Abstract
Peritoneal carcinomatosis arising from small bowel adenocarcinoma (PCSBA) carries a dismal prognosis. Presently, limited data have been published on the outcome of PCSBA treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). This series represents the largest series published to date examining our experience with 17 patients. From 1995 to 2011, 17 patients underwent HIPEC with mitomycin for PCSBA. Patients in this study were identified from a prospectively maintained database. Twenty HIPEC procedures were performed on 17 patients with a mean age of 52.2 years. Patients have achieved a mean overall postoperative survival of 18.4 months after progression on chemotherapy with an overall postoperative one- and three-year survival of 52 and 23 per cent, respectively. The mean total length of hospital stay was 10 days. There was no treatment-related mortality. Six patients were readmitted to the hospital within 30 days of discharge (35%). Eight patients (47%) experienced postoperative complications, in which two patients had major postoperative complications in the form of intra-abdominal abscess requiring interventions (12%). HIPEC has encouraging survival results for patients with PCSBA compared with similar patients treated with conventional treatments. However, even with such advancement in management, treatment for small bowel adenocarcinoma still remains a challenge.
Despite the several different types of malignant tumors of the small bowel, such lesions are rare, representing only 1 to 3 per cent of all gastrointestinal malignancies.1–4 Of these different types, small bowel adenocarcinoma (SBA) is the most common histologic variant.4–6 It is estimated that 2500 patients are diagnosed with SBA each year in the United States.4 SBA has historically been known for its poor prognosis with a median overall survival ranging from 12 to 20 months.1–4 With current treatment, survival for patients with SBA has remained relatively unchanged over the last 20 years.7
SBA tumors are difficult to identify at an early stage by most endoscopic or imaging modalities. Its vague presentation and low index of suspicion by physicians also contribute to diagnostic challenges.4, 5, 8 Consequently, at the time a definitive diagnosis is made, more than one-fourth of SBA cases have regional lymph node and/or peritoneal dissemination.1 These late presentations are associated with a median overall survival of only 8 to 20 months and a five-year survival rate ranging from 10 to 26 per cent.1–3 Peritoneal carcinomatosis (PC) has been identified as the most common form of disease progression for SBA.1 The late presentation, along with a lack of consensus on standard treatment, makes the prognosis grim. PC is usually resistant to conventional treatments including systemic chemotherapy, radiation, or abdominal resection alone.8
As a result of the poor results of traditional treatment, aggressive therapies, including cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC), have become more attractive options that have been gaining momentum in the last few decades.9–13 Multiple studies have demonstrated that HIPEC substantially improves outcomes, and quality of life in PC, secondary to a variety of gastrointestinal malignancies compared with historic series and best-available systemic therapies, including those of appendiceal and colorectal origins.12, 13 However, there is a paucity of data published to date regarding the outcome of PC secondary to SBA (PCSBA) treated with HIPEC.11, 14 This series represents the largest series published to date, which examines our experience with 17 consecutive patients diagnosed with PCSBA who were treated with HIPEC at our institution.
Methods
From November 1995 to June 2011, 17 patients from Wake Forest University Baptist Hospital, who underwent HIPEC at least once for PC as a result of primary SBA, were identified from a prospective database. The project was approved by Institutional Review Board. Patients were selected for the HIPEC operation based on several criteria, which included, but ere not limited to, medical fitness for the rigors of HIPEC procedure and state of disease. Preoperative images, including computed tomography, magnetic resonance imaging, and positron emission tomography scans, were used to rule out any extra-abdominal disease and determine the resectability of PC. Our techniques for HIPEC have been described elsewhere and are briefly described subsequently.12–14 All patients underwent surgical resection based on their symptoms and extent of disease on imaging modalities; additional therapies varied among the 17 patients. All had PC at the time of HIPEC. Cytoreductive surgery (CS) consisted of the resection of the primary tumor as well as the resection of all tissue or nonvital organs seeded with gross tumor. Resection of involved peritoneum was also performed as deemed safe and feasible in each patient. Peritonectomies were performed for bulk disease only but were not performed on a routine basis. Lysis of adhesion was also routinely performed if necessary to maximize the surface area of abdominal organs in preparation for chemotherapy.
