Highlights
* Habituation of fetal and neonatal visual evoked cortical responses. * Magnetoencephalographic recordings. * Neonates showed response decrement upon stimulus repetition. * The response rate in fetuses was lower than in the neonates.
Keywords: Habituation, Response recovery, Fetal and neonatal evoked responses, Magnetoencephalography
Abstract
In this study we aimed to develop a habituation paradigm that allows the investigation of response decrement and response recovery and examine its applicability for measuring the habituation of the visually evoked responses (VERs) in neonatal and fetal magnetoencephalographic recordings.
Two paradigms, one with a long and one with a short inter-train interval (ITI), were developed and tested in separate studies. Both paradigms consisted of a train of four light flashes; each train being followed by a 500 Hz burst tone. Healthy pregnant women underwent two prenatal measurements and returned with their babies for a neonatal investigation.
The amplitudes of the neonatal VERs in the long-ITI condition showed within-train response decrement. An increased response to the auditory dishabituator was found confirming response recovery. In the short-ITI condition, neonatal amplitude decrement could not be demonstrated while response recovery was present. In both ITI conditions, the response rate of the cortical responses was much lower in the fetuses than in the neonates. Fetal VERs in the long-ITI condition indicate amplitude decline from the first to the second flash with no further decrease. The long-ITI paradigm might be useful to investigate habituation of the VERs in neonates and fetuses, although the latter requires precaution.
1. Introduction
Habituation, a basic form of learning, constitutes one of the most frequently used paradigms to investigate pre- and postnatal development. Habituation can be simply defined as a response decrement that occurs as a result of repeated stimulation. There is sufficient evidence that the ability to habituate reflects an intact central nervous system (CNS) with normal functioning (Leader et al., 1982) and that habituation can be detected before birth (Madison et al., 1986a, Kuhlman et al., 1988, Smith et al., 1991, Groome et al., 1993, van Heteren et al., 2001a, van Heteren et al., 2001b, Bellieni et al., 2005). In addition, lack of habituation has been linked to several high-risk conditions in which the fetuses were exposed to increased risk of neurological damage (Doherty and Hepper, 2000, Allister et al., 2001). Some studies even suggested that habituation can be regarded as a predictor of postpartum cognitive development (Madison et al., 1986b, Gaultney and Gingras, 2005). Early identification of habituation deficits therefore could have an increased clinical significance by allowing detection of altered developmental trajectory. Prior research examining habituation in fetuses focused mainly on behavioural outcomes (Madison et al., 1986b, Groome et al., 1993, Doherty and Hepper, 2000, van Heteren et al., 2001b, Bellieni et al., 2005) or heart rate parameters (Goldkrand and Litvack, 1991, Smith et al., 1991, van Heteren et al., 2001a). Although it is believed that these outcomes reflect cortical processing of the stimulus, they are indirect observations of the brain activity, and should be therefore interpreted with caution.
Electroencephalography (EEG) and magnetoencephalography (MEG) are widely used tools to measure the evoked responses (ERs) to external stimulation, and hence, to assess brain response directly. It is well established that novel sensory stimulation causes widespread cerebral activation as part of the so-called orienting reaction (Sokolov and Vinogradova, 1975) in which attention is (re)directed to the stimulus and a neuronal model of the stimulus is constructed (Naatanen and Picton, 1987). Upon stimulus repetition, novelty being lost, the orienting reaction is inhibited and the development of a neuronal model suppressed. This leads to decrement in response amplitude, which can be seen and measured at the level of the cortical ERs. In adults, decrement in ER amplitudes with stimulus repetition has been shown repeatedly (Prosser et al., 1981, Woods and Elmasian, 1986, Bourbon et al., 1987, Lasky et al., 1996, Noguchi et al., 2004, Rosburg et al., 2006). In these studies, researchers used a so-called short-term habituation paradigm. This paradigm differs significantly from the long-term paradigm, which has been dominantly used in the behavioural studies on habituation. Specifically, in the short-term paradigm, the number of presented stimuli is fixed from the beginning and the inter-stimulus interval (ISI) is typically short. Moreover, the stimuli are usually presented in form of repeated trains accompanied by an inter-train interval (ITI) of longer duration, or a changed stimulus, that is inserted between them to overcome fatigue and/or enforce dishabituation. The criterion for the occurrence of habituation is the decrement in response from the first stimulus to the subsequent ones, and not the response cessation, as in the case of long-term paradigms. In addition, habituation per se involves two other criteria, namely: response recovery (an increase response to a changed stimulus or dishabituator) and dishabituation (an increased response to a previously habituated stimulus following insertion of a dishabituator) (Thompson and Spencer, 1966).
In adults, numerous studies have applied habituation paradigms – with or without respect to all three criteria – to investigate the response decrement or habituation of ERs. In children and especially in newborns, there are a limited number of studies that address this issue (Atkinson et al., 1988, Lasky, 1997, McGee et al., 2001, Oelkers-Ax et al., 2005, Sheridan et al., 2008). Recently, Gonzalez-Frankenberger et al. (2008) investigated habituation in newborns with EEG detection of ER to visual flashes, showing that neural mechanisms of visual habituation are normally present during the first month of life (Gonzalez-Frankenberger et al., 2008). While EEG constitutes a suitable technology to investigate directly the brain responses in newborns and older infants, it is not applicable to measure fetal brain activity. Over the past few years, fetal magnetoencephalography (fMEG) has been successfully used to detect and understand fetal evoked magnetic brain responses (Preissl et al., 2005, Sheridan et al., 2010). Based on this technology, it is now possible to detect noninvasively the fetal and neonatal evoked brain responses elicited by visual and auditory stimulation (Eswaran et al., 2002b, Draganova et al., 2005, Lowery et al., 2006, Draganova et al., 2007). In a previous study, Sheridan et al. (2008) successfully applied a short-term habituation paradigm on human fetuses and neonates in a MEG setting (Sheridan et al., 2008). Their results indicated that all of the neonates with a detectable visual evoked response (VER) revealed a decrease in response amplitude, but that fetuses showed a response cessation after the first stimulus rather than a continuous response decrement. The authors suggested the usefulness of the short-term habituation paradigm for assessing of the decrement in response amplitude of the VERs in newborns and fetuses (Sheridan et al., 2008). However, the question whether the response decrement occurred due to habituation versus fatigue, due respectively to habituation vs. refractory effects of the neural network generating the evoked potentials, could not be completely addressed. Starting from this premise, the present study aimed to (i) develop a short-term habituation paradigm that allows the investigation of both response decrement and response recovery, (ii) to examine the degree to which these mechanisms are influenced by different stimulation intervals. To examine the applicability of the paradigms for measuring habituation of VER in neonatal and fetal magnetoencephalographic recordings we used two experimental conditions (referred to as the short and long ITI condition).
This study could provide a better understanding of early habituation and its neural correlates, and thus could contribute to an improved assessment of human neonatal and prenatal brain development.
2. Materials and methods
2.1. Participants
Twenty-six healthy pregnant women with fetuses ranging in gestational age (GA) from 30 to 38 weeks agreed to participate in the first prenatal study. Three of them were excluded because they wore metal dental retainers that interfered with the MEG signal. From the remaining 23 subjects, 19 came back for a second fetal measurement, 18 within 2 weeks and 1 approximately 5 weeks after the first recording. We thus collected 42 fetal datasets from 23 subjects. For the short ITI study, 14 healthy pregnant women with fetuses ranging in GA between 32 and 37 were recruited. Eleven came back for a second prenatal measurement within 2 weeks after the first recording. Thus, for the short ITI study, a total of 25 fetal datasets from 14 subjects were gathered.
Sixteen of the mothers from the long ITI study, and 10 from the short ITI study, returned with their babies for a neonatal investigation. The newborn recordings in both studies were carried out between 6 and 35 days after delivery. Demographic data of the mothers and relevant medical information of the newborns are summarized in Table 1. The study was approved by the local Institutional Review Board, and written informed consent was obtained from each mother or their legal representative.
Table 1.
Demographic data of the mothers, fetuses and newborns.
| Subjects | Characteristics | Long ITI condition |
Short ITI condition |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % (N) | M | SD | Range | % (N) | M | SD | Range | ||
| Fetuses | Sex | ||||||||
| Female | 60.9 (14) | 57.2 (8) | |||||||
| Male | 39.1 (9) | 42.8 (6) | |||||||
| GA at 1st visit (weeks) | 33.04 | 1.37 | 30–35 | 34.07 | 0.88 | 32–35 | |||
| GA at 2nd visit (weeks) | 35.21 | 1.36 | 32–38 | 36.20 | 0.17 | 34–37 | |||
| Newborns | Sex | ||||||||
| Female | 68.7 (11) | 50 (5) | |||||||
| Male | 31.3 (5) | 50 (5) | |||||||
| GA at Delivery (weeks) | 40.4 | 1.76 | 37–42 | 41.3 | 1.49 | 39–44 | |||
| Chron. age (days) | 17.13 | 6.12 | 6–32 | 19.60 | 9.19 | 6–35 | |||
| Birth weight | |||||||||
| Apgar scores | |||||||||
| 1 min | 7.45 | 1.88 | 2–9 | 8.44 | 0.49 | 8–9 | |||
| 5 min | 9.14 | 4.43 | 7–10 | 9.22 | 0.42 | 9–10 | |||
| Mothers | Age (years) | 27.91 | 5.51 | 19–39 | 28.27 | 5.28 | 19–37 | ||
| Education level | |||||||||
| Under HSD | 8.7 (2) | – | |||||||
| HSD | 52.2 (12) | 28.6 (4) | |||||||
| Bachelor | 34.8 (8) | 35.7 (5) | |||||||
| Master | – | 28.6 (4) | |||||||
| PhD | 4.34 (1) | 7.1 (1) | |||||||
| Working | |||||||||
| Yes | 65.2 (15) | 92.8 (13) | |||||||
| No | 34.8 (8) | 7.2 (1) | |||||||
%: percentage; N: number of subjects; M: mean; SD: standard deviation; GA: gestational age; Chron. age: chronological age; HSD: high school degree.
