Abstract
Falls are common in older adults. The most common cause of falls is tripping while walking. Simulation studies demonstrated that older adults may be restricted by lower limb strength and movement speed to regain balance after a trip. This review examines how modeling approaches can be used to determine how different measures predict actual fall risk and what some of the causal mechanisms of fall risk are. Although increased gait variability predicts increased fall risk experimentally, it is not clear which variability measures could best be used, or what magnitude of change corresponded with increased fall risk. With a simulation study we showed that the increase in fall risk with a certain increase in gait variability was greatly influenced by the initial level of variability. Gait variability can therefore not easily be used to predict fall risk. We therefore explored other measures that may be related to fall risk and investigated the relationship between stability measures such as Floquet multipliers and local divergence exponents and actual fall risk in a dynamic walking model. We demonstrated that short-term local divergence exponents were a good early predictor for fall risk. Neuronal noise increases with age. It has however not been fully understood if increased neuronal noise would cause an increased fall risk. With our dynamic walking model we showed that increased neuronal noise caused increased fall risk. Although people who are at increased risk of falling reduce their walking speed it had been questioned whether this slower speed would actually cause a reduced fall risk. With our model we demonstrated that a reduced walking speed caused a reduction in fall risk. This may be due to the decreased kinematic variability as a result of the reduced signal-dependent noise of the smaller muscle forces that are required for slower. These insights may be used in the development of fall prevention programs in order to better identify those at increased risk of falling and to target those factors that influence fall risk most.
Keywords: Orbital stability, Local instability, Falls risk, Dynamic walking, Falling
1. Introduction
Falls are very common in older adults; about one in three adults aged over 65 years falls at least yearly (Tinetti, Speechley, & Ginter, 1988). These falls can have significant consequences, as about 29% of falls in those aged over 75 years result in serious injuries, like hip fractures (Tinetti et al., 1988). The economic costs of falls are also substantial. In the United States, the average cost of hospitalization after a fall-related injury was US$17,483 in 2004 (Roudsari, Ebel, Corso, Molinari, & Koepsell, 2005). Falls in older adults often lead to a downward spiral, where physical activity and capacity become reduced (Tinetti & Williams, 1998). First time fallers consequently often become frequent fallers (Tinetti et al., 1988). Primary prevention is therefore essential, as this may prevent or delay the onset of repetitive falls.
There have been three main motivations in fall prevention research: 1) identifying who is at increased fall risk; 2) identifying factors that contribute to fall risk; and 3) exploring interventions that may reduce fall risk. Fall risk factors may include factors that are correlated with fall risk (such as age) but not necessarily cause falls. These are useful to identify individuals at increased fall risk. To help determine why certain individuals are at increased fall risk, the underlying biomechanical and/or physiological factors that cause falls need to be identified. Identification methods are required to enroll older adults at increased fall risk in fall prevention programs as early as possible and preferably before they fall, as early enrollment in fall prevention programs can reduce falls in older adults (Lord et al., 2003; Skelton & Beyer, 2003). Therefore, determining fall risk factors is required to inform the development of targeted fall prevention programs that can effectively address those factors with the most influence on fall risk.
Determining fall risk in humans requires either longitudinal studies, or studies that require inducing actual falls or perturbing balance in a possibly frail participant group. However, the former is expensive and time consuming and the latter is complex and potentially dangerous. It is also difficult to identify the causal mechanisms that lead to increased fall risk experimentally because as people age, many potentially contributing factors (such as increased neuronal noise, loss of strength, decreased response time, reduced cognition) change simultaneously. Many of these factors cannot be manipulated easily experimentally. Conversely, computational models allow total control over the system, do not get injured and do not learn or adapt. Simulation studies are therefore essential to initially explore physical and/or physiological factors that contribute to increased fall risk. This could inform the development of fall prevention measures and would need to be tested in humans in exploratory trials followed by randomized control trials (Campbell et al., 2000).
The purpose of this paper is to review how dynamic walking models can be used to identify factors that contribute to increased risk of falling in older adults. We first review mechanisms of trip recovery (§2). Because most falls are caused by tripping, a better understanding of tripping mechanisms is essential to improve fall prevention programs. We then review approaches to identify fall risk (§3). We review several measures thought to be related to fall risk and describe simulation studies that directly related these measures to actual fall risk. We then review mechanisms that contribute to fall risk (§4), i.e., neuronal noise and walking speed. We investigated the relationship between walking speed and fall risk to provide insights into whether slowing down gait might effectively decrease fall risk, as this has been debated in literature. We complete this review with sections on limitations of simulations studies (§5), implications of the findings of these studies (§6) and conclusions (§7).
2. Trip recovery mechanisms
One of the most common causes of falls in older adults is tripping while walking (Campbell, Reinken, Allan, & Martinez, 1981; Tinetti et al., 1988). Older adults are more likely to fall after a trip than younger adults (Pijnappels, Bobbert, & van Dieën, 2005; Schillings, Mulder, & Duysens, 2005). An understanding of the underlying causes why older adults are more likely to fall due to a trip will allow addressing these factors in fall prevention programs.
At the instant of a trip, the body’s forward momentum of walking is transformed into angular momentum. To prevent a fall, this angular momentum needs to be controlled. The two main strategies to recover from a trip, used by both younger and older adults, are an ‘elevating’ and a ‘lowering’ strategy (Eng, Winter, & Patla, 1994). An elevating strategy is used in response to perturbations encountered in the early part of the swing phase of walking, while a lowering strategy is used in response to perturbations encountered later in swing (Schillings, van Wezel, Mulder, & Duysens, 2000). With trip recovery experiments, we showed that older adults moved from using a lowering instead of an elevating strategy earlier in swing than younger adults (Roos, McGuigan, & Trewartha, 2010). In elevating strategies, younger adults increased their step length when perturbed later in swing, while older adults used a shorter step length that did not increase when perturbed later in swing (Roos et al., 2010). From these experimental data we cannot identify what prevented older adults from using an elevating strategy where younger adults did.
