Abstract
BACKGROUND
Many studies have found that risk of childhood asthma varies by month of birth, but few have examined ambient aeroallergens as an explanatory factor.
OBJECTIVE
To examine whether birth during seasons of elevated ambient fungal spore or pollen concentrations is associated with risk of early wheezing or blood levels of Th1- and Th2-type cells at 24 months of age.
METHODS
514 children were enrolled before birth and followed to 24 months of age. Early wheezing was determined from medical records and Th1 and Th2-type cells were measured in peripheral blood using flow cytometry. Ambient aeroallergen concentrations were measured throughout the study period and discrete seasons of high spore and pollen concentrations were defined.
RESULTS
A seasonal pattern was observed, with birth in the fall-winter (the fungal spore season) associated with increased odds of early wheezing (adjusted odds ratio = 3.1; 95% confidence interval: 1.3, 7.4). Increasing mean daily concentrations of basidiospores and ascospores in the first three months of life were associated with increased odds of wheeze, as were increasing mean daily concentrations of total and specific pollen types. Levels of Th1 cells at age 24 months were positively associated with mean fungal spore concentrations and negatively associated with mean pollen concentrations in the first three months of life.
CONCLUSIONS
Children with higher exposure to fungal spores and pollen in the first three months of life were at increased risk of early wheezing. This association was independent of other seasonal factors, including ambient PM2.5 levels and lower respiratory infections.
Keywords: asthma, wheeze, birth season, mold, pollen, epidemiology
INTRODUCTION
Multiple studies have found month of birth to be associated with the risk of allergic sensitization[1–4] or asthma[5–12] later in life, an observation suggesting that exposure to seasonal allergens in the perinatal period may contribute to the development of atopic disease. However, previous studies have shown little consistency as to which months are associated with the highest asthma risks and whether these months are associated with higher ambient concentrations of specific aeroallergens, such as fungi or pollen. Additionally, month of birth may represent exposures other than aeroallergens, including respiratory syncytial virus (RSV)[5], air pollution, and residential dampness, all of which have been associated with development of early childhood wheeze and asthma.[13]
Aeroallergen exposure is clearly associated with asthma exacerbations,[14, 15] but the role of such exposures in disease induction is less clear. The initial priming of T-cells to respond to allergens is thought to occur during late gestation and the neonatal period, and early-life exposure to ambient allergens may reinforce T-cell switching to favor a predominance of Th2 cells.[16] Kihlström et al.[17, 18] found that children born in the three months before a season of extremely high birch pollen were at increased risk of asthma at age five, but children born after this season (whose mothers were exposed during pregnancy) were not. A cohort study of children at risk for atopic disease found that higher concentrations of Penicillium spp. and total fungal spores in living room air of infants 2–4 months of age were associated with an increased risk of wheeze and persistent cough at age 12 months.[19, 20] Another longitudinal study reported that higher spore concentrations in living room air collected when infants were 2–3 months old were associated with increased risks of lower respiratory infection at one year of age[21] and of allergic rhinitis at age five.[22] Recent work also implicates early-life exposure to airborne particulate matter (PM) with development of asthma and allergen sensitivity in young children in the Netherlands.[23]
In the present analysis we examine whether the association between month of birth and early wheezing at age two could be explained by high ambient fungal spore or pollen exposure in the first three months of life. Additionally, we measured levels of Th1- and Th2-type cells in peripheral blood at age 24 months to determine whether cytokine profiles were associated with early life aeroallergen exposure.
METHODS
Participants and Recruitment
Subjects were children in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a birth cohort study that is investigating the effects of environmental exposures on the health of low-income, predominantly Mexican immigrant families in an agricultural region of California. Pregnant women were recruited between October 1999 and October 2000 through collaborating prenatal clinics. Women were eligible to participate if they were less than 20 weeks’ gestation, 18 years or older, spoke English or Spanish, qualified for low-income government health insurance (Medicaid), and planned to deliver at the county hospital. Of 1,130 eligible women, 601 agreed to participate, and 536 newborns were enrolled in the study. The present analysis was limited to 514 children with complete medical record data through 24 months of age. Blood samples for measurement of Th1 and Th2 cytokines were available for 236 of these children at the 24-month visit (median age: 23.9 months; IQR: 0.9 months; range: 21.7–29.0 months). Study procedures were approved by the University of California, Berkeley Committee for the Protection of Human Subjects.
