Abstract
Objective
Integrins contribute to vascular morphogenesis through regulation of adhesion and assembly of the extracellular matrix. However the role of β1-integrin in the mature vascular wall is less clear.
Approach and Results
We sought to determine the function of β1-integrin in mature smooth muscle cells in vivo using a loss of function approach by crossing a tamoxifen-inducible sm22αCre line to a floxed β1-integrin transgenic line. Adult mice lacking smooth muscle β1-integrin survived only ten weeks post-induction. The deletion of β1-integrin resulted in profound loss of vasomotor control. Histological analysis revealed progressive fibrosis in arteries with associated apoptosis of smooth muscle cells that was not rescued by adventitial stem cells. Smooth muscle cell apoptosis was detected in arteries with dead cells replaced primarily by collagen. Despite the catastrophic effects on vascular smooth muscle, the deleted visceral smooth muscle remained viable with the exception of a short portion of the colon, indicating that vascular but not visceral smooth muscle is particularly sensitive to changes in β1-integrin.
Conclusions
This study reveals an essential function of β1-integrin in the maintenance of vasomotor control and highlights a critical role for β1-integrin in vascular, but not visceral, smooth muscle survival.
Keywords: adhesion, apoptosis, extracellular matrix, vascular fibrosis, other vascular biology
INTRODUCTION
Vascular smooth muscle cells (SMC) provide critical support to the cardiovascular system through their ability to sense and respond to changes in transmural pressure 1,2. This function requires intimate association and interaction of SMC with extracellular matrix (ECM) proteins. In fact, deletion or mutations in some of these proteins can lead to vascular dysfunction and aneurysms 3–8. The integrin family of ECM receptors resides at the interface between SMC and the ECM; thus, it is anticipated that these molecules, along with the EMC, partake in the maintenance of vascular homeostasis. While, much has been clarified about the contribution of integrins during vascular development, the individual (or combined) role of these proteins in adult vascular homeostasis in vivo is not well understood 1.
β1-integrin is a particularly important member of the integrin family of heterodimeric receptors because it can pair with each of 10 different integrin α subunits in vascular SMCs 1,9. In development, deletion of β1-integrin from smooth muscle results in aneurysms at the branch points of the aortic arch and it alters adhesion of isolated smooth muscle cells in vitro 4,5. In addition to its role in the assembly of the vascular wall, β1-integrin is thought to contribute to vasoregulation, serving as a mechanosensor in adult smooth muscle. For example, in isolated resistance arterioles, activation of integrins by ligand peptides can regulate Ca2+ channels on the plasma membrane triggering SMC contraction and vasoconstriction 10–12.
The contribution of β1-integrin in the regulation of Ca2+ channels represents one potential role of this protein in vascular wall physiology. A second function for β1 integrin is to provide mechanical stability through its ability to anchor several elements of the cytoskeleton providing critical support (tensegrity) to SMC13,14. Integrins, together with other ECM receptors are able to transmit forces experienced by the external environment to the cytoskeleton15. Within the cell, the combination of stiff microtubules and the more flexible microfilaments and intermediate filaments provide increasing resistance to increased force14 offering instantaneous responses to changes in stress. Integrins are also known to provide biochemical signals that regulate adhesion, migration and survival. It is intriguing that these respective models are often described to the exclusion of the other. Instead both the active signaling and the biomechanical tensegrity are likely to work in tandem to provide vasomotor control, particularly in the mature vascular wall. To further explore the biological contribution of β1-integrin in vascular structure and function, it is essential to evaluate these properties in mature and physiologically reactive vessels.
In the present study, we tested the hypothesis that β1-integrin is required to maintain the structure and function of vascular SMC in an adult mammalian system. For this purpose, a β1-integrin exon3 floxed mouse was crossed to the tamoxifen-inducible sm22αCre-ERT2 mouse16,17. Mice were allowed to reach five weeks of age at which point β1-integrin deletion was induced using a 14-day tamoxifen regimen. The consequences of this deletion varied in severity according to vessel diameter, but eventually resulted in apoptosis of vascular SMC. Compared to controls, systemic arteries exhibited extensive loss of vascular SMC with impaired force production and increased synthesis of ECM. In arterioles supplying the cremaster muscle, constriction to norepinephrine and dilation to acetylcholine were muted. These findings demonstrate an essential role for β1-integrin invascular SMC survival in conduit arteries and in the regulation of tissue blood flow in the microcirculation.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
The β1-integrin promoter is constitutively active in adult smooth muscle
In adult vessels both endothelial and smooth muscle cells express high levels of β1-integrin protein (Figure 1A). A β1-integrin reporter mice further confirmed that this gene is highly active in adult vessels. These reporter mice are double transgenic animals expressing a Cre transgene (sm22αCre or VE-Cadherin) and flox β1-integrin (β1fl) that when deleted enables lacZ gene expression in the cell specific cell type that expresses Cre-recombinase (Supplemental Figure I). Examination of β1-integrin promoter activity in the reporter mice using β-galactosidase (β-gal) activity in embryonic and mature smβ1fl/wtmice indicated high promoter activity in smooth muscle of the dorsal aorta during development and in the adult (Figure 1B and 1C). The same analysis performed on mice expressing the endothelial specific VE-cadherin-Cre showed only sporadic β-gal positive cells (Figure 1D). Both endothelial cells and smooth muscle maintain some level of β1-integrin protein; however, SMC appear to require continuous promoter activity in the majority of cells. The persistence of β1-integrin promoter activity into maturity coupled with the continued presence of the protein in the adult led us to further inquire about the biological function of β1-integrin in mature smooth muscle using an inducible loss of function approach.
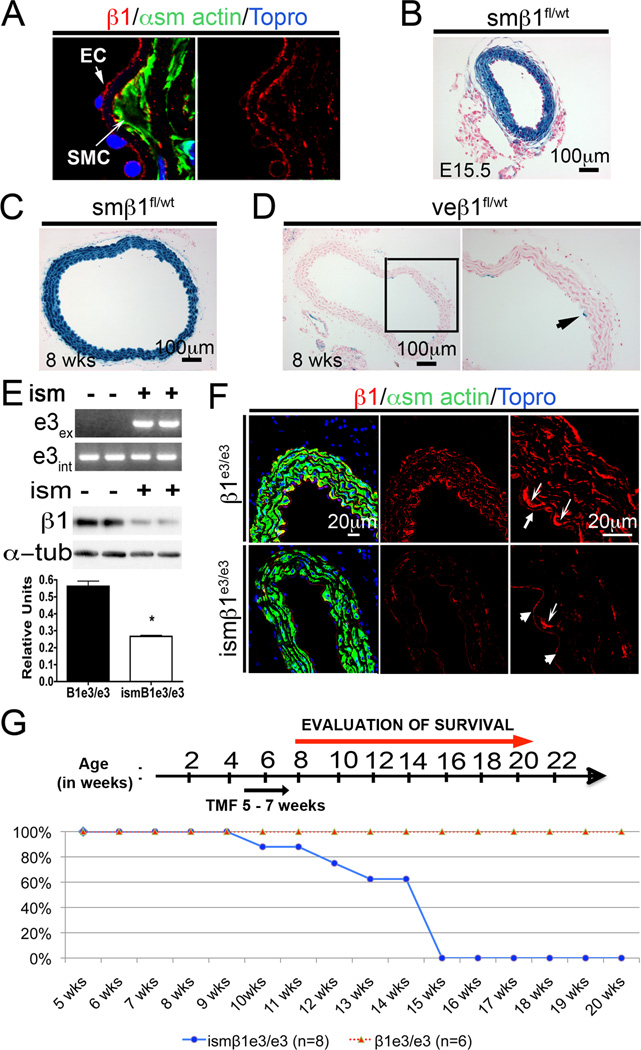
Figure 1. Deletion of β1-integrin in smooth muscle.