2.2. Stimuli
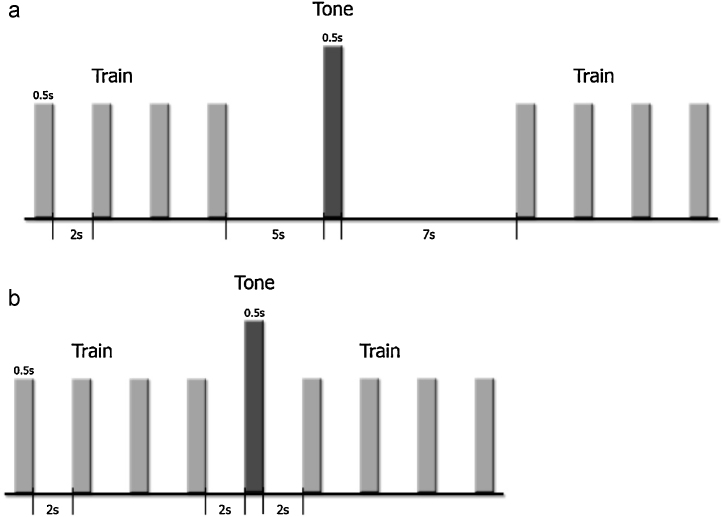
In both studies, we kept the same stimulation paradigm for the fetal and neonatal measurements. The paradigm in both studies consisted of a train of four light flashes with an ISI (offset-to-onset) of 2 s followed by a 500 Hz tone, this sequence being repeated 90 times. The tone inserted between the trains of light flashes was meant to account for response recovery. The flashes and the tones were presented each for 500 ms. The ITI differed in the two studies, as can be seen in Fig. 1.
Fig. 1.
Illustration of the stimulation paradigms: (a) long ITI and (b) short ITI. Train of four sequential light flashes, followed by a 500-Hz tone. The ISI within the train was 2 s and the ITI was 12.5 s (including the dishabituator) for the first experimental condition and 4.5 s for the second one.
In the long ITI study, the ITI was 12.5 s, the tone being presented 5 s after the onset of the 4th light flash. This design resulted in a 30-min recording with a total of 450 stimuli. In the short ITI study, the ITI was 4.5 s, the tone being presented 2 s after the 4th flash. This schedule yielded a 20-min recording with a total of 450 stimuli. Acoustic and visual stimuli were controlled by the STIMPRO software (UAMS, Little Rock, AR). The speaker delivering the sound and the light source were located outside the magnetically shielded room (Vakuumschmelze, Hanau, Germany). The sound stimulus was delivered to the inside of the shielded room through a 3.7-m length of flexible Tygon tubing that ended with an air-filled bag. For the fetal measurements the sound intensity measured at the end of the tube in the air was 110 dB. Considering an attenuation of sounds through the maternal abdomen of approximately 30 dB (Lecanuet et al., 1998), we expected an intensity of approximately 80 dB to have reached the fetuses. The light stimulus was delivered through a 3.4 m fiber-optic cable that ended with a 3 cm × 5 cm woven panel (Stocker Yale, Salem, USA) and had the same intensity for fetuses and newborns. The other end of the cable was divided into four sub-bundles each illuminated by high-power LED arrays. The light output measured at the panel was 35 mW at 630 nm, red (Wilson et al., 2009).
For the prenatal recordings, the air-filled bag and the woven panel were secured with an elastic belt and fixed over the maternal abdomen. In the postnatal setting, the acoustic and visual emitters were attached to the ceiling at a distance of approximately 75 cm from the newborn's head. The sound intensity for newborns measured at the end of the tube in the air was 80 dB.
2.3. Data acquisition
The fetal and neonatal evoked brain responses were recorded with an MEG System called SARA (SQUID Array for Reproductive Assessment, VSM MedTech, Port Coquitlam, Canada), which consists of 151 primary magnetic sensors spaced approximately 3 cm apart and covering an area of 850 cm2 (Eswaran et al., 2002a). By design, the system fits the shape of the maternal abdomen, and allows, among other things, the noninvasive and direct investigation of the functional development of the fetal brain. And by replacing the mother's seat with a cradle that enables the newborn to lie with the head touching the lower center of the sensor array, the system can be successfully used to conduct measurements on newborns. During the neonatal studies, one of the newborn's parents was seated next to the cradle and was instructed to interact with the newborn only if it became agitated. The sessions were monitored by a camera, making it possible for the investigator to record neonatal position, state, movements and parental intervention in a qualitative way. The measurements were discontinued when the newborns cried or moved extensively. In the present neonatal studies, all the babies were lying on their back, with their head slightly turned to one of the sides.
Before each prenatal measurement, an ultrasound was performed to estimate fetal weight, size, position and level of activity (Hadlock et al., 1991). After the ultrasound, the subjects were accompanied to the shielded room and seated comfortably in front of the sensor array. At this point, the fetal head position and the distance between the head and the sensors were determined using a portable ultrasound. Subsequently, the fiber-optic stimulus light panel and the air-filled bag were placed directly on the abdomen overlaying the fetal head. A marking system consisting of four coils was used to digitally record the location of the fetal head as identified by the ultrasound examination. The subjects were instructed to sit as still as possible during the recording, and to promptly inform the investigator about eventual discomforts using the speaker system located in the shielded room. At the end of the fMEG measurements, an ultrasound exam was performed to determine changes of the fetal head position and of the distance between the head and the sensors.
2.4. Data analysis
Data were recorded with a sampling rate of 312.5 Hz. After attenuating the interfering cardiac signals by orthogonal projection (Vrba et al., 2004, McCubbin et al., 2006) the datasets were split into 2 s segments (1 s prestimulus and 1 s poststimulus) corresponding to each of the five stimuli (first flash, second flash, third flash, fourth flash and the tone). To mark and exclude artefacts, automatic threshold detection was applied (MEG amplitude single channel threshold 2 pT). Averages of the segments corresponding to each of the five stimuli were separately computed. A minimum of 70 artefact-free averaged segments for the prenatal studies and 50 such segments for the neonatal studies were the criteria to consider the datasets for further analysis. For consistency, we used the abbreviations VERs (visually evoked responses) and AERs (auditory evoked responses). However; in order to differentiate between VERs within the train of four light flashes; we used ‘ERs to the 1st; 2nd 3rd or 4th flash’.
A two-step analysis was used to identify the occurrence of the evoked brain responses. The first step consisted of visual detection of the most prominent wave (peaked component) arising between 100 and 650 ms post stimulus. The peaked components were selected if the following criteria were met: (a) they were evident in channels that approximately overlapped the location of the fetal/neonatal head; (b) the amplitude present in at least one selected channel was greater than 7 fT. For the VERs, an additional criterion was applied. If the peaks on the subsequent flashes appeared more than 150 ms earlier or later than the peaked response to the first flash, these were considered different components of the evoked response and therefore disregarded. In the second step, we conducted a bootstrap test (Govindan et al., 2008). ERs were identified by visual inspection and then confirmed or rejected by bootstrap. Those that were confirmed by bootstrap test were considered detectable ERs and therefore included in the statistical analysis. The cortical responses for each of the five stimuli were determined separately and characterized by their latencies and amplitudes. To quantify the peak amplitude of each response we selected five channels with the best signal-to-noise ratio and calculated the root mean square (RMS) over them at the peak latency. Assuming that the VERs to all of the four light flashes are elicited in the same brain region, similar channels were selected for all visual stimuli. Since auditory and visual stimuli usually activate different brain regions (Kandel, 2009), we considered a waveform as an auditory cortical response even if it occurred in neighbouring channels selected for the visual responses. The peak amplitude of the AERs was quantified in the same way as those of the VERs. Based on the dipole pattern of magnetic fields, the peaks of the evoked responses measured with MEG can be either positive or negative. This polarity cannot be interpreted in terms of functionality as in EEG data.
2.5. Statistical analysis
In this section we first describe the statistical procedure that was developed in our group to assess the ERs (boot strap analysis) and then present the statistical tests used to evaluate the behaviour of the amplitude and latency of the detected ERs.
2.5.1. Statistical assessment of the ERs
To statistically assess the occurrence of the ERs we conducted a bootstrap test. Here, the null hypothesis was that the peak identified in the average of the original data was due to random chance and the alternate hypothesis was that the peak obtained was an answer to the stimuli. In order to test the null hypothesis, we generated surrogate data by shuffling 1 s blocks of data and performed event-related averaging (as done for the original data) on the block-shuffled data. Based on earlier work by Schreiber and Schmitz (2000) to reject the null hypothesis at level we synthesized N = 2K/−1 surrogates, where 2 in this equation denotes the two sided testing to include the positive and negative values of the average and K is a positive integer (Schreiber and Schmitz, 2000). In our calculations we set K = 1 and used 50 different surrogates, which together correspond to α = 0.04. If the peak in the average of the original data was greater than the Kth maximum or less than the Kth minimum of the averages of all the surrogates, then the null hypothesis could be rejected (we refer to these as detectable ERs).
