Abstract
The demand for total hip replacement (THR) surgery is increasing in the younger population due to faster rehabilitation and more complete restoration of function. Up to 2009, metal-on-metal (MoM) hip joint bearings were a popular choice due to their design flexibility, post-operative stability and relatively low wear rates. The main wear mechanisms that occur along the bearing surface of MoM joints are tribochemical reactions that deposit a mixture of wear debris, metal ions and organic matrix of decomposed proteins known as a tribolayer. No in-depth electrochemical studies have been reported on the structure and characteristics of this tribolayer or about the parameters involved in its formation.
In this study, we conducted an electrochemical investigation of different surfaces (bulk-like: control, nano-crystalline: new implant and tribolayer surface: retrieved implant) made out of two commonly used hip CoCrMo alloys (high-carbon and low-carbon). As per ASTM standard, cyclic polarization tests and electrochemical impedance spectroscopy tests were conducted. The results obtained from electrochemical parameters for different surfaces clearly indicated a reduction in corrosion for the tribolayer surface (Icorr: 0.76 μA/cm2). Further, polarization resistance (Rp:2.39±0.60MΩ/cm2) and capacitance (Cdl:15.20±0.75 μF/cm2) indicated variation in corrosion kinetics for the tribolayer surface, that attributed to its structure and stability in a simulated body environment.
Keywords: MoM hip implant, CoCrMo alloy, Tribolayer, Electrochemistry, Corrosion kinetics
1. Introduction
Over the past few decades, metal-based hip joint bearings have largely supplanted polyethylene and ceramic alternatives as successful clinical therapies for diseased and traumatized hips. At the same time, total hip arthroplasty (THA) and hip resurfacing (HR) procedures have been widely accepted as treatments that restore function and allow the patient to resume normal daily activities. By 2030, the need for primary THA surgeries will have doubled from the current 250K that are performed annually (Kurtz et al., 2007). By considering, an younger enthusiastic patient population, implants that exhibit low wear rates, allow operative flexibility and demonstrate post-operative stability are widely preferred. In this respect, metal-on-metal (MoM) bearings have been an attractive solution satisfying these criteria (Bozic et al., 2009). However, increasing failure rates sometimes leading to revision surgery have caused declining confidence in both surgeons and patients. Once, dominating the marketplace, utilization of MoM fell from 40% to under 10% when compared with all THA bearing alternatives.
MoM joints are exposed to a complex in vivo environment with mechanical and electrochemical degradation mechanisms that influence the longevity of the device. The wear of MoM joints is of particular concern because particulate debris and release of Co/Cr ions can lead to adverse tissue reactions including necrosis, hypersensitivity and pseudotumors (Clayton et al. 2008; Pandit et al., 2008). Although numerous grooves and scratches on the surfaces of explanted MoM joints point to an abrasive wear mechanism, it has been demonstrated that in well functioning joints the main mechanisms leading to surface degradation are mild surface fatigue and tribochemical reactions (Catelas et al., 2011; Wimmer et al., 2010; Wimmer et al., 2003). When these wear mechanisms predominate, the MoM interface operates within the ultra-low wear regime (Fischer et al., 2012). It was shown that in situ microstructural alterations are key factors in the wear of MoM joints (Buscher et al., 2005). Further, a newly implanted MoM joint generates a nano-crystalline layer in vivo at the immediate subsurface zone due to the accumulation of plastic strain. The grain size in this subsurface zone correlates strongly with the size of wear particles generated in MoM hips (Pourzal et al., 2011). Thus, in situ formation of a nano-crystalline layer brings about mild surface fatigue by allowing the formation of very fine wear debris. Larger particles would promote three-body wear and subsequently increase the wear rate.
In addition to wear, various corrosion mechanisms that release metal ions from the bulk material may harm the surrounding tissue (Clayton et al., 2008; Pandit et al., 2008; Hallab et al., 2001). Mechanical articulations can accelerate the corrosion process as in the case of fretting-corrosion or sliding-corrosion (called as tribocorrosion) (Antunes et al., 2009; Hallab et al., 2004; Mathew et al.,2011; Mathew et al., 2009; Yan et al., 2006; Jemmely et al., 2000) The destruction or breakage of the passive oxide film causes a direct contact with the surrounding medium and the virgin metal surface, promoting tribocorrosive events that can be enhanced by the presence of adhering micro-organisms (Souza et al., 2010).
In 2003, it was reported that tribochemical reactions in the MoM joints result in the formation of a tribolayer, consisting of a carbonaceous material that forms in situ in the primary contact areas of the head and cup of the joint (Wimmer et al., 2003). The origin of the carbonaceous matter is proteinaceous stemming from the intermediate fluid, i.e. the synovial fluid. Such proteins may decompose due to mechanical shear, increased contact temperatures, local changes in pH, and catalytic dehydration due to the availability of metal ions. The latter may explain the presence of graphitic carbon in the tribolayer (Liao et al., 2011). The tribolayer not only covers the metal implant surface, but also interacts with it in the presence of shear forces in that carbonaceous material is incorporated into the nano-crystalline layer (Wimmer et al., 2003). This process has been described as mechanical mixing (Rigney et al., 2000). Hence, this newly formed material is a metallo-organic composite making a patchy appearance on the bearing surface. At this point, a better understanding of the electrochemistry of the tribolayer, and its interaction with the nano-crystalline zone are required to improve the performance of MoM joints.
Previous corrosion studies of the CoCrMo alloy primarily studied the formation of the passive film under biologically simulated environment (Hodgson et al., 2004; Metiko-Hukovic et al., 2007; Valero Vidal et al., 2011; Williams et al., 1988; Martin et al., 2013). However, recent reports of tribolayers formation raise new questions. What are the electrochemical properties of this newly formed in situ interface? Does the tribolayer promote or inhibit corrosion? The occurrence of a tribolayer was reported for both high carbon and low carbon CoCrMo alloys, Hence, we attempted to investigate the corrosion behavior of the tribolayer on a high-carbon (HC) and low-carbon (LC) CoCrMo alloy. Three different surface conditions were electrochemically tested: (1) bulk-like (control) (2) nano-crystalline and (3) tribolayer. It was hypothesized that the presence of a tribolayer will reduce corrosion of CoCrMo alloys.
2. Materials and Methods
A schematic diagram of the experimental design in the current investigation is presented in Fig. 1. The diagram explains the input variables (alloys and surfaces), tests methods, output variables and objective of the current study.
Figure 1.
Diagram of the experimental design – Input variable (alloys and surfaces), tests methods and output variables.