A) Immunofluorescence staining of β1-integrin (β1-red), α smooth muscle actin (green) and topro to observe nuclei (blue) in the dorsal aorta from a wild-type adult mouse. B) Histological section of a β-gal stained dorsal aorta from embryonic day 15.5 (E15.5) smβ1fl/wt embryo. In this mouse model, the reporter β-gal indicates β1-integrin promoter activity in smooth muscle. C) Histological section of β-gal stained dorsal aorta from 8 week old smβ1fl/wt mouse. As previously, β-gal corresponds to β1-integrin promoter activity in smooth muscle. D) Histological section of β-gal stained dorsal aorta from veβ1fl/wt (VE-cadherin Cre). β-gal corresponds to β1-integrin promoter activity in endothelial cells. E) Upper panel: PCR confirmation of Exon 3 allele (e3int) and Exon 3 excision (e3ex) for the β1-integrin gene. Excision of Exon 3 results in presence of product. Lower panel: Western blot analysis of dorsal aorta from control= β1e3/e3 (−) and tamoxifen treated mice= ismβ1e3/e3 (+). Graph: Quantification of Western blot showing β1-integrin levels normalized to α-tubulin. ism= induced smooth muscle deletion. Note that the dorsal aorta contains endothelial cells and residual levels of adventitia that are not deleted for β1-integrin using the sm22αCre-ERT2. F) Immunofluorescence for β1-integrin (red), α-smooth muscle actin (green) and topro (blue) in the β1e3/e3 and ismβ1e3/e3 aortas. Thick arrows show expression of β1-integrin in the endothelium, which is not changed between β1e3/e3 and ismβ1e3/e3. Thin arrows show highest expression of β1-integrin in the first smooth muscle cell layer next to the endothelium. Compare reduction of β1-integrin in smooth muscle, in contrast to the similar intensity of protein in the endothelium between the two groups. G) Timeline of injection regimen and survival curve. Below, the survival curve of β1e3/e3 and ismβ1e3/e3. Note that control and experimental groups (β1e3/e3 and ismβ1e3/e3) were injected with tamoxifen using identical time and dose. The number of animals evaluated in this experiment has been indicated in the figure.
Conditional deletion of β1-integrin in smooth muscle
Conditional deletion of β1-integrin was achieved by crossing a β1e3/e3floxed mouse to the sm22α-CreERT2(ism: inducible smooth muscle Cre) mouse model 16,17. In order to delete β1-integrin, a two-week induction regimen was initiated at 5 weeks of age on ismβ1e3/e3and β1e3/e3controls. PCR for both the intact allele (e3int) as well as the excised allele (e3ex) were performed on dorsal aortas from β1e3/e3and ismβ1e3/e3mice injected for 2 weeks and aged for an additional 2 weeks. PCR confirmed recombination in the aorta of ismβ1e3/e3mice (Figure 1E). Western blot analysis and immunofluorescence staining for β1-integrin verified the decrease in protein levels (Figure 1E and F). Note that cells in the intima and adventitia continue to express β1-integrin (Figure 1F) offering an explanation for the residual levels of protein noted in the Western.
To assess recombination efficiency, we crossed the sm22α-CreERT2 to a Rosa26R (R26R) transgenic mouse model. Cre-mediated recombination in the ismR26R offspring showed increasing β-gal positive SMCs with increasing doses of tamoxifen (5 to 14) with minimal differences between 14 and 28 doses (Supplemental Figure IIa)18. Vascular SMC in vessels of the abdominal muscle, dorsal aorta, heart and lung were examined at the 14 dose time point to determine the degree of recombination occurring in the ismβ1e3/e3mice (Supplemental Figure IIb). Using this double transgenic model, recombination was also observed in the visceral smooth muscle of the lung, bladder and esophagus. No β-gal positive cells were identified in cardiac or skeletal muscle when animals were exposed to tamoxifen beyond 4 weeks of age (Supplemental Figure IIb). The status of β1 integrin protein after 14 doses of tamoxifen (once per day) was evaluated in bladder, dorsal aorta, small and large intestine by both Western blot and immunofluorescence (Supplemental Figure III). Combined our findings corroborated previous studies showing the sm22α-CreERT2 to be efficient and specific in the smooth muscle compartment of the adult 17.
Upon verification of the model and deletion approach, we proceeded to investigatethe effect of β1-integrin inactivation in mature smooth muscle. For these experiments, young adult ismβ1e3/e3and β1e3/e3mice were treated with tamoxifen for two weeks and observed. Mice from the ismβ1e3/e3group, but not from the control cohort, die between 9 and 15 weeks of age, which corresponds to 4–10 weeks after the initial tamoxifen treatment (Figure 1G). These findings demonstrated an essential requirement for β1-integrin in the SMC compartment and highlighted its critical role in survival.
β1 deletion mutes contractile vascular response
Previous in vitro experiments identified the contribution of β1-integrin in vasoregulation. Specifically, peptides associated with β1-integrin ligands were shown to be vasoactive and inhibition of this receptor changes vascular reactivity10–12. To determine the effects of β1-integrin deletion on vascular function, we studied the contractile properties of isolated vascular rings and the reactivity of arterioles controlling blood flow in the cremaster muscle of anesthetized mice. These functional studies were performed six weeks after the first tamoxifen injection.
Responses to norepinephrine (NE, 3×10−5M) and to 80mM extracellular [K+] (K80) were significantly reduced (P < 0.05) for ismβ1e3/e3versus β1e3/e3in three of the four arteries studied (Figure 2A). While maximal active tension was maintained in the aorta of ismβ1e3/e3, the superior mesenteric artery (SMA) was 61%, the superior mesenteric artery branch (SMA Br) was 11% and the femoral artery (FA) was 1% of values recorded for β1e3/e3controls. A similar pattern was observed for NE-induced contractions (Figure 2A, right panel). With no significant difference for aorta, peak active tensions for SMA and SMABr of ismβ1e3/e3were 29% and 7% of responses in β1e3/e3, respectively, while responses of ismβ1e3/e3FA were abolished.
Figure 2. Reactivity of vessel rings and arterioles is reduced upon deletion of β1-integrin.