2.5.2. Statistical analysis of ER amplitudes and latencies
Statistical analyses employed SAS version 9.1 (the SAS Institute, Cary, NC, USA). In each fetus or neonate, all raw amplitudes were normalized by expressing them as a percentage of the first-flash-VER amplitude, while the trend in VER amplitudes with flash number was quantified as a rank correlation. Rank correlations were summarized as medians, quartiles, and ranges, and tested for differences from zero by Wilcoxon signed-rank test. Raw and normalized data for each flash and the tone were summarized by ITI type and natal status as means, standard deviations, medians, and ranges. Data for neonates were analyzed using mixed-models repeated-measures ANOVA, in which the best-fitting covariance structures were determined by Akaike's information criterion. ANOVA post hoc analysis consisted of (a) polynomial contrasts to analyze VER trend with flash number, and (b) Dunnet's post hoc procedure to compare VER means to the AER mean. Data for fetuses were too sparse for mixed-models analysis, and were analyzed via Wilcoxon signed-rank tests instead. An α = 5% significance level was used despite the multiple comparisons in order to avoid inflating Type II error in this modestly powered observational study.
3. Results
The analyses of the amplitude and latency of the ERs are separately reported for fetuses and neonates for each of the two experimental conditions.
3.1. Long ITI condition
3.1.1. Neonates
Three out of 16 neonatal recordings were not included in the analysis because the measurements were stopped within the first 5 min of recording due to newborn crying. Nine out of 42 fetal recordings were excluded from the analysis because the number of the averaged segments after artefact detection and exclusion was lower than the predetermined acceptance limit. The reason for the low number of artefact-free segments included maternal movements and maternal and fetal heart residuals. The behavioural states of all 13 neonates included in the analysis were determined to be quiet or active sleep. Eleven newborns showed detectable VERs to, at least, the first flash.
3.1.1.1. Amplitude analysis
The RMS values of the amplitudes of the ERs, and their correlation coefficients with the flash number for the neonates are shown in Table 2.
Table 2.
Amplitudes and latencies of neonatal ERs for the long ITI condition. Neonatal age (“Age”; in days), number of triggers (“Tri”) recorded, response amplitudes (“Ampl”; RMS values in fT) with each numbered flash, Spearman's rank correlations (“Rank Corr”) of amplitude with flash number, and latencies (“Lat”; in ms) with each numbered flash.
| Subject ID | Age | Tri | Ampl 1st | Ampl 2nd | Ampl 3rd | Ampl 4th | Ampl Tone | Rank Corr | Lat 1st | Lat 2nd | Lat 3rd | Lat 4th | Lat Tone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S 07 | 14 | 51 | 37.86 | n.s.* | 23.31 | n.s.* | 46.40 | – | 0.253 | – | 0.381 | – | 0.432 |
| S 08 | 21 | 72 | 29.48 | n.s.* | n.s.* | 28.86 | 48.39 | – | 0.320 | – | – | 0.464 | 0.294 |
| S 10 | 19 | 72 | 35.74 | 32.96 | 33.92 | 29.20 | 27.54 | −.80 | 0.314 | 0.314 | 0.317 | 0.317 | 0.442 |
| S 11 | 23 | 55 | 36.92 | 39.77 | 42.47 | 39.57 | 47.74 | .40 | 0.314 | 0.314 | 0.314 | 0.314 | 0.160 |
| S 12 | 6 | 82 | n.s.* | 38.22 | n.s.* | 31.31 | 19.30 | – | – | 0.448 | – | 0.438 | 0.438 |
| S 13 | 12 | 89 | 21.30 | 12.31 | 19.85 | 18.11 | 35.41 | −1.0 | 0.304 | 0.544 | 0.410 | 0.429 | 0.253 |
| S 14 | 19 | 88 | n.s.* | n.s.* | 15.24 | 8.66 | 15.32 | – | – | – | 0.371 | 0.240 | 0.131 |
| S 15 | 20 | 84 | 33.89 | 36.85 | 34.15 | 14.16 | 51.80 | −.40 | 0.294 | 0.253 | 0.275 | 0.275 | 0.144 |
| S 18 | 12 | 74 | 73.35 | 74.82 | 41.66 | 28.28 | 56.53 | −.80 | 0.275 | 0.406 | 0.278 | 0.288 | 0.144 |
| S 19 | 32 | 87 | 35.37 | 21.59 | 20.10 | 26.58 | 49.93 | −.40 | 0.115 | 0.115 | 0.115 | 0.115 | 0.115 |
| S 21 | 18 | 84 | 21.09 | n.s.* | 23.09 | 27.98 | 33.97 | 1.0 | 0.426 | – | 0.282 | 0.339 | 0.349 |
| S 28 | 25 | 89 | 20.38 | 16.48 | 13.58 | 16.30 | 35.63 | −.40 | 0.246 | 0.314 | 0.365 | 0.240 | 0.282 |
| S 30 | 13 | 56 | 41.95 | 56.46 | 47.16 | 23.48 | 49.64 | −.80 | 0.192 | 0.192 | 0.202 | 0.192 | 0.112 |
Visually selected responses that were over the threshold detection but proved non-significant after the bootstrap test.
The rank correlations between the VER amplitude and the flash number had a median value of −0.4, which was not significantly different from zero (signed-rank P = 0.23). The interquartile range was −0.8 to +0.4, and the full range was −1.0 to +1.0.
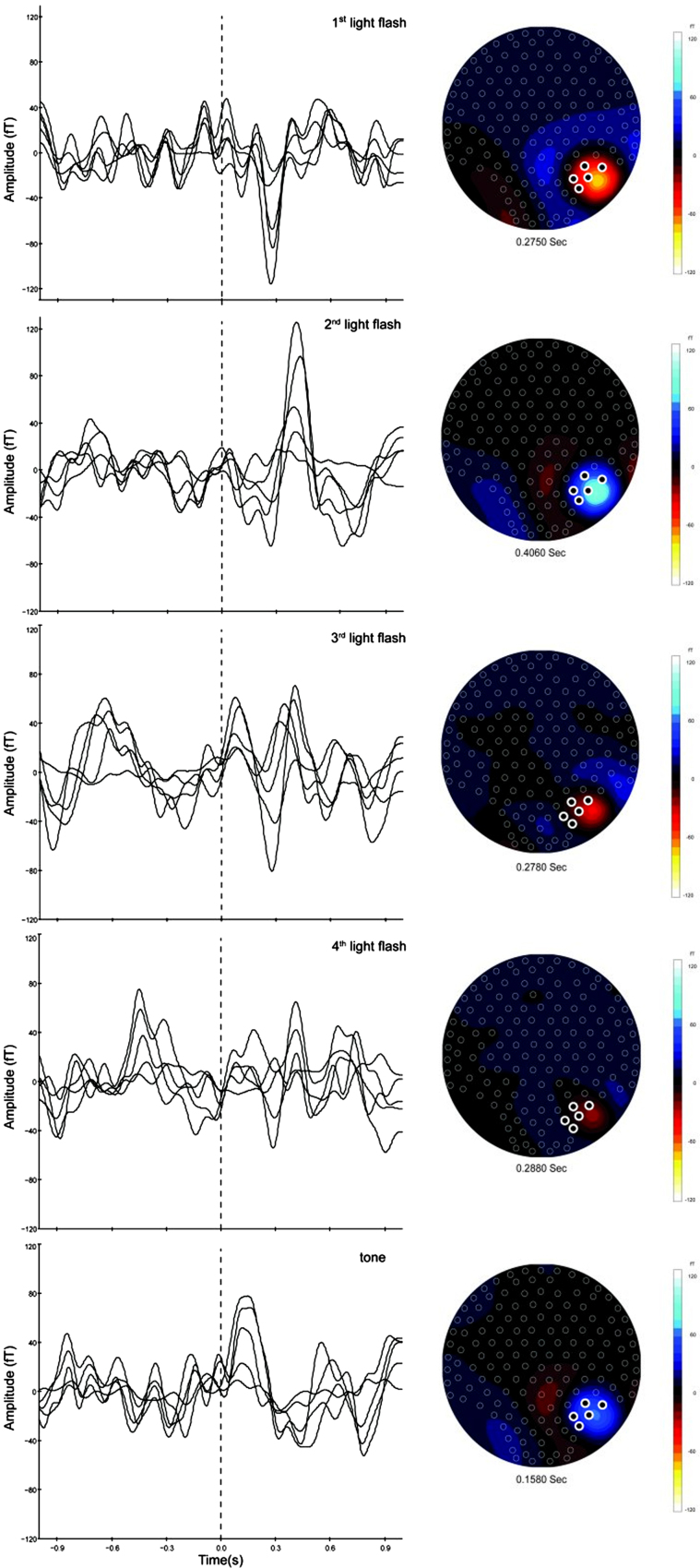
Amplitude variations were investigated both before and after normalizing to the amplitude of the first flash. Fig. 2 illustrates the averaged ERs (74 trials) corresponding to the five stimuli on a 12-day-old newborn. In the left panel, an overlay of the selected channels over the head is shown. The corresponding isofield maps of the magnetic fields and the location of the selected channels are depicted in the right panel of the figure.
Fig. 2.
Left panel: averaged evoked responses (74 triggers) to each of the four visual stimuli and to the tone of a 12-day-old newborn. An overlay of the selected channels is illustrated. Right panel: isofield maps of the magnetic fields corresponding to the evoked responses. Selected channels are highlighted.
A mixed-models repeated-measures ANOVA was used to analyze the differences between the raw amplitudes of the neonatal VERs. A homogeneous 1st-order autoregressive structure provided the best fit to the within-train covariances, and yielded 13.2 fT for the common SD and 63% for the correlation between adjacent stimuli. The fitted model yielded means (95% confidence intervals (CIs)) in fT of 34.85 (26.99–42.71) for the first flash, 35.75 (27.33–44.17) for the second flash, 29.08 (21.37–36.80) for the third flash and 24.40 (16.88–31.93) for the fourth one. The downward trend in raw amplitudes with flash number was tested by polynomial contrast analysis. This showed that the trend was statistically significant for a linear component (F1,38 = 5.72, P = 0.02) and not significant for deviation from linearity (F2,38 = 0.81, P = 0.45). The AER raw amplitudes had a mean (95% CI) in fT of 39.82 (32.42–47.21). This mean was compared to the above VER means by Dunnett's post hoc procedure, and found to be significantly higher than that from the fourth flash (P < 0.001) and third flash (P = 0.04), but not the second flash (P = 0.80) or first flash (P = 0.68).
