Abstract
OBJECTIVES
Diagnosis and treatment for a life threatening illness such as cancer is known to be psychologically impactful. However, little is known about the influence that non-cancer life stressors have on the quality of life (QOL) of ovarian cancer patients. The goal of the present study was to examine associations between non-cancer life stressors and QOL in 123 women with invasive epithelial ovarian cancer who were followed prospectively and longitudinally for one year.
METHODS
Mixed models for repeated measures were used to examine the relationship between life stressors and QOL pre-surgery and one year later, while adjusting for age, cancer stage, depressive symptoms, anxiety, and chemotherapy status (at one year). Prospective associations between QOL pre-surgery and one-year QOL were also examined.
RESULTS
Number and severity of life stressors were unrelated to QOL of participants before surgery. At one year, however, participants experiencing a greater number of life stressors reported poorer concurrent physical well-being (PWB) (p = 0.015), functional well-being (FWB) (p < 0.0001), social well-being (SWB) (p = 0.0003), and total QOL (p<0.0001). Similar effects were found for life event severity. Finally, experiencing a greater number of life stressors pre-surgery predicted poorer overall QOL one year post-diagnosis (p < 0.0001).
CONCLUSIONS
Non-cancer life stressors can substantially impact long-term QOL of ovarian cancer patients, adjusting for medical variables such as chemotherapy and cancer stage, thus highlighting the importance of evaluating the stress burden of patients in ongoing cancer care.
Keywords: ovarian cancer, life stress, life events, quality of life, depression, anxiety
Introduction
Given that most ovarian cancer patients have advanced stage disease [1], it is not surprising to find accompanying stress, depression, anxiety, and poorer overall quality of life (QOL) [2–6]. These experiences can have a substantial impact and persist for prolonged periods of time following initial diagnosis [6–8]. As a result, significant attention has been paid to understanding how patients cope with the stress associated with cancer diagnosis [9–11].
Although being diagnosed with a potentially life-threatening condition constitutes a major life stressor [12], cancer patients frequently report experiencing many other types of stressors (e.g., financial difficulties, relationship problems, illnesses and deaths of family members) [11]. Given the overwhelming emotions involved in facing a diagnosis of cancer, however, the impact that contextual (non-cancer) life stressors have on the QOL of cancer patients has been understudied, despite the fact that such stressors may substantially affect patients’ ability to cope and their clinical trajectories.
Life stressors are linked to exacerbation of chronic medical conditions and mental health problems in a variety of populations [13–14]. Non-cancer life stressors have been linked to disturbances in QOL in breast and prostate cancer, and melanoma patients [11, 15–16] as well as to melanoma patients’ survival [17]. Financial and economic stressors in particular have been associated with decreased QOL for low-income women with gynecologic and breast cancers [18]. Many reports have not differentiated between cancer-related and non-cancer related stressors [19–21]; thus, little is known about the extent of non-cancer life stressors among ovarian cancer patients and the impact that such stressors may have on QOL.
The goal of the present study was to examine associations between non-cancer life stressors, overall QOL, and physical, functional, social, and emotional well-being in women with invasive epithelial ovarian cancer who were followed longitudinally over one year. The study was unique in that it examined non-cancer life stressors at two time-points: in the year pre-surgery and during the year between surgery and one-year follow-up. This study design also permitted us to examine prospective associations between life stressors occurring pre-surgery and QOL at one-year. Because we were interested in the effects of life stress independent of its potential effects on negative mood, all primary analyses controlled for symptoms of depression and anxiety.
Method
Participants
Following Institutional Review Board approval (at Universities of Iowa, Miami, and Washington University), women over 18 years of age with a new diagnosis of a pelvic or abdominal mass suspected to be ovarian cancer were recruited for the study. Inclusion was confirmed by histologic diagnosis with primary invasive epithelial ovarian, primary peritoneal, or primary fallopian tube cancer as part of a larger study examining biobehavioral factors and QOL in ovarian cancer [22]. Patients were excluded for primary cancer of another organ site, a non-epithelial ovarian tumor, an ovarian tumor of low malignant potential, neoadjuvant chemotherapy, systemic corticosteroid medication use in the last 4 months, or comorbidities known to alter the immune response. Thus, 123 women with histologically confirmed epithelial ovarian cancer who had life stress data at both time points and one-year QOL data were included in analyses.
Patients completed psychosocial questionnaires between their initial pre-operative appointment and surgery, and again at the one-year follow-up. Medical information was abstracted from patient charts.