Data Collection
Pediatric medical records were collected from all facilities where the child had received care between birth and 24 months of age. A single registered nurse abstracted relevant information onto standardized forms. A child was considered to have early wheezing if medical records indicated a clinician’s diagnosis of asthma at any time between birth and 24 months of age. Because many of these children may not continue to have asthma at later ages[24], we considered this diagnosis to represent “early wheezing” rather than asthma.
Standardized interviews were administered to the mothers during pregnancy, after delivery, and when the children were approximately six months (median: 6.4 months; IQR: 1.0 month; range: 5.5–11.9 months) and 12 months (median: 12.4 months; IQR: 1.5 months; range: 11.1–22.8 months) of age. Interviews assessed demographic data and child exposures (e.g., duration of breastfeeding, second-hand smoke, frequency of colds, and presence of pets in the home), and were conducted in English or Spanish by bilingual, bicultural interviewers. Home inspections were conducted by trained inspectors when the children were 6 and 12 months of age to assess environmental exposures (e.g., wall moisture measurements, visible fungal growth, evidence of cockroach or rodent infestation, and presence of a gas stove).
Blood was analyzed for Th1- and Th2-associated cytokines if the sample was received at the study laboratory within 48 hours of collection. Methods for Th1 and Th2 analysis have been described previously.[25] Briefly, 500 μl of whole blood were activated for four hours with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma-Aldrich, St. Louis, MO) to stimulate cytokine production. Fluorescent stain was then applied with antigen-specific antibodies for CD4+ T-lymphocytes (PerCP, Becton Dickinson, Franklin Lakes, NJ), IFN-γ (IFN-γ /FITC, Becton Dickinson), and IL-4 (IL-4/PE, Becton Dickinson). With flow cytometry, the CD4+ population was identified and gated; these cells were then examined for expression of IFN-γ and IL-4. Cells that stained positive for IFN-γ were classified as Th1 cells, and those that stained positive for IL-4 were classified as Th2 cells. Th1 and Th2 percentages were defined as the proportion of CD4+ cells identified as IFN-γ or IL-4 positive, respectively.
Measurement of ambient spore and pollen air concentrations
The study area (Salinas Valley, California) is a valley approximately 60 miles long and 10 miles wide, with a Mediterranean climate characterized by mild, rainy winters and dry summers. We measured fungal spore and pollen concentrations between October 1999 and July 2003, a period that encompassed the in utero period and first 24 months of life for all study children. The time frame of interest was exposure during the first three months of life.
Airborne spores and pollen were collected with a Hirst-type sampler[26] (Seven-Day Recording Volumetric Spore Trap; Burkard Manufacturing Co. Ltd., Rickmansworth, UK) located in the city of Salinas and placed 10 m above the ground to avoid over-representation of local vegetation. An AAAAI-certified analyst read three slides per week throughout the study period.
Twenty-seven spore and 48 pollen groups were identified. Seven fungal groups were observed on more than half of all sampling days and accounted for 82% of the annual spore concentrations: Cladosporium spp. (44%), basidiomycetes (25%), ascomycetes (6%), Aspergillus or Penicillium spp. (4%), Botrytis spp. (1%), smuts or myxomycetes (1%), and Alternaria spp. (0.5%). Ten plant groups were observed on more than 10% of all sampling days and accounted for 75% of the annual pollen concentrations: cypress (32%), oak (11%), unidentified pollen grains (8%), pine (7%), grass (6%), nettle/pellitory (4%), mulberry (4%), alder (4%), elm (3%), sage/wormwood (2%), and plantain (2%).
For each child, mean daily spore and pollen concentrations during the first three months of life were calculated for the four identified spore and seven identified pollen types that accounted for more than 3% of the total. The four spores and seven pollen also were summed to create variables for total spore and total pollen concentration in the first three months of life.
Measurement of PM concentrations
PM data were obtained from the Monterey Unified Air Pollution Control District (MBAPCD), which operated an air pollution monitoring station within ¼ mile of the study’s aeroallergen station. For 24 hours every sixth day, the MBAPCD station measured PM with aerodynamic diameters ≤ 10 μm (PM10) and ≤ 2.5 μm (PM2.5) with high-volume Sierra-Andersen gravimetric samplers (Thermo Scientific, Waltham, MA). For each child, we calculated the mean 24-hour PM2.5 and PM10 concentration during the first three months of life.