2.1 Sample Preparation
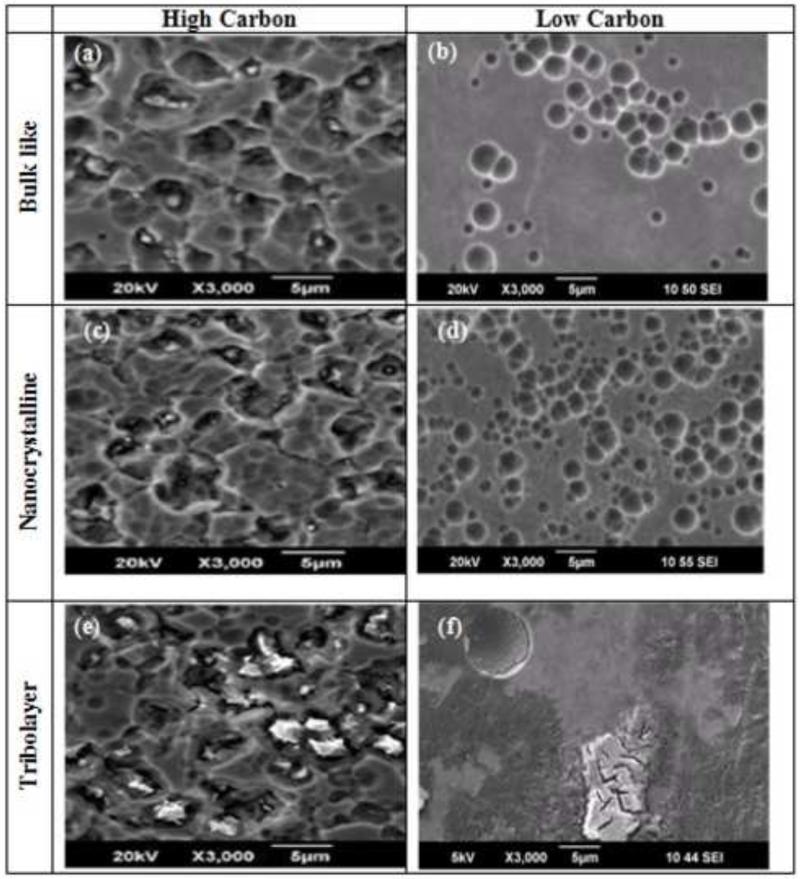
High carbon (HC) and low carbon (LC) CoCrMo disks, 29 mm in diameter and 12 mm in thickness, were machined from rods of CoCrMo wrought alloy (ATI Allvac, Monroe, NC, USA) with specification in ASTM Standard F 1537-07. The chemical composition of both alloys can be found in Table 1. Three surface conditions (Fig. 2 & SEM images are in Fig. 3) were tested: (i) bulk-like (control surface) (ii) nano-crystalline (polished: as out of box) and (iii) tribolayer (run-in: as retrieval). The surfaces were prepared as followed:
Bulk-like (control): In order to achieve a reference state, a sample surface with a bulk-like grain size was desired and to obtain this texture. Therefore, electrochemical polishing was used. After initial mechanical polishing, approximately 150 μm of the surface was removed using a standard electro-polishing set-up (Electropol, Struers, Ballerup, Denmark). This gave a bulk-like surface grain size of 8 to 12 μm. Areas of plastic deformation that are induced by the mechanical polishing process were also removed (Fig. 3 (a & b)). It is critical to consider that such a surface condition is for establishing a reference condition only and is not present in an in vivo environment. In a hip joint, a bulk-like microstructure at the surface would be quickly transformed to a nano-crystalline microstructure.
Nano-crystalline: A nano-crystalline subsurface zone forms at the surface of CoCrMo implants due to the introduction of cyclic shear stress (Buscher et al., 2005). Since mechanical polishing generates shear stresses, the result is a fine nano-crystalline surface layer on polished samples (Fig. 3 (c & d). The final polishing step used 1 μm diamond crystals to achieve a smoothness that is representative of a new implant (Ra< 0.04 μm). The resulting grain structure within the nano-crystalline subsurface zone was less than 50 nm. This surface condition was typical of a new MoM hip joint, which has a grain size in the range of 20-80 nm (Buscher et al., 2005; Pourzal et al., 2011).
Tribolayer: This type of sample surface was generated by the same standard metallographic polishing methods as the nano-crystalline surface described above. In order to generate the tribolayer at the surface, a polishing lubricant was used that consisted of mixing the 1 μm diamond crystals with bovine calf serum (BCS, 30 g/L protein). The SEM micrographs are provided in Fig. 3 (e & f). The aim was to form a tribolayer similar in appearance to those observed in previously reported investigation (Wimmer et al., 2010; Wimmer et al., 2003). Polishing was performed with a half-automated polisher (LaboPol-1, Struers, Ballerup, Denmark). The polishing fluid was added in a circular flow with a flexible tube pump. After six hours of polishing, a tribolayer was achieved that resembled the primary contact area of a MoM hip during steady-state. All test samples were cleaned with propanol in an ultrasonic bath for 10 minutes, then rinsed with distilled water for another 10 minutes and finally dried using warm air prior to testing.
Table 1.
Chemical composition of high and low carbon CoCrMo alloy composition
| Samples | Diameter (mm) |
Thickness (mm) |
Chemical composition (%wt) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Co | Cr | Mo | Si | Mn | Al | |||
| High carbon CoCrMo |
29 | 7 | 0.241 | 64.60 | 27.63 | 6.04 | 0.66 | 0.70 | 0.02 |
| Low carbon CoCrMo |
29 | 7 | 0.034 | 64.96 | 27.56 | 5.70 | 0.38 | 0.60 | <0.02 |
Figure 2.
Details of three surface conditions. Method of preparation, schematic and TEM micrographs
Figure 3.
SEM images of non-corroded surface: HC (a) and LC (b) Bulk-like surfaces; HC (c) and LC (d) Nano-crystalline surfaces; and HC (e) and LC (f) Tribolayer surfaces.
2.2 Experimental details
Electrochemical tests were conducted in a custom made three-electrode electrochemical cell with a known area of 0.38 cm2 as per ASTM standard G61. The exposed area of the CoCrMo surface, a graphite rod and a calomel electrode (SCE) represented the working electrode, auxiliary electrode and reference electrode, respectively. A potentiostat (G 700, Gamry Inc., Warminster, PA, USA) was used to carry out the electrochemical measurements for each surface condition (n = 4). A total volume of 150 ml of BCS (simulate synovial fluid, composition: NaCl: 9(g/L): EDTA: 0.2 (g/L), Tris: 27(g/L), protein: 30 g/L) was used as an electrolyte for each experiment while the temperature was maintained at 37° C.
Initially, to monitor the electrochemical stabilization of the system, the open circuit potential (OCP) was monitored for a period of 3600 seconds. An electrochemical impedance spectroscopy (EIS) test was subsequently performed. The EIS measurements were conducted in the frequency range from 100K Hz to 0.005 Hz with an AC sine wave amplitude of 10 mV applied to the electrode at its corrosion potential (Ecorr). The samples were then anodically polarized during the potentiodynamic test from −0.8 V to +1.8 at a scanning rate of 2 mV/s.