Psychosocial Assessments
Contextual Life Stressors
A modified version of the Life Experiences Survey (LES) [23,24] was administered to assess major life stressors over the past 12 months. The LES asks participants to indicate whether specific stressors (e.g., death of a close family member, loss of a job, relationship problems) occurred during the year before the assessment and to rate how stressful the experiences were on a 5-point Likert-type scale. The modified version of the scale used in the present study included 28 questions [24] and was utilized to minimize participant burden. These stressors have been shown to correlate with adverse health outcomes [25].
Quality of Life
The Functional Assessment of Cancer Therapy (FACT-G) [26] is a 27-item self-report measure assessing QOL in cancer patients. The FACT-G contains subscales that assess physical (PWB), social/family (SWB), emotional (EWB), and functional (FWB) well-being. Participants indicate their functioning over the last 7 days on a 5-point scale, ranging from 0 (not at all) to 4 (very much). The scale has good reliability and validity [26, 27]. Higher FACT-G scores indicate better QOL; means of the general US adult female population are approximately 80 (S.D=18.6). Five-point score changes score are considered clinically significant [28].
Depressed Mood
The Center for Epidemiologic Studies Depression Scale (CES-D) is a 20-item scale that measures depressive symptoms [29, 30]. Items are answered on a 4-point scale, from 0 (rarely) to 3 (most or all of the time), and indicate frequency of each item in the past week. Scores of 16 and above are associated with clinical depression. A four-factor structure has been identified for the CES-D, and these factors have been used independently to characterize different facets of depression [31]. In this study, the depressive mood subscale was used as a covariate in primary analyses.
Anxious Mood
The Profile of Mood States short form (POMS-SF), which lists 37 mood-related adjectives assessed over the past week was used as an indicator of anxious mood. Adjectives are rated on a 5-point scale from 0 (not at all) to 4 (extremely). The tension/anxiety subscale, including items such as “tense”, “nervous”, and “anxious” was used here to adjust for effects of anxiety. [32]
Demographic and Clinical Information
Demographic information was provided by self-report. Clinical information was obtained from medical records.
Statistical Analyses
Version 9.3 of SAS (SAS, Cary, NC) was used to analyze data. All distributions were examined for outliers and assumptions of non-normality. Descriptive statistics were used to examine levels of life stressors and QOL variables at baseline and one-year. Paired t-tests were used to test for changes in QOL from baseline to one-year. Correlations and ANOVAs were used to test for relationships between demographic variables and life stressors. Longitudinal analyses used a mixed model for repeated measures to examine associations between number of life stressors and QOL at baseline and one-year. Fixed effects in the model included stage, age, CESD depression subscale, POMS anxiety subscale, life stress exposure (baseline or year 1), time, and stress X time interactions. This model allowed estimation of the relationship of life stress with QOL at baseline and at year 1, as well as comparison of the slopes by testing the stress x time interaction effect. Linear regressions were used to test for prospective associations between pre-surgery life stressors and one-year QOL, adjusting for baseline QOL, chemotherapy (yes/no) at one-year and the covariates listed above. BMI and education were also examined as possible covariates in these models. When BMI was included as a potential covariate, it was not significantly associated with any QOL scale (p-values ranged from 0.19 to 0.96) and the effects of life stress remained significant. Similarly, when education (as a proxy for socioeconomic status [SES]) was included in the models as a covariate, it was not associated with any QOL score (p-values ranged from 0.53 to 0.96), and the effects of life stress were still significant. Therefore, for parsimony, neither BMI nor education was included in final models.