With a simple inverted pendulum model we were able to investigate how leg positioning and lower leg force capacity influenced successful recovery of a trip when perturbed in different phases of swing (Roos et al., 2010). This could not be determined experimentally as it would have involved inducing actual falls, a large number of tripping trials and one cannot alter physical characteristics. With our simulations we demonstrated that recovery step length and recovery limb force were limiting factors to perform an elevating strategy in late swing (Roos et al., 2010). We therefore suggested that older adults were unable to perform an elevating strategy in the later mid-swing phase due to a combination of insufficient recovery limb strength, response time and movement speed. This would restrict their recovery step length and the time to position their recovery leg optimally. This agreed with a simulation study by de Boer, Wisse, and van der Helm, (2010), which demonstrated that strategy selection was based on minimizing the cost of producing torque in the recovery limb. Our finding that recovery success was dependent on step length, step time and leg strength agreed with an earlier simulation study (Hsiao & Robinovitch, 1999) and the dependence on reaction time agreed with previous experimental (Smeesters, Hayes, & McMahon, 2001b) and simulation studies (van den Bogert, Pavol, & Grabiner, 2002). With our simulations we were able to demonstrate that recovery strategy selection was controlled by these measures and showed how they needed to be combined for successful recovery from a trip (Roos et al., 2010). Increased lower limb strength would allow a slower response time and a faster response time would allow decreased lower limb strength to successfully recover from a trip.
Experimental studies demonstrated that although both younger and older adults adopted the same strategies to recover from a trip in terms of center of mass motion, they used their arms differently (Pijnappels, Kingma, Wezenberg, Reurink, & van Dieën, 2010; Roos, McGuigan, Kerwin, & Trewartha, 2008). During elevating strategy recoveries, older adults used their arms in a ‘protective’ manner; reaching both arms forward as if to anticipate a potential fall (Roos et al., 2008). Younger adults used their arms in a more ‘preventive’ manner; elevating the center of mass of the body and reducing the body’s forward angular momentum (Roos et al., 2008). This would provide more time for appropriate positioning of the recovery limb. Pijnappels et al. (2010) further investigated the role of arm movements in younger adults and showed that arm movements were active responses to the trip and helped to achieve an appropriate body orientation for placement of the recovery foot, however by affecting mainly transverse plane body rotation.
Overall, this work provided evidence related to determining the mechanisms that underlie trip responses and distinguish successful from unsuccessful recovery. With the use of a simulation model we were able to demonstrate that recovery step length and recovery limb force were limiting factors for successful performance of an elevating strategy in late swing. These insights could not have been derived from experiments alone. They can be used to better inform the design of fall prevention programs, as they demonstrated that lower limb strength, response time and movement speed combined influence successful recovery from a trip.
3. Predicting fall risk
To enroll older adults at risk of falling into fall prevention programs the factors that contribute to fall risk need to be identified and appropriate measures for fall risk are needed that can easily be measured in a clinical setting. Falls in older adults are most common while walking (Campbell et al., 1981; Tinetti et al., 1988). We therefore sought to identify measures that could predict falls during walking, without having to actually perturb people. As stated in section 1, simulation studies provide an excellent tool to initially explore such measures.
3.1 Gait variability
Gait variability is increased in people with high fall risk (Hausdorff, Rios, & Edelberg 2001; Maki, 1997; Owings and Grabiner, 2004; Richardson, Thies, DeMott, & Ashton-Miller, 2005). This increased gait variability may predict future falls (Brach et al., 2010; Hausdorff et al., 2001; Maki, 1997). However, evidence as to which variability measures best distinguish those who are at increased risk of falling from those who are not is conflicting (Brach, Berlin, Vanswearingen, Newman, & Studenski, 2005; Moe-Nilssen and Helbostad, 2005; Owings and Grabiner, 2004). In particular, step width variability was found to differ between groups by Owings and Grabiner (2004) and by Brach et al. (2005), but was found not to differ between groups by Moe-Nilssen and Helbostad (2005). Brach et al.’s finding may however be influenced by their choice of coefficient of variation (CV) as their variability measure. Specifically, their finding that very low step width variability was related to fall history may have been an artefact caused instead by an increased mean step width, which would artificially reduce the CV without any true decrease in step width variability.
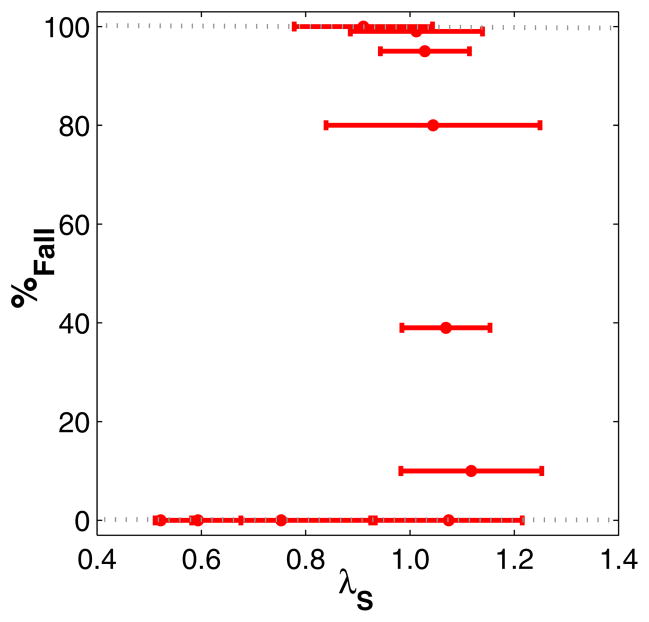
We therefore further investigated if and how gait variability could be used to identify actual risk of falling (Roos & Dingwell, 2010). As this cannot easily be investigated experimentally (see Section 1), we used simulations with a 3D dynamic walking model based on (Kuo, 1999). This model walked dynamically and therefore did not follow a pre-determined walking pattern. The model was laterally unstable and was only able to walk in a stable manner when we included a lateral step controller (Kuo, 1999). This was the only control that was applied to this model. When perturbations of increasing size were applied to this lateral step controller, kinematic variability increased and the model fell over more often (Roos & Dingwell, 2010). However, we demonstrated with our dynamic walking model that the relationship between fall risk and gait variability was highly nonlinear (Fig. 1A) and that gait variability measures essentially paralleled changes in fall risk (Roos & Dingwell, 2010). The increase in fall risk with a certain increase in gait variability was therefore greatly influenced by the initial level of gait variability (which depends on a person’s physiology and external factors) (Roos & Dingwell, 2010). Such insights could not have been obtained through experiments alone.