The corrosion parameters (Icorr, corrosion current density; Ecorr, corrosion potential) were obtained from the potentiodynamic polarization curves using Tafel’s method (Hodgson et al., 2004; Martin et al., 2013). EIS data were used to study electrochemical kinetics through Nyquist and Bode plots. EIS results were fitted to an equivalent circuit that was modeled using ZView2 (Zygo New View 6300, Middlefield, CT, USA) to determine the polarization resistance (Rp) and the double layer capacitance (Cdl). A modified Randle’s simple equivalent circuit that was proposed was composed of electrolyte resistances (Rs) in series with a constant phase element (CPE) and in parallel with the polarization resistance (Rp), and is presented in Fig. 4. The impedance of a CPE is defined by ZCPE = 1/((jw)n C), where j = √(−1), w = 2πf, and the exponent ‘n’ of the CPE is related to non-equilibrium current distribution due to surface roughness (Hodgson et al., 2004; Hirschorn et al., 2010) (CPE is used instead of the ideal capacitance in order to take into account the capacitance of the space charge with a passive film). The parameter ‘C’ is a constant, representing true capacitance of the oxide barrier layer.
Figure 4.
Diagram for the three-element Randle’s equivalent circuit used to determine the polarization resistance (Rp). Rs represents the solution resistance and Cdl represents the double layer capacitance
2.3 Surface characterization
In order to identify the differences in the corrosion behavior of the three surface conditions, their surfaces were analyzed after testing. The samples were cleaned using the same procedure described previously. The global surface topography was analyzed by means of white light interferometry (NewView6000, Zygo Corp., USA). The surface roughness (Ra) values were determined and are displayed in Table 3. For detailed surface characterization, a scanning electron microscope (SEM-JSM -5600 model, JOEL Co., Japan) was employed.
Table 3.
Surface roughness parameters (Ra and rms values) before and after corrosion exposure
| Material | Before testing | After testing |
|---|---|---|
| Sample | Ra / μm | Ra /μm |
| Bulk-like (HC) | 0.033 ± 0.005 | 0.424 ± 0.066 |
| Nano-crystalline (HC) | 0.004 ± 0.0001 | 0.207 ± 0.165 |
| Tribolayer (HC) | 0.018 ± 0.006 | 0.444 ± 0.034 |
| Bulk-like (LC) | 0.015 ± 0.001 | 0.126 ± 0.014 |
| Nano-crystalline (LC) | 0.004 ± 0.00003 | 0.170 ±0.010 |
| Tribolayer (LC) | 0.0107 ± 0.002 | 0.117 ± 0.010 |
HC: high carbon, LC: Low carbon, Ra: Roughness parameter
3. RESULTS
3.1 Potentiodynamic polarization measurements
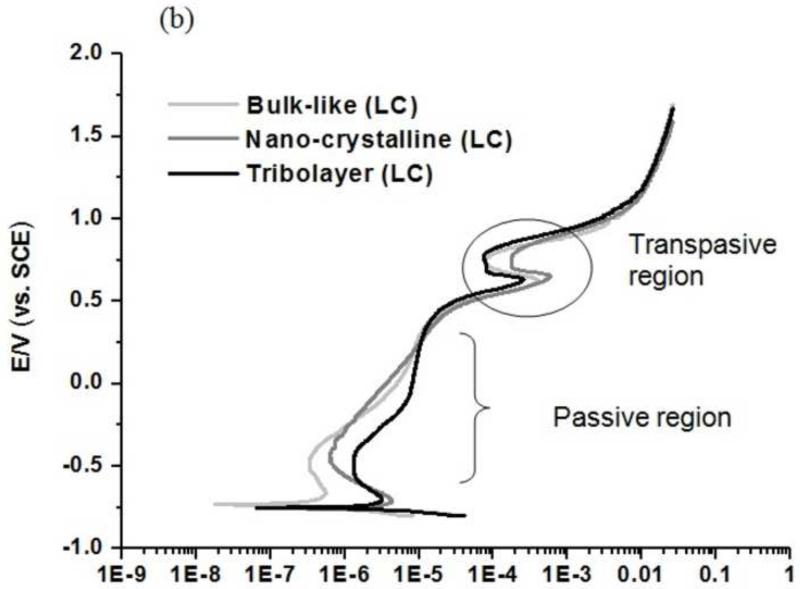
The polarization curves obtained for the HC and LC surface conditions are shown in Fig. 5(a) and (b) respectively. The created surface conditions exhibited significant differences in corrosion mechanisms, passivation and repassivation kinetics. The tribolayer surface demonstrated a more favorable behavior than the nano-crystalline surface characterized by a lower corrosion current and a passive region (marked in Fig. 5(a & b)). There is a transpassive region is observed at a potential range of +0.75 to + 0.8 V vs SCE (marked in Fig. 5), irrespective surfaces and alloy content. The presence of transpassive region is reported by previous studies (Hodgson et al., 2004; Metiko-Hukovic et al., 2007; Valero Vidal et al., 2011). Further, in the case of low carbon alloy the transpassive region is more evident, and other details are discussed in later section 4.1. Overall, the bulk-like surface exhibited the best behavior, which could be attributed to the formation of a strong passive film from the electrochemical preparation process prior to testing. It is interesting to note that the current density for the tribolayer on LC CoCrMo alloy is higher than for the nano-crystalline layer, particularly in the passive regime Fig. 5 (a & b). This is possibly due to the differences in the material chemistry of the formed tribolayer on both alloys.
Figure 5.
Polarization curves for three surfaces for high carbon (HC) and low carbon (LC) alloy: (a) HC- Bulk-like surfaces; Nano-crystalline surfaces and Tribolayer surfaces (b) LC- Bulk-like surfaces; Nano-crystalline surfaces and Tribolayer surfaces
The Ecorr and Icorr values for the different CoCrMo surfaces are shown in Table 2. No significant difference was observed for the Ecorr parameters either between the carbon composition of the alloys or the surface conditions. The tribolayer and bulk-like surfaces exhibited lower Icorr values when compared to the nanocrystalline surface. Hence, it can be stated that the the Icorr behavior was significantly affected by the variation in the surface conditions in both alloys. (Burstein et al., 2007; Yang et al., 1994; Truc et al., 2002; Amin et al., 2009; Frateur et al., 2006; Barril et al., 2004)
Table 2.
