Abstract
The objective of this study was to evaluate the long term performance of cell-free vascular grafts made from a fast-degrading elastic polymer. We fabricated small arterial grafts from microporous tubes of poly(glycerol sebacate) (PGS) reinforced with polycaprolactone (PCL) nanofibers on the outer surface. Grafts were interpositioned in rat abdominal aortas and characterized at 1 year post-implant. Grafts remodeled into “neoarteries” (regenerated arteries) with similar gross appearance to native rat aortas. Neoarteries mimic arterial tissue architecture with a confluent endothelium and media and adventita-like layers. Patent vessels (80%) showed no significant stenosis, dilation, or calcification. Neoarteries contain nerves and have the same amount of mature elastin as native arteries. Despite some differences in matrix organization, regenerated arteries had similar dynamic mechanical compliance to native arteries in vivo. Neoarteries responded to vasomotor agents, albeit with different magnitude than native aortas. These data suggest that an elastic vascular graft that resorbs quickly has potential to improve the performance of vascular grafts used in small arteries. This design may also promote constructive remodeling in other soft tissues.
1. INTRODUCTION
Autografts remain the gold standard for small diameter vascular grafts, but donor site morbidity and limited autograft availability warrants the search for an effective alternative.[1, 2] Nonresorbable synthetic grafts and allogeneic grafts have poor patency at diameters less than 6 mm.[3] Tissue engineering strategies aim to improve graft patency by culturing cells in resorbable scaffolds. Tissue engineered vascular grafts have shown great promise in animal studies[4–12] and arteriovenous shunt clinical trials.[13] However, high fabrication costs and long production times for patients limit the clinical adoption of these grafts. Devitalized tissue engineered grafts eliminate patient waiting periods[14–16] and perform well in large animals,[14, 15] but production time and costs remain high. Approaches using shorter periods of in vitro maturation may reduce fabrication costs and have shown promise in animals[17–30] and in humans,[31, 32] but these treatments are still limited by the cost and time of cell harvest and seeding.
To minimize production costs and eliminate patient wait time, we developed a resorbable vascular graft that does not require cell seeding or culture prior to implantation. We use a composite vascular graft made from two parts: (1) a microporous tube of fast-degrading, elastomeric poly(glycerol sebacate) (PGS), and (2) a thin sheath of polycaprolactone (PCL) nanofibers for mechanical support.[33] This design emphasizes rapid resorption to maximize the rate of host remodeling and uses an elastomer with a Young’s modulus similar to arteries, setting it apart from other cell-free resorbable grafts which use slower degrading materials.[34–41] We hypothesized that (1) a fast-resorbing design would improve graft performance by minimizing the host’s exposure to foreign material, thereby minimizing chronic inflammation, and (2) an elastomer will promote the formation of an arterial-like extracellular matrix. This report describes the long-term stability and constructive host remodeling of the composite grafts in a small animal model.
2. MATERIALS AND METHODS
2.1 Fabrication of composite vascular grafts
Grafts were fabricated as previously described.[33] Briefly, PGS prepolymer was synthesized in house.[42] The PGS core of the composite graft was fabricated by casting a PGS prepolymer solution into a fused salt template within a tubular mold. To cross-link the PGS prepolymer, constructs were heat cured at 150 °C under vacuum for 24 h to produce a PGS-salt template. To fabricate the PCL sheath, PCL (Mn 80 kDa; Aldrich, St. Louis, MO) was dissolved in 2,2,2-trifluoroethanol at 14% weight/volume and electrospun onto a rotating PGS-salt template at 20 RPM. Salt was removed from PGS-PCL-salt composites by immersing them in deionized water. Composite grafts were lyophilized (Labconco Freezone 4.5, Kansas City, MO) and stored in a desiccator at ambient temperature until use. Grafts were sterilized with ethylene oxide prior to implant.
2.2 Implantation
Rats were cared for in compliance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh following NIH guidelines for the care and use of laboratory animals (NIH publication No. 85–23 rev. 1985). We used male Lewis rats (body weight: 200–250 g, n = 5, Charles River Laboratories, Boston, MA) for experiments. We performed interpositional implantation in rat abdominal aortas as follows. Rats were anesthetized by isoflurane inhalation (5% for induction, then 2–3% for maintenance). A midline abdominal incision was made to expose the abdominal aorta. The aorta was separated from the inferior vena cava, the infrarenal abdominal aorta was cross-clamped, and then a 4-mm segment was transected. The composite graft (8–10 mm long) was placed in the gap and connected to the native aorta by end-to-end anastomosis with 9-0 suture. Rats received no anticoagulation or antiplatelet treatment preoperatively or postoperatively. After implantation, grafts resorbed and transformed into “neoarteries” (regenerated arteries). At one year post-implant, neoarteries were characterized in situ, then rats were sacrificed to explant grafts for ex-vivo analysis (n = 5).