Fig. 1.
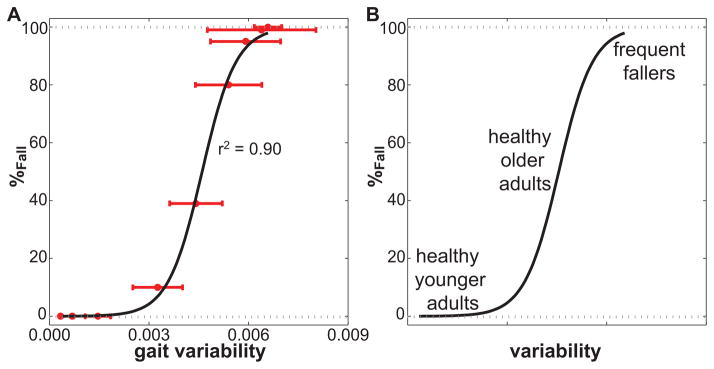
Fig. 1A. Gait variability (combined variability of state variables as in (Roos & Dingwell, 2010)) versus probability of falling (%Fall). The horizontal error bars indicate ±1 SD of the gait variability. The solid black line is a sigmoidal function that was fit to the data. The correlation of the fit to the data was very high: r2 = .90. This figure is adapted and modified from Fig. 7A of (Roos & Dingwell, 2010)). B) We speculated that younger adults, older adults and frequent fallers could be placed at different variability levels in order to predict how changes in their gait variability would influence their fall risk
We therefore demonstrated that specific increases in kinematic variability may not always lead to increased fall risk, as might be assumed based on previous experimental studies (Brach et al., 2010). We showed that at low variability levels fall risk remained low and at higher variability levels fall risk remained very high regardless of modest changes in variability levels. Only at the intermediate variability levels increases in variability resulted in increased fall risk. Further research is still required to determine if, and/or how, these results might translate directly to humans. We expect however that younger adults, older adults and frequent fallers could be placed at different variability levels in order to predict how changes in their gait variability would influence their fall risk (Fig. 1B). Younger adults, with low gait variability, would exhibit only negligible changes in fall risk in response to small increases in gait variability. Healthy older adults exhibit increased gait variability (Kang & Dingwell, 2008). Similar increases in gait variability could for them result in significant increases in fall risk. This agrees with findings by Brach et al. (2010) on community dwelling older adults. Frequent fallers are likely to have high kinematic variability, and additional increases in variability may not further increase their fall risk. This nonlinear relationship between gait variability and fall risk may also explain the contradicting conclusions of some experimental studies. Brach et al. (2005) for example showed low and high variability to be associated with fall history, while Beauchet et al. (2009) demonstrated the opposite and concluded low and high variability were related to increased gait stability. Both studies however used CV as measure of variability, it can therefore not be said for certain if their low variability was caused by a high mean step width or actually a low variability.
This study (Roos & Dingwell, 2010) agreed with and extended previous work by Su and Dingwell (2007) and Byl and Tedrake (2009) who applied external perturbations to similar dynamic walking models and investigated their kinematic variability and fall risk respectively. They found, in agreement with our results, that variability increased with the magnitude of the external perturbations that were applied. However, their models did not fall over, and they were therefore not able to investigate actual fall risk. The mean first passage times used by Byl and Tedrake (2009) were however direct measures of fall risk.
Our findings also agreed with experimental studies, indicating they are expected to at least qualitatively translate to humans. McAndrew, Dingwell, and Wilken (2010) showed that step width and step length variability increased when human subjects were subjected to continuous random mechanical or visual perturbations (extrinsic source of noise), comparable to the noise we added to the lateral step controller in our simulations (intrinsic source of noise). When humans were externally stabilized, step width variability decreased (Donelan, Shipman, Kram, & Kuo, 2004). The external stabilization resulted in a reduced gait variability and its effects would therefore be comparable to those of reducing the noise applied to the step controller in our simulations. Similar to our simulation results at the intermediate levels, increases in kinematic variability in elderly humans predict increased fall risk (Brach et al., 2005; Moe-Nilssen & Helbostad, 2005; Owings & Grabiner, 2004).
A recent simulation study with a 2D passive dynamic walking model (Bruijn, Bregman, Meijer, Beek, & van Dieën, 2011) confirmed our findings. Gait variability was in this study however induced by external noise (bumpy slope) and not by applying noise to a controller (simulating neuronal noise, the main intrinsic source of gait variability in humans (Hamacher, Singh, van Dieën, Heller, & Taylor, 2011). They demonstrated a linear relationship between the logarithm of step time variability (a different measure of variability than kinematic state variability) and one divided by the maximum perturbation the model could withstand (Bruijn et al., 2011). Transforming this back to fall risk and step time variability, would result in a similar trend to what we showed for medium and high variability levels and probability of falling (Fig. 1). Therefore, similar changes in step time variability would result in larger changes in fall risk for the smaller variability levels than for the higher variability levels, confirming our previous findings (Roos & Dingwell, 2010). To investigate whether variability could be useful to predict fall risk, it is important to investigate the sensitivity of fall risk to absolute changes in variability measures.