Evolution of electrochemical parameters from Tafel’s estimation and Electrochemical impedance spectroscopy modeling. Ecorr: Corrosion potential, Icorr: corrosion current, Rs: Solution resistance Rp: Polarization resistance, Cdl: Capacitance, Eq=0: open circuit potential
| Material | Electrochemical Parameters | |||||
|---|---|---|---|---|---|---|
| Tafel’s estimation | EIS parameters | |||||
| Sample | Ecorr /V (vs. SCE) |
Icorr (μA/cm2) |
Rs (Ω) | Rp – (MΩ/ cm2) |
Cdl-(μF/ cm2) |
Chi-squared values (average) |
| Bulk-like (HC) | −0.540 ± .244 |
0.29 ± 0.46 | 68.77 ± 1.70 |
4.57 ± 2.07 |
10.90 ± 0.33 |
0.001 |
| Nano- crystalline (HC) |
−0.751 ± .011 |
1.86 ± 0.21 | 66.21 ± 0.15 |
4.01 ± 0.94 |
19.21 ± 0.79 |
0.002 |
| Tribolayer (HC) |
−0.729 ± .011 |
0.76 ± 0.09 | 71.83 ± 1.23 |
2.39 ± 0.60 |
15.20 ± 0.75 |
0.001 |
| Bulk-like (LC) | −0.706 ± .022 |
0.17 ± 0.12 | 73.04 ± 1.79 |
7.86 ± 1.58 |
12.10 ± 4.20 |
0.001 |
| Nano- crystalline (LC) |
−0.741 ± .013 |
2.49 ± 0.46 | 66.93 ± 1.29 |
3.50 ± 0.76 |
18.20 ± 2.00 |
0.002 |
| Tribolayer (LC) |
−0.729 ± .018 |
0.77 ± 0.17 | 70.56 ± 0.72 |
2.86 ± 0.44 |
17.00 ± 1.80 |
0.001 |
HC: high carbon, LC: low carbon, Ecorr: Corrosion potential, Icorr: corrosion current, Rs: Solution resistance, Rp: Polarization resistance, Cdl: Capacitance
3.2 Electrochemical impedance spectroscopy (EIS) results
Electrochemical impedance spectroscopy (EIS) was employed to study in situ changes in the passive film/electrolyte-interface on different CoCrMo surfaces.
(a) Nyquist and Bode plots
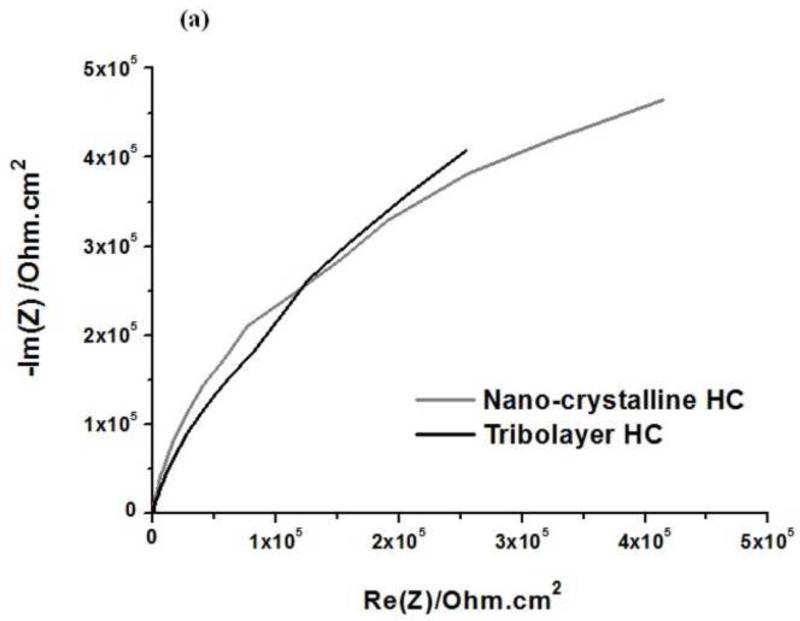
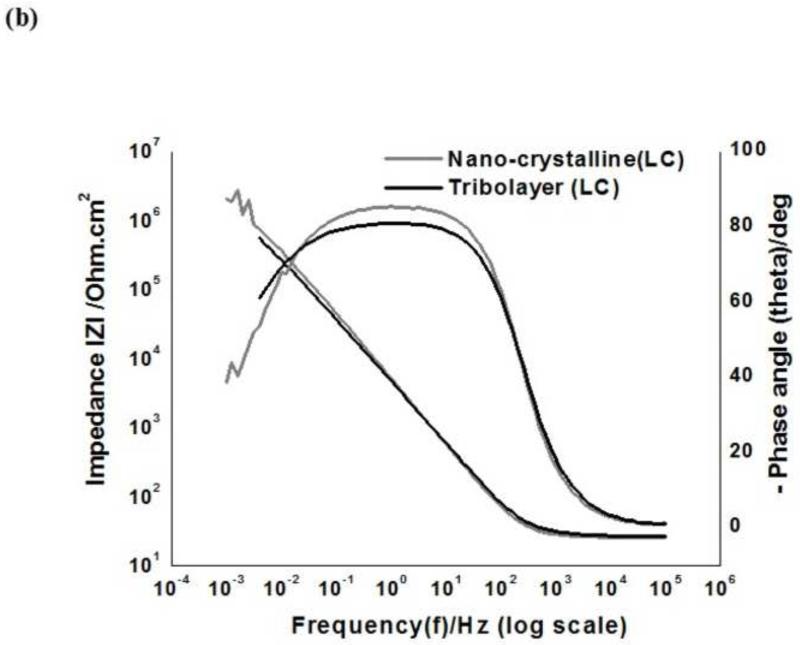
The EIS data are presented through Nyquist plots (Real impedance (Re(Z) vs. Imaginary impedance (Im(Z)), (Fig. 6 (a & b)) and Bode plots(Impedance IZI and phase angle (theta) vs frequency (f)) (Fig. 7 (a&b)) for HC and LC nano-crystalline and tribolayer surfaces. As the focus of the current study on the tribolayer surface, the plots for the bulk-like surface are not included.
Figure 6.
Electrochemical impedance spectroscopy (EIS) results-Nyquist plots (Re(Z) vs Im(Z))- for nano-crystalline and tribolayer surfaces: (a) High carbon (HC) (b) Low carbon (LC)
Figure 7.
Electrochemical impedance spectroscopy (EIS) results-Bode plots-(Impedance (Z) and phase angle (theta) vs, frequency (f)) for nano-crystalline and tribolayer surfaces: (a) High carbon (HC) (b) Low carbon (LC)
The Nyquist plots for both surfaces resulted in a quarter-circle. The Bode plots, demonstrate one time constant at intermediate frequencies. At low frequencies, the time constant is not perceptible, which suggests that it may virtually overlap with the intermediate frequency constant. It is clear from the spectra that the impedance values in the mid to lower frequency regions increases for the tribolayer surface compared to nano-crystalline. At high frequency, tribolayer exhibit, high capacitance. The increase in the low frequency impedance values together with a more ideal capacitive behavior with time indicate that the passive film on CoCrMo become more protective, as observed in the polarization results (Metiko-Hukovic et al., 2007; Valero Vidal et al., 2010).
The Bode phase angle plot shows that both alloys, regardless of carbon content, exhibit a very similar maximum relative angle. On the other hand, the observed greatest width of the phase angle at the intermediate frequency zone implies capacitive behavior better than corrosion behavior due to the decrease in the surface’s susceptibility to corrosion. The tribolayer surface (Fig. 7(b)) demonstrated a greater width compared to the nano-crystalline surface.