2.3 Arterial impedance
In a non-survival surgery, rats were anesthetized with isoflurane inhalation (5% for induction, then 2–3% for maintenance), then ventilated by the insertion of a tracheal cannula attached to a rodent ventilator (Harvard Apparatus, South Natick, MA) set to 90 breaths/min with a tidal volume of 8 ml/kg body weight. The chest was opened with a midline incision and then a four-electrode pressure-volume catheter (Scisence Inc., London, Ontario, Canada) was placed through the left ventricular apex and fed into the aortic arch.
Simultaneous Doppler signal (20 MHz probe) and pressure were captured via digital signal processing workstation (DSPW; Indus Instruments, Houston, TX). Within DSPW, Doppler signals were semi-automatically traced by an operator and saved with a sampling rate of 1 kHz. The signals were then analyzed for impedance using custom software (CVfunction, Matlab). Within CVfunction, Doppler signals were converted to velocity and subsequently flow, assuming an aortic diameter at site of measurement of 0.25 cm. Average representative pressure and flow waveforms were selected. Impedance was calculated as the ratio of pressure and flow (P/Q) after first converting the signals into the frequency domain via the Fast Fourier Transform routine within Matlab (MathWorks, Natick, MA). The first eight harmonics of the impedance spectra were analyzed.
2.4 In vivo dynamic mechanical compliance
Immediately following arterial impedance measurement, aortic pressure and neoartery inner diameter were measured simultaneously to determine the dynamic mechanical compliance of neoarteries. Aortic pressure was measured using the same pressure-volume catheter used in arterial impedance measurement. To measure neoartery inner diameter, hair was removed from the abdominal skin, then the abdominal aorta was imaged transcutaneously using a 21 MHz ultrasound linear probe (MS 250) connected to a high frequency small animal imaging system (Vevo2100, Visualsonics, Canada) in B-scan mode. The change in inner diameter of neoarteries due to blood pressure was measured from B-scan images. Dynamic mechanical compliance was calculated from Equation 1:
| (1) |
where Dinner represents inner diameter, PSystole represents aortic pressure at systole, and PDiastole represents aortic pressure at diastole.
2.5 Characterization of the vasoactive properties
The following were obtained from Sigma-Aldrich, St. Louis, MO: L-phenylephrine hydrochloride, norepinephrine, acetylcholine chloride, sodium nitroprusside dihydrate, serotonin, and forskolin. Animals were humanely euthanized. Functional arterial myography assays were performed as previously published with several modifications.[43] The neoarteries or native aortas were dissected free, cleaned of surrounding connective tissue, and placed in standard Krebs buffer (composition in mM: NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; dextrose, 11.1; Na2Ca EDTA, 0.029; pH 7.4). Aorta segments (2 mm in length) were mounted in a dual wire myograph system (Multiple Myograph Model 610 M, DMT, Denmark). The rings were allowed to equilibrate in Krebs buffer with 95% O2 - 5% CO2 at 37 °C at the optimized passive tension. Single dose challenges to phenylephrine (PE) and serotonin (5-HT) were performed. Rings were then preconstricted to serotonin (5-HT) and allowed to reach a steady-state plateau. Single dose challenges to acetylcholine (Ach) and forskolin were then determined. In other experiments log-dose response curves of preconstricted rings to the NO prodrug sodium nitroprusside (SNP) were obtained. Concentration-response curves were plotted as percentage of relaxation using all points on the concentration-response curve by nonlinear regression curve fitting using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Data represents results from 12 individual neoartery ring assays, derived from explants from 3 animals.
2.6 Histology and immunofluorescence
All microscope images presented are representative fields from at least three different animals. Neoartery images are all taken from the middle 4 mm of the remodeled graft. Detailed description of histology and immunofluorescence staining is available in Supplementary Methods.
2.7 En face immunofluorescence microscopy
Neoartery explants were cut into 1 to 2 mm long rings, then cut longitudinally into segments. Segments were fixed in 2% paraformaldehyde for 2 h at room temperature. Segments were then immunostained as whole mounts by blocking in 2% bovine serum albumin (BSA; EMD Chemicals, Gibbstown, NJ) with 0.1% Triton-X100 (Sigma) for 6 h at 4 °C, incubating in primary antibodies overnight at 4 °C, washing with 0.5% BSA for 5 h at 4 °C, incubating in secondary antibodies overnight at 4 °C, then rinsing again with 0.5% BSA. Segments were stained for either (1) endothelial cells, or (2) perivascular nerves. For endothelial cell staining, primary and secondary antibodies were mouse anti-rat CD31 (1:250; Millipore MAB1393) and goat anti-mouse AlexaFluor 594 (1:200; Invitrogen), respectively. For nerve staining, segments were co-stained with two primary antibodies: rabbit anti-rat protein gene product 9.5 (PGP 9.5; 1:250, Millipore Ab9724) and mouse anti-rat β-Tubulin (1:1000; Sigma T5293). Secondary antibodies were goat anti-rabbit AlexaFluor 594 (1:200; Invitrogen) and goat anti-mouse AlexaFluor 488 (1:200; Invitrogen), respectively. Dual staining was used to reduce effects of nonspecific staining which are amplified in whole mount imaging. To prepare stained whole mounts for imaging, segments were mounted in gelvatol on glass slides, flattened under a coverslip, and stored at 4 °C protected from light until imaging. Glass slides were imaged en face using an Olympus Fluoview 1000 confocal microscope (Center Valley, PA), and Z-stacked images were flattened using MetaMorph imaging software (Molecular Devices, Downingtown PA).