3.2 Dynamic stability
We demonstrated that increases in gait variability similar to those seen in aging humans could predict fall risk (Roos & Dingwell, 2010). The relationship between variability and fall risk was however not straightforward. Hence, gait variability might not be the most useful measure to identify those at increased risk of falling. Therefore, we further explored measures that may predict fall risk, using the same 3D dynamic walking model. Stability measures were originally developed for deterministic mechanical systems, but have been applied to assess stability of human gait for almost 20 years (Dingwell & Cusumano, 2000; Dingwell & Kang, 2007; Granata & Lockhart, 2008; Hurmuzlu & Basdogan, 1994; Hurmuzlu, Basdogan, & Stoianovici, 1996; Sloot et al., 2011). None of these studies however looked at the relationship between stability measures and actual fall risk.
3.2.1 What is stability?
In theoretical mechanics, stability is how a system responds to perturbations. During walking, humans can be perturbed minimally or they can experience perturbations large enough to cause falls. The first type of perturbations concern ‘local’ (and ‘orbital’) instability, which quantifies a system’s response to infinitesimally small perturbations (Dingwell & Cusumano, 2000). The second concerns ‘global’ stability, which is the set of the largest possible perturbations a system can withstand without falling over. Although there are no theoretical reasons that ‘local stability’ should necessarily predict ‘global stability’, several studies suggest such a relationship would exist. Orbital instability has for example been found to be increased in fall prone older adults (Granata & Lockhart, 2008) and in post-polio patients (Hurmuzlu et al., 1996).
Orbital stability reflects the cycle-to-cycle tendency of the state variables of a periodic system to return to a specified trajectory (limit cycle) after small perturbations away from the limit cycle. Orbital stability is therefore only strictly defined for systems that exhibit periodic behavior. Maximum Floquet ultipliers are used to estimate orbital stability (Hurmuzlu & Basdogan, 1994). A system is orbitally stable when the maximum Floquet multipliers have magnitude less than 1, and is unstable when they have magnitudes larger than 1. More detailed descriptions of how these measures are calculated can be found in Hurmuzlu and Basdogan (1994) and Su and Dingwell (2007).
In contrast to orbital stability, local stability reflects how the state variables of a system respond to very small perturbations in real time. Local stability is therefore primarily intended for systems that exhibit a periodic behavior (no limit cycle) (Dingwell & Cusumano, 2000). Local divergence exponents are used to quantify local stability. They quantify the divergence rate of neighboring trajectories in the state space. For human walking, two local divergence exponents were introduced; a short-term ( ) and a long-term local divergence exponent ( ) (Dingwell & Cusumano, 2000; Dingwell et al., 2001).
Human gait is strongly periodic, but not exactly. The 3D dynamic walking model that we used in our studies behaved periodically, but became slightly aperiodic when simulated neuronal noise was applied to the lateral step controller. Using both stability measures for such “pseudo-periodic” or “nearly-periodic” systems can provide additional, and potentially complimentary, insights into the dynamics of these systems.
3.2.2 Can stability measures predict fall risk?
To determine if local or orbital stability measures can predict fall risk we used the simulations as in Roos and Dingwell (2010) where we applied neuronal noise of increasing amplitude to a 3D dynamic walking model. The noise amplitude was varied such that the model did not fall over at the smallest noise levels and fell over all the time at the largest amplitudes. This allowed us to evaluate the full range of fall risk.
The short-term local divergence exponent ( ) was an early predictor for fall risk, as it started to increase before the probability of falling increased (Roos & Dingwell, 2011) (Fig. 2). It therefore predicted fall risk in a different manner than gait variability in these same simulations (Roos & Dingwell, 2010) and may be a more useful predictor for increased risk of falling than kinematic variability measures because it appears more sensitive to earlier changes in system dynamics that are not captured by measures of variability.
Fig. 2.
Short-term local divergence exponent ( ) versus probability of falling (%Fall) with the horizontal bars indicating ±1 SD of . This figure is adapted and modified from Fig. 6B of Roos and Dingwell (2011).
The other two stability measures that were assessed (long-term ( ) local divergence exponents and maximum Floquet multipliers (maxFM)) were not good predictors of fall risk (Roos & Dingwell, 2011). Orbital dynamic stability changed only little with increasing neuronal noise, and the changes occurred too late to predict increased fall risk. This agrees with a previous study that showed that orbital stability measures were less sensitive to experimental perturbations (and therefore instability in general) than the short-term local divergence exponent (McAndrew, Wilken, & Dingwell, 2011). The orbital stability measures were however more specific, as they only increased for movements in the same directions as the perturbations applied (McAndrew et al., 2011). Similarly, Kang and Dingwell (2008) found increases in local but not orbital stability for healthy older adults. However, orbital stability was increased in fall prone older adults (Granata & Lockhart, 2008). It may therefore be that increased local instability indicates that something is changing in the system (possibly related to fall risk) and that perhaps increased orbital instability, while less sensitive to changes in general, may more specifically indicate changes that are more directly related to increased fall risk.
Our findings agreed with previous findings that short-term local instability, but not orbital stability, changed when a 2D dynamic model was perturbed with increasing magnitude (Su & Dingwell, 2007). That study did however not investigate actual fall risk, as the model was not perturbed enough to make it fall over. Also, that model was passively stable, as it was 2D, and therefore did not require any control. Recent studies with a 2D dynamic model (Bruijn, Bregman, Meijer, Beek, & van Dieën, 2012; Bruijn et al., 2011) confirmed our findings that short-term local divergence exponents were good predictors of fall risk and that Floquet multipliers and long-term local divergence exponents were not good predictors of fall risk. Another simulation study by Hobbelen and Wisse (2007) also confirmed that Floquet multipliers were unrelated to the disturbance rejection of their walking model.
The relationship between short-term local divergence exponents and fall risk as quantified by Bruijn et al. (2012) (i.e., the maximum perturbation their model could withstand), however differed from the relationship we demonstrated with our 3D dynamic walking model (Roos & Dingwell, 2011). In our simulations, the short-term divergence exponent was an early predictor of fall risk, while in Bruijn et al. (2012) it was most sensitive when fall risk had already increased. This could either be caused by mechanical differences between the different walking models, or by the methods used to increase fall risk in each study. Our 3D model was laterally unstable and required active control (similar to humans), while the 2D model was laterally stable and required no control. To increase fall risk we applied noise of increasing amplitude to the lateral step controller of the model (i.e., simulating neuronal noise), while Bruijn et al. (2012) changed physical properties of the model itself (foot radius and hip spring stiffness) to simulate physical changes with ageing. Each study thus applied very different methods to increase fall risk that may be relevant to different underlying mechanisms that could cause increases in fall risk in humans. In spite of these different approaches, both studies showed clear links between short-term local divergence exponents and fall risk.