HIPEC was administered intraoperatively after CS. Patients were cooled to a core temperature of 34 to 35°C by passive measures. Inflow and outflow catheters for peritoneal perfusion were placed percutaneously into the abdominal cavity, and the abdominal skin incision was temporarily closed, with a running cutaneous suture, to maintain a closed space for the peritoneal perfusate and to prevent leakage. A perfusion circuit with 3 L of lactated Ringer’s solution was established at a flow rate of approximately 1000 mL/min by a roller pump managed by a pump technician. The circuit was run through the roller pump and a heat exchanger, and perfusate temperature was strictly monitored by temperature probes on the inflow and outflow catheters. Once outflow temperatures reached 39°C, 30 mg mitomycin C was added to the perfusate. After 60 minutes, an additional 10 mg was added and total perfusion time equaled 120 minutes. The abdomen was gently massaged throughout the perfusion to optimize drug distribution. After completion of the perfusion, the peritoneum was washed with several liters of lactated Ringer’s solution; the abdomen was opened and requisite anastomoses and stomas created before final closure if needed.
Survival time was calculated based on the time between the initial HIPEC treatment and the last known date of clinical follow-up for surviving patients or the date of death for the patients who died. Median survival was estimated in months using the Kaplan-Meier method. Median survival after diagnosis was calculated based on the time in months between the date of diagnosis of SBA and the date of last follow-up or date of death. Additionally, Kaplan-Meier survival analyses were performed using SAS 9.2 (SAS Institute) software to generate a survival curve.
Results
There were 10 females and seven males. All patients in this series were diagnosed with a primary SBA of various locations and had progressed to PC at the time of HIPEC. Three of the 17 patients had a repeat HIPEC (11, 21, and 20 months after the initial HIPEC procedure). The mean age at the time of the operation was 52.2 years (range, 32 to 65 years). Primary locations of SBA differed among the 17 patients; one was in the duodenum (6%), nine in the jejunum (47%), and seven in the ileum (35%). With one of 17 patients having primary SBA originating from the duodenum (compared with the jejunum or ileum), the duodenal site was less likely to be the site of origin (P = 0.05; 80% power, using a normal approximation). Demographic, histologic, and survival data along with perioperative and postoperative variables are shown in Table 1.
Table 1.
Demographic, Histologic, and Survival Data of 17 Patients with Variables in Perioperative and Postoperative Outcome
| Patient No. | Age at HIPEC (years) | Gender | Primary Site | Interval between Primary Diagnosis and HIPEC (months) | Survival after Primary Diagnosis (months) | Survival after HIPEC (months) | Number of Visceral Resections | Duration of HIPEC (hours) | Length of ICU Stay (days) | Length of Hospital Stay (days) | Clavien-Dindo Classification (grade)18 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | Jejunum | 6 | 36 | 30 | 2 | 11 | 1 | 11 | I |
| 2 | 37 | M | Ileum | 10 | 55 | 45 | 3 | 8 | 0 | 6 | I |