2.8 En face multiphoton microscopy
Neoartery explants were cut, fixed, and mounted as done for en face immunofluorescence, but without incubation in BSA or antibodies, and saline was used instead of gelvatol for mounting. Collagen and elastin autofluorescence within the neoartery wall was measured as previously described using an Olympus Fluoview FV1000-MPE Multiphoton microscope with a 1.12 NA 25x MPE water immersion objective.[44] Excitation was set to 870 nm, and the emission range was set to 400–500 nm and 525–575 nm for collagen and elastin, respectively. To image collagen and elastin close to the abluminal (outer) surface, explants were placed within the wells of hanging drop slides, coverslipped, and turned with the abluminal surface facing the objective. A schematic depicting each en face view is available in Supplementary Figure 1.
2.9 Elastin quantification
Neoartery explants were cut into ring segments as described for en face imaging. Each segment was minced finely, weighed to obtain wet weight, and then digested three times with 0.25 M oxalic acid at 95 °C for 1 h to extract mature insoluble elastin into the solution supernatant. Elastin content in each extract was measured using a Fastin Elastin assay (F2000; Biocolor, Carrickfergus, UK) according to manufacturer’s instructions, then pooled and normalized to sample wet weight to yield the mass of insoluble elastin per tissue wet weight (μg/mg). Sample size: n = 3 for neoarteries and native aortas. All samples were tested in duplicate during the same assay run, except for one neoartery for which there was insufficient tissue to run in duplicate.
2.10 Statistical Analysis
Comparisons between two groups were made with a two-tailed Student’s t-test using IBM SPSS Statistics 19 software (Armonk, NY). Comparisons between three groups were made using a one-way analysis of variance test followed by Tukey’s Honestly Significant Difference (HSD) post hoc test using GraphPad Software (LaJolla, CA).
3. RESULTS
3.1 Grafts remodel into “neoarteries” resembling native arteries
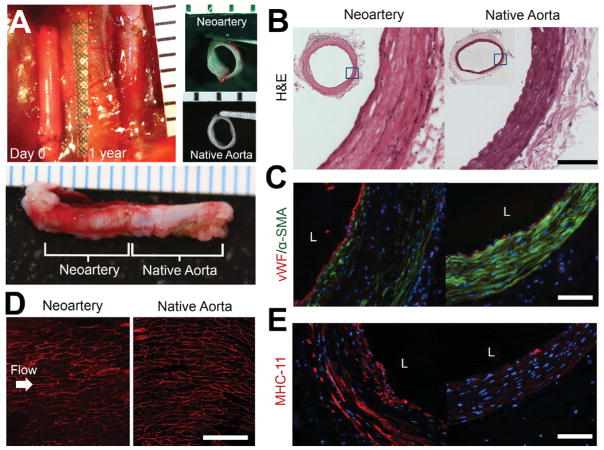
By one year post-implant, grafts transformed into tissues grossly resembling native aortas (Fig. 1A) with similar tissue architecture and no visible graft material residues (Fig. 1B). “Neoarteries” (regenerated arteries) showed a confluent endothelium as demonstrated by complete coverage of the luminal surface with cells positive for von Willebrand Factor (vWF) (Fig. 1C) and CD31 (Fig. 1D). Endothelial cells in neoarteries had cobblestone-like morphology and alignment in the direction of blood flow, as seen in native aortas (Fig. 1D). The middle layer of the neoartery vascular wall contains many smooth muscle-like cells with elongated nuclei which stain positively for α-SMA, resembling the native arterial medial layer (Fig. 1C). Smooth muscle-like cells are also positive for myosin heavy chain 11 (MHC-11), suggesting a mature, contractile phenotype. (Fig. 1E) Regenerated arteries showed an adventitia-like layer outer layer of less dense, α-SMA-negative tissue (Fig. 1B,C,E). Interestingly, neoarteries contain some cells which are negative for α-SMA and MHC-11 in the vascular media-like layer, including cells with elongated nuclei (Fig. 1C,E). In sparse, unrepresentative sections of neoarteries, the majority of cells in the media-like vascular layer are negative for smooth muscle markers (Supplementary Fig. 2). Neoarteries also contain some α-SMA and MHC-11 positive cells adjacent to the endothelium which are not elongated in the circumferential direction (Fig. 1C,E, Supplementary Fig. 2).