Our conclusions on stability measures generally agree with a recent review on measures to estimate gait stability (Bruijn, Meijer, Beek, & van Dieën, 2013). They concluded that short-term divergence exponents had a good construct validity and predictive and convergent validity for identifying individuals who are at increased risk of falling due to unstable gait. Long-term divergence exponents and Floquet multipliers also had a good construct validity, but did not work as well in practial application to predict actual fall risk. Our overall findings on fall risk measures agree with the conclusions of a recent review paper on experimental studies that investigated measures to distinguish between fallers and non-fallers (Hamacher et al., 2011). They concluded that both variability and stability measures could be used to distinguish fallers from non-fallers, and cross-fertilization of both methods is needed (Hamacher et al., 2011). We should also highlight that further research is needed on the sensitivity of these measures, to ensure they can identify increased fall risk before the first fall.
4. Mechanisms that contribute to instability and fall risk
To help determine why certain individuals experience increased fall risk, factors that contribute to an increased fall risk need to be identified. Fall risk increases with age. However, as people age neuronal noise levels also increase and walking speed decreases. We therefore investigated the extent to which each of these mechanisms might possibly contribute to fall risk.
4.1 Neuronal noise
With age, force production becomes more discretized due to reduced and larger motor units (Fling, Knight, & Kamen, 2009; Ling, Conwit, Ferrucci, & Metter, 2009). Likewise, sensory accuracy degrades due to deteriorated muscle spindles and proprioception (Shaffer & Harrison, 2007). Increased gait variability in older adults is likely caused, at least in part, by this increase in neuronal ‘deafness’ with age (Shaffer & Harrison, 2007). It is unknown whether this neuronal ‘deafness’ could directly lead to increased fall risk, or if it increases fall risk through increased gait variability.
Although a relationship between fall risk and gait variability had been demonstrated, it was unknown whether increased variability directly causes falls. Theoretically, there is no absolute requirement that increased variability would be equated with greater instability (i.e., risk of falling). Increased variability observed in a system’s output (e.g., movement kinematics) may cause increased fall risk, or it may not. For example, variations in movement kinematics caused by increased physiological noise in the neuromotor system could be disruptive (i.e., destabilizing). Conversely, fluctuations in movement kinematics introduced by a control system correcting for deviations and/or perturbations could contribute to increased variability, while at the same time reduce fall risk.
We investigated the causal mechanism of neuronal noise amplitude on fall risk (Roos & Dingwell, 2010). As these relationships cannot easily be investigated experimentally (see Section 1), we used simulations with a 3D dynamic walking model based on (Kuo, 1999). In experiments with humans, we cannot manipulate either the physiological noise levels or that actions of the controller, much less manipulate these independently. In a computational model, we can control both independently to determine how each may contribute to fall risk.
We showed that at low noise levels, increases in noise amplitude were easily counteracted by the lateral step controller and fall risk remained low. At the higher noise levels, the model exceeded its capacity to prevent falls and so fall risk remained very high regardless of modest changes in noise and variability levels. Only at the intermediate noise levels could increases in neuronal noise not be easily counteracted by the lateral step controller. This resulted in increased kinematic variability, which led to increased fall risk. Therefore increased neuronal noise amplitude may cause increased fall risk, but this would depend on the base level of neuronal noise.
4.2 Walking speed
Walking speed generally decreases with increasing age. Older adults with increased fall risk walk slower than healthy older adults (Lazaro, Gonzalez, Latorre, Fernandez, & Ribera, 2011; Maki, 1997) and tend to have a more cautious gait (Kesler et al., 2005; Hallemans, Ortibus, Meire, & Aerts 2010; Tersteeg, Marple-Horvat, & Loram, 2012). It is unknown whether older adults walk slower to reduce their fall risk (as suggested by Maki, (1997), Rogers, Cromwell, and Newton (2005), Tsai and Lin (2013)), or because they have reduced strength and/or slower response times etc. that generally come with older age and so these then lead to both slow gait speed and increased fall risk. It is further unknown whether the slow gait speed itself could cause falls, as slower walking speeds have been shown to sometimes predict increased fall risk (Bergland, Jarnlo, & Laake, 2003; Taylor, Delbaere, Mikolaizak, Lord, & Close, 2013).
Theoretical evidence is inconclusive as to whether a slower gait would reduce fall risk. Experimental studies showed that gait variability increased with slower gait speeds (Dingwell & Marin, 2006; Kang & Dingwell, 2008a). Increased gait variability has been associated with increased fall risk (Hausdorff et al., 2001; Maki, 1997; Owings & Grabiner, 2004), creating a paradox of why older adults walk slower than younger adults (Dingwell & Marin, 2006).
The empirical investigation of true fall risk requires introducing actual falls and/or longitudinal follow-up studies. An acceptable alternative may be provided by stability measures. Several studies aimed to provide a better understanding of the relationship between walking speed and such stability measures. Most of these studies showed that reducing walking speed would lead to more stable walking (Dingwell, Kang, & Marin, 2007; England and Granata, 2007; Kang and Dingwell, 2008a; Manor, Wolenski, & Li 2008). One research group found the relationship between walking speed and stability to be more complex, and dependent on the way these measures were calculated (Bruijn, van Dieën, Meijer, & Beek, 2009; Bruijn et al., 2010).
We recently further investigated the relationship between walking speed, stability measures and actual fall risk, using our 3D dynamic walking model (Roos & Dingwell, 2010, 2011). The model was perturbed so that it actually fell over, so the relationship between gait speed and actual fall risk could be investigated (Roos & Dingwell, 2013). This provided further insights into this relationship, as we previously showed that stability measures do not always correlate with fall risk (Roos & Dingwell, 2011).