A) Contractile responses of vessel rings of the aorta, superior mesenteric artery (SMA), the superior mesenteric artery branch (SMA Br) and the femoral artery (FA) to 80 mM K+ (K80; left panel) and to norepinephrine (NE, 3×10−5 M). Note difference in ordinate scales between panels. * ismβ1e3/e3 (n=6 each) is significantly different from β1e3/e3 (n=7 each), P < 0.01. B) Top row: Concentration-response curves of first-(1A), second-(2A) and third-(3A) order arterioles in cremaster muscles of β1e3/e3 (●) and ismβ1e3/e3 (■) mice (n=6 per group) to ACh. * ismβ1e3/e3 significantly different from β1e3/e3, P < 0.05. Middle row: Concentration-response curves of 1A, 2A and 3A in cremaster muscles of β1e3/e3 (●) and ismβ1e3/e3 (■) mice to NE (n=6 per group). Bottom row: Data for ismβ1e3/e3 were separated into responders (▲, n=3) or non-responders (△, n=3) to NE relative to β1e3/e3 (●, n=6). Note difference in ordinate scales between panels for ACh vs. NE and for arteriolar branch orders. * ismβ1e3/e3 non-responders significantly different from β1e3/e3, P < 0.01; † ismβ1e3/e3 non-responders significantly different from β1e3/e3 and from ismβ1e3/e3 responders, P < 0.01. Response curves to NE were terminated when 3A constricted to closure.
A trend similar to the one observed in vessel ring assays was also noted in cremaster arteriolar preparations. These showed loss of vascular responses to NE in ismβ1e3/e3(Figure 2A and 2B). The physiological responses of the superior mesenteric artery at 6 weeks post-tamoxifen treatment can be compared to the histological status of the same artery at the identical time point (Supplemental Figure IVc). At this post-deletion time, the mesenteric artery shows significant loss of smooth muscle, identified in red by the trichrome staining, and progressive fibrosis in green (Supplemental Figure IVc). Such loss of SMCs is consistent with impaired response to vasoconstrictors. In contrast to the mesenteric artery, the aorta displayed no functional alterations at 6 weeks post-treatment (Figure 2A) and histological examination revealed only minimal loss of SMCs in the layer near to the adventitia (Supplemental Figure IVd). Overall the findings indicate that deletion of β1 integrin has more devastating effects in arteries of intermediate than in those of larger size like the aorta.
Endothelial cell-dependent vasodilation to acetylcholine (ACh) and vascular SMC-dependent vasoconstriction to NE was examined in cremaster arterioles in vivo (Figure 2B). First order (1A), second order (2A) and third-order (3A) arterioles were examined under resting conditions, and in the presence of cumulative concentrations of ACh and NE. Although baseline diameter in 1A arterioles was significantly smaller in ismβ1e3/e3vs. β1e3/e3(P < 0.05), 2A and 3A arterioles were not different between groups (Table 1). During superfusion with sodium nitroprusside (SNP) and ACh, the maximum diameters of 1A, 2A and 3A were each significantly (P < 0.05) smaller in ismβ1e3/e3vs. β1e3/e3(Table 1). Spontaneous vasomotor (i.e., myogenic) tone was greater in 2A and 3A than in 1A for both β1e3/e3and ismβ1e3/e3mice, but was less in 2A and 3A of ismβ1e3/e3versus β1e3/e3(P < 0.01; Table I). Across branch orders, ACh had no effect on the diameter of arterioles in ismβ1e3/e3mice (Figure 2B, top 3 panels). In contrast, arteriolar diameters increased with progressively higher concentrations of ACh in β1e3/e3and were significantly greater (P < 0.05) than ismβ1e3/e3at the highest concentrations (Figure 2B, top 3 panels). For 1A in ismβ1e3/e3, a cumulative increase in NE concentration had no significant effect on diameter through 10−5 M. For 1A in β1e3/e3, NE was not studied above 3 × 10−7 M due to complete vasoconstriction of 3A. In 2A and 3A of β1e3/e3, vasoconstriction increased with cumulative addition of NE and 3A closure occurred with ≤ 10−6 M (Figure 2B, middle panels). For 2A and 3A of ismβ1e3/e3, constriction to NE appeared to be attenuated versus β1e3/e3(Figure 2B, middle panels) but there were no statistically significant differences between respective groups. Upon closer examination, distinct response profiles emerged for 2A and 3A in the ismβ1e3/e3group. In half of these mice, there was no vasomotor activity in response to NE while responses in the other half were similar to β1e3/e3(Figure 2B, lower 3 panels). This difference between ismβ1e3/e3 responders and non-responders was significantly different. Together these data indicate that deletion β1-integrin from vSMCs diminishes both contraction and relaxation capacities.
Table I.
Arteriolar diameters and spontaneous vasomotor tone
| β1e3/e3 | ismβ1e3/e3 | |||||
|---|---|---|---|---|---|---|
| 1A | 2A | 3A | 1A | 2A | 3A | |
| Diameter, µm | ||||||
| Rest | 59 ± 5 | 14 ± 2 | 6 ± 1 | 44 ± 1* | 11 ± 1 | 7 ± 1 |
| Maximum | 61 ± 5 | 32 ± 3 | 22 ± 3 | 45 ± 2* | 19 ± 3* | 12 ± 2* |
| Vasomotor tone | 5 ± 1 | 57 ± 6 | 68 ± 4 | 3 ± 1 | 35 ± 9* | 36 ± 6* |
Arteriolar diameters and spontaneous vasomotor tone in cremaster muscle preparations of β1e3/e3 and ismβ1e3/e3 mice. Maximum diameters were recorded during superfusion with ACh + SNP (10 µM each). Vasomotor tone is expressed as “% maximum diameter” calculated as: [(maximum diameter - resting baseline diameter)/maximum diameter] × 100. Values are means ± SE (n=6 per cell).
Arteriolar diameter of ismβ1e3/e3 significantly different from corresponding branch of β1e3/e3, P < 0.01.
Progressive degeneration of the tunica media
The lack of vasomotor responses to ACh and NE indicated significant smooth muscle dysfunction. In order to assess the cellular changes associated with deletion of β1 integrin, coronary vessels from ismβ1e3/e3 and β1e3/e3 controls were evaluated at 5 days, 2 weeks, 3 weeks, 6 weeks and 10 weeks from the first tamoxifen injection (Figure 3A). At 5 days the coronary arteries of ismβ1e3/e3 resembled controls. At 2 and 3 weeks the vSMCs of the ismβ1e3/e3 appeared less compact. By 6 weeks the coronary arteries had significantly lost vSMC coverage which was even more pronounced at the 10-week time point. Although β1-integrin reduction was apparent by Western blot analysis and microarray as early as 2 weeks after treatment in mesenteric arteries (Supplemental Figure IVa and IVb), histological deterioration of the vSMC layer was only evident at 6 weeks. From 6 weeks progressive medial degeneration similar to that seen in the coronary arteries was noted in the mesenteric arteries (Supplemental Figure IVc). Matrix proteins occupied the space vacated by smooth muscle. Surprisingly, this loss of smooth muscle was not associated with an inflammatory response.