Figure 1. Gross morphology and tissue architecture of neoarteries resemble native arteries.
A. Gross morphology of neoarteries. Top left: transformation of graft into neoartery in situ over the course of 1 year. Nondegradable sutures (black) mark the graft location. Top Right: Transverse view of explanted neoarteries resembles that of native aortas. Bottom: Longitudinal view of explanted neoarteries resembles the adjacent native aorta. All ruler ticks are 1 mm. B. H&E stained transverse sections of the middle of neoarteries show similar tissue architecture with native aortas, with no visible graft material residues. Scale bar 100 μm. C. Neoartery sections immunostained for von Willebrand factor (vwf, red) and α-smooth muscle actin (α-SMA, green). The luminal surface of neoarteries is completely covered by vWF positive cells (red), suggesting a confluent endothelium. Neoarteries contain a media-like middle layer of the vascular wall rich in α-SMA positive cells with circumferentially elongated nuclei, similar to vascular smooth muscle found in native aortas. The outermost layer of neoarteries lacks α-SMA, resembling native adventitia. Some cells in the media-like layer are negative for α-SMA, and some cells adjacent to the endothelium are α-SMA positive but not circumferentially elongated. Scale bar 100 μm. L indicates vessel lumen. Nuclei stained with DAPI (blue). D. En face view of the luminal surface of neoarteries shows complete coverage by CD31 positive cells with cobblestone-like morphology and alignment parallel to the direction of blood flow, an arrangement similar to that found in native aortas. Neoarteries were cut open longitudinally and imaged as whole mounts using confocal microscopy and z-stack flattening. Arrow indicates the direction of blood flow. Scale bar 100 μm. E. Immunostaining for smooth muscle myosin heavy chain 11 (MHC-11, red). Many elongated cells in the media-like layer are positive for MHC-11, suggesting a mature, contractile smooth muscle phenotype. The distribution of MHC-11 appears similar to that of α-SMA. MHC-11 positive cells in neoarteries stain brighter than those in native aortas at the same exposure. Scale bar 100 μm. L indicates vessel lumen. Nuclei stained with DAPI (blue).
3.2 Neoarteries resist common modes of late-term vascular graft failure
One-year patency was unchanged from the three-month patency of 80%,[33] suggesting resistance to late-term failure. The only occluded graft was likely closed by acute thrombosis, as the non-vascular tissue architecture at the mid-graft suggests little constructive remodeling occurred before occlusion, and the tissue occluding the graft lumen does not resemble neointimal hyperplasia (Supplementary Fig 3). Patent grafts showed no statistically significant difference in inner diameter compared with native arteries, suggesting resistance to both dilation and narrowing by neointimal hyperplasia (Fig. 2A). Neoarteries showed no evidence of mineralization or calcification, as they are negative for von Kossa staining (Fig. 2B). Macrophages are present only in the adventia-like region of regenerated arteries at 1 year post-implant, likely associated with a small amount of remnant PCL (Fig. 2C).
Figure 2. Neoarteries resist common modes of late-term graft failure.
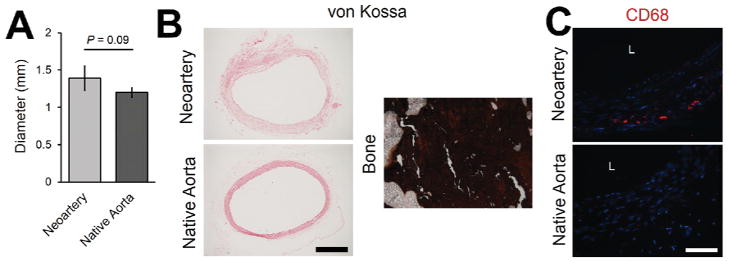
A. Neoartery inner diameter is not significantly different from that of age-matched native aortas, suggesting resistance to dilation and narrowing caused by neointimal hyperplasia. B. Neoarteries are negative for von Kossa staining (dark brown indicates a positive stain), suggesting resistance to calcification. Bone (rabbit ulna) was stained with von Kossa as a positive control for calcified tissue. Scale bar 500 μm. C. Neoarteries contain cells positive for macrophage marker CD68, concentrated mostly in the outermost layer of the neoartery wall. Scale bar 100 μm. Nuclei stained with DAPI (blue).
