Abstract
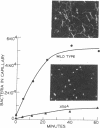
The source of energy for bacterial motility is the intermediate in oxidative phosphorylation, not ATP directly. For chemotaxis, however, there is an additional requirement, presumably ATP. These conclusions are based on the following findings. (i) Unlike their parents, mutants of Escherichia coli and Salmonella typhimurium that are blocked in the conversion of ATP to the intermediate of oxidative phosphorylation failed to swim anaerobically, even when they produced ATP. When respiration was restored to the mutants, motility was simultaneously restored. (ii) Carbonylcyanide m-chlorophenylhydrazone, which uncouples oxidative phosphorylation, completely inhibited motility even though ATP remained present. (iii) Arsenate did not inhibit motility in the presence of an oxidizable substrate, though it did reduce ATP levels to less than 0.3% (iv) Arsenate completely inhibited chemotaxis under conditions where motility was normal.
Keywords: oxidative phosphorylation, electron transport, flagella
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J., Dahl M. M. A method for measuring the motility of bacteria and for comparing random and non-random motility. J Gen Microbiol. 1967 Feb;46(2):161–173. doi: 10.1099/00221287-46-2-161. [DOI] [PubMed] [Google Scholar]
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Genetics of motility in Escherichia coli: complementation of paralysed mutants. Genetics. 1967 Jul;56(3):363–373. doi: 10.1093/genetics/56.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B. An S-adenosylmethionine requirement for chemotaxis in Escherichia coli. Can J Microbiol. 1972 Nov;18(11):1695–1701. doi: 10.1139/m72-263. [DOI] [PubMed] [Google Scholar]
- Barlow G. H., Blum J. J. On the "Contractility" of Bacterial Flagellae. Science. 1952 Nov 21;116(3021):572–572. doi: 10.1126/science.116.3021.572-a. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavari B. Z., Avi-Dor Y. Effect of carbonyl cyanide m-chlorophenylhydrazone on respiration and respiration-dependent phosphorylation in Escherichia coli. Biochem J. 1967 May;103(2):601–608. doi: 10.1042/bj1030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. M., Butlin J. D., Crane F. L. Reconstitution of the energy-linked transhydrogenase activity in membranes from a mutant strain of Escherichia coli K12 lacking magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1973 Apr;132(4):689–695. doi: 10.1042/bj1320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M. A., Doetsch R. N. Effect of respiratory inhibitors on the motility of Pseudomonas fluorescens. J Bacteriol. 1969 Feb;97(2):806–811. doi: 10.1128/jb.97.2.806-811.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerstedt R. H. An automated method for ATP analysis utilizing the luciferin-luciferase reaction. Anal Biochem. 1973 Apr;52(2):449–455. doi: 10.1016/0003-2697(73)90047-x. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Boos W. Energy coupling of the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1973 Jun 25;248(12):4429–4435. [PubMed] [Google Scholar]
- SHERRIS J. C., PRESTON N. W., SHOESMITH J. G. The influence of oxygen and arginine on the motility of a strain of Pseudomonas sp. J Gen Microbiol. 1957 Feb;16(1):86–96. doi: 10.1099/00221287-16-1-86. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Motility tracks: technique for quantitative study of bacterial movement. Appl Microbiol. 1969 Apr;17(4):584–588. doi: 10.1128/am.17.4.584-588.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]