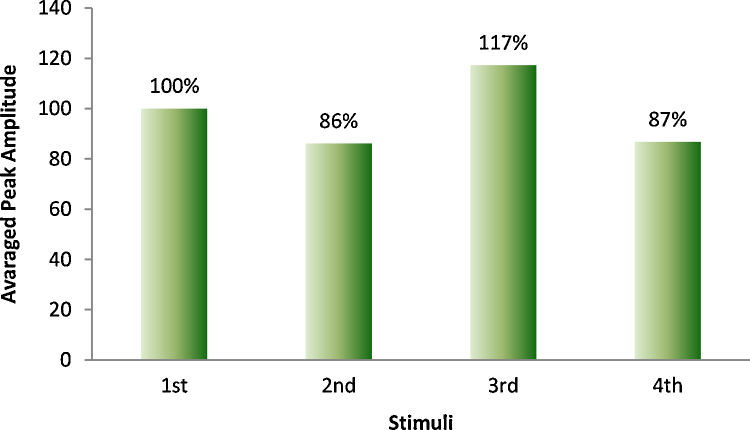
Normalized amplitudes were analyzed via repeated-measures ANOVA. The intra-subject covariance structure was fit best using homogeneous compound symmetry, which yielded 28.6% for the common SD and 25% for the correlation among all stimuli. Fig. 3 illustrates the normalized amplitudes of the four consecutive flashes with respect to the first flash. In neonates, and relative to that from the first flash (defined as 100%), the normalized VER amplitude was virtually unchanged on the second flash (mean = 102%, 95% CI: 0–124%; P = 0.86), mildly decreased on the third flash (mean = 88%, 95% CI: 69–106%; P = 0.19), and significantly decreased on the fourth flash (mean = 79%, 95% CI: 60–97%; P = 0.03). Polynomial contrast analysis of the downward trend among normalized, relative amplitude through all the four flashes indicated the presence of a linear component (F1,24 = 6.87, P = 0.015) with no significant evidence for deviation from linearity (F2,24 = 0.37, P = 0.70). The normalized amplitudes of the AERs had a mean (95% CI) of 135% (117–153%), which was significantly elevated (P = 0.0005) over the first-flash normalized VER amplitude.
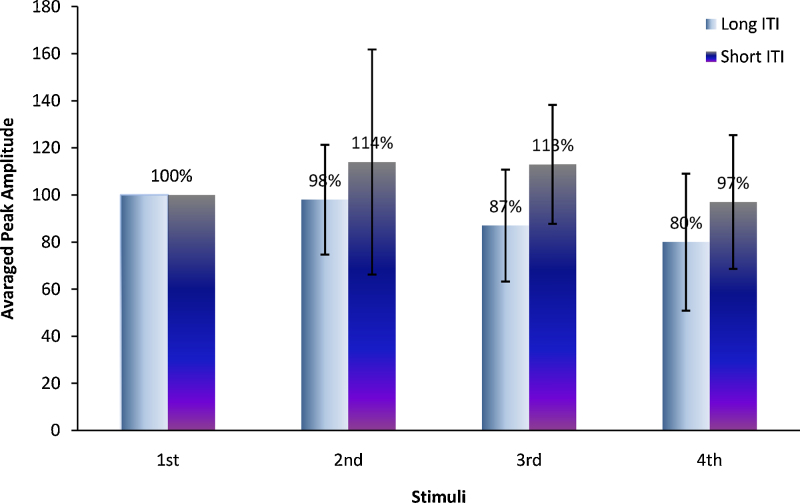
Fig. 3.
Normalized neonatal response amplitudes of the four consecutive flashes with respect to the first flash for the long and short ITI experimental condition.
3.1.1.2. Latency analysis
The latencies of neonatal ER are listed in Table 2. To analyse latencies for neonates, we used a repeated-measures ANOVA with autoregressive covariance structure. The fitted model rendered latency means (95% CIs) were 289 ms (range: 229–348 ms) for the first flash, 321 ms (range: 259–382 ms) for the second flash, 321 ms (range: 263–379 ms) for the third flash and 314 ms (range: 257–372 ms) for the fourth one. Although averaged latencies for the subsequent flashes were longer than the latency for the first flash, the polynomial-contrast analysis detected no significant trend. The neonate AERs had a mean latency (95% CI) of 241 ms (range: 184–297 ms). This was compared to the above mean latencies for each flash via Dunnett's post hoc procedure and found to be marginally or significantly shorter compared to three of them (P values of 0.38, 0.051, 0.013, and 0.002 for 1st through 4th flashes, respectively).
3.1.2. Fetuses
Nine out of 42 fetal recordings were excluded from the analysis because the number of the averaged segments after artefact detection and exclusion was lower than the predetermined acceptance limit. The reason for the low number of artefact-free segments included maternal movements and maternal and fetal heart residuals. A total of 33 fetal recordings from 23 subjects were included in the analysis.
3.1.2.1. Amplitude analysis
Detectable VERs to the first flash were found in 15 out of 33 (45.4%) artefact-free fetal recordings. Evident VERs on one or two of the subsequent light flashes were identified in 11 cases. In 6 (40%) of the datasets containing VERs to the first flash, AERs have been also determined. Table 3 presents the RMS values of the peak amplitudes of the responses. The cases in which no detectable ERs could be identified were denoted either by “<7” indicating that the amplitudes were below the detection threshold or by “n.s.” meaning that the responses have been visually selected and therefore were over the detection threshold but proved non-significant after the bootstrap test.
Table 3.
Amplitudes and latencies of fetal ERs for the long ITI condition. Fetal age (“Age”; in weeks of gestation), number of triggers (“Tri”) recorded, response amplitudes (“Ampl”; RMS values in fT) with each numbered flash, and latencies (“Lat”; in ms) with each numbered flash.
| Subject ID | Age | Tri | Ampl 1st | Ampl 2nd | Ampl 3rd | Ampl 4th | Ampl Tone | Lat 1st | Lat 2nd | Lat 3rd | Lat 4th | Lat Tone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S 07 | 33 | 80 | 6.87 | <7** | n.s.* | n.s.* | 8.78 | 0.547 | – | – | – | 0.221 |
| S 08 | 30 | 85 | 11.10 | 11.27 | <7** | 11.46 | n.s.* | 0.355 | 0.396 | – | 0.211 | – |
| S 11 | 34 | 71 | 8.66 | <7** | 11.39 | 7.51 | 7.52 | 0.582 | – | 0.211 | 0.227 | 0.307 |
| S 13 | 33 | 84 | 9.10 | n.s.* | 10.67 | <7** | n.s.* | 0.118 | – | 0.144 | – | – |
| S 13 | 35 | 80 | 9.89 | <7** | <7** | <7** | <7** | 0.218 | – | – | – | – |
| S 15 | 33 | 76 | 11.30 | 8.03 | n.s.** | 9.46 | 9.72 | 0.493 | 0.525 | – | 0.550 | 0.227 |
| S 17 | 35 | 89 | 7.47 | <7** | <7** | <7** | 6.50 | 0.550 | – | – | – | 0.512 |
| S 17 | 37 | 84 | 8.63 | 5.38 | 5.41 | <7** | <7** | 0.330 | 0.6 | 0.294 | – | – |
| S 19 | 35 | 87 | 5.58 | 5.52 | <7** | <7** | <7** | 0.496 | 0.486 | – | – | – |
| S 21 | 33 | 77 | 5.98 | n.s.* | <7** | 9.59 | 6.32 | 0.531 | – | – | 0.320 | 0.450 |
| S 22 | 33 | 88 | 8.14 | <7** | <7** | 6.39 | <7** | 0.501 | – | – | 0.412 | – |
| S 23 | 34 | 68 | 6.84 | <7** | <7** | <7** | <7** | 0.232 | – | – | – | – |
| S 27 | 35 | 74 | 9.23 | 7.92 | <7** | <7** | n.s.* | 0.454 | 0.429 | – | – | – |
| S 29 | 33 | 77 | 6.40 | n.s.* | 7.52 | n.s. | 15.56 | 0.435 | – | 0.365 | – | 0.189 |
| S 30 | 35 | 86 | 8.99 | 7.76 | 8.65 | <7** | n.s.* | 0.429 | 0.454 | 0.454 | – | – |
Visually selected responses that were over the threshold detection but proved non-significant after the bootstrap test.
Amplitudes below the detection threshold of 7 fT.