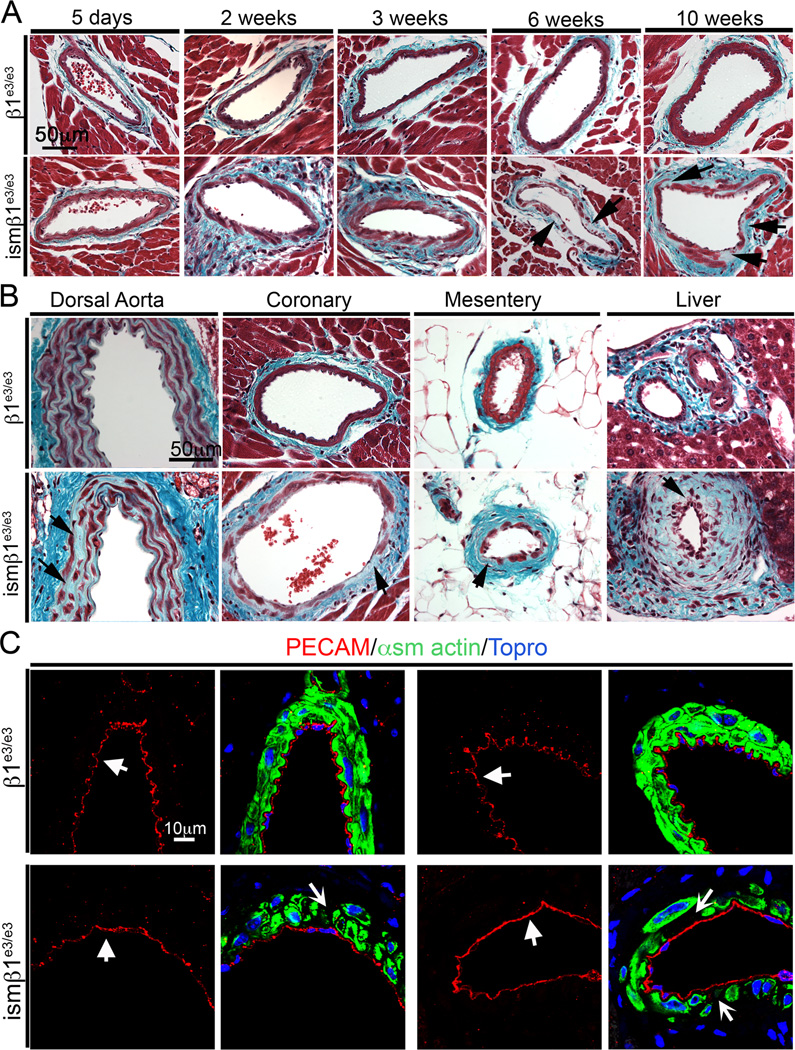
Figure 3. Absence of β1-integrin results in progressive vascular fibrosis.
A) Trichrome stained mouse coronary arteries from β1e3/e3 and ismβ1e3/e3 at 5 days, 2 weeks, 3 weeks, 6 weeks and 10 weeks after initiation of deletion (first tamoxifen injection). Arrows at 6 weeks and 10 weeks indicate loss of smooth muscle and increase of ECM. The trichrome stain identifies cells in red and extracellular matrix in a blue-green color. These are representative images from the cohort evaluated (n=4–6 per group). B) Trichrome staining of representative vessels from dorsal aorta, coronary arteries, mesenteric arteries and hepatic arteries of β1e3/e3 and ismβ1e3/e3 at 6 weeks after initiation of deletion (first tamoxifen injection). Arrows indicate areas with loss of smooth muscle. These are representative images from the cohort evaluated (n=4–6 per group). C) Immunofluorescence staining of PECAM to reveal endothelial cells (red and indicated by thick arrow), α-smooth muscle actin (green) and topro (blue) in the coronary arteries of β1e3/e3 and ismβ1e3/e3 animals at 6 weeks after initiation of deletion (first tamoxifen injection). The arrows in the second and last panel of ismβ1e3/e3 indicate gaps between smooth muscle cells. The images shown are representative from analysis of n=3–6 mice per control and experimental group.
Recent reports indicate that a stem cell population resides within the adventitia. It has been proposed that these cells may contribute to vascular wall repair 19,20 and give rise to SMC. To assess potential stem cell investment during the degeneration of the media layer, ismβ1e3/e3 animals were crossed to animals carrying the R26R transgene. As the cre recombinase does not mark adventitial cells, if adventitial stem cells were able to integrate into the tunica media, those cells would be negative for β-gal. Following the 2-week injection regimen, animals were aged an additional 4 weeks and the tissue was stained for β-gal (Supplemental Figure V). These representative sections indicate that β-gal positive cells deleted for β1 integrin were not replaced by β-gal negative stem cells after tamoxifen injections ended. This failure to rescue vSMCs was further corroborated by the progressive degeneration and fibrosis of the tunica media (Figure 3A and Supplemental Figure IVc).
The coronary vasculature provided insight regarding the effects of β1-integrin deletion; however, arteries supplying the heart may be subjected to physical stress not experienced by other vascular beds. To assess the systemic consequence of β1-integrin deletion in vSMCs, additional vascular networks as well as other SMC populations were evaluated. At 6 weeks, the dorsal aorta showed signs of tunica media degeneration, although the effect was not as significant as that observed in medium-sized arteries (Figure 3B and Supplemental Figure IVd). The mesenteric arteries and arteries of the liver exhibited a marked degradation of the tunica media (Figure 3B). Further, all vascular beds examined showed some occluded vessels (particularly arterioles), especially the liver (Supplemental Figure VIa and VIb). While we anticipated the coronary vasculature to be affected by β1-integrin deletion, we found that the effect was systemic and it was characterized by progressive vascular fibrosis with concurrent loss of SMC (Figure 3A and 3B).
As endothelium-dependent dilation in arterioles was consistently impaired by β1-integrin deletion (Figure 2B), we examined the physical integrity of the endothelium of vessels with mutant SMC. Immunofluorescence of α-smooth muscle actin (green) and PECAM (red) illustrates that deletion of β1-integrin clearly affects the tunica media, while the endothelium remains intact (Figure 3C).