Seasonal Patterns of Exposure
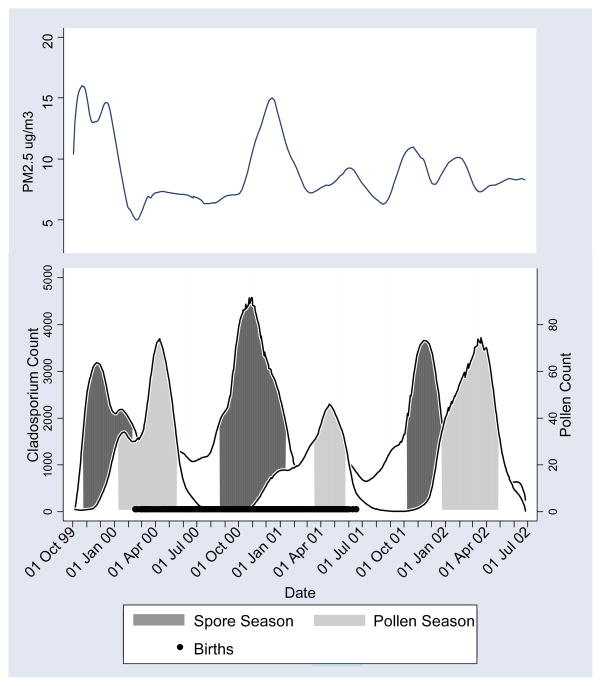
To determine seasonal patterns of ambient exposures, we plotted daily concentrations of spores and pollen over the study period. A Lowess smooth[27] was fit to each scatter-plot, and high-concentration days were defined as those in which the fitted values were ≥40% of the maximum fitted value. High concentration days were clustered in time, resulting in three distinct periods each year: 1) high spore/low pollen concentration, termed the “spore season”, 2) high pollen/low spore concentration, termed the “pollen season”, and 3) periods with low concentrations of both. These seasons are shown in Figure 1, with the period of participants’ births (February 16, 2000 through June 18, 2001) shown in black along the x-axis. Children were categorized according to season of birth, with low spore/low pollen as the reference category. There was very little overlap between the spore and pollen seasons; however, four children born between February 16 and March 1, 2000 overlapped the end of the spore season and the beginning of the pollen season. These children were classified as pollen season births because their primary early life exposure would be pollen. (Changing these children to spore season births did not change results markedly.)
Figure 1.
Lowess smoothing of daily spore and pollen concentration and mean PM2.5 levels. Salinas Valley, California, 1999–2002.
We also examined seasonal patterns of PM air pollution using Lowess plots. Figure 1 shows that the periods of elevated PM2.5 tended to coincide with those of high spore concentrations. No seasonal patterns were observed for PM10, but this is not surprising since the components of PM10 (PM2.5 and PM10-2.5) have different temporal peaks, which would tend to reduce PM10 seasonality.
Data Analysis
We used logistic regression to examine the association between early wheezing and mean ambient spore and pollen concentrations in the first three months of life. Spore and pollen concentrations were negatively correlated and no children (including the 4 children born in the overlap of the spore and pollen seasons) simultaneously experienced high spore and pollen levels in the first three months of life; therefore, total spore and pollen exposure were not included in the same model. Separate models also were created for individual spore and pollen types. Because of the wide difference in ranges of spore and pollen concentrations, odds ratios were standardized to an increase in units equal to the interquartile range (IQR) for each exposure.
Associations between Th1 and Th2 profiles at age 24 months and mean spore and pollen concentrations were examined with linear regression. Th1, Th2, and the ratio of Th1:Th2 were log-transformed and three influential (outlier or leverage) points were excluded. Th1 and Th2 were analyzed both as absolute levels and as percentage of total CD4+ cells; as the results were similar by both approaches, we present only the latter.
To explore the possibility of confounding, we examined other early life exposures (Table 1) that we hypothesized might be associated with early wheezing. Variables of particular interest were: physician-diagnosed respiratory infections; visible mold, dampness, or evidence of cockroaches or rodents in the home; and PM2.5 exposure, all of which may display seasonal patterns. Exposures associated with both the exposure (season of birth) and outcomes (wheezing or Th1/Th2) were included as covariates in the multiple regression models. Covariates were kept in the model if they had a p-value <0.1 or if their exclusion changed the coefficient for the main effect by 10% or more. Because exposure to PM2.5 and fungal spores were highly correlated (r = 0.6), we regressed spore concentration on PM2.5 and used the residuals from this regression in the models that contained spore variables.
Table 1.