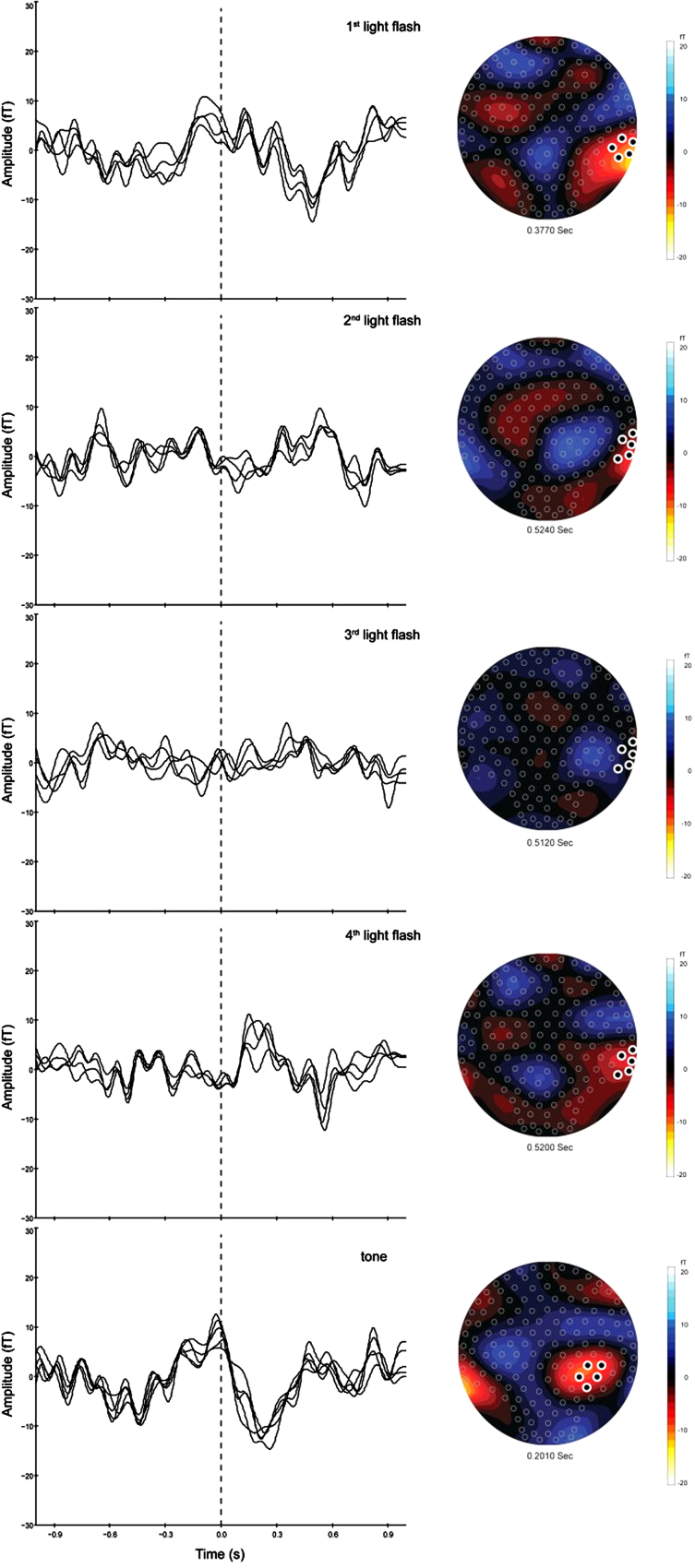
A typical response from a fetus at 33 weeks GA is shown in Fig. 4 illustrating averaged responses of 76 trials for each of the visual and auditory stimuli. The left panel shows an overlay of selected channels covering the fetal head. The corresponding isofield maps and the location of the selected channels are shown in the right panel.
Fig. 4.
Left panel: averaged evoked responses (76 triggers) to each of the four visual stimuli and to the tone of a fetus (33 weeks GA). Right panel: isofield maps of the magnetic fields corresponding to the evoked responses. Selected channels are highlighted.
The distance between the fetal head and the sensor array was determined by ultrasound measurement at the beginning and end of each fMEG recording. We were therefore able to investigate the correlation between sensor distance and fetal response rates. We conducted a group comparison in relation to the sensor distance between fetuses showing detectable ERs to the first light and fetuses showing no detectable responses. The results showed no significant differences between the groups (t29 = 0.72, p = 0.4 for pre measurement, and t29 = 1.08, p = 0.2 for post measurement).
The peak amplitudes of the fetal VERs to the first flash had an average of 8.27 fT (5.57–11.29; SD 1.75), and were smaller than the neonatal cortical responses. In those cases where we detected fetal VERs on the first and second flash as well (N = 6) we conducted Wilcoxon signed-rank tests to compare both raw and normalized amplitudes. The differences between amplitudes of first and second flashes had values of 1.36 in raw fT and 13.95% in normalized units, both of which nearly reached significance (P values of 0.07 and 0.06, respectively). Fig. 5 illustrates the normalized amplitudes of the four consecutive flashes in respect to the first flash. However, foetuses responding to the first flash did not necessarily respond to all consecutive flashes. Therefore the rate of detected cortical response for the 2nd, 3rd and 4th flash was (i) lower and (ii) not equally distributed between the flashes; that is, a fetus might or might not respond to any of the 2nd, 3rd or 4th flashes.
Fig. 5.
Normalized fetal response amplitudes of the four consecutive flashes with respect to the first flash for the long ITI experimental condition.
We applied the same statistical test to compare VERs (first flash) amplitudes to those of the AERs (N = 6). Some of these subjects were different from those included in the previous comparison of VERs (first and second flash). Results for both raw and normalized amplitudes proved non-significant, yielding a median difference of 0.98 fT for raw amplitudes (P = 0.9) and 3.66% for normalized amplitudes (P = 0.7).
In 41% (in 8 out of 18 datasets) of the fetal recordings showing no evident response to the first flash, ERs were determined to the 2nd and/or 3rd flash.
Additionally, we categorized fetuses depending on their responses to one or both modalities. Fetuses showing detectable VERs to the first flash but no detectable AERs to the tone were considered unimodal; whereas those showing detectable responses to both types of stimuli were considered bimodal. There was a significant correlation between these categories and the GA (p < 0.05; Spearman Rank Correlation), meaning that older fetuses showed more bimodal responses than younger ones.
3.1.2.2. Latency analysis
Fetal VERs to the first flash occurred at an average latency of 414 ms (118–582, SD 138) after the stimulus onset. Comparison of the latencies of the first two flashes (6 datasets) yielded a median difference of 0.02 ms that was not statistically significant (P = 0.1). However, latencies of the tone were significantly shorter than the latencies of the first flash (median difference = 0.27 ms; P = 0.02).
3.2. Short ITI condition
3.2.1. Neonates
A total of 10 neonatal measurements were included in the analysis. VERs to the first light flash could be found in seven newborns. The latencies, the RMS values of the amplitudes of the neonatal ERs and their correlation coefficients with the flash number are shown in Table 4.
Table 4.
Amplitudes and latencies of neonatal ERs for the short ITI condition. Neonatal age (“Age”; in days), number of triggers (“Tri”) recorded, response amplitudes (“Ampl”; RMS values in fT) with each numbered flash, and latencies (“Lat”; in ms) with each numbered flash.
| Subject ID | Age | Tri | Ampl 1st | Ampl 2nd | Ampl 3rd | Ampl 4th | Ampl Tone | Lat 1st | Lat 2nd | Lat 3rd | Lat 4th | Lat Tone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S 07 | 21 | 50 | 39.08 | 65.68 | 25.55 | 54.93 | 46.93 | 0.256 | 0.256 | 0.198 | 0.374 | 0.221 |
| S 09 | 22 | 90 | 24.33 | 13.19 | 36.32 | 15.66 | 34.47 | 0.272 | 0.435 | 0.138 | 0.333 | 0.390 |
| S 11 | 27 | 88 | 38.85 | 46.16 | 44.21 | 39.09 | 32.50 | 0.278 | 0.264 | 0.243 | 0.147 | 0.275 |
| S 12 | 26 | 50 | 40.80 | 70.07 | 48.72 | 35.52 | 83.64 | 0.285 | 0.448 | 0.224 | 0.256 | 0.208 |
| S 13 | 26 | 89 | 18.60 | n.s.* | 21.68 | n.s.* | 52.74 | 0.333 | – | 0.317 | – | 0.307 |
| S 14 | 6 | 84 | 22.69 | 19.07 | 28.19 | 16.53 | 28.93 | 0.272 | 0.307 | 0.282 | 0.301 | 0.317 |
| S 15 | 13 | 90 | 17.87 | 15.67 | 18.47 | 20.83 | 24.81 | 0.365 | 0.365 | 0.388 | 0.227 | 0.323 |
Visually selected responses that were over the threshold detection but proved non-significant after the bootstrap test.
3.2.1.1. Amplitude analysis
To investigate the amplitude variations repeated measures analysis were carried out both before and after normalizing to the amplitude of the first flash.
A mixed-models repeated-measures ANOVA with heterogeneous compound-symmetry covariance structure was used to analyse the differences between the raw amplitudes of the neonatal VERs. A heterogeneous compound-symmetry structure gave the best fit to the intra-train covariances, and yielded a common correlation parameter of 63% along with respective SDs of 9.5 fT, 21.8 fT, 12.1 fT, and 14.9 fT for the 1st through 4th flashes plus a SD of 19.5 fT for the tone. The fitted model gave raw VER-amplitude means (95% CIs) in fT of 27.05 (20.24–33.86) for the first flash, 31.76 (16.64–46.87) for the second flash, 28.89 (20.48–37.30) for the third flash and 25.51 (15.18–35.84) for the fourth flash. Polynomial contrasts analysis yielded no evidence for either linear trend (F1,26 = 0.33, P = 0.57) or other pattern of differences among flashes (F2,26 = 0.66, P = 0.52). AER raw amplitudes were generally higher, with a mean (95% CI) of 40.13 (27.10–53.15) fT. Assessment of the amplitude differences for AER versus each VER via Dunnett's post hoc procedure yielded statistical significance on the fourth flash (P = 0.037), suggesting response recovery to the dishabituator. Raw-amplitude differences between the tone and the flashes were marginally significant on the first flash (P = 0.057), but not significant on the second or third (P = 0.47 and 0.11, respectively).