To gain a better understanding of β1-integrin in SMCs in general, we also examined the condition of visceral smooth in several organs. Analysis of the ismR26R intestine showed that cre recombinase was fairly penetrant, as indicated by β-gal positive cells in the small and large intestine (Supplemental Figure VIIb). Surprisingly, histology of the small intestine showed no difference between ismβ1e3/e3 and β1e3/e3 controls despite significant decreases in β1-integrin protein (Supplemental Figure IIIa and IIIb and Supplemental Figure VIIc). The finding was also true for most of the colon, with the exception of a small region at the junction with the caecum (region 4 in Supplemental Figure VIIc). In the latter region, SMC loss and fibrosis was evident and similar to the effect noted in the vasculature. Visceral SMCs in the bladder and lung (not shown) between knockout and control animals were morphologically identical (Supplemental Figure VIIa). We were intrigued by the different outcomes of β1 integrin deletion in vascular versus visceral SMC populations. In order to gain insight into this difference, we evaluated levels of β1 integrin, β3 integrin, β5 integrin, CD36, galactosidase β1 (GLB1), ribosomal protein SA (RPSA) and serum response factor (SRF) by Western blot in the bladder, dorsal aorta, mesenteric arteries, small intestine and large intestine of two wild type mice (Supplemental Figure VIII). The prediction was that differential integrin expression patterns (or other receptors) could explain such a difference. We found that levels of β1 integrin were highest in bladder and similar between vascular and intestinal smooth muscle. A similar pattern emerged for β3 and β5 integrins. Furthermore, other receptors (CD36, GLB1) were more specific to the vascular smooth muscle. These findings are in accordance with recent work demonstrating that vascular and visceral smooth muscle cells exhibit unique expression profiles 21 and such differences may in turn contribute to differences in vulnerabilities to loss of β1-integrin found here. Remarkably, despite effective deletion of β1-integrin in visceral smooth muscle the relative lack of consequences is in striking contrast to the devastating effect of β1-integrin deletion in vascular smooth muscle.
ECM expression is increased in the vasculature
Histological evaluation indicated that SMC loss in ismβ1e3/e3 vessels was associated with a corresponding increase in ECM. Given the large spectrum of ECM molecules secreted by SMC, several of which are ligands for β1 integrin heterodimers, we performed microarray analysis and obtained information on ECM and other transcriptional changes triggered by loss of β1 integrin. For these experiments, we used RNA isolated from mesenteric arteries at the 2-week time point. This time was selected because the cells were beginning to show stress (Figure 3A), but had yet to undergo apoptosis. The microarray data indicates that transcripts for most ECM molecules were increased (Figure 4A). To further validate the findings and gain information on location, we performed immunofluorescence for fibrillar collagen, fibronectin, elastin and laminin. The analysis was done in n=3–5 independent mice per group (β1e3/e3 and ismβ1e3/e3), representative images are show in Figure 4B–E. Sections of both coronary vessels and the dorsal aorta were examined by confocal microscopy (Figure 4B–4E and Supplemental IXa–IXd). In the tunica media, fibrillar collagens (I, III and IV) together with laminin replaced the SMCs in both the coronary vasculature and the dorsal aorta (Figure 4B and 4E). While fibronectin was the predominant ECM within the adventitia of coronary vessels and dorsal aorta, the protein was not found to populate the media upon deletion of β1-integrin (Figure 4C). There was little difference in elastin staining between wild type and deleted vessels (Figure 4D) despite the changes in elastin transcripts. Changes in ECM production in the media are often associated with de-differentiation of vSMCs (synthetic versus secretory phenotypes). Nevertheless, the microarray data did not indicate statistically significant changes in expression of myosin or calponin. Immunostaining of smooth muscle myosin, α-smooth muscle actin, vimentin and α-tubulin were consistent with the microarray findings of a relatively intact cytoskeleton in a differentiated SMC (Supplemental Figure X and Supplemental Table I). Overall, these findings reveal differential deposition of matrix proteins with a preponderance of fibrillar collagens in the tunica media of ismβ1e3/e3. In turn the increased deposition of collagen within the vascular wall is likely to contribute to the altered functional response of vessels in mutant mice (Figure 2).
Figure 4. Increase in extracellular matrix (ECM) deposition upon deletion of β1-integrin.
A) Microarray analysis of mesenteric arteries showing the fold change in several ECM molecules representing ismβ1e3/e3 over β1e3/e3 at 2 weeks post-deletion (red arrows indicate ECM validated by immunofluorescence, * indicates p<0.1 for a 1.5 fold change). Microarray was performed on n=3 animals per group. Both groups were treated with tamoxifen. B–E) Immunofluorescence staining of fibrillar collagen (B); fibronectin (C); elastin (D) and laminin (E) (all in red) with α-smooth muscle actin (green) and topro (blue) in the coronary arteries of β1e3/e3 and ismβ1e3/e3 animals at 6 weeks after first tamoxifen injection (arrows indicate excess ECM). Note robust increase in fibrillar collagen (antibody recognizes collagens I, III and IV). Figure shows representative images from coronary arteries from β1e3/e3 and ismβ1e3/e3 animals (n=3 per group).
Vascular SMC undergo apoptosis
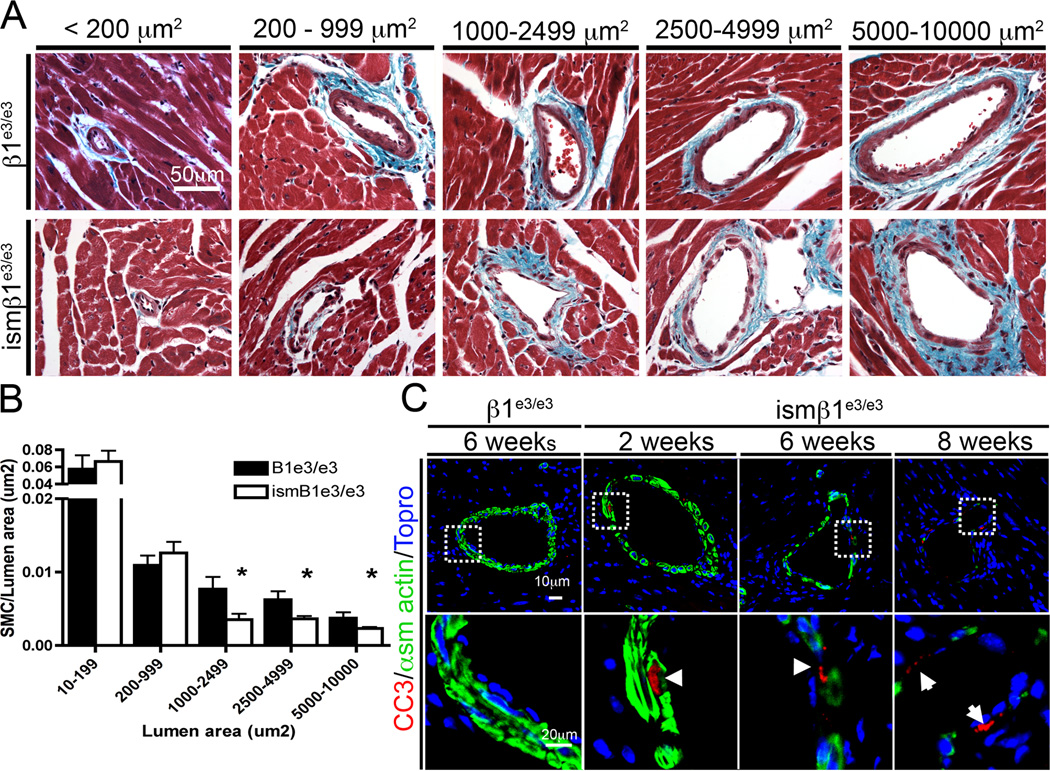
In addition to increased ECM, the coronary vessels of ismβ1e3/e3 mice clearly depicted loss of SMC. As β1-integrin has been shown to function in cell survival in vitro, we evaluated SMC loss in ismβ1e3/e3 mice. A systematic analysis of SMC content was applied in ismβ1e3/e3 and β1e3/e3 mice using lumen area to classify vessels according to size. As anticipated, we found loss of smooth muscle in ismβ1e3/e3 mice, however at the time point examined (6 weeks post-deletion) only vessels from ismβ1e3/e3 mice with a lumen area >1000µm2 were found to have a significant reduction in SMC coverage compared to β1e3/e3 (P>0.05) (Figure 5A and 5B). These data indicates that SMC in medium-sized arteries are more sensitive to loss of β1-integrin and are consistent with the functional data from arterial rings (Figure 2A). To determine whether this loss in smooth muscle was due to apoptosis, heart sections were stained for cleaved caspase 3 (CC3=red) with α-smooth muscle actin (green) marking the tunica media. No cleaved caspase positive clusters were identified in the β1e3/e3 control (228 SMC counted across three animals). In contrast >40 positive clusters were seen among the ismβ1e3/e3 vessels examined (260 SMCs across three animals). Images of coronary arteries from 2, 6 and 8 weeks after the first injection further confirm the progressive loss of SMC attribute to apoptosis (Figure 5C). The prevalence of apoptosis within the tunica media indicates that β1-integrin is in fact critical to SMC survival. This finding is in sharp contrast to the lack of apoptosis in visceral smooth muscle, with the unique exception of a small region in the colon (Supplemental Figure VIIc).