Results
Patient Characteristics
Participants had a mean age of 57.5 years (range: 27–88), and were predominately non-Hispanic Caucasians. The majority of patients had advanced stage (65.9%) and high-grade disease (83.7%). (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Ovarian Cancer Patients (N = 123) |
|---|---|
| Age, years (Mean, S.D.,) | 57.52 (11.67) |
| Body Mass Indexa (Mean, S.D.) | 27.94 (6.3) |
| Ethnicity (N =123) | |
| Non-Hispanic | 94.3% (116) |
| Hispanic | 5.7% (7) |
| Race | |
| American Indian/Alaska native | 0.8% (1) |
| Asian | 0.8% (1) |
| Black/African American | 2.4% (3) |
| White/Caucasian | 95.9% (118) |
| Educationb | |
| Less than eighth grade | 2.5% (3) |
| Some high school | 3.3% (4) |
| High school graduate | 33.3% (40) |
| Trade school/some college | 32.5% (39) |
| College graduate | 19.2% (23) |
| Postgraduate | 9.2% (11) |
| Marital status | |
| Single | 9.75% (12) |
| Divorced | 9.75% (12) |
| Widowed | 8.2% (10) |
| Married/Living with partner | 71.5% (88) |
| Separated | 0.8% (1) |
| Cancer Stage | |
| I | 25.2% (31) |
| II | 6.5% (8) |
| III | 57.7% (71) |
| IV | 8.2% (10) |
| Missing | 2.4% (3) |
| Cancer Grade | |
| Low | 16.3% (20) |
| High | 83.7% (103) |
| Tumor histology | |
| Serous | 74.0% (91) |
| Endometrioid | 13.0% (16) |
| Mucinous | 3.3% (4) |
| Clear cell | 4.0% (5) |
| Other | 5.7% (7) |
| Residual disease | |
| Yes | 25.3% (31) |
| No | 74.7% (92) |
N=122,
N=120
Life stressors and QOL over time
At the time of surgery, 17.9% of patients reported having no life stressors in the past year, 57.7% reported 1–3 stressors, and 24.4% of patients reported having 4 or more stressors. Similar frequencies were reported at one-year, with 22.0% of patients reporting no life stressors in the past year, 55.3% reporting 1–3 stressors, and 22.7% of patients reporting 4 or more stressors. Stressor types included spousal sickness and death; divorce; death of parents, close friends, or family members; job loss; caretaking for parents or in-laws with Alzheimer’s or other diseases; financial difficulties; and sexual assault, incarceration, and military deployment of adult children. (Table 2). Patients reporting higher levels of life stressors at baseline had significantly higher levels of stressors at one-year, (p <0.0001). Number and severity of life stressors were unrelated to income (p-values > 0.65) and education (p-values > 0.54). Younger patients (less than median age of 59) reported significantly more life stressors (p-values < 0.001) and stressors of greater severity (p-values < 0.002) both at surgery and one year later.
Table 2.
Means and standard deviations for life stress and quality of life pre-surgery and at one-year following surgery
| Variable | Baseline | One Year | ||||
|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | |
| FACT Total | 118 | 74.7 | 13.5 | 118 | 82.7 | 14.5 |
| Physical Well-Being | 120 | 20.0 | 6.1 | 120 | 23.0 | 4.7 |
| Functional Well-Being | 120 | 18.2 | 5.2 | 120 | 21.3 | 5.6 |
| Social Well-Being | 121 | 20.6 | 3.2 | 121 | 19.9 | 3.6 |
| Emotional Well-Being | 122 | 15.9 | 4.6 | 122 | 18.7 | 4.4 |
| CESD-depression subscale | 123 | 3.7 | 3.3 | 123 | 2.7 | 3.6 |
| POMS-SF anxiety | 117 | 9.94 | 5.93 | 119 | 6.36 | 5.52 |
| Life Stressors (Number) | 123 | 2.3 | 2.2 | 123 | 2.2 | 2.2 |
| Life Stressors (Severity) | 123 | 5.9 | 6.6 | 123 | 5.4 | 6.7 |
|
| ||||||
| Life Stressors (Type) | N | % | Number | N | % | Number |
| Relationship problems | 123 | 32.5 | (40) | 123 | 27.6 | (34) |
| Work problems | 123 | 29.3 | (36) | 123 | 23.6 | (29) |
| Financial Problems | 123 | 30.9 | (38) | 123 | 27.6 | (34) |
| Death | 123 | 26.0 | (32) | 123 | 32.5 | (40) |
| Illness | 123 | 33.3 | (41) | 123 | 31.7 | (39) |
| Violence | 123 | 1.6 | (2) | 123 | 2.4 | (3) |
| Trauma | 123 | 28.5 | (35) | 123 | 24.4 | (30) |
At the time of surgery, the FACT total score (M = 74.7; SD=13.5) was below means for a general female cancer population (M = 82.1; SD=16.3), indicating clinically impaired QOL [28]. Clinically and statistically significant improvements in QOL were evident from pre-surgery to one-year (p < 0.0001), with average gains of about 8 points [28]. (Table 2).