Selected characteristics of study population and associations with early wheezing (N = 514)
| N (%) | Early Wheezing
|
|
|---|---|---|
| Unadjusted OR (95% CI) | ||
| Baby’s gender | ||
| Boy | 255 (49.6) | 1.0 |
| Girl | 259 (50.4) | 0.7 (0.4, 1.4) |
| Maternal Country of Birth | ||
| United States | 69 (13.5) | 1.0 |
| Mexico | 433 (84.4) | 0.6 (0.2, 1.4) |
| Other | 11 (2.1) | 0.9 (0.1, 8.0) |
| Family Income | ||
| Above poverty level | 171 (33.3) | 1.0 |
| At or below poverty level | 343 (66.7) | 2.5 (1.0, 6.2)* |
| Family History of Asthma | ||
| No | 417 (82.3) | 1.0 |
| Yes | 90 (17.8) | 1.4 (0.6, 3.2) |
| Breastfeeding Duration | ||
| Never breastfed | 28 (5.5) | 1.0 |
| ≤ 6 months | 272 (53.5) | 1.2 (0.3, 5.4) |
| > 6 months | 208 (40.9) | 0.7 (0.1, 3.2) |
| Maternal Smoking during Pregnancy | ||
| No | 483 (94.2) | 1.0 |
| Yes | 30 (5.9) | 1.6 (0.5, 5.4) |
| Child Exposed to Tobacco Smoke (0–12 months) | ||
| No | 431 (92.1) | 1.0 |
| Yes | 37 (7.9) | 3.0 (1.2, 7.9)* |
| Signs of Cockroaches in home (0–12 months) | ||
| No | 140 (33.5) | 1.0 |
| Yes | 278 (66.5) | 0.9 (0.4, 1.9) |
| Signs of rodents in home (0–12 months) | ||
| No | 251 (60.1) | 1.0 |
| Yes | 167 (40.0) | 1.8 (0.9, 3.8) |
| Moderate-to-severe visible mold in home (0–12 months) | ||
| No | 148 (35.4) | 1.0 |
| Yes | 270 (64.6) | 0.8 (0.4, 1.7) |
| Any wall >17% moisture (6 months) | ||
| No | 273 (72.4) | 1.0 |
| Yes | 104 (27.6) | 0.3 (0.1, 1.0)* |
| Gas stove in home (0–12 months) | ||
| No | 91 (19.4) | 1.0 |
| Yes | 377 (80.6) | 3.8 (0.9, 16.4) |
| Any pets (0–12 months) | ||
| No | 302 (67.0) | 1.0 |
| Yes | 149 (33.0) | 1.9 (0.9, 3.9) |
| Diagnosis of lower respiratory infection (0–12 months)1 | ||
| No | 350 (68.1) | 1.0 |
| Yes | 164 (31.9) | 4.0 (2.0, 8.2)* |
| Mean daily PM2.5 in the first three months of life | ||
| < 8 μg/m3 | 230 (44.8) | 1.0 |
| 8 – 12 μg/m3 | 218 (42.4) | 1.7 (0.8, 3.7) |
| ≥ 12 μg/m3 | 66 (12.8) | 2.4 (0.9, 6.4) |
| Season of birth | ||
| Low spore/low pollen | 203 (22.0) | 1.0 |
| Spore | 195 (37.9) | 2.8 (1.2, 6.5)* |
| Pollen | 116 (22.6) | 1.6 (0.6, 4.4) |
Diagnosis of bronchitis, bronchiolitis, pneumonia or croup noted in medical records
p-value < 0.05
RESULTS
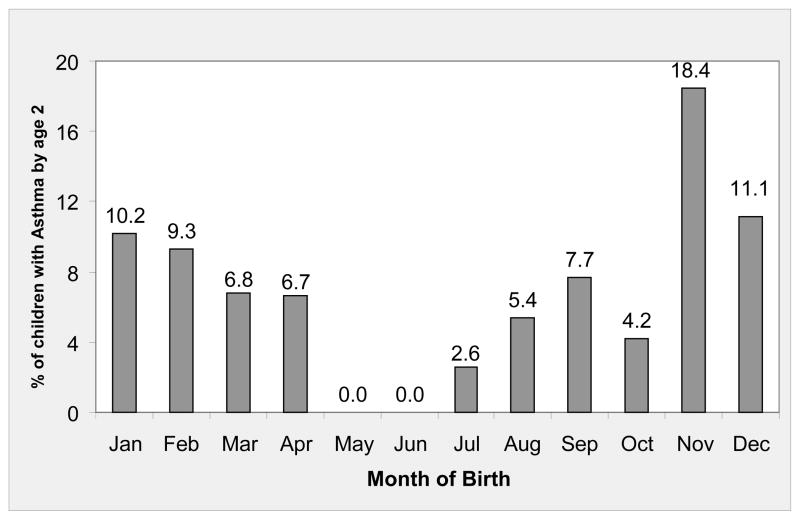
Table 1 shows the characteristics of the study population. Children were predominantly low income and of Mexican descent. Thirty-five children (6.8%) had a diagnosis of early wheezing by 24 months of age; a clear pattern was observed with month of birth (Figure 2). The unadjusted odds ratio for early wheezing for children born during the spore season relative to children born outside of the spore and pollen seasons was 2.8 (95% CI: 1.2, 6.5) (Table 1), an association that persisted after controlling for confounders (aOR: 3.1, 95% CI: 1.3, 7.4)(not shown). Poverty status, tobacco smoke exposure, and lower respiratory tract infection in the first year of life also were associated with increased odds of early wheezing, and higher PM2.5 exposure, pets, gas stove, or rodents in the home were of borderline statistical significance. Wall moisture measured at age six months was associated with reduced odds of early wheezing.