(b) EIS model
The EIS parameters (solution resistance (Rs), polarization resistance (Rp) and double layer capacitance (Cdl) are presented in Table 2. The fitted and the experimental data were in good agreement (average Chi-squared values are in acceptable range ≤0.002- Table 2). Considering the Rp parameter, the carbon content of the alloy made a significant difference only in the bulk-like surface; the low carbon alloy exhibited exceptional high values when compared to the high carbon alloy. The tribolayer surface condition had the lowest average Rp, while the nano-crystalline surface condition had slightly higher values. There was no significant difference in Cdl observed between high and low carbon alloys. The lowest Cdl values were achieved with the bulk-like surface condition, although the values of the LC alloy had a large variation. The highest Cdl was observed for the nano-crystalline surface condition. The tribolayer surface had a slightly lower average Cdl value compared to the nano-crystalline surface. One interesting observation was that the tribolayer surface did not exhibit a significant reduction in the Cdl value, as one would have expected based on its superior corrosion behaviour from the cyclic polarization curves. More details of this observation are addressed in a later section.
3.3 Surface characterization results
In this study, the surfaces were analyzed using a white light interferometer and an SEM. In Fig. 8 (a-f), the topographies for HC and LC alloys of the three surface conditions after testing are presented. Table 3 displays the Ra and RMS values before and after testing. The differences in topography are clearly evident between the HC and LC alloys and the surface conditions. The bulk-like and tribolayer surfaces show slightly higher roughness values. After testing, the topography of all HC samples appears relatively consistent when looking at the surface reconstruction plots in Fig. 8. However, the tribolayer HC samples exhibited random surface peaks along the corroded surface.
Figure 8.
White light interferometry scans of corroded: (a) HC-CoCrMo alloy – bulk-like; (b) LC CoCrMo alloy - bulk-like; (c) HC CoCrMo alloy - nanocrystalline; (d) LC CoCrMo alloy – nano-crystalline; (e) HC CoCrMo alloy - tribolayer surfaces; (f) LC CoCrMo alloy - tribolayer surfaces
SEM micrographs of corroded surfaces for nano-crystalline, bulk-like and tribolayer samples of HC and LC are shown in Fig. 9 (a-h). For all samples, the tests were conducted under high anodic conditions (+1.8 V vs. SCE). Generally, all three surfaces exhibited an abundance of pits. Once the pitting potential has been reached, the influence of thin surface layers like the tribolayer (<100 nm) and the nano-crystalline zone (<400 nm) are marginal. The SEM can only be used to investigate fine traces that point to acting corrosion mechanisms.
Figure 9.
SEM images of corroded surfaces: (a) HC-CoCrMo alloy - bulk-like;; (b) LC CoCrMo alloy - bulk-like; (c) HC CoCrMo alloy - nanocrystalline; (d) LC CoCrMo alloy - nanocrystalline; (e) HC CoCrMo alloy - tribolayer surfaces; (f) LC CoCrMo alloy - tribolayer surfaces
4. DISCUSSION
4.1 Variations in electrochemical behavior
(a) Polarization curves and electrochemical parameters
On the basis of the potentiodynamic and EIS measurements, it can be stated that the bulk-like and the tribolayer surface conditions show an overall better corrosion behavior than the nano-crystalline surface. This is primarily reflected in the Icorr values (Table 2) that directly correlate to the corrosion rate at the surface. The corrosion tendency of the surfaces, as indicated by the Ecorr parameter (Table 2) is similar between the three surface conditions. In general, Ecorr and Icorr values, which were estimated by Tafel’s slope method, reflect the kinetics of the corrosion process and are independent of the exposure time. The similar Ecorr values of the surfaces indicate that the electron transfer trends of all three surface states are within the same range. The low Icorr values of the tribolayer samples indicate the corrosion inhibiting characteristics of the tribolayer surface (Hodgson et al., 2004; Valero Vidal et al., 2010). In the case of high carbon (HC), tribolayer surface also exhibit early passivation behavior an indicative of increased corrosion resistance compared to nanocrystalline (Fig. 5(a)-marked region). The composition and structure of the passive film could be the reason for the better corrosion resistance (Hodgson et al., 2004). However, in case of low carbon the tribolayer exhibit weaker behavior, after an initial period and getting back again at higher potential of +0.25 vs SCE (Fig. 5(b)). This shows that carbon content has an important role in passivation kinetics and chemical nature of the passive film.
There is also evidence of an interesting peak at the potential range of +0.75 to +0.8 V vs SCE, for all the surfaces of high carbon (HC) and low carbon (LC) (Fig. 5 (a &b)). Similar observation was reported by previous investigators (Hodgson et al., 2004; Metiko-Hukovic et al., 2007; Valero Vidal et al., 2010) and potential role of H2O oxidation due to the transpassive oxidation of Cr3+ to Cr6+. Such Cr oxidation peak were high for tribolayer surface, which is in agreement with the increased protective nature of the oxide film. The peak region is more evident and clear in the case of low carbon alloy than high carbon (marked in the Fig. 5 (a & b)), possible indication of role of carbon content, as in the case of passivation kinetics.
The superior corrosion behavior of the bulk-like samples must be viewed critically (Fig. 5 (a &b)). During the electrochemical polishing process, the sample surface is exposed to an acidic solution. Under such circumstances, the surface is able to grow a thicker passive film that prevents the surface from corroding (see SEM images -Fig 3 (a&b)). Charge exchange only proceeds due to diffusion processes. Therefore, it is expected that the bulk-like surface hardly undergoes any corrosive processes until the breakdown potential. Once the passive film has been removed, corrosion is promoted in the same way as for the nano-crystalline surface. Slight differences in pitting behavior are evident for the bulk-like surface as can be seen from the polarization curves (Fig. 5 (a&b)) and SEM images (Fig. 9 (a&b)).
(b) Electrochemical impedance spectroscopy (EIS) results
The Nyquist plots (Fig. 6) for both surfaces resulted in a quarter-circle which is attributed to the time constant of the charge transfer and the double layer capacitance. Bode plots (Fig. 7), at low frequencies; the time constant is not perceptible, which suggests that it may virtually overlap with the intermediate frequency constant. It is clear that, the nano-crystalline surface exhibits slightly higher impedances, than the tribolayer surface. However, the tribolayer surface (Fig. 7(a&b)) demonstrated slightly depressed and wide curve compared to the nano-crystalline surface. This behavior is possibly caused by the alteration of the metal surface due to the presence of carbonaceous materials in the tribolayer which retards the relaxation time linked to the electrical characteristics of the surface. The overlapping effects of such relaxation periods during the corrosion process influence the charge transfer process (Mathew et al., 2011; Souza et al., 2010; Hirschorn et al., 2010).
Further, the EIS plots (Fig. 6 & 7) indicate the better corrosion behavior of the LC CoCrMo alloy compared to the HC alloy. The plots did not show a significant difference between the surface conditions of nano-crystalline and tribolayer surfaces. This could be due to the composition of the tribolayer and the presence of a proteinaceous electrolyte which may possibly inhibit the current flow that might further hinder film formation. The existing tribolayer was also patchy in appearance, with a non-homogeneous mixture of decomposed proteins, corrosion products and metal particles along the surface of the alloy (Wimmer et al., 2010). Therefore, the low Rp values (Table 2) could be related to the peculiar nature of the surface that generates local variation in charge transfer and dielectric characteristics.