Number of Life Stressors and QOL
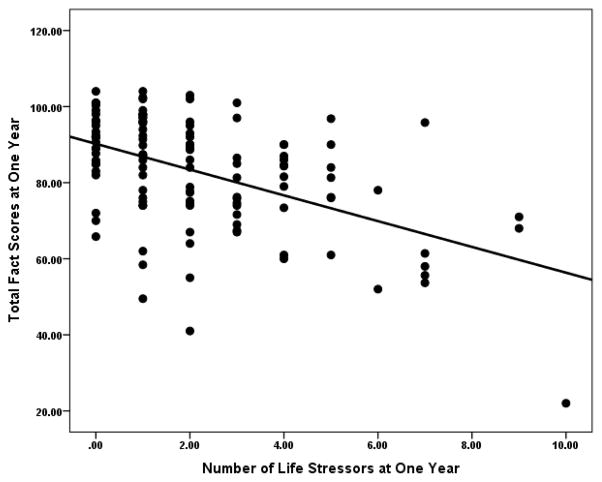
A mixed model for repeated measures was used to examine the relationship between number of life stressors, total QOL, and QOL subscales at the time of surgery and one-year, and changes in these associations over time. At the time of surgery, adjusting for effects of stage, age, anxiety and depressive mood, number of life stressors was unrelated to total QOL or to any of the QOL subscales (all p-values > 0.20). In contrast, at one-year, adjusting for the same variables plus presence/absence of chemotherapy at one-year, patients reporting more stressors reported significantly poorer physical (PWB: p=0.015), functional (FWB: p < 0.0001), social (SWB: p=0.0003), and total QOL (p<0.0001) (Figure 1). Each additional stressor between surgery and one-year was associated with a 1.96-point decrease in total QOL at one-year (95% C.I.: −2.86, −1.06: p<0.0001). (Table 3). The association between life stress and total QOL was significantly different at baseline and one-year as indicated by a comparison of slopes at these two time-points (p=0.006), highlighting the differences in effects of contextual stressors at these time-points.
Figure 1.
Association between number of stressful life events at one year and quality of life at one year.
Table 3.
Regression models predicting quality of life at pre-surgery and one year from number of concurrent life stressorsa
| Outcome | Pre-Surgery | Year 1 | Compare slopes p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | SE | 95% C.I. | p- value | Standar dized β | Slope | SE | 95% C.I. | p-value | Standardi zed β | ||
| FACT Total | −0.50 | 0.43 | −1.34, 0.34 | 0.240 | −0.08 | −1.96 | 0.46 | −2.86, −1.06 | < 0.0001 | −0.29 | 0.006 |
| PWB | −0.20 | 0.21 | −0.60, 0.21 | 0.337 | −0.07 | −0.54 | 0.22 | −0.97, −0.10 | 0.015 | −0.25 | 0.196 |
| FWB | −0.24 | 0.19 | −0.61, 0.13 | 0.201 | −0.11 | −0.94 | 0.20 | −1.34, −0.54 | < 0.0001 | −0.36 | 0.003 |
| EWB | 0.02 | 0.13 | −0.24, 0.27 | 0.890 | 0.01 | 0.13 | 0.14 | −0.14, 0.40 | 0.355 | 0.06 | 0.499 |
| SWB | −0.01 | 0.13 | −0.27, 0.25 | 0.958 | −0.005 | −0.52 | 0.14 | −0.80, −0.24 | 0.0003 | −0.31 | 0.002 |
All pre-surgery models adjust for age, cancer stage, anxiety, and depressive mood. All one-year models additionally adjust for chemotherapy status.
FACT = Functional Assessment of Cancer Therapy; PWB = Physical well-being; FWB = Functional well-being; EWB = Emotional well-being; SWB = Social well-being.
Although the association between number of stressors and emotional well-being was not significant at either time-point, because of the considerable overlap of EWB with the constructs of depression and anxiety, the EWB model was also examined in secondary analyses without adjusting for mood. In this model, number of life stressors significantly predicted EWB both at surgery (p=0.049) and at one-year (p=0.002), suggesting that life stress is an important contributor to EWB when overlapping constructs are removed from analyses.
Type of Life Stressors and QOL
Mixed models were also used to examine the effects of different types of stressors (i.e., relationship, work, financial, death), adjusting for covariates listed above. Patients reporting relationship problems had poorer total QOL at both time of surgery (QOL decrement = 3.69 ± 2.1 points, p=0.081) and at one-year (QOL decrement = 6.6 ± 2.1 points, p=0.002) compared to patients not reporting relationship problems. Participants reporting major work problems at one-year also had lower total QOL (QOL decrement=5.2 ± 2.14 points, p=0.016) compared to participants without major work problems. These associations were not observed at the time of surgery. Experiencing financial problems or death of a friend or family member was not associated with poorer QOL at either time-point (p> 0.37).