In repeated-measures ANOVA, the within-train covariances of the normalized amplitudes showed best fit to a homogeneous 1st-order autoregressive structure with common SD of 50% and negatively valued correlation parameter of–67%. Fig. 3 illustrates the four consecutive flashes after normalization to the first. Neonatal cortical responses to the 2nd, 3rd and 4th flash were not significantly different than those to the first one (P = 0.50, 0.50, and 0.51, respectively). The means (95% CIs) relative to the first flash were: 114% (72–156%) for the second flash, 113% (73–153%) for the third, 87% (46–128%) for the fourth flash. However, the difference between tone and first flash was significant, with a mean (95% CI) of 157% (117–197%; P = 0.008). Polynomial contrast analysis of the downward trend among normalized amplitudes through all four flashes showed no evidence for linear trend (F1,26 = 0.70, P = 0.42) or higher-order difference (F2,26 = 1.19, P = 0.33).
3.2.1.2. Latency analysis
To investigate the behaviour of the latencies, repeated-measures ANOVA was conducted. A homogeneous compound-symmetry structure, with common SD of 82 ms and correlation parameter of −0.15%, was found to give best fit to the within-train covariances. The fitted model rendered means (95% confidence limit) of 294 ms (range: 231–358 ms) for the first flash, 324 ms (range: 265–384 ms) for the second flash, 244 ms (range: 185–304 ms) for the third flash and 284 ms (range: 224–344 ms) for the fourth one. There was no evidence for a downward trend of the latencies with later flashes. The averaged latency of neonatal AERs was 276 ms (range: 220–333 ms). There was no evidence for a downward trend of the latencies with later flashes.
3.2.2. Fetuses
A total of 23 fetal datasets from 14 subjects were included in the analysis. Two of the fetal datasets were excluded from the analysis because the number of the averaged segments after artefact detection was lower than the acceptance criteria. Detectable VERs to the first flash were found in five out of 21 (23.8%) artefact-free fetal recordings. In two out of these five cases evident VERs on one or two of the subsequent light flashes were identified. In none of the five datasets containing VERs to the first flash could AERs be determined. Table 5 presents the RMS values of the peak amplitudes and latencies of the fetal responses. Similarly to the long ITI study, the cases in which no detectable ERs could be identified were denoted either by “<7” indicating that the amplitudes were below the detection threshold or by “n.s.” meaning that the responses have been visually selected and therefore were over the detection threshold but proved non-significant after the bootstrap test.
Table 5.
Amplitudes and latencies of fetal ERs for the short ITI condition. Fetal age (“Age”; in weeks of gestation), number of triggers (“Tri”) recorded, response amplitudes (“Ampl”; RMS values in fT) with each numbered flash, and latencies (“Lat”; in ms) with each numbered flash.
| Subject ID | Age | Tri | Ampl 1st | Ampl 2nd | Ampl 3rd | Ampl 4th | Ampl Tone | Lat 1st | Lat 2nd | Lat 3rd | Lat 4th | Lat Tone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S 03 | 35 | 88 | 7.86 | n.s.* | <7** | <7** | n.s.* | 0.192 | – | – | – | – |
| S 04 | 35 | 89 | 5.96 | 10.32 | <7** | <7** | <7** | 0.520 | 0.620 | – | – | – |
| S 06 | 37 | 90 | 9.17 | <7** | 7.85 | 6.39 | <7** | 0.410 | – | 0.378 | 0.403 | – |
| S 14 | 35 | 88 | 5.96 | n.s.* | <7** | <7 | <7** | 0.362 | – | – | – | – |
| S 15 | 35 | 76 | 5.25 | <7** | <7** | <7 | n.s.* | 0.224 | – | – | – | – |
Visually selected responses that were over the threshold detection but proved non-significant after the bootstrap test.
Amplitudes below the detection threshold of 7 fT.
The peak amplitudes of the fetal VERs to the first flash with an average of 6.82 fT (range) were smaller than the neonatal cortical responses. Fetal VERs to the first flash occurred at an average latency of 330 ms (95% CI: −432 ms to +1093 ms) after the stimulus onset. Due to the low response rate and the high number of missing values, no further statistical analysis was carried out on fetal data of the short ITI study.
4. Discussion
In the present study, we investigated whether neonates and fetuses show cortical response decrement upon stimulus repetition, and whether this pattern can be attributed to habituation.
Results indicate that amplitude decrease of neonatal VERs following stimulus repetition can be interpreted as habituation, since the insertion of a novel stimulus between the trains of repeated stimuli produced response recovery and the effects of refractoriness could be partially ruled out. The amplitudes of the neonatal VERs in the long ITI condition showed within-train response decrement confirming results from Sheridan et al. (2008). In addition, an increased response to the auditory dishabituator was found, confirming the cross-modal response recovery. For neonates, the short ITI condition revealed mostly different results. While response recovery to the dishabituator was present, amplitude decrement with repetition of visual stimuli could not be demonstrated. A significant decrease in amplitudes from the AERs to the VERs elicited by the first light flash was found with no further amplitude decrement with consecutive flashes. The primary aim of shortening the ITI in the second experimental condition was to reduce recording time and thus minimize the risk of maternal movement due to eventual discomfort, while at the same time keeping the number of trials high. Simultaneously, we had the possibility to investigate the effects of ITI manipulation on habituation responses. In the longer ITI condition, dishabituation was more enforced compared to the shorter ITI condition due to the presence of both a dishabituator and longer break after the train of stimuli. This could explain the discrepancies between the relatively different amplitude patterns of the VERs found in the two conditions. In the short ITI condition, the neonates did not show response decrement from the first flash onward as did neonates in the long ITI condition. The stronger dishabituation effects during the interval between the sequences of flashes present in the longer ITI condition could have allowed a steeper response decrement from the first flash onward.
Moreover, earlier studies showed that by using paradigms in which a stimulus is presented repeatedly at different ISIs, relative refractory periods or recovery cycles of visual or auditory processing networks generating ERs can be assessed (Coch et al., 2005). It is generally argued that, similarly to single nerve cells, neural networks generating ERs exhibit a recovery period immediately after their excitatory period. For the visual system, studies using different ISIs reported recovery-cycle periods for adults ranging between 200 ms (Skrandies and Raile, 1989) and 6 s (Lehtonen, 1972, Uusitalo et al., 1996). Several studies suggested that the recovery of relative amplitudes in the mature auditory modality may not be achieved with ISIs shorter than 3 s and that substantial refractory period may extend to more than 10 s (Sams et al., 1993, Loveless et al., 1996, Budd et al., 1998). Little is known about the developmental course of the refractory effects. The very few reports on children showed, however, that the recovery periods of infantile neuronal networks are longer than those of the adult ones, and that only starting with school age do the refractory properties of auditory and visual cortical networks begin to look similar (Coch et al., 2005).
During these recovery periods, further stimulation will either elicit a weaker response or no response at all (Wastell and Kleinman, 1980). Consequently, an ISI manipulation had an evident effect upon amplitudes of the ER components, with longer ISI being associated with larger components. Therefore, a possible explanation for the lack of evidence for neonatal response decrement of VERs in the short ITI condition could be that, in this paradigm, the effect of response decrement was less pronounced and less detectable compared to response recovery due to presumable noise interference. Moreover, previous studies suggested that amplitude decrements resulting from habituation may be superimposed by or interactive with those associated with neural refractoriness. Thus, a clear distinction between amplitude decrement due to habituation vs. refractoriness effects is difficult and, in our case, limited by small sample size. Future studies could exploit the effects of longer or shorter ISI on the amplitude decrement of the ER elicited by repeated stimulation proving an opportunity to better understand and distinguish between the two phenomena (habituation vs. refractoriness).
The fetal visual response rates to the first light flash were different between the two experimental conditions. In the long ISI study, the visual fetal response rate was 39%, compared to only 23% in the short ISI study. Both response rates are comparable and a little higher than the respective results reported by Sheridan et al. (2008). Then again, compared to earlier findings of previous fMEG studies on VERs (Eswaran et al., 2002b), the current response rates are lower. One explanation for this could be that, in the current study, we did not control for the eye position of the fetus. In their work, Eswaran and colleagues showed that, in all of the recordings containing detectable VERs, the fetal eyes were visible (as shown by ultra-sound measurement) and were in a position that allowed direct reception of the light stimuli (Eswaran et al., 2005). Another explanation for the lower response rates in our study could be the low number of averaged trials (70 vs. 180), which decreased the signal-to-noise ratio and made the detection of fetal responses considerably more difficult. However, fetal visual responses, when detected, indicate amplitude decline from the first to the second flash with no further decrease among consecutive flashes. The fetal data in the long ISI condition revealed thus a response decrement from the first to the second flash followed by response cessation rather than a successive response decrement. One reason for the low detectable response rates for the rest of the flashes in the train could be the relatively low peak amplitudes detected for the first two stimuli, which makes any further amplitude decrease undetectable, being very likely below the noise level of the device. The same rationale can be invoked for the low response rates found in the short ITI condition. Not least, we can invoke further reasons for the considerably lower fetal response rates compared to those of the neonates. For example, (a) the increased distance between the fetal head and the sensors can correlate with attenuation of the responding brain signal, and (b) the maternal tissues lying between the fetus and sensors can cause attenuation of the light flashes reaching the fetus. With respect to (a), our results showed that distance between the fetal head and sensors at the beginning and end of the measurement did not influence the detection rate of fetal ERs. However, the visual signals transmitted to the fetus are attenuated in a complex manner, mainly due to interfering biological tissues, being many orders of magnitude weaker than the visual signals reaching the neonate. The different light intensities transmitted to the fetuses and neonates could account for the lower fetal response rate found in our study. Future studies should overcome this limitation by delivering an optimized light flash (within the maximum permissible exposure) during fetal recordings.