3.3 Nerves in the perivascular space of neoarteries
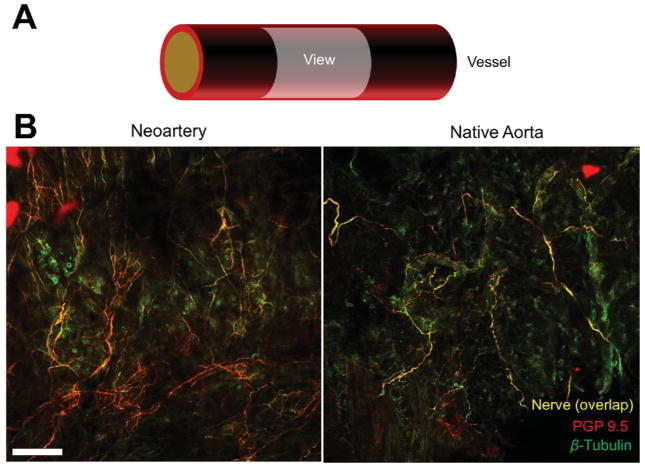
We searched regenerated vessels for perivascular nerves to (1) investigate whether neoartery tone could be regulated by the nervous system, and (2) determine whether nerves can regenerate and traverse the implant site. We found nerves on the adventitial surface of neoarteries with similar morphology and distribution to those found in native arteries (Fig. 3). Nerves were positive for protein gene product 9.5, a nerve-specific marker commonly used to identify perivascular nerves.
Figure 3. Regeneration of nerves in neoarteries.
A. Schematic of en face imaging of the adventitial surface of neoarteries. Neoarteries were cut open, immunostained for protein gene product 9.5 (PGP 9.5) and β-Tubulin, and imaged as whole mounts using confocal microscopy with z-stack flattening. B. Nerves cover the adventitial surface of neoarteries with similar morphology to those in native aortas. Nerves appear yellow from the overlap of nerve-specific marker PGP 9.5 (red) and nerve non-specific marker β-Tubulin (green), which stains nerves brightly but is also expressed ubiquitously. The co-staining technique was used to reduce effects of nonspecific staining which are amplified in whole mount imaging. Scale bar 100 μm.
3.4 Neoartery extracellular matrix composition and mechanical properties resemble that of native arteries
Proper matrix composition and structure is crucial for the long-term mechanical stability of arteries. Excitingly, we found that regenerated arteries contain the same quantity of cross-linked elastin as found in native arteries (P = 0.80; Fig. 4A). Furthermore, neoarteries contain fibrillin-1, a crucial component of functional elastic fibers (Fig. 4B). However, elastin and fibrillin-1 are less uniformly distributed in the media-like layer of neoarteries than in native aortas. Regenerated arteries contain collagens III and I as well, both found in native aortas, but their organization also differs from native aortas (Fig. 4B, Supplementary Fig. 4). Despite differences in matrix organization, we found no significant difference in the dynamic mechanical compliance of neoarteries compared to healthy aortas in vivo, suggesting protection from compliance-mismatch induction of neointimal hyperplasia (Fig. 4C, Video 1). Lastly, analysis of the impedance spectra showed similar results in control and grafted aortas between the first and eighth harmonic in a limited dataset (data not shown).
Figure 4. Extracellular matrix and mechanical properties of neoarteries resemble native arteries.
A. Elastin quantification using a Fastin Elastin assay showed that neoarteries contain the same amount of cross-linked elastin per tissue wet weight as native aortas. B. Transverse cross-sections of neoarteries immunostained for vascular extracellular matrix proteins. Neoarteries contain elastin, fibrillin-1, collagen III, and collagen I. Elastin and fibrillin-1 are less uniformly distributed in the media-like layer of neoarteries than in native aortas. Collagen III appears ubiquitously distributed in neoarteries, whereas in native arteries it localizes to vascular media. Collagen I is less bright in the neoartery adventitia compared with adventitia in native aortas. L indicates vessel lumen. Scale bar 100 μm. Nuclei stained with DAPI (blue). C. In vivo dynamic mechanical compliance of neoarteries shows no statistically significant difference from native aortas. Compliance was measured in vivo using ultrasound to record neoartery inner diameter and a manometer implanted in the aorta to record pressure. Compliance was taken as % change in diameter normalized to the difference between systolic and diastolic pressure.