Figure 5. Inactivation of β1-integrin results in vascular smooth muscle cell apoptosis.
A) Trichrome stained histological sections of coronary arteries representing the five-lumen area categories analyzed for SMC loss in β1e3/e3 and ismβ1e3/e3 animals. B) Quantification of the ratio of vascular SMC (vSMC) to lumen area (µm2). As in (A) vessels were grouped into five different categories according to the lumen size. For each group a minimum of five and a maximum of seven vessels were evaluated in each of the control and experimental cohorts. The vessels analyzed represent at least three β1e3/e3 animals and three ismβ1e3/e3 animals (* indicates P>0.05). C) Immunofluorescence staining of cleaved caspase3 (CC3 in red), α-smooth muscle actin (green) and topro (blue) in the coronary arteries of β1e3/e3 and ismβ1e3/e3 animals at the time from initial injection specified. For these experiments n=4 animals were evaluated. Arrows indicate apoptotic cells.
Compensatory response by visceral but not vascular smooth muscle
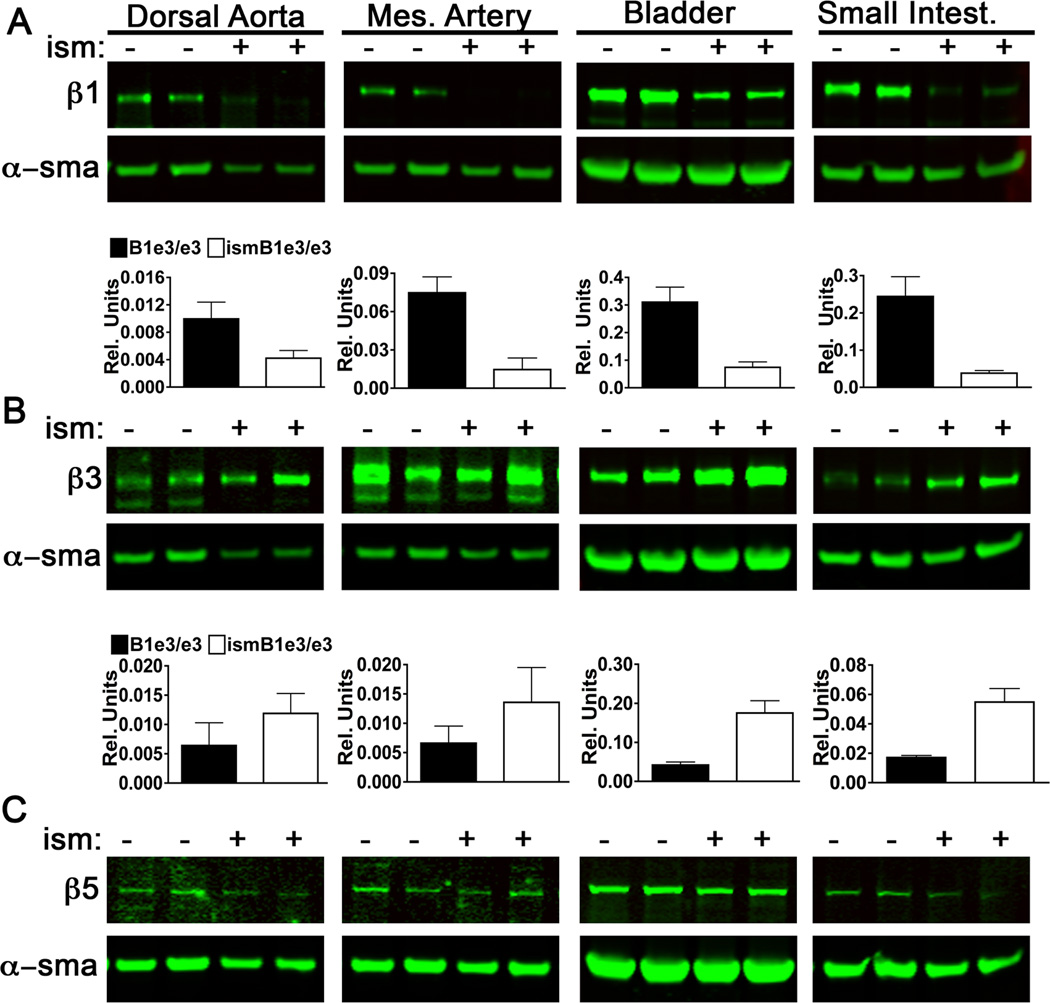
The striking differences between the phenotype of vascular and visceral smooth muscle let us to further consider potential compensatory differences in integrin expression. As discussed previously, β1, β3, and β5 integrins are expressed by both vascular and visceral SMC at similar proportions. Meaning, SMC of both types have high levels of β1 and β3, followed by lower levels of β5 (Supplemental Figure VIII). Upon loss of β1 integrin in vascular SMC, we observed a slight trend, but no significant increase in β3 integrin (Figure 6 A and B). Conversely visceral SMC exhibited a three-fold increase in β3 upon lost of β1 integrin (Figure 6 A and B). No changes were detected in the level of expression of β5 upon β1 integrin deletion in either visceral or vascular (Figure 6C).
Figure 6. Loss of ß1 integrin alters expression of visceral but not vascular ß3 integrin.