| 3 | 61 | F | Jejunum | 12 | 64 | 52 | 5 | 7.5 | 1 | 13 | I |
| 4 | 47 | F | Jejunum | 20 | 51 | 31 | 3 | 7.5 | 2 | 11 | I |
| 5 | 53 | F | Jejunum | 46 | 57 | 11 | 3 | 6.5 | 0 | 6 | IIIa |
| 6 | 54 | F | Jejunum | 16 | 21 | 5 | 3 | 8.3 | 2 | 20 | I |
| 7 | 60 | M | Ileum | 23 | 28 | 5 | 4 | 7 | 1 | 13 | I |
| 8 | 59 | M | Ileum | 12 | 15 | 3 | 3 | 9 | 2 | 24 | I |
| 9 | 55 | M | Duodenum | 21 | 40 | 19 | 2 | 7 | 1 | 7 | I |
| 10 | 64 | F | Jejunum | 6 | 33 | 27 | 3 | 8.2 | 0 | 6 | I |
| 11 | 32 | M | Ileum | 7 | 12 | 5 | 6 | 5.5 | 0 | 15 | I |
| 12 | 58 | M | Ileum | 2 | 47 | 45 | 3 | 8.2 | 2 | 7 | IIIa |
| 13 | 40 | F | Ileum | 7 | 21 | 14 | 3 | 5.5 | 0 | 7 | I |
| 14 | 65 | M | Jejunum | 33 | 35 | 2 | 3 | 6.5 | 0 | 6 | I |
| 15 | 63 | F | Ileum | 7 | 31 (alive) | 24 | 1 | 10 | 1 | 6 | I |
| 16 | 44 | F | Jejunum | 35 | 42 | 7 | 3 | 7.35 | 1 | 9 | I |
| 17 | 60 | F | Jejunum | 6 | 14 (alive) | 8 | 4 | 5.3 | 0 | 7 | I |
| Mean | 52.2 | 17.1 | 37.1 | 18.4 | 3.2 | 7.55 | 0.82 | 10.2 |
HIPEC, hyperthermic intraperitoneal chemotherapy; ICU, intensive care unit; F, female; M, male.
The chronology of receiving chemotherapy treatment varied among the patients. Thirteen of 17 received chemotherapy before their HIPEC (76%); five patients received chemotherapy after their HIPEC (29%); this included three of the patients who did not have chemotherapy before surgery. Seven of the 13 patients who received preoperative chemotherapy received it within three months of their HIPEC (41% of the entire group, 54% of patients receiving chemotherapy).
The number of visceral organs resected was different in each patient. Fifteen of 17 patients underwent omentectomy (88%); 13 had semicolectomy (76%); 12 had small bowel resection (71%); four had splenectomy (24%); three appendectomy and cholecystectomy (18%); three patients had liver resection (18%); two pancreatectomy (12%); and one nephrectomy, one hysterectomy, and one oophorectomy (6%). Fourteen patients had either R0 or R1 resections, and the rest of the patients had R2 resections for their peritoneal carcinomatosis at the time of HIPEC.
The median survival after HIPEC procedure was 18.4 months; the one-year survival was 52 per cent (± 12%) with a three-year survival rate of 23 per cent (± 11%) postoperatively. Six patients developed recurrence after their initial CS and HIPEC (35%). The Kaplan-Meier curve shows overall survival of 17 patients status post-HIPEC operations. Two patients are still alive, one of whom has no evidence of disease; however, 15 died of progressive intra-abdominal disease. A median overall survival after diagnosis of 37 months was achieved in the study, excluding the two patients who we are still following at our institution, with a 1-, 2-, 3-, and 5-year overall survival of 94.1, 75.3, 48.3, and 6.9 per cent, respectively (Fig. 1A–B).
Fig. 1.
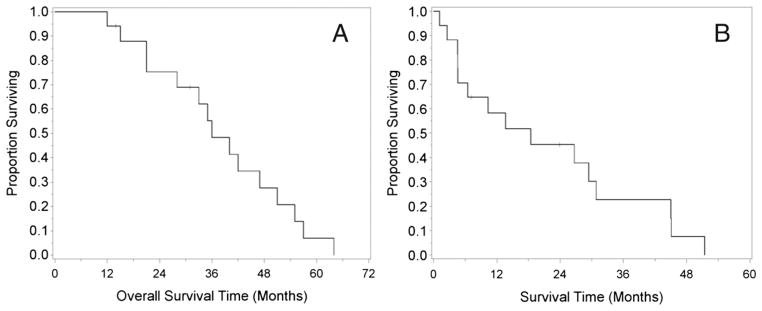
(A) Kaplan-Meier analysis of survival since initial diagnosis in 17 patients with PC originating from SBA. (B) Kaplan-Meier analysis of postoperative survival in seventeen patients with PC originating from SBA. Two patients, 15 and 17, are still alive at 24 months and 7 months, respectively. PC, peritoneal carcinomatosis; SBA, small bowel adenocarcinoma.