Figure 2.
Physician-diagnosed early wheezing, according to month of birth. Salinas Valley, California, 1999–2001.
In adjusted analyses, there was only a weak association between total spore concentration in the first three months of life and early wheezing (Table 2). However, basidiospore and ascospore exposure each were associated with increased odds of wheezing (aOR per IQR of exposure: 2.1 and 2.8, respectively). Higher total pollen concentration in the first three months was associated with increased odds of early wheezing (aOR = 2.0; 95% CI: 1.1, 3.9). Increasing concentrations of cypress, oak, pine, alder, and mulberry pollen in the first three months of life were also associated with greater odds of early wheezing, of which the associations for cypress, pine, and alder were statistically significant.
Table 2.
Adjusted odds ratios for association of wheeze at age 2 years associated with mean daily ambient spore and pollen concentrations in first 3 months of life
| Median (Range) | Adjusted OR (95% CI) | |
|---|---|---|
| Fungi1 | ||
| Cladosporium spp. (OR per 1776 units)3 | 1238 (610, 4180) | 0.9 (0.5, 1.6) |
| Basidiospores (OR per 930 units) | 550 (45, 1470) | 2.1 (1.0, 4.4)* |
| Ascospores (OR per 203 units) | 271 (82, 603) | 2.8 (1.3, 5.9)* |
| Asperguillus/Penicillum spp.(OR per 145 units) | 114 (25, 348) | 0.8 (0.5, 1.5) |
| Total spores (OR per 2695 units) | 2150 (1070, 6238) | 1.2 (0.7, 2.0) |
| Pollens2 | ||
| Cypress (OR per 11 units) | 4.4 (0, 29) | 2.2 (1.2, 3.9)* |
| Oak (OR per 8 units) | 0.5 (0, 16) | 1.6 (0.9, 2.6) |
| Pine (OR per 4 units) | 1.7 (0, 6) | 2.8 (1.3, 6.0)* |
| Grass (OR per 4 units) | 0.8 (0, 12) | 1.0 (0.7, 1.6) |
| Alder (OR per 2 units) | 1.0 (0, 4) | 4.7 (1.3, 17.9)* |
| Elm (OR per 2 units) | 0.2 (0, 6) | 1.0 (0.7, 1.4) |
| Mulberry (OR per 2 units) | 0 (0, 7) | 1.6 (1.0, 2.6) |
| Nettle (OR per 1 units) | 1.9 (1, 4) | 0.9 (0.5, 1.6) |
| Total pollen (OR per 27 units) | 18.6 (2, 61) | 2.0 (1.1, 3.9)* |
Separate models for each fungal spore, controlling for gas stove in home, respiratory infection in first year of life, and PM2.5 in first three months of life (residuals independent of spores).
Separate models for each pollen, controlling for gas stove in home, respiratory infection in first year of life, and PM2.5 in first three months of life.
Units equivalent to IQR of exposure for each spore or pollen type
p-value< 0.05
The median level of Th1-type cells in this population was 3.4% of total CD4+ lymphocytes (IQR: 3.07; range: 0.03 – 21.6). The median level of Th2-type cells was 0.9% of total CD4+ cells (IQR: 0.8; range: 0.4 – 4.1). Previously, we have shown that early wheezing was associated with higher Th2 levels in this population.[28]
The associations between Th1, Th2 and Th1:Th2 ratio and ambient spore and pollen concentrations in the first months of life are shown in Table 3, with beta coefficients representing the percent change in Th1, Th2 or Th:Th2 associated with a unit increase in spore or pollen concentration equal to the IQR. Th1:Th2 ratio was positively associated with number of colds, and negatively associated with early PM2.5 exposure and having a gas stove in the home (not shown). Thus, analyses controlled for these variables. Ambient fungal spore concentration in the first three months of life was positively associated with Th1 levels and Th1:Th2 ratio. Early life pollen concentration was negatively associated with Th1 levels at age 2.
