compact, homogeneous and porous structure. Besides, EDX analysis revealed that iRoot SP and MTA Fillapex had high solubility and Ca+ ion release in contrast with AH Plus sealer2. Consistent with this study, our findings suggest a porous structure in the outer surface of iRoot SP after setting. The porosity of iRoot SP enables the continuity of water penetration2 which provides Ca+ ion release over time and the biocompatibility of calcium-silicate based materials is attributed to the formation of hydroxyapatite as crystals in the presence of Ca+ ions during setting reactions16.
Studies with iRoot SP have reported the material's sealing ability9,28, antibacterial activity27, cytotoxic evaluations9,30, and biological responses29, but there is limited information available about MTA Fillapex12,15,17. In vivo studies reported that salicylate-based root canal sealers that contain calcium hydroxide showed an incremental reduction in the inflammatory reaction over 48 hours6. One of the main components of MTA Fillapex is salicylate resin. In the present study, the SEM analysis demonstrated the severe cytotoxic potential of MTA Fillapex. Cell destruction was visible on day 3; however, over a 24-hour interval, the attachment of round-shaped cells to the surface was observed. The solubility of MTA Fillapex was found to be similar to iRoot SP which was more than the solubility of AH Plus after setting2. AH Plus sealer is an epoxy-resin based material and decreased cytotoxicity potential at longer setting times was observed with AH Plus8. The cytotoxic effect of epoxy-resin based sealers which was limited over time, can be explained with its low solubility results. On the other hand, the high cytotoxic potential of MTA Fillapex observed in the present study can be explained by the continuity of resin component activation due to the material's high solubility. Also, low MTA content (13.2%) might enable the material, incapable of releasing favorable amounts of Ca+ ions, to provide the biocompatibility.
The antibacterial activity of MTA Fillapex was also attributed to its resin component which was detected during the setting periods but did not continue after setting11. In the present study, MTA Fillapex showed severe toxicity over the 14-day experimental period. According to Scelza, et al.17 (2012), MTA Fillapex strongly affected cell viability and was not affected by time. However, the study included time points at 1-day and 7-day intervals, and extracts of the materials were used to analyze three different cell viability parameters17. Waltimo, et al.22 (2001) described the earlier periapical repair of teeth obturated with calcium hydroxide including salicylate resin-based sealers. This in vivo study was designed to compare the effects of calcium hydroxide containing zinc oxide eugenol (ZOE) and salicylate-based root canal sealers on the periapical healing of teeth with apical periodontitis, based on clinical and radiological evaluations performed once per year for a four-year period22. On the contrary, in the present in vitro study, the findings suggest an increased severity of cytotoxicity at the end of the two weeks related to MTA Fillapex. Cytotoxicity is only one aspect of biocompatibility and therefore cytotoxicity tests alone cannot characterize a material as biocompatible or not. According to an example mentioned in a biocompatibility review by Wataha23 (2012), ZOE yielded clinically favorable results when applied with a dental barrier onto the dental pulp, although it appeared to be highly cytotoxic under in vitro conditions23.
Based on the visualization of material-cell interactions, AH Plus Jet was detected to have a reduced potential for cytotoxicity on hTGSCs that was dependent on the number and shape of surface-attached cells, consistent with previous studies8. During the first day, a few round-shaped cells attached to the surface in the AH Plus Jet group, but the number of attached cells increased by the end of the two weeks, and these cells exhibited a significant change in cell morphology. Besides, according to the MTS results, only on the 3rd day, AH Plus Jet was found to be more cytotoxic when compared to iRoot SP but, at the end of the two weeks, iRoot SP and AH Plus Jet were similar in terms of the cytotoxicity parameters. These results are inconsistent with those of Zhang, et al.30 (2010) in which mouse fibroblasts were used with endodontic material extracts as toxicity targets. According to their results, when AH Plus and iRoot SP extracts were exposed to L-929 for 2 hours, AH Plus was found to be more cytotoxic than iRoot SP. In contrast, Loushine, et al.9 (2011) reported an ongoing, mildly cytotoxic effect of EndoSequenceBC sealer, which is also a bioceramic root canal sealer, after six weeks. However, AH Plus sealer became non-cytotoxic by the end of the same time interval.
Various studies have demonstrated the biocompatibility and hard tissue deposition capacity of MTA7. MTA is composed of calcium silicates and is able to set in an aqueous environment. The inventors of iRoot SP drew attention to its chemical similarities to MTA26. The similarities between iRoot SP and MTA in terms of their chemical compositions might enable iRoot SP to induce regeneration. Previously, Zhang, et al.30 (2010) attributed their biocompatibility results to the similarities between the main components in iRoot SP, calcium phosphate, calcium silicates, zirconium oxide and calcium hydroxide, and dental hard tissue.