The extent of disease, the magnitude of cytoreduction, the duration of the operation, and health status of the patients all made morbidity and mortality significant. The mean duration of the HIPEC procedure was 7.5 hours (range, 6.5 to 11 hours), and total length of hospital stay was 10 days (range, 6 to 24 days). There was no treatment-related mortality. Six patients were readmitted to the hospital within 30 days of discharge (35%). Eight patients (47%) experienced postoperative complications, in which four patients had neutropenia (23%); two patients had major postoperative complications in the form of intra-abdominal abscess requiring interventions (12%) as documented in Table 1.
Discussion
PC from SBA is still a diagnostic and treatment challenge to physicians as a result of its rarity and poor prognosis when compared with PC originating from other gastrointestinal sources.15 Several studies have been published on the natural history of PC derived from gastrointestinal malignancies, which have found a median survival of only 3.1 to seven months in patients not being aggressively treated with HIPEC.8 Treatments for PC with systemic chemotherapy and conventional surgery have been attempted in the past but have shown limited efficacy. Systemic chemotherapy agents are hampered by peritoneal barrier. In the study published by Koo et al.,16 an overall survival of 11.8 months was achieved in patients with advanced SBA who received chemotherapy alone. However, the use of adjuvant chemotherapy with 5-fluorouracil and a platinum agent could potentially add to survival benefit. Overman et al.3 demonstrated a higher response rate and progression-free survival using the two-agent combination compared with other chemotherapy regimens; and their experience with overall survival rate was not different from other authors’ reports, which was approximately 12 months. The use of using systemic chemotherapy in combination with HIPEC remains uncertain; thus, it needs to be further evaluated with larger prospective clinical data. Conventional surgery is frequently limited to diverting ileostomy and/or draining tube gastrostomy, which, although of palliative value, have limited use. However, with more aggressive treatment such as HIPEC, overall survival can be extended to as long as 57 months.14
The three parts of the small bowel, duodenum, jejunum, and ileum, are not represented equally in incidence in this series. Primary SBA occurs most commonly in the duodenum, which accounts for half of all documented cases; but the majority of PCs arose from either the jejunum or ileum, as shown in our series,1 which is consistent with the literature. The site of primary cannot be explained by chance alone. For any carcinoma in the gastrointestinal tract to disseminate through the peritoneum, it has to penetrate the full thickness of the bowel wall to have access to the peritoneum. However, because the duodenum is predominantly retroperitoneal, it naturally forms a barrier that makes peritoneal dissemination difficult, thus explaining its rarity.
In our group’s experience, treatment of PC from mesothelioma or the gastrointestinal tract with HIPEC extends median survival time while maintaining a good quality of life.15, 17 Most of the experience with HIPEC has been published on PC that originated in the appendix, colon, or stomach. The rarity of small bowel carcinoma makes experience with PC from this site extremely limited. A literature search identified three published studies on the outcome of HIPEC in patients with PCSBA,11, 15, 17 all of which showed similar outcomes. In the first of these studies, we had previously published a series of six patients treated with HIPEC.17 Marchettini11 reported a series of six patients as well with similar outcomes. Chua et al.15 showed similar results with their data and from the meta-analysis of all three series published at the time. In this series, we have expanded our patient population from the initial six patients published in our first series16 to include all patients with SBA who underwent HIPEC in the past 15 years. At 17 patients, this is the largest that has been reported. Our patients had an overall postoperative survival of 27.6 months after HIPEC if they had the operation within 12 months of initial diagnosis. The same survival benefit dropped to 11.4 months if they underwent the procedure longer than one year after diagnosis. Furthermore, reducing tumor burden after extensive cytoreduction is expected to correlate with better overall survival.