Western blots from tissue lysates isolated from ismβ1e3/e3 and β1e3/e3 (2 each shown in Western) for dorsal aorta, mesenteric artery (Mes Artery), bladder and small intestine (Small Intest.). Normalized densitometry is also shown and the graph includes evaluation of four biological replicates. A) β1 integrin Western blots with densitometry normalized to α-smooth muscle actin (α-sma) +/− SEM. p-values: 0.076 (Dorsal Aorta), 0.0081 (Mes. Artery), 0.0065 (Bladder) and 0.0085 (Small Intest.). B) β3 integrin with densitometry normalized to α-smooth muscle actin (α-sma) +/− SEM and p-values: 0.0063 (Bladder) and 0.0049 (Small Intest.). C) β5 integrin Western blots of indicated tissue lysates. Upon normalization no differences were detected in the level of expression between the two groups for this integrin.
The findings indicate a potential mechanism for the lack of phenotype in visceral SMC namely functional compensation by β3 integrin. Interestingly vascular SMC were not able to mount such a response upon lost of equivalent levels of β1 integrin. The reason(s) for this difference is unclear and highlights the strict dependency of vascular SMC for this receptor.
DISCUSSION
Previous reports have shown an essential role for β1-integrin in the assembly of the vascular wall during development 4,5. Here we examined the contribution of β1-integrin in adult SMC by selectively inactivating this gene in young adult mice. We demonstrate that deletion of β1-integrin results in a reduced contractile response of arterial rings, impaired constriction and dilation of arterioles in vivo, progressive systemic fibrosis and eventual death of SMC.
Animals with induced deletion for β1 integrin in SMCs remained physically active for several weeks, but deletion eventually resulted in their demise. Although it is difficult to determine the exact cause of death, the ismβ1e3/e3 surviving to 10 weeks post-tamoxifen treatment showed a number of fibrotic vessels with partially or fully occluded lumens. In addition, mice became bloated from intestinal impaction. This is likely due to the fibrosis of a small area of the colon that is sensitive to β1-integrin deletion and undergoes apoptosis (Supplemental Figure VIIc region 4). At 10 weeks ismβ1e3/e3 mice appeared to have less adipose tissue and become moribund at approximately the same time. We did not see any obvious signs of aneurysms or dissections in any of the animals examined.
Although we noted a clear effect of β1-integrin deletion in vascular tone of arterioles in vivo, phenotypes varied according to vessel caliber. Remarkably, contractile responses of the aorta showed little consequence to the loss of β1-integrin. This may be due to the high elastic content of the aorta, storing energy during systole and releasing it during diastole to facilitate driving blood flow to the periphery 22. It is also possible that this vessel experiences less recombination (Supplemental Figure IIa and IIb). In contrast, medium size arteries rely mostly on the contractile function of smooth muscle, which showed extensive SMC apoptosis and a significantly depressed response in ismβ1e3/e3 animals (Figure 2A and Figure 5).
The absence of constriction to NE in ismβ1e3/e3 mice is consistent between the 1A arterioles and the vascular rings from the SMA Br and FA (Fig. 2A and 2B) of these mice when compared to β1e3/e3. That such differences were observed to K80 as well as NE of arterial rings points to a generalized functional deficit in the ability of SMC to contract either in response to membrane depolarization or adrenoreceptor activation, respectively. The loss of contraction to NE in 2A and 3A that was manifested in half of the ismβ1e3/e3 group (Figure 2B) suggests that the penetrance of this functional deficit in arteriolar SMC was ~50% at the 6 week time point.
Our findings in arterioles of the cremaster muscle illustrate that endothelium-dependent vasodilation to ACh was impaired, along with vasoconstriction to NE, despite the presence of an intact endothelium. In vascular rings, contractile responses to NE as well as depolarizing K+ were dramatically compromised in muscular arteries. In turn, impaired dilation and lack of constriction in arterioles would underscore poor regulation of blood pressure and tissue blood flow while impaired constriction of muscular arteries would contribute further to such an effect. The consistency across vascular branch orders implies that β1-integrin is important for vasomotor control.
Across branch orders, arterioles of ismβ1e3/e3 have diminished endothelium-dependent vasodilation. When SNP (an NO donor) was added together with ACh to obtain maximal diameters, dilation of 2A and 3A (Table 1) indicates that vSMCs of these arterioles in ismβ1e3/e3 can still relax to exogenous NO, albeit to a lesser extent than β1e3/e3. With similar resting diameters for 2A and 3A between groups, the reduced tone (i.e., smaller maximal diameters) in 2A and 3A of ismβ1e3/e3 reflects the inability of SMC to relax and/or remodel to smaller diameters. Collectively, the reduction in maximal diameter across branch orders is consistent with arteriolar remodeling following β1-integrin deletion. That deficits in arteriolar function, including impaired vasodilation, are due to defective SMC rather than impaired endothelium is consistent with the lack of inflammatory response seen throughout the vasculature, with intact endothelial cells maintaining this protective role despite SMC apoptosis.
In addition to impaired functional responses from ismβ1e3/e3 vessels, histological evidence showed a progressive degradation of the tunica media. This deterioration continued after tamoxifen injections had ceased. The design of this experiment also enabled us to examine potential repopulation of the tunica media through adventitial stem cells. Sca1+ (vascular stem cells) have been observed in the adventitia of the aortic root and express some smooth muscle differentiation markers 20. In our early studies we applied a 28-day injection regimen and found significant smooth muscle degeneration continuing until the animals were found moribund at approximately 8 weeks from the first tamoxifen injection (data not shown). The 2-week injection protocol was later found to be sufficient to induce significant deletion. The ismβ1e3/e3 animals survived for several weeks following the final injection. From the last injection until death the medial layer continued to degenerate with no visible gain of potential cells from the adventitia. β-gal staining of ismR26R mice compared with ismβ1e3/e3; R26R mice indicate that unmarked cells of the adventitia do not repopulate the vascular media. In fact, there was neither invasion of adventitial cells nor any inflammatory cells into the media. Data from Clarke and colleagues, also suggests that vascular SMC apoptosis does not stimulate a compensatory or replacement mechanism 23. It is possible that the lack of recovery may reflect the timing of β1-integrin deletion. The Sca1+ progenitor population is reduced in maturation, but these data indicate, that at least within this time frame, adventitial stem cells are not active in mature vessels and do not contribute towards the healing of an impaired vascular wall 20. Alternatively, the fibrotic condition may disrupt the stem cell differentiation seen in ApoE-deficient mice 24.