CONCLUSIONS
In the present study, severe cytotoxic results were detected with MTA Fillapex over two weeks, even though its composition is based on MTA. However, the possible clinical effects of MTA Fillapex should be investigated in vivo. Based on the results of this study, iRoot SP and AH Plus Jet appear to be more suitable as root canal sealers, and further studies should be performed in order to investigate the biological effects of iRoot SP.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank M. Müge Yazici and Neslihan Taşli from the Genetics and Bioengineering Department of Yeditepe University for their help with the SEM experiments and cell preparation, respectively.
REFERENCES
- 1.Accorinte ML, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan Jr E, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De- Deus G, Miranda CES, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45:419–428. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Hargreaves KM. Pathways of the pulp. 9th ed. St. Louis: Mosby Elsevier; 2006. [Google Scholar]
- 4.Dahl JE. Toxicity of endodontic filling materials. Endodontic Topics. 2005;12:39–43. [Google Scholar]
- 5.De-Deus G, Coutinho-Filho T. The use of white Portland cement as an apical plug in a tooth with a necrotic pulp and wide-open apex: a case report. Int Endod J. 2007;40:653–660. doi: 10.1111/j.1365-2591.2007.01269.x. [DOI] [PubMed] [Google Scholar]
- 6.Desai S, Chandler N. Calcium hydroxide-based root canal sealers: a review. J Endod. 2009;35:475–480. doi: 10.1016/j.joen.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues and biomineralization of cementoblasts. J Endod. 2009;35:513–519. doi: 10.1016/j.joen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Lodiene G, Morisbak E, Bruzell E, Ørstavik D. Toxicity evaluation of root canal sealers in vitro. Int Endod J. 2008;41:72–77. doi: 10.1111/j.1365-2591.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 9.Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, et al. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37:673–677. doi: 10.1016/j.joen.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod. 2010;36:647–652. doi: 10.1016/j.joen.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80–83. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Morgental RD, Vier-Pelisser FG, Oliveira SD, Antunes FC, Cogo DM, Kopper PM. Antibacterial activity of two MTA-based root canal sealers. Int Endod J. 2011;44:1128–1133. doi: 10.1111/j.1365-2591.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 13.Morsczeck C, Schmalz G, Reichert TE, Völlner F, Saugspier M, Viale-Bouroncle S, et al. Gene expression profiles of dental follicle cells before and after osteogenic differentiation in vitro. Clin Oral Invest. 2009;13:383–391. doi: 10.1007/s00784-009-0260-x. [DOI] [PubMed] [Google Scholar]
- 14.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128–150. doi: 10.1111/j.1365-2591.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 15.Sagsen B, Ustün Y, Demirbuga S, Pala K. Push-out bond strength of two new calcium silicate-based endodontic sealers to root canal dentine. Int Endod J. 2011;44:1088–1091. doi: 10.1111/j.1365-2591.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 17.Scelza MZ, Linhares AB, Silva LE, Granjeiro JM, Alves GG. A multiparametric assay to compare the cytotoxicity of endodontic sealers with primary human osteoblasts. Int Endod J. 2012;45:12–18. doi: 10.1111/j.1365-2591.2011.01941.x. [DOI] [PubMed] [Google Scholar]
- 18.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 19.Simon S, Cooper P, Smith A, Picard B, Ifi CN, Berdal A. Evaluation of a new laboratory model for pulp healing: preliminary study. Int Endod J. 2008;41:781–790. doi: 10.1111/j.1365-2591.2008.01433.x. [DOI] [PubMed] [Google Scholar]
- 20.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 21.Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225–228. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 22.Waltimo TM, Boiesen J, Eriksen HM, Ørstavik D. Clinical performance of 3 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:89–92. doi: 10.1067/moe.2001.116154. [DOI] [PubMed] [Google Scholar]
- 23.Wataha JC. Predicting clinical biological responses to dental materials. Dent Mater. 2012;28:23–40. doi: 10.1016/j.dental.2011.08.595. [DOI] [PubMed] [Google Scholar]
- 24.Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, et al. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implicationsin neo-vascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2010;10:105–113. doi: 10.1038/tpj.2009.40. [DOI] [PubMed] [Google Scholar]
- 25.Yalvac ME, Ramazanoglu M, Tekguc M, Bayrak OF, Shafigullina AK, Salafutdinov II, et al. Human tooth germ stem cells preserve neuro-protective effects after long-term cryo-preservation. Curr Neurovasc Res. 2010;7:49–58. doi: 10.2174/156720210790820181. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Lu D. Premix biological hydraulic cement paste composition and using the same. Dec 04, 2008. US 20082990993 A1. [Google Scholar]
- 27.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–1055. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Li Z, Peng B. Assessment of a new root canal sealer's apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e79–e82. doi: 10.1016/j.tripleo.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Li Z, Peng B. Effects of iRoot SP on mineralizationrelated genes expression in MG63 cells. J Endod. 2010;36:1978–1982. doi: 10.1016/j.joen.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J. 2010;43:769–774. doi: 10.1111/j.1365-2591.2010.01733.x. [DOI] [PubMed] [Google Scholar]