The prognosis in patients with PCSBA is poor, and there is scarcity of data on how best to treat these patients. Difficult questions continue to arise on what the best treatment option is for PCSBA. Early treatment with HIPEC may prolong survival. Whether a repeat HIPEC would improve outcome in patients with primary SBA is unclear. However, the three patients in this series who underwent a repeat HIPEC, and other studies published on PC arising from other gastrointestinal malignancies, support such a possibility. Additional prospective studies need to be investigated in this patient population. Nonetheless, it seems that treating PC originating from SBA with HIPEC is a reasonable surgical option for patients diagnosed at an advanced stage. In comparison, after HIPEC in patients with advanced disease who had already undergone chemotherapy (with an average survival of 12 months3, 17), the average post-HIPEC survival of 22 months had been achieved at all three centers reporting results, as shown in Table 2, doubling the survival time of what had been accomplished by medical therapy alone. A total of 76 per cent of patients in our series received chemotherapy before their operation; and all of them had an unfavorable response to their treatment or disease had progressed. HIPEC lengthened their survival and quality of life by an average of more than 18 months. Ideally, this finding would be confirmed by a randomized trial; however, the rarity of this disease makes such a study implausible. Although the results of HIPEC is not a curative treatment for PCSBA, results from this largest published experience as well as multiple centers point to HIPEC being the most effective surgical option for this population.
Table 2.
Published Series of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis Originating from Small Bowel Adenocarcinoma
| Source | Patients (no.) | Mean Age (years) | Institution† | Survival Status Post-HIPEC (months) |
|---|---|---|---|---|
| Jacks et al. | 6 | 47.7 | WFU | 30.1 |
| Marchettini | 6 | 45.7 | WHC | 27.0 |
| Chua et al. | 7 | 44.7 | UNSW | 21.2 |
| Sun et al.* | 17 | 52.2 | WFU | 18.4 |
| Total | 30 | 49.1 | 22.2 |
Current series includes the six patients published in our group’s original series.16
WFU, Wake Forest University; WHC, Washington Hospital Center; UNSW, University of New South Wales.
Acknowledgments
We thank Mary Cromer for her ongoing database maintenance and Joan Feder for her editorial insights.
Footnotes
Presented at the Annual Scientific Meeting and Postgraduate Course Program, Southeastern Surgical Congress, Jacksonville, FL, February 9–12, 2013.
References
- 1.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–26. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 2.Locher C, Malka D, Boige V, et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology. 2005;69:290–4. doi: 10.1159/000089678. [DOI] [PubMed] [Google Scholar]
- 3.Overman MJ, Kopetz S, Wen S, et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038–45. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 4.Talamonti MS, Goetz LH, Rao S, et al. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564–70. doi: 10.1001/archsurg.137.5.564. [DOI] [PubMed] [Google Scholar]
- 5.North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg. 2000;66:46–51. [PubMed] [Google Scholar]
- 6.Frost DB, Mercado PD, Tyrell JS. Small bowel cancer: a 30-year review. Ann Surg Oncol. 1994;1:290–5. doi: 10.1007/BF02303567. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States. Changes in epidemiology, treatment and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 8.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–63. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Witkamp AJ, de Bree E, Kaag MM, et al. Extensive cytoreductive surgery followed by intra-operative hyperthermic intra-peritoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979–84. doi: 10.1016/s0959-8049(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27:239–43. doi: 10.1053/ejso.2000.1038. [DOI] [PubMed] [Google Scholar]
- 11.Marchettini P, Sugarbaker PH. Mucinous adenocarcinoma of the small bowel with peritoneal seeding. Eur J Surg Oncol. 2002;28:19–23. doi: 10.1053/ejso.2001.1196. [DOI] [PubMed] [Google Scholar]
- 12.Shen P, Levine EA, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 13.McQuellon RP, Loggie BW, Lehman AB, et al. A long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–62. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 15.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 16.Jacks SP, Hundley JC, Shen P, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. J Surg Oncol. 2005;91:112–7. doi: 10.1002/jso.20296. [DOI] [PubMed] [Google Scholar]
- 17.Chua TC, Koh JL, Yan TD, et al. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. J Surg Oncol. 2009;100:139–43. doi: 10.1002/jso.21315. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]