Severity of Life Stressors and Quality of Life
Pre-surgery, severity of life stressors was unrelated to any QOL variable (p-values > 0.098). At one-year, patients with more severe stressors had poorer PWB (p=0.029), FWB (p <0.0001), SWB (p=0.002), and total QOL (p< 0.0001) adjusting for covariates as above. (Table 4). When the EWB model was examined without adjusting for anxious and depressive mood, severity of life stressors was associated with EWB both at the time of surgery (p=0.044) and at one-year (p=0.002).
Table 4.
Regression models predicting quality of life at pre-surgery and one year from concurrent life stressor severitya
| Outcome | Pre-Surgery | Year 1 | Compare slopes p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | SE | 95% CI | p-value | Standardi zed β | Slope | SE | 95% CI | p-value | Standar dized β | ||
| FACT Total | −0.18 | 0.15 | −0.46, 0.11 | 0.228 | −0.09 | −0.60 | 0.15 | −0.90, −0.30 | 0.0001 | −0.28 | 0.015 |
| PWB | −0.08 | 0.07 | −0.22, 0.06 | 0.271 | −0.09 | −0.16 | 0.07 | −0.31, −0.02 | 0.029 | −0.23 | 0.323 |
| FWB | −0.11 | 0.06 | −0.24, 0.02 | 0.098 | −0.14 | −0.30 | 0.07 | −0.43, −0.16 | <0.0001 | −0.36 | 0.017 |
| EWB | 0.04 | 0.04 | −0.06, 0.11 | 0.535 | 0.04 | 0.04 | 0.05 | −0.05, 0.13 | 0.427 | 0.057 | 0.858 |
| SWB | 0.008 | 0.044 | −0.08, 0.10 | 0.862 | 0.016 | −0.15 | 0.05 | −0.24, −0.06 | 0.002 | −0.28 | 0.002 |
All pre-surgery models adjust for age, cancer stage, anxiety, and depressive mood. All one year models additionally adjust for chemotherapy status.
FACT = Functional Assessment of Cancer Therapy; PWB = Physical well-being; FWB = Functional well-being; EWB = Emotional well-being; SWB = Social well-being.
Prospective Effects of Life Stressors at Pre-Surgery on One-year QOL
We also examined the extent to which life stressors reported at the time of surgery were prospectively associated with one-year QOL. Adjusting for covariates as above, participants experiencing more non-cancer stressors at baseline reported significantly poorer overall QOL at one-year, such that each additional life stressor at baseline was associated with a 1.41-point (± 0.22) decrease in QOL at one-year (p < 0.0001). Adjusting for the same covariates, severity of life stressors pre-surgery had a possible trend towards an association with poorer total QOL at one-year (p=0.0715) (Table 5). When examining the effects of different types of life stressors pre-surgery on QOL at one-year, only work problems were prospectively associated with poorer total one-year QOL, at a marginal level (p=0.068). Participants reporting major work problems pre-surgery had an overall QOL approximately 5.1 points lower than patients without work-related problems.
Table 5.
Regression models predicting total quality of life at one year from number and severity of life stressors at time of surgerya
| Number of Stressors | ||||||
|---|---|---|---|---|---|---|
| Parameter | DF | Estimate | Standardized Estimate | Standard Error | t | P |
| Intercept | 1 | 59.165 | 11.882 | 4.98 | <.0001 | |
| FACT Baseline | 1 | 0.370 | 0.340 | 0.109 | 3.38 | 0.001 |
| Age | 1 | 0.051 | 0.042 | 0.107 | 0.47 | 0.6365 |
| Stage 3–4b | 1 | −0.123 | −0.004 | 2.838 | −0.04 | 0.9655 |
| Year 1 Chemotherapyc (Yes) | 1 | −6.174 | −0.180 | 3.090 | −2.00 | 0.0484 |
| Baseline Anxiety (POMS-SF) | 1 | 0.042 | 0.017 | 0.240 | 0.18 | 0.8608 |
| Baseline Depression (CES-D) | 1 | −0.798 | −0.182 | 0.483 | −1.65 | 0.1019 |
| Number of Life Stressors | 1 | −1.414 | −0.221 | 0.581 | −2.43 | 0.0167 |
| Stressor Severity | ||||||
|---|---|---|---|---|---|---|
| Parameter | DF | Estimate | Standardized Estimate | Standard Error | t | P |
| Intercept | 1 | 57.579 | 12.109 | 4.76 | <.0001 | |
| QOL Baseline | 1 | 0.375 | 0.345 | 0.111 | 3.38 | 0.001 |
| Age | 1 | 0.056 | 0.046 | 0.111 | 0.50 | 0.6171 |
| Stage 3–4b | 1 | −0.481 | −0.016 | 2.865 | −0.17 | 0.867 |
| Year 1 Chemotherapyc (Yes) | 1 | −5.655 | −0.164 | 3.111 | −1.82 | 0.072 |
| Baseline Anxiety (POMS-SF) | 1 | 0.076 | 0.031 | 0.243 | 0.31 | 0.7556 |
| Baseline Depression (CES-D) | 1 | −0.929 | −0.212 | 0.483 | −1.93 | 0.0569 |
| Severity of Life Stressors | 1 | −0.359 | −0.169 | 0.197 | −1.82 | 0.0715 |
Adjusted for baseline QOL, age, cancer stage, anxiety, and depression at pre-surgery, and chemotherapy at year 1; n = 110
Stages 1 and 2 are reference group