As in Roos and Dingwell (2010, 2011) we applied randomly distributed noise to the lateral step controller of the model (simulating the effects of neuronal noise, which is an intrinsic source of variability). We also applied signal dependent noise (Harris & Wolpert, 1998) to the slope the model walked down. Since the propulsion for this model was provided by gravity, this effectively mimicked motor output noise applied to the ‘push-off force’ (Su & Dingwell, 2007) and was therefore an extrinsic source of variability (Roos & Dingwell, 2013).
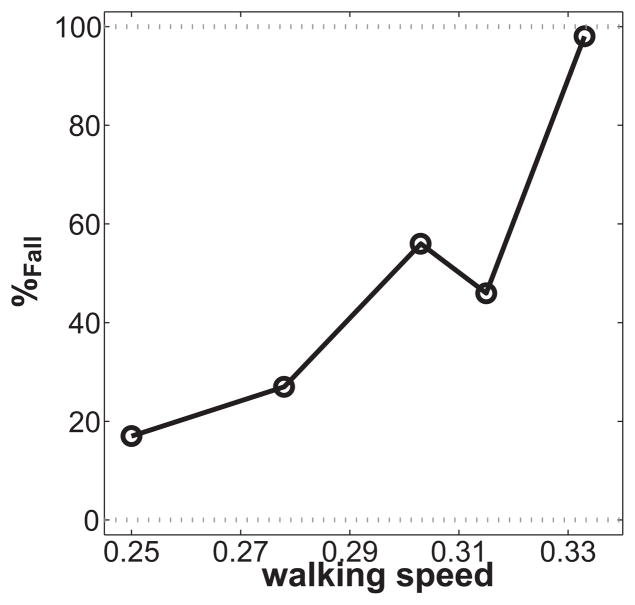
The model fell over more often when walking speed increased (Fig. 3) and the probability of falling increased faster with speed than the signal dependent noise amplitude did.
Fig. 3.
Probability of falling (%Fall) of the 3D dynamic walking model versus non-dimensional walking speed. Walking speed was non-dimensionalized. Data are from Roos and Dingwell (2013).
We showed that for a 3D dynamic walking model with simulated neuronal noise and signal dependent ‘push-off’ noise the probability of falling increased with walking speed. Our results suggest that older adults decrease their walking speed to reduce fall risk. Our conclusions partly agree with a simulation study performed by Hobbelen and Wisse (2008), who concluded that their model’s robustness to perturbations during walking was smallest at the slowest and fastest walking speeds. They however tested their model at a slower range of walking speeds. The decline in fall risk they found at their faster speeds actually corresponds to the slower of the range of speeds we performed our simulations at, and is therefore largely in agreement.
5. Limitations of simulation studies
Our simulation studies provided insight into the relationships between fall risk and neuronal noise, gait variability and walking speed (Kang & Dingwell, 2008b, 2009; Roos & Dingwell, 2010, 2011). The 3D dynamic model used in our studies was laterally unstable, which resulted in very realistic behavior, as humans are also most sensitive to lateral perturbations during gait (Bauby & Kuo, 2000; Dean, Alexander, & Kuo, 2007; Donelan et al., 2004; McAndrew et al., 2010; McAndrew et al., 2011). However, like all models, our model was a simplification and did not include all factors that could influence fall risk, such as muscle strength and response time. Some other limitations of the dynamic walking model were that it did not include a double support phase, that the controller noise was added instantaneously and not continuously like in humans, and that it did not include reflex responses. Further limitations have been described previously (Roos & Dingwell, 2010, 2011, 2013). The model was however sufficiently complex to answer our research questions. Further research is required to investigate how this research would translate to humans.
6. Implications
Improving lower limb strength and movement speed could potentially reduce fall risk in older adults as these were limiting factors for using similar trip recovery strategies as younger adults in more challenging trips (Roos et al., 2010). Short-term local divergence exponents could potentially be used as a fall risk measure for humans as they were good early predictors for increased fall risk in our dynamic walking model and (Roos & Dingwell, 2011). Slowing down gait is one of the several possible strategies that can be adopted to reduce fall risk (Roos & Dingwell, 2013). These findings may be used to inform the development of fall prevention programs. Such programs may prevent (or at least significantly delay) the first fall and consecutive falls.
7. Conclusions
We explored mechanisms of trip recovery. With an inverted pendulum model we demonstrated that reduced lower limb strength and movement speed may prevent older adults from using similar trip recovery strategies as younger adults in more challenging trips (Roos et al., 2010). We also showed with experimental data that older adults used a more ‘protective’ trip recovery strategy than younger adults, anticipating a potential fall (Roos et al., 2008).
We further used a dynamic walking model to investigate measures that could predict fall risk. With our walking model we showed that the relationship between gait variability and fall risk was complex and that more useful measures of fall risk were needed (Roos & Dingwell, 2010). We demonstrated that short-term local divergence exponents were good early predictors for increased fall risk in our dynamic walking model (Roos & Dingwell, 2011).
Using the same dynamic walking model, we investigated mechanisms that could contribute to fall risk and validated the idea that age-related increases in neuromuscular noise are a potential cause of increased falls and/or gait instability. We showed that walking slower may reduce fall risk. This may be due to the decreased kinematic variability that results from the reduced signal-dependent noise of the smaller muscle forces that are required for slower gait (Roos & Dingwell, 2013).