3.5 En face comparison of elastin and collagen architecture
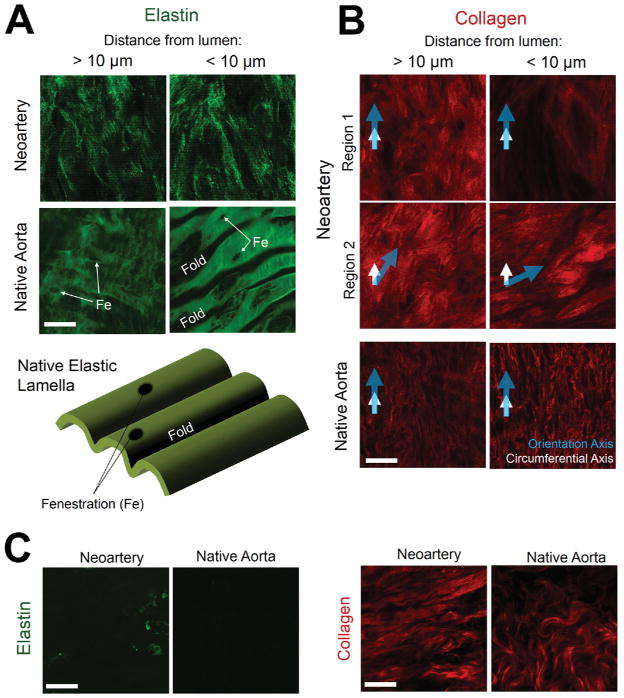
Elastin in neoarteries was arranged as a fine fibrous network, in contrast to the fenestrated lamellae seen in native aortas (Fig. 5A). Throughout the majority of the neoartery wall, collagen was aligned in the circumferential direction similar to native medial collagen (Fig. 5B, left). However, regenerated collagen lacked the crimped fibril morphology seen in native aortas and showed weaker fiber alignment. Interestingly, the axis of collagen alignment changed near the luminal surface in some regions of neoarteries. (Fig. 5B, right). Near the abluminal surface, elastin is largely absent in neoarteries, similar to native adventitial matrix (Fig. 5C, left). Collagen near the albumen is crimped resembling native adventitial collagen, but crimp is still less than in native arteries, and neoartery collagen is aligned more strongly than that of the native adventitia (Fig. 5C, right)
Figure 5. Elastin and collagen architecture in neoarteries and native aortas.
All images are en face views of matrix autofluorescence imaged using multiphoton microscopy. Detailed depiction of en face view shown in Supplementary Fig. 1. Scale bars 25 μm. A. Elastin architecture within the neoartery wall, imaged > 10 μm from the luminal surface (left), or within 10 μm of the lumen (right). Elastin is arranged as a fibrous network in neoarteries, in contrast to the fenestrated lamellae seen in native aortas. “Fe” indicates fenestrations in elastic lamellae in native aortas. “Fold” notes where the lamellae drops below the viewing plane (schematic at bottom of A). B. Collagen architecture within the neoartery wall, viewed from the luminal side. Collagen is less aligned and less crimped than collagen seen in native aortas. Two regions from the same vessel segment (Region 1 and Region 2) are shown to demonstrate variation in collagen architecture within the same neoartery. Regions 1 and 2 represent different circumferential locations at the same longitudinal position near the middle of the regenerated vessel. In both regions, collagen at > 10 μm from the luminal surface is oriented in the circumferential direction, as seen in the vascular media of native aortas. However, collagen at < 10 μm from the lumen can either retain its orientation (Region 1) or substantially shift its orientation (Region 2), the latter contrasting with collagen near the intima of healthy native aortas. Arrows indicate the circumferential direction (white) and direction of orientation (blue). C. Collagen and elastin architecture near the abluminal surface of neoarteries. Left: Neoarteries contain little elastin near the abluminal surface, similar to the adventitia of native aortas. Right: Neoartery collagen near the abluminal surface is undulated resembling native adventitial collagen, but has higher undulation frequency than native arteries. Neoartery collagen alignment is stronger than that in native adventitia.
3.6 Neoarteries respond to physiologic vasodilators and vasoconstrictors
We used standard dual pin wire myography to determine whether neoarteries could respond to vasomotor signals. Neoarteries constricted in response to both the selective α1-adrenergic receptor agonist phenylephrine (PE) and the 5-hydroxytryptamine receptor activator serotonin (5-HT), though the magnitude of constriction was less than that of native aortas (32.5 +/− 2.78 vs. 1616 +/− 836.6 mg and 42.2 +/− 25.8 vs. 3485 +/− 1161 mg for PE and 5-HT, respectively) (Fig. 6A). Regenerated vessels displayed vasodilation to the endothelial-specific activator acetylcholine (Ach) (Fig. 6B), suggesting the presence of signaling capability between endothelial cells and contractile smooth muscle. Neoarteries also dilated in response to endothelium-independent vasodilators including the andenylyl cyclase activator forskolin (Fig. 6C) and the direct soluble guanylyl cyclase (sGC) activator sodium nitroprusside (SNP) (Fig. 6D). However, due to small sample size we cannot completely rule out the potential for passive relaxation to contribute to our observed results. Nonetheless, regenerated vessels showed no substantial dilation in situ at the time of explant, suggesting the absence of passive relaxation in vivo. Taken together these data demonstrate that the neoarteries are sensitive to a range of physiologically relevant activators and can translate these signals into vasoactivity, suggesting that vascular cells within neoarteries express healthy phenotypes.