Previous studies also highlighted the passive behavior of the CoCrMo alloy is due to a formation an oxide film highly enriched with Cr (90%Cr oxides) on the alloy surfaces. The passive and transpassive behavior of the alloy is hence dominated by the alloying elements Cr. In passive region, Cr is present in the film, however, in the transpassive region, strong thickening of the oxide film takes place, combined with a change in the composition of the film and strongly increased dissolution rate (Hodgson et al., 2004; Metiko-Hukovic et al., 2007; Valero Vidal et al., 2010). A recent work (Martin et al., 2013) reported a such film formation at this potentials (around +0.75 vs SCE) as the mass gain was observed on the EQCM (Electrochemical Quartz Crystal Microgram) study.
The reported capacitance values are indicative of actual interfacial capacitance, which is highly sensitive to environmental variation Hence, the low polarization resistance (Rp) and capacitance values for tribolayer surface are not surprising results. It should also be noted that the EIS results indicate the corrosion kinetics at metal/solution interfaces and are more related to the formation and growth rate of passive film (Mathew et al. 2011; Hirschorn et al., 2010). The slight differences in Rs values (Table 2) can be attributed to the differences in the metallurgical nature of the surfaces and the ion concentration of the electrolyte. In addition, the tribolayer itself contains metal ions (stable or unstable forms) and wear debris (nano particles) which can potentially interfere the electrochemical process/kinetics. Hence it is difficult to identify the reason behind the peculiar electrochemical nature of the tribolayer surface.
4.2 Variation in the surface appearance and potential causes
The distinctions in the corrosion behavior between the three surface conditions were further investigated by surface topography techniques (SEM and white light interferometry, Figs 8 & 9). The topography of the nano-crystalline samples shows the lowest roughness before testing, which is a direct result of the well-defined mechanical polishing procedure. The increase in roughness values of the bulk-like sample (Table 3) is the result of a slightly uneven material removal during electro-polishing due to different orientations of the surface grains. Also, the roughness of the tribolayer samples can be explained by the discontinuous nature of the tribofilm that occurs in patches. Except for the tribolayer surfaces, the difference in carbon content for the HC and LC alloys relieved a slightly higher roughness for the HC samples that can be attributed to the high amount of carbides at the surface of HC samples. Carbides are more resistant to mechanical and electro-polishing than the CoCrMo matrix and thus may form surface asperities.
The white light interferometry images, Fig. 8, exhibited negative surface peaks that appear as blank spots in the images because the peaks are out of the measuring range of the interferometer. A possible explanation could be the occurrence of deep pits along the surface (Burstein et al., 2007). The bulk-like samples (Fig. 8(a&b)) showed a clear difference in surface topography compared to the nanocrystalline and tribolayer surfaces (Fig. 9 (c-f)). The latter two surfaces demonstrated ridges formed due to the different extent of corrosive attacks between remains of dendritic structures within the surface microstructure, whereas the bulk-like surface appeared to have a smooth and even topography similar to the HC bulk-like sample. LC samples exhibited a considerably lower number of blank measuring spots.
The SEM images in Fig. 9 (a-f) show that after corrosion, all three surfaces exhibited an great quantity of pits. Further, two different types of pits were distinguishable along the surfaces: (1) circular shaped intra-granular pits within the alloy matrix and (2) randomly shaped pits in between grains. These types of pits are the result of, pitting corrosion, or corrosion at phase boundaries with the consequence of hard phase (e.g. carbides) detachment. HC samples exhibited both types of pits which are also reflected in the higher roughness values measured after corrosion tests. The bulk-like (Fig. 9 (a & b)) and nano-crystalline (Fig. 9 (c & d)) samples hardly had any hard phases (mainly carbides) remaining at the surface. However, the tribolayer samples (Fig. 9 (e & f)) still exhibited numerous hard phases even though its phase boundaries appeared strongly affected by corrosive processes. The remaining hard phases must also account for the surface peaks within the topography of HC tribolayer samples.
The LC samples exhibited only circular pits, which can simply be explained by the absence of hard phases within this type of alloy. The number of circular pits appeared higher for LC samples, possibly explained by the higher density of twins, and thus the higher surface energy compared with the HC alloy. Interestingly, pitting for the tribolayer surface seemed to be localized to certain areas. It may be assumed that areas without pitting were initially covered by the tribolayer, resulting in the prevention of pitting. Compared with each other, bulk-like and nano-crystalline surfaces did not show any significant differences. Also, all samples showed carbonaceous residues on their surfaces, however, the tribolayer surfaces demonstrated thicker carbonaceous films in localized areas.
The appearance and topography of the tribolayer surfaces were clearly different from the other surfaces (Fig. 9(e & f)). There are also significant differences in the appearance of HC tribolayer surface (Fig. 9(e) and LC Tribolayer surface (Fig. 9(f)). The HC Tribolayer surface (Fig. 9(e)) exhibited patches with hard phases at their surfaces, while no hard phases remained within the nano-crystalline surfaces (Fig. 9(c)). It appears that the areas with phase boundaries were covered by an initial tribolayer and thus exhibited enhanced corrosion protection. At such phase boundaries, corrosion was promoted at a lower rate; thus hard phases remained anchored to the surface
The LC nano-crystalline samples (Fig. 9(d)) alloys when compared to the LC tribolayer (Fig. 9(f)) surfaces exhibited strong pitting corrosion over the entire surface, whereas the latter exhibited pitting only in patches. Areas that were protected by a tribolayer did not promote the occurrence of pits, while areas without a tribolayer showed very strong pitting. It can be assumed that the formation of local galvanic elements between tribolayer and tribolayer-free regions further promote localized pitting corrosion (Burstein et al., 2007). The observed inferior corrosion behavior of LC vs. HC CoCrMo alloys has been consistent with the surface characterization.
4.3 Schematic model of a tribolayer
A schematic model of the tribolayer is shown in Fig. 10. It demonstrates that the tribolayer hinders electrochemical processes from corroding the surface of the alloy. The enhanced corrosion resistance is possibly related to inhibited oxygen transport through the carbonaceous film. A recent study has demonstrated that this carbonaceous film partly consists of graphitic material (Liao et al., 2011). Considering the electrically noble nature of graphite, this phenomenon may be partially responsible for the decrease in the corrosion rate in the tribolayer areas. It may also explain the slow corrosion kinetics resulting in low Rp values (Table 2). However, it was also shown that the tribolayer is the origin of wear particles and their detachment into surrounding tissue. Such wear particles may also participate in the electrochemical process within the tribolayer and EDL and result in particle oxidation. This was confirmed in several studies that have shown that wear particles emitted from MoM joints consist mainly of chromium oxide (Yang et al., 1994). The presence of metal ions and wear particles within the tribolayer could possibly increase the corrosion kinetics, as the results indicate, through increasing Cdl values (Table 2). In addition, the comparative study between tribolayer and nanocrystalline surfaces clearly indicate that the role of passive film on the improved corrosion resistance still valid and considered together with tribolayer formation.
Figure 10.