The paradigms used in our study are passive and were developed such that changes in the level of arousal should not influence the habituation rates. However, we cannot exclude that both neonatal and fetal ERs changed during the habituation procedure and that this changes may be due to the different arousal states of the subjects. According to the literature there are no clear evidences about the influence of the sleep–wakefulness cycle on the amplitude and latency of evoked potentials in newborns and infants. While several studies reported no differences between ERs recorded in infant during sleep and awake time, for both auditory and visual modalities (Barnet, 1966, Baitch and Levi, 1988, van Sweden et al., 1994) more recent work showed lower amplitudes and longer latencies for P2-N3 in sleep compared to the awake condition (Apkarian et al., 1991, Mercuri et al., 1995, Shepherd et al., 1999, Benavente et al., 2005). This issue should be addressed in future fMEG studies by identifying sleep/awake states of fetuses and neonates and by investigating their influence on habituation of cortical responses.
With our current work we extended previous research, by attempting to dissociate the differences between response decrement as an indicator of habituation and sensory fatigue in the fetuses and neonates. For this, we examined the response recovery to an auditory stimulus inserted between the trains of repeated light flashes. Although we did find evidence for response recovery in neonates, this was not the case in fetuses. The failure to find response recovery to AERs in fetuses has to be interpreted with caution since the number of subjects included in this analysis was very low (N = 6). Nevertheless, we could speculate that a decrement in fetal response to repeated visual stimulation might not necessarily reflect true habituation effects but rather be a consequence of sensory fatigue.
To completely rule out sensory fatigue, a more complex paradigm which also allows the assessment of dishabituation (beside response decrement and response recovery to a dishabituator) needs to be developed fulfilling all the habituation criteria proposed by Thompson and Spencer (1966). Consequently, the paradigm should consist of a sequence of repeated stimuli wherein a dishabituator is placed on the second to last position. In such a short term auditory habituation paradigm our group indeed found evidences for both dishabituation and habituation of fetal AERs (Muenssinger et al., under review). These findings together with the present results corroborate the fact that the development and maturation of human visual and auditory systems show different sequential order, with the visual system reaching functionality later.
Interestingly, when categorizing fetuses depending on their responses to one or both modalities we found a significant association with their gestational age. The results suggested that older fetuses show more detectable bimodal responses (AERs and VERs). This finding is in line with Bahrick and Lickliter's (2000) intersensory redundancy hypothesis, which states that information presented redundantly and in temporal synchrony across two sense modalities selectively recruits attention and facilitates perceptual differentiation more effectively than does the same information presented unimodally. In a study it was shown that 5-month-old infants could discriminate between five element rhythms when the rhythm information was presented bimodally, but not when it was presented unimodally, either visually or acoustically (Bahrick and Lickliter, 2000).
The ability to habituate to repeated stimulation is one of the basic conditions for the brain to handle the overwhelming complexity of the sensory array. Previous studies provided evidence for a clinical relevance of the assessment of habituation effects in fetuses and newborns. Lack of fetal habituation has been found in mothers with different risk conditions, such as diabetes (Doherty and Hepper, 2000), depression (Allister et al., 2001) or stress (Sandman et al., 2003), the underlying neural mechanisms being not yet completely understood. Future studies should therefore focus on the application of the proposed habituation paradigm on high-risk populations, in which fetuses are exposed to increased risk of neurological damage. Early and noninvasive detection of eventual detours from the typical developmental trajectory would benefit prenatal interventions.
5. Conclusions
Overall, our findings indicate that neural mechanisms of visual habituation are already present in the first month of prenatal life and suggest that prenatal cortical response decrement upon stimulus repetition can be also detected. Thus, the proposed paradigm (long ITI) might be useful to investigate habituation of the VERs in both neonates and fetuses, the later requiring more precaution.
Conflict of interest statement
The authors declare no competing interests.
Acknowledgements
The present work was supported by Grants: R01-NS36277-10, NINDS, National Institutes of Health, USA and DFG BI 195-50, Deutsche Forschungsgemeinschaft. We would like to thank Jessica Temple for collecting part of the data, Dr. Carolin Sheridan for her advice related to the study design and Adrian Furdea for his useful comments and his help in preparing the figures.
References
- Allister L., Lester B.M., Carr S., Liu J. The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Dev. Neuropsychol. 2001;20:639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- Apkarian P., Mirmiran M., Tijssen R. Effects of behavioural state on visual processing in neonates. Neuropediatrics. 1991;22:85–91. doi: 10.1055/s-2008-1071422. [DOI] [PubMed] [Google Scholar]
- Atkinson J., Hood B., Wattam-Bell J., Anker S., Tricklebank J. Development of orientation discrimination in infancy. Perception. 1988;17:587–595. doi: 10.1068/p170587. [DOI] [PubMed] [Google Scholar]
- Bahrick L.E., Lickliter R. Intersensory redundancy guides attentional selectivity and perceptual learning in infancy. Dev. Psychol. 2000;36:190–201. doi: 10.1037//0012-1649.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitch L.W., Levi D.M. Evidence for nonlinear binocular interactions in human visual cortex. Vision Res. 1988;28:1139–1143. doi: 10.1016/0042-6989(88)90140-x. [DOI] [PubMed] [Google Scholar]
- Barnet A.B. Visual responses in infancy and their relation to early visual experience. Clin. Proc. Child. Hosp. Dist. Columbia. 1966;22:273–278. [PubMed] [Google Scholar]
- Bellieni C.V., Severi F., Bocchi C., Caparelli N., Bagnoli F., Buonocore G., Petraglia F. Blink–startle reflex habituation in 30–34-week low-risk fetuses. J. Perinat. Med. 2005;33:33–37. doi: 10.1515/JPM.2005.005. [DOI] [PubMed] [Google Scholar]
- Benavente I., Tamargo P., Tajada N., Yuste V., Olivan M.J. Flash visually evoked potentials in the newborn and their maturation during the first six months of life. Doc. Ophthalmol. 2005;110:255–263. doi: 10.1007/s10633-005-0818-0. [DOI] [PubMed] [Google Scholar]
- Bourbon W.T., Will K.W., Gary H.E., Jr., Papanicolaou A.C. Habituation of auditory event-related potentials: a comparison of self-initiated and automated stimulus trains. Electroencephalogr. Clin. Neurophysiol. 1987;66:160–166. doi: 10.1016/0013-4694(87)90185-4. [DOI] [PubMed] [Google Scholar]
- Budd T.W., Barry R.J., Gordon E., Rennie C., Michie P.T. Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int. J. Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Coch D., Skendzel W., Neville H.J. Auditory and visual refractory period effects in children and adults: an ERP study. Clin. Neurophysiol. 2005;116:2184–2203. doi: 10.1016/j.clinph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Doherty N.N., Hepper P.G. Habituation in fetuses of diabetic mothers. Early Hum. Dev. 2000;59:85–93. doi: 10.1016/s0378-3782(00)00089-x. [DOI] [PubMed] [Google Scholar]
- Draganova R., Eswaran H., Murphy P., Huotilainen M., Lowery C., Preissl H. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28:354–361. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Draganova R., Eswaran H., Murphy P., Lowery C., Preissl H. Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Hum. Dev. 2007;83:199–207. doi: 10.1016/j.earlhumdev.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Eswaran H., Lowery C.L., Wilson J.D., Murphy P., Preissl H. Fetal magnetoencephalography—a multimodal approach. Brain Res. Dev. Brain Res. 2005;154:57–62. doi: 10.1016/j.devbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Eswaran H., Preissl H., Wilson J.D., Murphy P., Robinson S.E., Rose D., Vrba J., Lowery C.L. Short-term serial magnetoencephalography recordings offetal auditory evoked responses. Neurosci. Lett. 2002;331:128–132. doi: 10.1016/s0304-3940(02)00859-5. [DOI] [PubMed] [Google Scholar]
- Eswaran H., Wilson J., Preissl H., Robinson S., Vrba J., Murphy P., Rose D., Lowery C. Magnetoencephalographic recordings of visual evoked brain activity in the human fetus. Lancet. 2002;360:779–780. doi: 10.1016/s0140-6736(02)09905-1. [DOI] [PubMed] [Google Scholar]
- Gaultney J.F., Gingras J.L. Fetal rate of behavioral inhibition and preference for novelty during infancy. Early Hum. Dev. 2005;81:379–386. doi: 10.1016/j.earlhumdev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Goldkrand J.W., Litvack B.L. Demonstration of fetal habituation and patterns of fetal heart rate response to vibroacoustic stimulation in normal and high-risk pregnancies. J. Perinatol. 1991;11:25–29. [PubMed] [Google Scholar]