Figure 6. Neoarteries display sensitivity to vasodilators and vasoconstrictors.
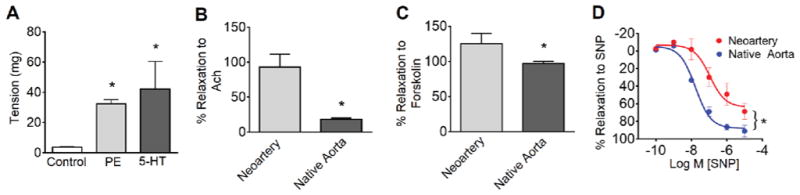
A. Response to physiologic vasoconstrictors. Neoarteries were freshly harvested, mounted on a standard dual pin myograph, resting tension established, then assessed in their response to vasoconstrictors. Neoarteries demonstrated constriction in response to both the selective α1-adrenergic receptor agonist phenylephrine (PE) and the 5-hydroxytryptamine receptor activator serotonin (5-HT). However, the magnitude of constriction was less than native arteries (32.5 +/− 2.78 vs. 1616 +/− 836.6 mg and 42.2 +/− 25.8 vs. 3485 +/− 1161 mg for PE and 5-HT, respectively). Results are the mean ± SEM of n=12 rings from 3 animals per treatment group. * P > 0.05. B–D. Response to physiologic vasodilators. Neoarteries were mounted as described in A, preconstricted with serotonin, then assessed in their response to vasodilators. Results are the mean ± SEM of n=12 rings from 3 animals per treatment group. * P < 0.05. B. Neoarteries relax in response to the endothelial specific activator acetylcholine (Ach, 10 μM), suggesting signaling capability between endothelial cells and contractile smooth muscle. C. Neoarteries relax in response to the cAMP activator (NO-independent) forskolin (10 μM). D. Neoarteries respond to the vascular smooth muscle cell specific activator sodium nitroprusside (SNP) in a dose-dependent manner.
4. DISCUSSION
An ideal vascular graft should conduct blood flow for the duration of a patient’s lifetime. Consequently, long-term in vivo performance is an important indicator of a new vascular graft’s clinical potential. A number of cell-free, resorbable vascular grafts have shown promising long-term results (> 3 months) in small animals. HYAFF-11 rat abdominal aorta grafts completely resorbed by 120 days and remodeled into artery-like tissues with elastic fibers.[35] Silk fibroin grafts showed good patency as rat abdominal aorta grafts, but some residual material and substantial inflammation were still present at 1 year post-implant.[38] PLA, PEUU, and PCL have also shown good long-term patency as rat abdominal aorta grafts, but graft resorption and cellular infiltration were limited due to slow material degradation.[39–41] Several designs also show good long-term performance in large animal models. Canine carotid artery replacements made from small intestinal submucosa (SIS) showed similar patency to saphenous vein grafts at 1 year post-implant.[34] Composite grafts made from PGA, PLA, and collagen also performed well as canine carotid artery replacements for 1 year, but showed limited cell infiltration and graft resorption.[36] HYAFF-11 grafts in porcine carotid arteries showed complete graft resorption and remodeling into artery-like tissues with elastic fibers over the course of 5 months, though intimal hyperplasia and thrombosis was observed in some implants.[37] Excitingly, decellularized tissue engineered blood vessels showed good patency at 6 months as an arteriovenous shunt in baboons and at 1 year as a carotid artery replacement.[15]
Our design departs from the above by using a faster resorbing material which enables more rapid host remodeling. The vast majority of our composite graft design is completely resorbed by 30 days post-implant, except for sparse PCL residues.[33] The advantage of this approach is that it minimizes the duration of host’s response to foreign material, which may promote neointimal hyperplasia,[45] calcification,[41, 46] and tissue regression[41] in the late term. Indeed, we found that our grafts showed no sign of tissue regression (Fig. 1), no significant narrowing from neointimal hyperplasia, (Fig. 2A), and resisted calcification at 1 year post-implant (Fig. 2B). Grafts also resisted dilation and aneurysm, common failure modes for resorbable synthetic and collagen based vascular grafts (Fig. 2). The only graft occlusion appeared to be thrombosis (Supplementary Fig. 3). The time of thrombosis is unknown, but it likely occurred during or shortly after surgery, as we did not use systemic heparinization during graft implantation. Systemic heparinization is standard practice for human surgery, and should substantially reduce the risk of thrombosis in composite grafts.