Acknowledgments
Funding for the studies presented in section 3 and 4 was provided by Grant #1-R21-EB007638 from the National Institutes of Health. Dr. Roos is an Academic Fellow funded by Arthritis Research UK (Grant No: 18461).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Annweiler C, Bridenbaugh S, Assal F, Kressig RW, Herrmann FR. Gait variability among healthy adults: Low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55:702–706. doi: 10.1159/000235905. [DOI] [PubMed] [Google Scholar]
- Bergland A, Jarnlo GB, Laake K. Predictors of falls in the elderly by location. Aging Clinical and Experimental Research. 2003;15:43–50. doi: 10.1007/BF03324479. [DOI] [PubMed] [Google Scholar]
- Brach JS, Berlin JE, Vanswearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fal history in older persons who walk at or near normal gait speed. Journal of NeuroEngineering and Rehabilitation. 2005;2(21) doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait & Posture. 2010;31:175–179. doi: 10.1016/j.gaitpost.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Bregman DJJ, Meijer OG, Beek PJ, van Dieën JH. Maximum Lyapunov exponents as predictors of global stability: A modelling approach. Medical Engineering and Physics. 2012;34:428–436. doi: 10.1016/j.medengphy.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, van Dieën JH, Meijer OG, Beek PJ. Is slow walking more stable? Journal of Biomechanics. 2009;42:1506–1512. doi: 10.1016/j.jbiomech.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, ten Kate WRT, Faber GS, Meijer OG, Beek PJ, van Dieën JH. Estimating dynamic gait stability using data from non-aligned inertial sensors. Annals of Biomedical Engineering. 2010;38:2588–2593. doi: 10.1007/s10439-010-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Bregman DJ, Meijer OG, Beek PJ, van Dieën JH. The validity of stability measures: A modelling approach. Journal of Biomechanics. 2011;44:2401–2408. doi: 10.1016/j.jbiomech.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieën JH. Assessing the stability of human locomotion: A review of current measures. Journal of the Royal Society Interface. 2013;10(83):20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byl K, Tedrake R. Metastable walking machines. The International Journal of Robotics Research. 2009;28:1040–1064. [Google Scholar]
- Campbell AJ, Reinken J, Allan BC, Martinez GS. Falls in old age: A study of frequency and related clinical factors. Age and Ageing. 1981;10:264–270. doi: 10.1093/ageing/10.4.264. [DOI] [PubMed] [Google Scholar]
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. British Medical Journal. 2000;321(7262):694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. Ieee Transactions on Biomedical Engineering. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- de Boer T, Wisse M, van der Helm FT. Mechanical analysis of the preferred strategy selection in human stumble recovery. Journal of Biomechanical Engineering. 2010;132(7):071012. doi: 10.1115/1.4001281. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10:848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Kang HG. Difference between local and orbital dynamic stability during human walking. Journal of Biomechanical Engineering-Transactions of the ASME. 2007;129:586–593. doi: 10.1115/1.2746383. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Kang HG, Marin LC. The effects of sensory loss and walking speed on the orbital dynamic stability of human walking. Journal of Biomechanics. 2007;40:1723–1730. doi: 10.1016/j.jbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. Journal of Biomechanics. 2006;39:444–452. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Experimental Brain Research. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait & Posture. 2007;25:172–178. doi: 10.1016/j.gaitpost.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Knight CA, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: Implications for size principle. Experimental Brain Research. 2009;197:125–133. doi: 10.1007/s00221-009-1898-y. [DOI] [PubMed] [Google Scholar]
- Granata KP, Lockhart TE. Dynamic stability differences in fall-prone and healthy adults. Journal of Electromyography and Kinesiology. 2008;18:172–178. doi: 10.1016/j.jelekin.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallemans A, Ortibus E, Meire F, Aerts P. Low vision affects dynamic stability of gait. Gait & Posture. 2010;32:547–551. doi: 10.1016/j.gaitpost.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Singh NB, van Dieën JH, Heller MO, Taylor WR. Kinematic measures for assessing gait stability in elderly individuals: A systematic review. Journal of the Royal Society Interface. 2011;9:1682–1698. doi: 10.1098/rsif.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of Physical Medicine and Rehabilitation. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Hobbelen DGE, Wisse M. A disturbance rejection measure for limit cycle walkers: The Gait Sensitivity Norm. IEEE Transactions on Robotics. 2007;23:1213–1224. [Google Scholar]
- Hobbelen DGE, Wisse M. Controlling the walking speed in limit cycle walking. International Journal of Robotics Research. 2008;27:989–1005. [Google Scholar]
- Hsiao ET, Robinovitch SN. Biomechanical influences on balance recovery by stepping. Journal of Biomechanics. 1999;32:1099–1106. doi: 10.1016/s0021-9290(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Hurmuzlu Y, Basdogan C. On the measurement of dynamic stability of human locomotion. Journal of Biomechanical Engineering-Transactions of the ASME. 1994;116:30–36. doi: 10.1115/1.2895701. [DOI] [PubMed] [Google Scholar]
- Hurmuzlu Y, Basdogan C, Stoianovici D. Kinematics and dynamic stability of the locomotion of post-polio patients. Journal of Biomechanical Engineering-Transactions of the ASME. 1996;118:405–411. doi: 10.1115/1.2796024. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. Journal of Biomechanics. 2008a;41:2899–2905. doi: 10.1016/j.jbiomech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait and Posture. 2008b;27:572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Changes in the dynamic stability of walking in active healthy older adults independent of changes in walking speed. Proceedings of the ASME International Mechanical Engineering Congress and Exposition 2008, Vol. 2009;2:381–384. [Google Scholar]
- Kesler A, Leibovich G, Herman T, Gruendlinger L, Giladi N, Hausdorff JM. Shedding light on walking in the dark: The effects of reduced lighting on the gait of older adults with a higher-level gait disorder and controls. Journal of NeuroEngineering and Rehabilitation. 2005;2:27. doi: 10.1186/1743-0003-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AD. Stabilization of lateral motion in passive dynamic walking. International Journal of Robotics Research. 1999;18:917–930. [Google Scholar]
- Lazaro M, Gonzalez A, Latorre G, Fernandez C, Ribera JM. Postural stability in the elderly: Fallers versus non-fallers. European Geriatric Medicine. 2011;2:1–5. [Google Scholar]