- Gonzalez-Frankenberger B., Harmony T., Ricardo-Garcell J., Porras-Kattz E., Fernandez-Bouzas A., Santiago E., Avecilla-Ramirez G. Habituation of visual evoked potentials in healthy infants and in infants with periventricular leukomalacia. Clin. Neurophysiol. 2008;119:2879–2886. doi: 10.1016/j.clinph.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Govindan R.B., Wilson J.D., Preissl H., Murphy P., Lowery C.L., Eswaran H. An objective assessment of fetal and neonatal auditory evoked responses. Neuroimage. 2008;43:521–527. doi: 10.1016/j.neuroimage.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groome L.J., Gotlieb S.J., Neely C.L., Waters M.D. Developmental trends in fetal habituation to vibroacoustic stimulation. Am. J. Perinatol. 1993;10:46–49. doi: 10.1055/s-2007-994700. [DOI] [PubMed] [Google Scholar]
- Hadlock F.P., Harrist R.B., Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- Kandel E.R. The biology of memory: a forty-year perspective. J. Neurosci. 2009;29:12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman K.A., Burns K.A., Depp R., Sabbagha R.E. Ultrasonic imaging of normal fetal response to external vibratory acoustic stimulation. Am. J. Obst. Gynecol. 1988;158:47–51. doi: 10.1016/0002-9378(88)90773-9. [DOI] [PubMed] [Google Scholar]
- Lasky R.E. Rate and adaptation effects on the auditory evoked brainstem response in human newborns and adults. Hear. Res. 1997;111:165–176. doi: 10.1016/s0378-5955(97)00106-8. [DOI] [PubMed] [Google Scholar]
- Lasky R.E., Maier M.M., Hecox K. Auditory evoked brain stem responses to trains of stimuli in human adults. Ear Hear. 1996;17:544–551. doi: 10.1097/00003446-199612000-00010. [DOI] [PubMed] [Google Scholar]
- Leader L.R., Baillie P., Martin B., Vermeulen E. The assessment and significance of habituation to a repeated stimulus by the human fetus. Early Hum. Dev. 1982;7:211–219. doi: 10.1016/0378-3782(82)90084-6. [DOI] [PubMed] [Google Scholar]
- Lecanuet J.P., Gautheron B., Locatelli A., Schaal B., Jacquet A.Y., Busnel M.C. What sounds reach fetuses: biological and nonbiological modeling of the transmission of pure tones. Dev. Psychobiol. 1998;33(3):203–219. doi: 10.1002/(sici)1098-2302(199811)33:3<203::aid-dev2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lehtonen J.B. Long-term recovery functions of occipital and vertex recorded visual evoked potentials. Acta Neurol. Scand. Suppl. 1972;51:451–453. [PubMed] [Google Scholar]
- Loveless N., Levanen S., Jousmaki V., Sams M., Hari R. Temporal integration in auditory sensory memory: neuromagnetic evidence. Electroencephalogr. Clin. Neurophysiol. 1996;100:220–228. doi: 10.1016/0168-5597(95)00271-5. [DOI] [PubMed] [Google Scholar]
- Lowery C.L., Eswaran H., Murphy P., Preissl H. Fetal magnetoencephalography. Semin. Fetal Neonatal. Med. 2006;11:430–436. doi: 10.1016/j.siny.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Madison L.S., Adubato S.A., Madison J.K., Nelson R.M., Anderson J.C., Erickson J., Kuss L.M., Goodlin R.C. Fetal response decrement: true habituation? J. Dev. Behav. Pediatr. 1986;7:14–20. doi: 10.1097/00004703-198602000-00003. [DOI] [PubMed] [Google Scholar]
- Madison L.S., Madison J.K., Adubato S.A. Infant behavior and development in relation to fetal movement and habituation. Child Dev. 1986;57:1475–1482. [PubMed] [Google Scholar]
- McCubbin J., Robinson S.E., Cropp R., Moiseev A., Vrba J., Murphy P., Preissl H., Eswaran H. Optimal reduction of MCG in fetal MEG recordings. IEEE Trans. Biomed. Eng. 2006;53:1720–1724. doi: 10.1109/TBME.2006.876619. [DOI] [PubMed] [Google Scholar]
- McGee T.J., King C., Tremblay K., Nicol T.G., Cunningham J., Kraus N. Long-term habituation of the speech-elicited mismatch negativity. Psychophysiology. 2001;38:653–658. [PubMed] [Google Scholar]
- Mercuri E., von Siebenthal K., Tutuncuoglu S., Guzzetta F., Casaer P. The effect of behavioural states on visual evoked responses in preterm and full-term newborns. Neuropediatrics. 1995;26:211–213. doi: 10.1055/s-2007-979756. [DOI] [PubMed] [Google Scholar]
- Muenssinger, J., Matuz, T., Schleger, F., Kiefer-Schmidt, I., Goelz, R., Wacker-Gussmann, A., Birbaumer, N., Preissl, H. Learning in the fetus and neonate: auditory habituation and dishabituation—an fMEG-study, under review. [DOI] [PubMed]
- Naatanen R., Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Inui K., Kakigi R. Temporal dynamics of neural adaptation effect in the human visual ventral stream. J. Neurosci. 2004;24:6283–6290. doi: 10.1523/JNEUROSCI.0655-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers-Ax R., Parzer P., Resch F., Weisbrod M. Maturation of early visual processing investigated by a pattern-reversal habituation paradigm is altered in migraine. Cephalalgia. 2005;25:280–289. doi: 10.1111/j.1468-2982.2004.00853.x. [DOI] [PubMed] [Google Scholar]
- Preissl H., Lowery C.L., Eswaran H. Fetal magnetoencephalography: viewing the developing brain in utero. Int. Rev. Neurobiol. 2005;68:1–23. doi: 10.1016/S0074-7742(05)68001-4. [DOI] [PubMed] [Google Scholar]
- Prosser S., Arslan E., Michelini S. Habituation and rate effect in the auditory cortical potentials evoked by trains of stimuli. Arch. Otorhinolaryngol. 1981;233:179–187. doi: 10.1007/BF00453642. [DOI] [PubMed] [Google Scholar]
- Rosburg T., Trautner P., Boutros N.N., Korzyukov O.A., Schaller C., Elger C.E., Kurthen M. Habituation of auditory evoked potentials in intracranial and extracranial recordings. Psychophysiology. 2006;43:137–144. doi: 10.1111/j.1469-8986.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Sams M., Hamalainen M., Hari R., McEvoy L. Human auditory cortical mechanisms of sound lateralization. I. Interaural time differences within sound. Hear. Res. 1993;67:89–97. doi: 10.1016/0378-5955(93)90236-t. [DOI] [PubMed] [Google Scholar]
- Sandman C.A., Glynn L., Wadhwa P.D., Chicz-DeMet A., Porto M., Garite T. Maternal hypothalamic–pituitary–adrenal disregulation during the third trimester influences human fetal responses. Dev. Neurosci. 2003;25:41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- Schreiber T., Schmitz A. Surrogate time series. Physica D. 2000;142:346–382. [Google Scholar]
- Shepherd A., Saunders K., McCulloch D. Effect of sleep state on the flash visual evoked potential. A case study. Doc. Ophthalmol. 1999;98:247–256. doi: 10.1023/a:1002471022790. [DOI] [PubMed] [Google Scholar]
- Sheridan C.J., Matuz T., Draganova R., Eswaran H., Preissl H. Fetal magnetoencephalography – achievements and challenges in the study of prenatal and early postnatal brain responses: a review. Infant Child Dev. 2010;19:80–93. doi: 10.1002/icd.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C.J., Preissl H., Siegel E.R., Murphy P., Ware M., Lowery C.L., Eswaran H. Neonatal and fetal response decrement of evoked responses: a MEG study. Clin. Neurophysiol. 2008;119:796–804. doi: 10.1016/j.clinph.2007.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrandies W., Raile A. Cortical and retinal refractory periods in the human visual system. Int. J. Neurosci. 1989;44:185–195. doi: 10.3109/00207458908986198. [DOI] [PubMed] [Google Scholar]
- Smith C.V., Davis S.R., Rayburn W.F., Nelson R.M. Fetal habituation to vibroacoustic stimulation in uncomplicated term pregnancies. Am. J. Perinatol. 1991;8:380–382. doi: 10.1055/s-2007-999420. [DOI] [PubMed] [Google Scholar]
- Sokolov E.N., Vinogradova O.S. Lawrence Erlbaum Associates; Hillsdale, NJ: 1975. The Neuronal Mechanisms of the Orienting Reflex. [Google Scholar]
- Thompson R.F., Spencer W.A. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Uusitalo M.A., Williamson S.J., Seppa M.T. Dynamical organisation of the human visual system revealed by lifetimes of activation traces. Neurosci. Lett. 1996;213:149–152. doi: 10.1016/0304-3940(96)12846-9. [DOI] [PubMed] [Google Scholar]
- van Heteren C.F., Boekkooi P.F., Jongsma H.W., Nijhuis J.G. Fetal habituation to vibroacoustic stimulation in relation to fetal states and fetal heart rate parameters. Early Hum. Dev. 2001;61:135–145. doi: 10.1016/s0378-3782(00)00130-4. [DOI] [PubMed] [Google Scholar]
- van Heteren C.F., Boekkooi P.F., Schiphorst R.H., Jongsma H.W., Nijhuis J.G. Fetal habituation to vibroacoustic stimulation in uncomplicated postterm pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;97:178–182. doi: 10.1016/s0301-2115(00)00543-1. [DOI] [PubMed] [Google Scholar]
- van Sweden B., van Dijk J.G., Caekebeke J.F. Auditory information processing in sleep: habituation to repetitive stimuli. Neuropsychobiology. 1994;30:143–147. doi: 10.1159/000119149. [DOI] [PubMed] [Google Scholar]
- Vrba J., Robinson S.E., McCubbin J., Lowery C.L., Eswaran H., Wilson J.D., Murphy P., Preissl H. Fetal MEG redistribution by projection operators. IEEE Trans. Biomed. Eng. 2004;51:1207–1218. doi: 10.1109/TBME.2004.827265. [DOI] [PubMed] [Google Scholar]
- Wastell D.G., Kleinman D. Potentiation of the habituation of human brain potentials. Biol. Psychol. 1980;10:21–29. doi: 10.1016/0301-0511(80)90004-6. [DOI] [PubMed] [Google Scholar]
- Wilson J.D., Adams A.J., Murphy P., Eswaran H., Preissl H. Design of a light stimulator for fetal and neonatal magnetoencephalography. Physiol. Meas. 2009;30:N1–N10. doi: 10.1088/0967-3334/30/1/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.L., Elmasian R. The habituation of event-related potentials to speech sounds and tones. Electroencephalogr. Clin. Neurophysiol. 1986;65:447–459. doi: 10.1016/0168-5597(86)90024-9. [DOI] [PubMed] [Google Scholar]