Rather than an inflammatory response, the loss of β1-integrin and cell death resulted in the production of ECM as the primary response to progressive degeneration of the tunica media. In fact, an interesting aspect of this phenotype is the transition of vascular SMCs to a “secretory” state, in this case in the absence of drastic dedifferentiation as noted in vitro and prior to initiation of apoptosis. The present data would indicate that lack of tension triggered by defective vSMC binding to the ECM results in significant synthesis and secretion of matrix proteins, perhaps in an attempt to regain the previous tensile state (Figure 4). The increase in matrix might indeed represent the mechanism described by Tomasek and colleagues where myofibroblasts secrete excess matrix until a set tension is achieved 25and an attempt of the defective smooth muscle to regain attachment with its adjacent matrix after losing tension from vSMC loss. It should be stressed; however, that the staining reveals deposited ECM and not necessarily measures secreted matrix proteins. The ability of transglutaminase to cross-link matrix proteins requires β1-integrin, thus it is possible that these deleted cells might also secrete fibronectin, but are unable to incorporate this protein in the remodeled matrix.
Developmental deletion of β1-integrin from smooth muscle is not associated with apoptosis, although the time frame of development deletion versus induced deletion is slightly different and perhaps the reason for the absence of apoptosis 4,5. Nonetheless, β1-integrin inactivation in development also resulted in abnormal ECM assembly and this information can provide important points of comparison with the adult deletion. During development, excess ECM was deposited in areas of high stress. In the adult, ECM deposition correlates with SMC loss. While β1-integrin was inactivated in all SMC populations, SMC death occurred mostly in large and medium sized arteries with the exception of the aorta, which may be buffered from stress by its elastin content in addition to lower recombination efficiency. The smallest vessels neither lost smooth muscle, nor accumulated excess ECM. In the vascular hierarchy, smaller vessels maintain a lower pressure than larger vessels 26. The data suggests there may be a correlation between the higher vascular pressure of medium arteries and cell death following β1-integrin deletion. This interpretation is further corroborated by the lack of apoptosis in the small intestine and bladder where pressure is generally at 20 mm Hg, while the colon sees spikes > 40 mm Hg 27,28.
In adult vascular tissues, the ultimate consequence of β1-integrin loss is apoptosis, which occurs in the absence of a robust inflammatory response. The lack of inflammation correlates with models of diptheria-toxin in which induced SMC apoptosis did not result in an inflammatory response 23,29. Curiously, diptheria toxin-induced apoptosis did not produce a fibrotic response. By comparison, a mouse carrying a mutated copy of lamin A, showed both SMC death and fibrosis 30. These differences between models may suggest that fibrosis following SMC death results from death in the context of an altered / stressed population of SMC (slow death), but that rapid apoptotic events do not induce ECM production.
In conclusion, this work illustrates the critical role β1-integrin in SMC survival and function in vivo. By considering the consequences of deletion in vivo we gain valuable insight into potential mechanisms of disease, possible therapies and their side effects. The ismβ1e3/e3 phenotype resembles the medial degeneration and vascular fibrosis seen in Raynaud’s Syndrome and CADASIL, yet lacks an inflammatory component. There are also similarities in the vascular phenotype between a lamin A mutant mouse model of progeria and the smooth muscle β1-integrin knockout 30. The model presented here provides a unique opportunity to distinguish the molecular mechanism driving the inflammatory response versus the mechanism of fibrosis. Further, these findings may offer insight into the side effects of anti-integrin therapies, which are being used for the treatment of Crohn’s disease and multiple sclerosis. With the knowledge that β1-integrin contributes to vasoregulation and cell survival, perhaps more specific therapies can be developed and adverse effects avoided based on our understanding of β1-integrin in adult vSMCs.
Supplementary Material
SIGNIFICANCE.
This work defines the role of β1 integrin in mature smooth muscle cells in vivo. Using a tamoxifen-inducible sm22αCre-recombinase to delete the itgb1 gene in a manner that bypasses developmental stages, we show that loss of β1 integrin in adult smooth muscle initially impairs vascular contractility/dilatory responses and eventually results in cell death. Following deletion of β1 integrin, vessels become fibrotic with investment of collagen and other extracellular matrix molecules but in the absence of an inflammatory response. Finally, this study highlights differences between vascular and visceral smooth muscle cells to loss of β1 integrin, indicating an important divergence in these two cell populations. We also highlight the relevance of in vivo studies to definitively determine the unique function integrins within the context of a given organ.
ACKNOWLEDGEMENTS
We would like to thank the contribution of the Tissue Procurement Core Laboratory Shared Resource (TPCL) for their support in histological processing of our samples.
Experiments other than those in Figure 2 were executed by LIA, KAT and JS. Experimental design and interpretation by LIA and KAT. Manuscript by KAT and LIA. Experiments on vessel rings were performed by AJW in the laboratory of RJK. Experiments on cremaster muscle arterioles were performed by PB in the laboratory of SSS. For the experiments presented in Figure 2: all investigators contributed to experimental design and data interpretation. AWJ and PB prepared the first draft of these results. SSS prepared the documents required for shipping and care of these animals for the University of Missouri and edited the final submission of this report. Transgenic sm22αCre-ERT2 mouse from RF.
SOURCES OF FUNDING
This research was supported by NIH grants RO1-HL085618 (LIA), R37-HL041026 and RO1-HL086483 (SSS), F32-HL097463 (PB), and RO1-HL095486 (RJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no disclosures.
REFERENCES
- 1.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 3.Shen D, Li J, Lepore JJ, Anderson TJ, Sinha S, Lin AY, Cheng L, Cohen ED, Roberts JD, Jr, Dedhar S, Parmacek MS, Gerszten RE. Aortic aneurysm generation in mice with targeted deletion of integrin-linked kinase in vascular smooth muscle cells. Circ Res. 2011;109:616–628. doi: 10.1161/CIRCRESAHA.110.239343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham S, Kogata N, Fassler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 5.Turlo KA, Noel OD, Vora R, Larussa M, Fassler R, Hall-Glenn F, Iruela-Arispe ML. An essential requirement for beta1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev Biol. 2012;365:23–35. doi: 10.1016/j.ydbio.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 7.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet. 1995;4(Spec No):1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 8.Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975;72:1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3-and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol. 2008;586:1699–1713. doi: 10.1113/jphysiol.2007.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010;584:2033–2042. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 15.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 16.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 19.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi JT, Rodriguez EH, Wang Z, Nuyten DS, Mukherjee S, van de Rijn M, van de Vijver MJ, Hastie T, Brown PO. Gene expression programs of human smooth muscle cells: tissue-specific differentiation and prognostic significance in breast cancers. PLoS Genet. 2007;3:1770–1784. doi: 10.1371/journal.pgen.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 26.Davis MJ, Ferrer PN, Gore RW. Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am J Physiol. 1986;250:H291–H303. doi: 10.1152/ajpheart.1986.250.2.H291. [DOI] [PubMed] [Google Scholar]
- 27.Rauch S, Krueger K, Turan A, You J, Roewer N, Sessler DI. Use of wireless motility capsule to determine gastric emptying and small intestinal transit times in critically ill trauma patients. J Crit Care. 2012 doi: 10.1016/j.jcrc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor LT, Jr, Vaughan ED, Jr, Felsen D. In vivo cystometric evaluation of progressive bladder outlet obstruction in rats. J Urol. 1997;158:631–635. [PubMed] [Google Scholar]
- 29.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 30.Varga R, Eriksson M, Erdos MR, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.