Table 3.
Adjusted regression of Th1, Th2, and Th1:Th2 ratio at age 24 months on total mean spore and pollen concentrations
| Th1
|
Th2
|
Th1:Th2
|
|
|---|---|---|---|
| Adjusted β1 (95% CI) | Adjusted β2 (95% CI) | Adjusted β3 (95% CI) | |
| Total spores (per 2695 units)4 | 22.6 (1.9, 47.5)* | −11.7 (−25.4, 4.6) | 38.8 (14.3, 68.6)* |
| Total pollen (per 27 units)4 | −20.3 (−33.5, −4.5)* | 0.8 (−14.4, 18.6) | −13.5 (−28.7, 4.8) |
Controlling for ≥2 colds in the first 6 months of life and PM2.5 in first three months of life.
Controlling for presence of gas stove in home.
Controlling for ≥2 colds in the first 6 months of life, PM2.5 in first three months of life, and gas stove in home.
Equivalent to IQR of exposure
p-value < 0.05
DISCUSSION
We found that children born during periods of high ambient fungal spore concentration (February 16, 2000-March 1, 2000 or August 21, 2000-January 10, 2001) were at greatest risk of early wheezing by age 24 months. Although high concentration of total ambient spores in early life was not associated with wheezing, high concentration of basidiospores and ascospores in the first three months was. Total pollen concentration in the first three months of life also was associated with increased odds of wheezing, with individual associations seen with cypress, pine, and alder pollen. These associations were independent of lower respiratory illnesses and PM2.5 exposure in the first three months of life, both of which also were associated independently with increased odds of wheezing. Control for other seasonal factors, such as dampness, cockroaches, and rodents in the home did not alter these findings.
Our finding of increased odds of wheezing with elevated ambient concentrations of certain fungi is somewhat consistent with Gent et al.,[20] who found higher indoor concentrations of Penicillium spp. and total fungi at two to four months of age to be associated with greater risk of wheezing at age one. Indoor fungi were not measured in this study, but ambient and indoor concentrations have been shown to be correlated and the relationship is seasonally dependent [29]. Indoor concentrations of Penicillium/Aspergillus (which cannot be distinguished and are reported as one group) often are elevated in damp homes [30], which may explain why our ambient measures were not associated with early wheezing.
To our knowledge, this is the first study to examine early-life exposure to multiple outdoor fungal groups. Basidiospores are often the most abundant outdoor allergen-bearing particle and, along with ascospores, are released during rainfall or as humidity increases [31], a finding that is consistent with the crude association of early wheezing and being born in the spore season, which coincides with the rainy season in the study area (Table 1). Gregory and Hirst first suggested basidiospores as possible allergen sources [32], but far less is known about the allergenicity of ascospores [30]. Cladosporium spp., although present in very high concentrations in this region during the spore season, were not associated with wheezing.
Other studies also support our finding of an association between early wheezing and pollen exposure in the first months of life. Kihlström et al.,[17, 18] observed increased risk of sensitization and allergic asthma among Swedish children exposed to unusually high levels of birch pollen in the first months of life. Although birch pollen was not detected in this region, a strong association was observed with alder pollen and early wheezing. Alder and birch are closely related and the major allergens from members of the birch family have been shown to cross-react [33].
Despite the observed associations of early wheezing and a number of antigen spores and pollens, our T-helper phenotype data do not offer any insight into the likelihood that the associations are related to atopic sensitization. The extent to which early exposure to antigens is important with respect to subsequent risks is not well established and undoubtedly related to a complex interplay of genetic predisposition, other environmental exposures, and the intensity and duration of exposure to specific allergens [34]. Given the developmental processes that govern T-helper maturation in children [35] and the fact that most of these children with early wheezing will not develop asthma later in childhood [24, 36], it is not surprising that a clear association between a T-helper phenotype and antigen exposure was not observed in this study.
We have assumed that the first three months of life represent the critical time period for exposure to ambient allergens, based on previous studies [34]. The sequential aeroallergen seasons dictate that children with high spore exposure between 0 and 3 months of age are likely to have high pollen exposure between 3 and 6 months of age. Thus, it is possible that the increased odds of wheezing associated with basidiospores and ascospores may actually represent an effect of exposure to pollen at slightly later ages. However, the seasonal pattern also offers the advantage of clearly differentiating children with high spore and pollen exposure because children born during the spore season have low pollen exposure and vice versa. No children were highly exposed to both spores and pollen in the first three months of life.