Diagram of the tribolayer surface, exhibiting the possible contributing factors for its improvement in corrosion resistance
Although the better corrosion behavior in the presence of proteins has been previously reported (Hodgson et al., 2004; Metiko-Hukovic et al., 2007; Valero Vidal et al., 2010; Frateur et al., 2006) the in situ behavior is much more complex. The generated wear particles that migrate from the metal surface to the surrounding tissue most likely influence corrosion kinetics. In addition, some of the metal particles directly bind to proteins in the medium inhibiting oxidation and make the particles electrochemically inactive (Yang et al., 1994) The exact effect of particle oxidation on the overall corrosion kinetics is quite difficult to identify. The interaction between metal ions, proteins, oxide film and metallo-organic complexes is still incompletely understood.
4.4 Clinical relevance of the implant in operation
This study examined the corrosion characteristics of three types of surface conditions on HC and LC CoCrMo alloys. The corrosion process that results in the release of metal ions into the surrounding medium is of great importance for understanding the biocompatibility of artificial joints. Contrasting and different opinions exist, though, as to whether a tribolayer confers benefit by preventing the release of metal ions and improving the corrosion resistance in an in vivo environment.
The nano-crystalline surface condition represents a new implant surface, as it is shortly after THR surgery. However, the implant surface will be altered during running-in to a tribolayer surface within the primary contact zones. This surface state is here represented by samples with a pre-applied tribolayer. It correlates to the primary contact areas of the bearing surface during steady-state. The reported results give new insights into corrosion mechanisms in MoM hip joints. It is well known that a CoCrMo alloy provides corrosion resistance due to passive oxide film formation under static conditions. Tribocorrosion studies, which consider wear and corrosion simultaneously, have shown that there is a synergism between the two that contributes significantly to the overall mass loss (Mathew et al., 2011, Yan et al., 2006). The role of the tribolayer is the key to understand such synergistic effects. In an earlier study it was stated that in well-functioning hips the tribolayer, including the mechanically mixed zone, can be considered as the origin of wear particles (Wimmer et al., 2010; Liao et al., 2011). Further, it was shown that wear particles grossly consist of chromium oxide with only a small number of cobalt-containing particles. It can be assumed that the oxidation of wear particles occurs mainly within the tribolayer and contributes to the detected electrochemical process. Since it is not clear if adverse tissue reactions in MoM hips are mainly caused by wear nanoparticles or metal ions, more studies are required to provide a better understanding of such electrochemical processes within the tribolayer. In clinical perspective, the study indicate the range of electrochemical processes behind the formation and inhomogeneous nature of the tribolayer implant surfaces, which is subjected to a complex in-vivo environment with an electrolyte with various chemical compositions, proteins, mechanical motions and stresses, and with other metals present that may alter the corrosion characteristics.
4.5 Limitations and future outlook
It is worthwhile to note some of the limitations of the current study. The proposed control sample that was referred to as the bulk-like surface did not resemble the bulk material of the CoCrMo alloy. The electrochemical polishing process employed to generate the bulk-like microstructure, resulted in an unnatural and much thicker than normal passive film. A bulk-like surface microstructure is unlikely for a MoM bearing surface, because once in contact in-vivo, it will change its microstructure to nano-crystalline. Its purpose was only to serve as a reference state, one without direct clinical relevance. Similarly, the tribolayer samples were created by a mechanical polishing process of the surface in the presence of bovine calf serum as a lubricant; however, concerns about the homogeneity and structure of the tribolayer still exist. The selection of an equivalent EIS circuit was such that it will yield a goodness of fit at a satisfactory level.
In order to further understand the properties of the tribolayer, it is necessary to address to what extent the tribolayer will be beneficial in the presence of articulations. It will be critical to expose the tribolayer to a tribocorrosive environment and determine how this affects any synergism between wear and corrosion. Further, electrochemical noise measurements would be helpful in understanding localized areas of corrosion, and in situ mass change could be estimated by electrochemical quartz micro-gravimetry (EQCM). Such novel approaches will be considered in future studies (Xie et al., 2003).
5.0 Conclusions
In this study, the electrochemical behavior of three surfaces was studied by focusing on our understanding of the corrosion kinetics of tribolayer surfaces, representing the surface of the implant while it is in operation in-vivo. Results unveiled that each surface has different susceptibility to corrosion in body or joint environment and corrosion kinetics largely depend on the structure and distribution of the tribolayer on the surface. The following observations were noted in the study:
The study clearly shows that the electrochemical behavior of HC and LC CoCrMo alloys are different as the surfaces conditions are varied (bulk-like, nanocrystalline, tribolayer).
Low Icorr values indicate inferior corrosion behavior of LC vs. HC CoCrMo alloys during anodic polarization.
The corroded surface of HC and LC CoCrMo alloys exhibit differences in SEM characterization. Irrespective of the variation in surface conditions and alloy composition, pitting corrosion behavior was consistently observed on the surface.
In the case of a tribolayer, the non-homogeneous appearance could be attributed to the low polarization resistance (Rp) values. The capacitance (Cdl) was also affected by local variations in charge transfer and dielectric characteristics that were due to the distinct nature of the tribolayer surface.
The unexpected trends in the polarization resistance and double layer capacitance indicated variation in corrosion kinetics when protein was present in the solution which influences the formation and growth of passive films (together with tribolayer).
The comparative study, between tribolayer and nanocrystalline surfaces clearly indicate that the role of passive film on the improved corrosion resistance still valid of the CoCrMo alloy and considered together with tribolayer formation and further investigation are required.
The findings on the superior electrochemical behavior of the tribolayer surfaces have clinical importance as such layers can potentially improve the implant performance.
Research highlights.
This study has great clinical importance, as currently metal-on-metal MoM joints arises several concerns in orthopedic community.
Tribolayer(s) are formed in MoM joints due to the mechanical motions and electrochemical reactions
This work reports the electrochemical behavior of the tribolayer(s) in (MoM) hip implant system made of CoCrMo alloys
Main question addressed in this study “Does the tribolayer promote or inhibit corrosion?”
The influence alloy composition on tribolayer formation were investigated
The findings on the superior electrochemical behavior of the tribolayer surfaces have clinical importance as such layers can potentially improve the implant performance.