- Ling SM, Conwit RA, Ferrucci L, Metter EJ. Age-associated changes in motor unit physiology: Observations from the Baltimore Longitudinal Study of Aging. Archives of Physical Medicine and Rehabilitation. 2009;90:1237–1240. doi: 10.1016/j.apmr.2008.09.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Castell S, Corcoran J, Dayhew J, Matters B, Shan A, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: A randomized, controlled trial. Journal of the American Geriatrics Society. 2003;51:1685–1692. doi: 10.1046/j.1532-5415.2003.51551.x. [DOI] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: Predictors of falls or indicators of fear? Journal of the American Geriatrics Society. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Manor B, Wolenski P, Li L. Faster walking speeds increase local instability among people with peripheral neuropathy. Journal of Biomechanics. 2008;41:2787–2792. doi: 10.1016/j.jbiomech.2008.07.006. [DOI] [PubMed] [Google Scholar]
- McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo-random oscillations of the support surface and visual field. Journal of Biomechanics. 2010;43:1470–1475. doi: 10.1016/j.jbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. Journal of Biomechanics. 2011;44:644–649. doi: 10.1016/j.jbiomech.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe-Nilssen R, Helbostad JL. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait & Posture. 2005;21:164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of Biomechanics. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieën JH. Push-off reactions in recovery after tripping discriminate young subjects, older non-falters and older fallers. Gait & Posture. 2005;21:388–394. doi: 10.1016/j.gaitpost.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Kingma I, Wezenberg D, Reurink G, van Dieën JH. Armed against falls: The contribution of arm movements to balance recovery after tripping. Experimental Brain Research. 2010;201:689–699. doi: 10.1007/s00221-009-2088-7. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. Gait analysis in a challenging environment differentiates between fallers and nonfallers among older patients with peripheral neuropathy. Archives of Physical Medicine and Rehabilitation. 2005;86:1539–1544. doi: 10.1016/j.apmr.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Rogers HL, Cromwell RL, Newton RA. Association of balance measures and perception of fall risk on gait speed: A multiple regression analysis. Experimental Aging Research. 2005;31:191–203. doi: 10.1080/03610730590915434. [DOI] [PubMed] [Google Scholar]
- Roos PE, McGuigan MP, Kerwin DG, Trewartha G. The role of arm movement in early trip recovery in younger and older adults. Gait & Posture. 2008;27:352–356. doi: 10.1016/j.gaitpost.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Roos PE, McGuigan MP, Trewartha G. The role of strategy selection, limb force capacity and limb positioning in successful trip recovery. Clinical Biomechanicsi. 2010;25:873–878. doi: 10.1016/j.clinbiomech.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Roos PE, Dingwell JB. Influence of simulated neuromuscular noise on movement variability and fall risk in a 3D dynamic walking model. Journal of Biomechanics. 2010;43:2929–2935. doi: 10.1016/j.jbiomech.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos PE, Dingwell JB. Influence of simulated neuromuscular noise on the dynamic stability and fall risk of a 3D dynamic walking model. Journal of Biomechanics. 2011;44:1514–1520. doi: 10.1016/j.jbiomech.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos PE, Dingwell JB. Influence of neuromuscular noise and walking speed on fall risk and dynamic stability in a 3D dynamic walking model. Journal of Biomechanics. 2013;46:1722–1728. doi: 10.1016/j.jbiomech.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudsari BS, Ebel BE, Corso PS, Molinari NAM, Koepsell TD. The acute medical care costs of fall-related injuries among the US older adults. Injury-International Journal of the Care of the Injured. 2005;36:1316–1322. doi: 10.1016/j.injury.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Schillings AM, Mulder T, Duysens J. Stumbling over obstacles in older adults compared to young adults. Journal of Neurophysiology. 2005;94:1158–1168. doi: 10.1152/jn.00396.2004. [DOI] [PubMed] [Google Scholar]
- Schillings AM, van Wezel BMH, Mulder T, Duysens J. Muscular responses and movement strategies during stumbling over obstacles. Journal of Neurophysiology. 2000;83:2093–2102. doi: 10.1152/jn.2000.83.4.2093. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Ageing of the somatosensory system: A translative perspective. Physical Therapy. 2007;87:193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Beyer N. Exercise and injury prevention in older people. Scandinavian Journal of Medicine and Science in Sports. 2003;13:77–85. doi: 10.1034/j.1600-0838.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Sloot LH, van Schooten KS, Bruijn SM, Kingma H, Pijnappels M, van Dieën JH. Sensitivity of local dynamic stability of over-ground walking to balance impairment due to galvanic vestibular stimulation. Annals of Biomedical Engineering. 2011;39:1563–1569. doi: 10.1007/s10439-010-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeesters C, Hayes WC, McMahon TA. Disturbance type and gait speed affect fall direction and impact location. Journal of Biomechanics. 2001a;34:309–317. doi: 10.1016/s0021-9290(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Smeesters C, Hayes WC, McMahon TA. The threshold trip duration for which recovery is no longer possible is associated with strength and reaction time. Journal of Biomechanics. 2001b;34:589–595. doi: 10.1016/s0021-9290(01)00005-7. [DOI] [PubMed] [Google Scholar]
- Su JL, Dingwell JB. Dynamic stability of passive dynamic walking on an irregular surface. Journal of Biomechanical Engineering-Transactions of the ASME. 2007;129:802–810. doi: 10.1115/1.2800760. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JCT. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait & Posture. 2013;37:126–130. doi: 10.1016/j.gaitpost.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Tersteeg MCA, Marple-Horvat DE, Loram ID. Cautious gait in relation to knowledge and vision of height: Is altered visual information the dominant influence? Journal of Neurophysiology. 2012;107:2686–2691. doi: 10.1152/jn.00875.2011. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk-factors for falls among elderly persons living in the community. New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1998;53:M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- Tsai YJ, Lin SI. Older adults adopted more cautious gait patterns when walking in socks than barefoot. Gait & Posture. 2013;37:88–92. doi: 10.1016/j.gaitpost.2012.06.034. [DOI] [PubMed] [Google Scholar]
- van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. Journal of Biomechanics. 2002;35:199–205. doi: 10.1016/s0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]