An additional possibility is that other factors with seasonal patterns, such as PM2.5 or RSV, are confounding the association of pollens and spores with wheezing. PM2.5 was associated with early wheezing in this population. However, analyses of pollen and spores controlled for this variable. We were not able to control for RSV in this analysis. Wu et al [5] recently showed that infants in Tennessee who were approximately 4 months of age at the peak of the RSV season were at highest risk of developing asthma by age 5, and that the seasonal birth pattern for asthma directly mirrored the pattern for RSV bronchiolitis. Like our study, Wu et al found the highest risk of asthma among children born in the fall. The authors attribute this increase in risk to RSV exposure rather than ambient allergen exposure, which was not measured. We do not have information on the seasonal pattern of RSV infection in this population. However, if the RSV peak in our study region occurs between December and February, as it did in the Tennessee study, RSV could be another possible explanatory factor for the birth season pattern of asthma risk observed.
Few studies have examined the roles of spore, pollen, and PM exposures in the early postnatal period in the development of childhood asthma or chronic wheezing. A strength of this study is that it linked date of birth to specific periods of elevated ambient allergens. We used outdoor measurements of a large number of fungal and plant groups reduced to epidemiologically relevant categories [33] and time-resolved to participant data. The number of children with early wheezing in this study was small (N=35), leading to imprecise estimation of the odds ratios. However, despite the small number of cases, we found several risk factors to be significantly associated with early wheezing. Unfortunately, atopy status as measured by skin prick testing was not available for these children; therefore we were unable to differentiate atopic from non-atopic wheezing. This is relevant, since a large number of non-atopic children with wheezing will lose their symptoms in later childhood [36].
In conclusion, we found that birth during periods of elevated spore or pollen concentration was associated with wheezing at age two years in this largely Mexican immigrant population. Our T-helper lymphocyte data do not provide evidence that the association of early aeroallergen exposure with wheezing is related necessarily to allergic sensitization in this group. Despite our lack of more specific data on atopic sensitization to aeroallergens, these latter findings are consistent with the expectation that most of our subjects will lose their wheeze in later childhood as has been observed in populations of largely European origin [36].
Acknowledgments
We gratefully acknowledge Z. Dyer, N. Kwaan, the CHAMACOS staff and students, and the CHAMACOS participants and their families, without whom this study would not have been possible.
FUNDING
Funding for this study was received from the National Institute of Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency.
Footnotes
COMPETING INTERESTS
None
Contributor Information
Kim G. Harley, School of Public Health, University of California, Berkeley, California, USA.
Janet M. Macher, California Department of Public Health, Richmond, California, USA.
Michael Lipsett, California Department of Public Health, Richmond, California, USA.
Paurene Duramad, School of Public Health, University of California, Berkeley, California, USA.
Nina T. Holland, School of Public Health, University of California, Berkeley, California, USA.
Steven S. Prager, Central Coast Allergy and Asthma, Salinas, California.
Jeannette Ferber, School of Public Health, University of California, Berkeley, California, USA.
Asa Bradman, School of Public Health, University of California, Berkeley, California, USA.
Brenda Eskenazi, School of Public Health, University of California, Berkeley, California, USA.
Ira B. Tager, School of Public Health, University of California, Berkeley, California, USA.