Not receiving chemotherapy at one year is reference group
FACT=Functional Assessment of Cancer Therapy, POMS-SF= Profile of Mood States Short Form, CES-D=Center for Epidemiological Studies Depression Scale
Discussion
The key findings of this prospective longitudinal study are that non-cancer-related stressors are strongly associated with QOL among ovarian cancer patients. Specifically, non-cancer life stressors occurring during the year since diagnosis had a clinically and statistically significant impact on QOL at one-year, adjusting for factors such as cancer stage, age, chemotherapy status, and mood. This association extended to physical, social, functional, and total QOL. In contrast, non-cancer life stress was unrelated to concurrent QOL at the time of initial cancer surgery. Additionally, the number of life stressors, and to a lesser extent, severity of life stress in the year pre-surgery was prospectively related to poorer QOL at one-year, suggesting that patients experiencing significant stress burden around the time of surgery may not be as resilient in their recovery from surgery and initial treatment as those experiencing less stress burden. Importantly, major non-cancer stressors were relatively common, with patients reporting 2–3 major life stressors (on average) at both baseline and at one-year. That magnitude of concurrent life stressors would be associated with approximately a 4- to 6-point decrease in total QOL scores at one-year – a magnitude of change previously associated with clinically significant alterations in QOL [28]. As such, it appears that exposure to major life stressors is associated with clinically significant QOL decrements, which may have implications for QOL trajectories among women with ovarian cancer.
Although there was no relationship between number or severity of stressors and EWB when depression and anxiety were included as covariates, because of substantial overlap between mood variables and EWB, we conducted secondary analyses without these variables. When mood covariates were removed, life stress predicted poorer EWB at both time-points, suggesting strong associations with deficits to emotional well-being as well.
Previous research among melanoma, breast, and prostate cancer patients found that non-cancer life stressors were associated with poorer QOL 3–4 months post-diagnosis, with disturbances largely in psychological or physical functioning [16]. Among breast cancer patients, higher levels of stress were linked with more psychological symptoms and worse QOL at 6- and 15-months [16]. In fact, among newly-diagnosed melanoma and breast cancer patients, non-cancer life stressors were stronger predictors of QOL at 3–4 months post-diagnosis than cancer type or treatment [33]. Another study of found that number of life stressors in the year prior to breast cancer diagnosis was unrelated to QOL at the time of surgery, but was associated with QOL at a 12-month follow-up [11]. The present findings are similar, suggesting that non-cancer stressors occurring in the year before surgery have less impact on QOL at the time of surgery, but may be strongly associated with QOL over the following year.
Major life stressors impact an individual’s goals, plans, and future aspirations [12]. Such stressors can include traumatic events as well as stressors called “entrapment events” which include caretaking or chronic financial difficulties that limit an individual’s control and options, and may cause biologic “wear and tear” [12,34–35]. Thus, non-cancer life stressors provide an emotional and cognitive context for shaping an individual’s response to cancer diagnosis and treatment. The number of life stressors was more strongly associated with QOL than their severity, suggesting that it may be the repeated and cumulative nature of the stress that most affects QOL [35].
Although speculative, it is possible that the lack of association between non-cancer life stressors and QOL pre-surgery is due to pre-occupation with issues related to cancer diagnosis and treatment at that time. Non-cancer life stressors may be less salient at this time. In contrast, at one-year, issues related to diagnosis and treatment may be less immediate, and patients’ reactions to contextual life issues may be more likely to influence their experience.