ACKNOWLEDGMENTS
This study was funded by an NIH RC2 (1RC2AR058993) grant. The support from the co-partners of this NIH grant, Prof. L. Marks and Prof. K. Shull and Dr. L. Yifeng (Northwestern University, Evanston, IL) is gratefully acknowledged. We thank Prof. Robert Urban and Ms. Deborah Hall for their assistance in SEM characterization. The CoCrMo alloy was kindly donated by ATI Allvac. The authors would like to thank Felix Liedtke for his support in sample preparation. Authors acknowledge the assistance of Mrs. B. Kishor for the editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amin MA, Mohsen Q, Hazzazi OA. Synergistic effect of I– ions on the corrosion inhibition of Al in 1.0;M phosphoric acid solutions by purine. Mater. Chemis. Phys. 2009;114:908–914. [Google Scholar]
- 2.Antunes RA, Oliveira MC. Corrosion processes of physical vapor deposition-coated metallic implants. Crit. Rev. Biomed Eng. 2009;37-6:425–460. doi: 10.1615/critrevbiomedeng.v37.i6.10. [DOI] [PubMed] [Google Scholar]
- 3.Barril S, Mischler S, Landolt D. Influence of fretting regimes on the tribocorrosion behaviour of Ti6Al4V in 0.9 wt.% sodium chloride solution. Wear. 2004;256:963–972. [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J. Bone Joint Surg. Am. 2009;91(1):128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 5.Burstein GT, Liu C. Nucleation of corrosion pits in Ringer’s solution containing bovine serum. Corros. Sci. 2007;49:4296–4306. [Google Scholar]
- 6.Büscher R, Täger G, Dudzinski W, Gleising B, Wimmer MA, Fischer A. Subsurface microstructure of metal-on-metal hip joints and its relationship to wear particle generation. J. Biomed. Mater. Res. B. Appl. Biomater. 2005;72:72, 206. doi: 10.1002/jbm.b.30132. [DOI] [PubMed] [Google Scholar]
- 7.Catelas I, Wimmer MA. New insights into wear and biological effects of metal-on-metal bearings. J. Bone Joint Surg. Am. 2011;93-2:76–83. doi: 10.2106/JBJS.J.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton RAE, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report. J. Bone Joint Surg. Am. 2008;90:1988. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 9.Fischer A, Weiss S, Wimmer MA. The tribological difference between biomedical steels and CoCrMo-alloys. J. Mech. Behav. Biomed. Mater. 2012;9:50–62. doi: 10.1016/j.jmbbm.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frateur I, Lartundo-Rojas L, Méthivier C, Galtayries A, Marcus P. Influence of bovine serum albumin in sulphuric acid aqueous solution on the corrosion and the passivation of an iron–chromium alloy. Electrochim. Acta. 2006;51:1550–1557. [Google Scholar]
- 11.Hallab NJ, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J. Bone Joint Surg. Am. 2001:83. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Hallab NJ, Messina C, Skipor A, Jacobs JJ. Differences in the fretting corrosion of metal-metal and ceramic-metal modular junctions of total hip replacements. J. Orthop. Res. 2004;22:250–259. doi: 10.1016/S0736-0266(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 13.Hirschorn B, Orazem M, Tribollet B, Vivier V, Frateur I, Musiani M. Determination of effective capacitance and film thickened from constant-phase-element parameters. Electrochimica Acta. 2010;55(21):6218–6227. [Google Scholar]
- 14.Hodgson AWE, Kurz S, Virtanen S, Olsson COA, Mischeler S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochemical Acta. 2004;40:2167–2178. [Google Scholar]
- 15.Jemmely P, Mischler S, Landolt D. Electrochemical modeling of passivation phenomena in tribocorrosion. Wear. 2000;237:63–76. [Google Scholar]
- 16.Kurtz SM, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 17.Liao Y, Pourzal R, Wimmer MA, Jacobs JJ, Fischer A, Marks LD. Graphitic tribological layers in metal-on-metal hip replacements. Science. 2011;334:1687–1690. doi: 10.1126/science.1213902. [DOI] [PubMed] [Google Scholar]
- 18.Martin EJ, Pourzal R, Mathew MT, Shull KR. Dominant role of molybdenum in the electrochemical deposition of biological macromolecules on metallic surfaces. Langmuir. 2013 Apr 16;29(15):4813–22. doi: 10.1021/la304046q. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew MT, Runa MJ, Laurent M, Jacobs JJ, Rocha LA, Wimmer MA. Tribocorrosion behavior of CoCrMo alloy for hip prosthesis as a function of loads: a comparison between two testing systems. Wear. 2011;271:1210–1219. doi: 10.1016/j.wear.2011.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew MT, Srinivasa Pai P, Pourzal R, Fischer A, Wimmer MA. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Advances in Tribology. 2009:1–12. Article ID 250986. [Google Scholar]
- 21.Metiko-Hukovic M, Babic R. Passivation and corrosion behaviours of cobalt and cobalt-chromium-molybdenum alloy. corrosion science. 2007;49:3570–3579. [Google Scholar]
- 22.Pandit H, Glyn-Jones s., McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J. Bone Joint Surg. Br. 2008;90-B:847. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 23.Pourzal R, Catelas I, Theissmann R, Kaddick C, Fischer A. Characterization of wear particles generated from CoCrMo alloy under sliding wear conditions. Wear. 2011;271:1658–1666. doi: 10.1016/j.wear.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigney DA. Transfer, mixing and associated chemical and mechanical processes during the sliding of ductile materials. Wear. 2000;245:1–9. [Google Scholar]
- 25.Souza JC, Henrique M, Oliveira R, Teughels W, Celis JP, Rocha LA. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling. 2010;26:471–478. doi: 10.1080/08927011003767985. [DOI] [PubMed] [Google Scholar]
- 26.Truc TA, Pebere N, Xuan Hang TT, Hervaud Y, Boutevin B. Study of the synergistic effect observed for the corrosion protection of a carbon steel by an association of phosphates. Corros. Sci. 2002;44:2055–2071. [Google Scholar]
- 27.Valero Vidal A, Igual Munoz A. Effect of physico-chemical properties of simulated body fluids on the electrochemical behaviour of CoCrMo alloy. Electrochemical Acta. 2011;56:8239–8248. [Google Scholar]
- 28.Valero Vidal C, Olmo Juan A, Igual Munoz A. Adsorption of bovine serum albumin on CoCrMo surface: Effect of temperauture and protein concentration. Colloids and Surfaces B: Biointerfaces. 2010;80:1–11. doi: 10.1016/j.colsurfb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Williams RL, Brown SA, Merritt K. Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials. 1988;9:181–186. doi: 10.1016/0142-9612(88)90119-6. [DOI] [PubMed] [Google Scholar]
- 30.Wimmer MA, Fischer A, Büscher R, Pourzal, Sprecher C, Hauert R, Jacobs JJ. Wear mechanisms in metal-on-metal bearings: The importance of tribochemical reaction layers. J. Orthop. Res. 2010;28-4:436–443. doi: 10.1002/jor.21020. [DOI] [PubMed] [Google Scholar]
- 31.Wimmer MA, Sprecher C, Hauert R, Tager G, Fischer A. Tribochemical reaction on metal-on-metal hip joint bearings: A comparison between in-vitro and in-vivo results. Wear. 2003;255:1007–1014. [Google Scholar]
- 32.Xie Q, Wang J, Zhou A. A novel dual-impedance-analysis EQCM system—investigation of bovine serum albumin adsorption on gold and platinum electrode surfaces. J. Colloid Interf. Sci. 2003;262:107–115. doi: 10.1016/S0021-9797(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 33.Yan Y, Neville A, Dowson D. Understanding the role of corrosion in the degradation of metal-on-metal implants. Proc. Inst. Mech. Eng. H. 2006;220:173–181. doi: 10.1243/095441105X63246. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Black J. Competitive binding of chromium, cobalt and nickel to serum proteins. Biomaterials. 1994;15:262–268. doi: 10.1016/0142-9612(94)90049-3. [DOI] [PubMed] [Google Scholar]