References
- 1.Wjst M, Dharmage S, Andre E, et al. Latitude, birth date, and allergy. PLoS Med. 2005;2:e294. doi: 10.1371/journal.pmed.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carosso A, Ruffino C, Bugiani M. The effect of birth season on pollenosis. Ann Allergy. 1986;56:300–3. [PubMed] [Google Scholar]
- 3.Croner S, Kjellman NI. Natural history of bronchial asthma in childhood. A prospective study from birth up to 12–14 years of age. Allergy. 1992;47:150–7. doi: 10.1111/j.1398-9995.1992.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 4.Korsgaard J, Dahl R. Sensitivity to house dust mite and grass pollen in adults. Influence of the month of birth. Clin Allergy. 1983;13:529–35. doi: 10.1111/j.1365-2222.1983.tb02634.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Dupont W, Griffin M, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aberg N. Birth season variation in asthma and allergic rhinitis. Clin Exp Allergy. 1989;19:643–8. doi: 10.1111/j.1365-2222.1989.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 7.Karachaliou FH, Panagiotopoulou K, Manousakis M, et al. Month of birth, atopic disease, and sensitization to common aeroallergens in Greece. Pediatr Allergy Immunol. 1995;6:216–9. doi: 10.1111/j.1399-3038.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 8.Schafer T, Przybilla B, Ring J, et al. Manifestation of atopy is not related to patient’s month of birth. Allergy. 1993;48:291–4. doi: 10.1111/j.1398-9995.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HR, Bailey PA, Bland JM. The effect of birth month on asthma, eczema, hayfever, respiratory symptoms, lung function, and hospital admissions for asthma. Int J Epidemiol. 1981;10:45–51. doi: 10.1093/ije/10.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Dik N, Tate RB, Manfreda J, et al. Risk of physician-diagnosed asthma in the first 6 years of life. Chest. 2004;126:1147–53. doi: 10.1378/chest.126.4.1147. [DOI] [PubMed] [Google Scholar]
- 11.Gazala E, Ron-Feldman V, Alterman M, et al. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41:1125–8. doi: 10.1002/ppul.20442. [DOI] [PubMed] [Google Scholar]
- 12.Linneberg A, Simonsen JB, Petersen J, et al. Differential effects of risk factors on infant wheeze and atopic dermatitis emphasize a different etiology. J Allergy Clin Immunol. 2006;117:184–9. doi: 10.1016/j.jaci.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 14.Schwindt CD, Tjoa T, Floro JN, et al. Association of atopy to asthma severity and medication use in children. J Asthma. 2006;43:439–46. doi: 10.1080/02770900600758234. [DOI] [PubMed] [Google Scholar]
- 15.Zhong W, Levin L, Reponen T, et al. Analysis of short-term influences of ambient aeroallergens on pediatric asthma hospital visits. Sci Total Environ. 2006;370:330–6. doi: 10.1016/j.scitotenv.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott SL, King B, Strong TL, et al. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy. 2003;58:1187–94. doi: 10.1034/j.1398-9995.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 17.Kihlström A, Lilja G, Pershagen G, et al. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. 2002;110:78–84. doi: 10.1067/mai.2002.125829. [DOI] [PubMed] [Google Scholar]
- 18.Kihlström A, Lilja G, Pershagen G, et al. Exposure to high doses of birch pollen during pregnancy, and risk of sensitization and atopic disease in the child. Allergy. 2003;58:871–7. doi: 10.1034/j.1398-9995.2003.00232.x. [DOI] [PubMed] [Google Scholar]
- 19.Belanger K, Beckett W, Triche E, et al. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am J Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- 20.Gent JF, Ren P, Belanger K, et al. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110:A781–6. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stark PC, Burge HA, Ryan LM, et al. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am J Respir Crit Care Med. 2003;168:232–7. doi: 10.1164/rccm.200207-730OC. [DOI] [PubMed] [Google Scholar]
- 22.Stark PC, Celedon JC, Chew GL, et al. Fungal levels in the home and allergic rhinitis by 5 years of age. Environ Health Perspect. 2005;113:1405–9. doi: 10.1289/ehp.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–88. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates [see comments] N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 25.Duramad P, McMahon CW, Hubbard A, et al. Flow cytometric detection of intracellular TH1/TH2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2004;13:1452–8. [PubMed] [Google Scholar]
- 26.Hirst JM. An automatic volumetric spore trap. Ann Appl Biol. 1952;39:257–265. [Google Scholar]
- 27.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Amer Stat Assoc. 1979;74:829–836. [Google Scholar]
- 28.Duramad P, Harley K, Lipsett M, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–22. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai FC, Macher JM. Concentrations of airborne culturable fungi and fungal spores in large U.S. office buildings from the BASE study. Atmos Environ. 2007;41:5181–91. [Google Scholar]
- 30.Levetin E. Bioaerosols. Boca Raton, FL: Lewis Publishers; 1995. Fungi; pp. 87–120. [Google Scholar]
- 31.Burge HA, Rogers CA. Outdoor allergens. Environ Health Perspect. 2000;108(suppl 4):653–9. doi: 10.1289/ehp.00108s4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory PH, Hirst JM. Possible role of basidiospores as air-borne allergens. Nature. 1952;170:414. doi: 10.1038/170414a0. [DOI] [PubMed] [Google Scholar]
- 33.Hjelmroos-Koski M, Macher J, Hammond S, et al. Considerations in the grouping of plant and fungal taxa for an epidemiologic study. Grana. 2006:261–87. [Google Scholar]
- 34.Prescott SL. Allergy: when does it begin and where will it end? Allergy. 2003;58:864–7. doi: 10.1034/j.1398-9995.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- 35.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 36.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]