Physiological Mechanisms Underlying the Life Stress-QOL Relationship
Potential mechanisms relating life stressors to QOL include stress hormones and inflammatory cytokines. Chronic stress has been associated with elevated levels of cortisol and norepinephrine [36,37] and the inflammatory cytokine interleukin-6 (IL-6). IL-6 has profound effects on neurovegetative symptoms [38], and has been associated with fatigue, sleep disturbance, and vegetative depression in ovarian cancer patients [39–41]. Cortisol has been linked with fatigue and debility in ovarian cancer [39]. Such symptoms would likely be reflected in poorer physical well-being and could also affect mood and activity levels, resulting in impairments in all aspects of QOL.
Limitations
Study limitations include use of self-report surveys which may limit a nuanced understanding of the impact and chronicity of relevant stressors. Retrospective reporting is subject to the possibility of biased recall; we do not know how this may have affected reporting of life events. Because of limited availability of data on income, education was used as the indicator of SES. Thus, the present findings lack a detailed approach to SES and may underestimate its influence on QOL. The relatively homogeneous study population may limit conclusions about the role of racial or ethnic factors.
Applications to Clinical Care
In light of present findings, screening for major life stressors and ongoing chronic difficulties as part of an initial clinical assessment would be highly relevant for a comprehensive patient care plan. It may also be important to assess patients’ coping strategies for dealing with life stressors, given previous research indicating that maladaptive coping strategies are associated with decreased QOL in gynecological cancer patients following chemotherapy [42,43]. Additionally, identification of social-environmental resources and support, including mental health resources, may be a useful adjunct for recovery for patients with high levels of stress.
Conclusions
The present data suggest that non-cancer stressors are strongly associated with QOL in women with ovarian cancer. Taking such effects into account may be important for improving clinical care for these patients.
Research Highlights.
Number and severity of life stressors were unrelated to participants’ QOL before surgery.
Greater number of life stressors at one-year post surgery was related to poorer concurrent quality of life.
Greater number of life stressors prior to surgery surgery predicted poorer overall QOL at one year.
Acknowledgments
This research was supported in part by NIH grants CA88293, CA104825, and CA140933 to SL.
We would like to thank Amina Ahmed, M.D., Lauren Clevenger, B.S., Brian Weinberg, B.S., and the participating patients for their contributions to this project.
Footnotes
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American CS. Cancer facts and figures. Atlanta, GA: 2012. [Google Scholar]
- 2.Norton TR, Manne SL, Rubin S, et al. Prevalence and predictors of psychological distress among women with ovarian cancer. J Clin Oncol. 2004;22:919–26. doi: 10.1200/JCO.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Bodurka-Bevers D, Basen-Engquist K, Camark CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecologic Oncology. 2000;78:302–8. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 4.Arden-Close E, Gidron Y, Moss-Morris R. Psychological distress and its correlates in ovarian cancer: a systematic review. Psychooncology. 2008;17:1061–72. doi: 10.1002/pon.1363. [DOI] [PubMed] [Google Scholar]
- 5.Schulman-Green D, Ercolano E, Dowd M, Schwartz P, McCorkle R. Quality of life among women after surgery for ovarian cancer. Palliat Support Care. 2008;6:239–47. doi: 10.1017/S1478951508000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves V, Jayson G, Tarrier N. A longitudinal investigation of psychological morbidity in patients with ovarian cancer. Br J Cancer. 2008;99:1794–801. doi: 10.1038/sj.bjc.6604770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price MA, Butow PN, Costa DS, et al. Prevalence and predictors of anxiety and depression in women with invasive ovarian cancer and their caregivers. Med J Aust. 2010;193(5 Suppl):S52–7. doi: 10.5694/j.1326-5377.2010.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 8.Hipkins J, Whitworth M, Tarrier N, Jayson G. Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol. 2004;9:569–81. doi: 10.1348/1359107042304542. [DOI] [PubMed] [Google Scholar]
- 9.Andersen BL, Andersen B, deProsse C. Psychological outcomes. J Consult Clin Psychol. 1989;57:692–7. doi: 10.1037//0022-006x.57.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter KM, Fowler JM, Maxwell GL, Andersen BL. Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med. 2010;39:79–90. doi: 10.1007/s12160-010-9160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, et al. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24:288–96. doi: 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GW, Harris TO. Social Origins of Depression: A study of Psychiatric Disorder in Women. New York: The Free Press; 1978. [Google Scholar]
- 13.Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Ann Rev Clin Psychol. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- 14.Chen E, Miller GE. Socioeconomic Status and Health: Mediating and Moderating Factors. Annu Rev Clin Psychol. 2013;9:723–49. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress: Risk factors for cancer-related fatigue. Clinical Psychological Science. doi: 10.1177/2167702613496243. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehto US, Ojanen M, Väkevä A, Aromaa A, Kellokumpu-Lehtinen P. Noncancer life stresses in newly diagnosed cancer. Support Care Cancer. 2008;16:1231–41. doi: 10.1007/s00520-008-0410-8. [DOI] [PubMed] [Google Scholar]
- 17.Lehto US, Ojanen M, Dyba T, Aromaa A, Kellokumpu-Lehtinen P. Impact of life events on survival of patients with localized melanoma. Psychother Psychosom. 2012;81:191–3. doi: 10.1159/000334486. [DOI] [PubMed] [Google Scholar]
- 18.Ell K, Xie B, Wells A, Nedjat-Haiem F, Lee P, Vourlekis B. Economic stress among low-income patients with cancer: Effects on quality of life. Cancer. 2008;112:616–25. doi: 10.1002/cncr.23203. [DOI] [PubMed] [Google Scholar]
- 19.Beatty L, Lee C, Wade TD. A prospective examination of perceived stress as a mediator of the relationship between life-events and QOL following breast cancer. Br J Health Psychol. 2009;14:789–804. doi: 10.1348/135910709X412459. [DOI] [PubMed] [Google Scholar]
- 20.Joseph HJ, Thibault GP, Ruttle-King J. Perceived stress and quality of life among prostate cancer survivors. Mil Med. 2006;171:425–9. doi: 10.7205/milmed.171.5.425. [DOI] [PubMed] [Google Scholar]
- 21.Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE. Screening and referral for psychosocial distress in oncologic practice: use of the Distress Thermometer. Cancer. 2008;113:870–8. doi: 10.1002/cncr.23622. [DOI] [PubMed] [Google Scholar]
- 22.Lutgendorf SK, De Geest K, Bender D, et al. Social Influences on Clinical Outcomes of Patients with Ovarian Cancer. J Clin Oncol. 2012;30:2885–90. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 24.Leserman J, Whetten K, Lowe K, et al. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–07. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 25.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–7. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Tulsky D, Gray G, Sarafian B, Lloyd S, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, Eckberg K, Purl S, Blendowski C, Goodman M, Barnicle M, Stewart I, McHale M, Bonomi P, Kaplan E, Taylor S, Thomas C, Harris J. The Functional Assessment of Cancer Therapy (FACT) scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 27.Basen-Enquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, Gershenson DM. Feliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 28.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 29.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Ensel WM. Measuring depression: The CES-D scale. In: Lin N, Dean A, Ensel W, editors. Social support, life events, and depression. New York: Academic Press; 1986. [Google Scholar]
- 31.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies depression scale. J Personality Assess. 1995;64:507–21. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 32.Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states (POMS-SF). psychometric information. Psychol Assess. 1995;7:80–3. [Google Scholar]
- 33.Lehto US, Ojanen M, Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16:805–16. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–96. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–79. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 36.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increases in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82:2458–65. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- 37.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen B. Price of adaptation--allostatic load and its health consequences. Arch Int Med. 1997;157:2259–68. [PubMed] [Google Scholar]
- 38.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414–26. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrepf A, Clevenger L, Christensen D, et al. Cortisol and Interleukin-6 in Ovarian Cancer Patients Following Primary Treatment: Relationships with Quality of Life. Brain, Behav Immun. 2012;30 (Suppl):S126–34. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, Cortisol, and Depressive Symptoms in Ovarian Cancer Patients. J Clin Oncol. 2008;26:4820–7. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clevenger L, Schrepf A, Christensen D, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun. 2012;26:1037–44. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutgendorf SK, Anderson B, Ullrich P, et al. Quality of life and mood in women with gynecologic cancer: A one year prospective study. Cancer. 2002;94:131–40. doi: 10.1002/cncr.10155. [DOI] [PubMed] [Google Scholar]
- 43.Costanzo ES, Lutgendorf SK, Rothrock NE, Anderson B. Coping and quality of life among women extensively treated for gynecologic cancer. Psychooncology. 2006;15:132–42. doi: 10.1002/pon.930. [DOI] [PubMed] [Google Scholar]