In addition to displaying encouraging long-term patency, our grafts remodeled into neoarteries demonstrating close resemblance to native aortas at 1 year post-implant. This design regenerated nerves in perivascular tissues (Fig. 3), and neoartery responsiveness to vasomotor agents suggests these nerves may regulate vascular tone (Fig. 6). Two other groups have also demonstrated vasomotor responsiveness in cell-free degradable vascular grafts.[47, 48] Our grafts achieve full regeneration of the amount of mature elastin seen in native tissue (Fig. 4A). Coupled with physiologic compliance in vivo and stable inner diameter, this data demonstrates that neoarteries are mechanically stable at late term despite complete or near-complete resorption of the original graft material.
Neoarteries still differ from native arteries. Macrophages are present in the adventitia, likely due to trace amounts of residual PCL (Fig. 2C). While no residual graft materials are visible, electrospun PCL nanofibers can persist longer than 18 months in vascular grafts.[41] The regenerated arteries showed no evidence of late-term failure, suggesting the macrophages are not affecting graft performance. Elastin and collagen architecture differ from native arteries. (Fig. 4B, 5) However, the regenerated extracellular matrix still exhibits in vivo compliance similar to native aortas and resist dilation. Burst pressure after 3 month host remodeling exceeded 2000 mmHg,[33] thus it wasn’t measured at 1 year post implantation. The magnitude of the response to vasoconstrictors is smaller than that of native arteries. Two characteristics of neoarteries may explain these differences. Firstly, most collagen is not crimped in the unloaded, ring-opened state, in contrast to native arteries. Consequently, the regenerated matrix may be partially stress-shielding the tissue from smooth muscle contractile forces. Secondly, neoarteries contain fewer cells capable of generating constrictive forces in the circumferential direction than do native aortas, as some elongated cells in neoarteries do not express smooth muscle markers, and some smooth muscle-like cells are not elongated in the circumferential direction (Supplementary Fig. 2). However, while neoarteries do not precisely mimic native vasoresponsiveness, response to vasomotor agents serves minimal physiologic function in the aorta or any arteries for which vascular grafts are used as bypasses or replacements. Thus the reduced capacity of neoarteries to change their tone should not impair their long term performance as conduits for blood flow. Rather than assessing neoartery function, myography serves to assess the overall health of arterial cellular and structural components. In this context, the observed vasoresponsiveness of neoarteries suggests a functional endothelium and the presence of vasoresponsive, contractile smooth muscle cells.
The disparity between neoarteries and native arteries is expected, as graft remodeling differs from natural artery development. In the embryo, primitive blood vessels remodel into arteries largely in response to changing hemodynamic forces over the course of development.[49] In contrast, these grafts are immediately exposed to adult hemodynamics. In this context, it is remarkable that cells in the neoarteries synthesized ECM proteins with composition resembling native aortas. While neoarteries differ from native arteries, these differences might not be clinically significant, as neoarteries matched native arteries mechanically and demonstrated good long-term performance in vivo.
Future work will address this study’s limitations. Without cell seeding, it may be challenging for grafts to endothelialize in humans. The rat model we used does not test graft endothelialization to a clinical standard, as rats have superior endothelialization capacity to large animals and humans.[50, 51] Large animal studies will provide better indication of this design’s clinical potential. The host must replace the graft with artery-like tissue quickly enough to prevent dilation, but many patients have reduced healing potential due to old age and disease. Localized delivery of bioactive reagents may be necessary to accelerate both endothelialization and graft remodeling to prevent graft failure. Macrophages persisting in the neoartery at 1 year post-implant might be avoided if the graft sheath was made from a material that resorbs faster than PCL, thereby reducing the sheath’s residence time in vivo. Quantifying total collagen content and staining for additional collagen types and subtypes could improve our understanding of neoartery health and mechanical stability. Further study is needed to determine whether passive relaxation contributed to vasodilation observed in neoarteries during myography. Finally, mechanisms of graft remodeling need to be studied to further accelerate graft remodeling.
5. CONCLUSION
This study demonstrates that a fast-resorbing, cell-free vascular graft can remodel in situ into tissues with good long-term stability and likeness to healthy arteries. These results suggest that this design is a promising strategy for improving the performance of vascular grafts used in small arteries. Additionally, fast resorbing elastic implants may be applicable to other regenerative applications, including wound dressings and soft tissue repair.
Supplementary Material
Neoarteries distend at physiologic pressures. Transverse view of neoartery taken in vivo using transcutaneous ultrasound, slowed to ¼ speed. Neoartery distends with systolic blood pressure similarly to native arteries.
Acknowledgments
We thank Jeffrey Baust for assistance with animal surgery, Callen Wallace for assistance with whole mount immunostaining, Nicole Myers for help with von Kossa staining, and Drs. Jiao Yu and Seunghan Ha for assistance with ultrasound.
FUNDING SOURCES
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award numbers R01HL089658, 2T32HL076124, and 1R21EB013353-01. The small animal imaging system (Vevo2100) is supported through the National Center for Research Resources of the National Institutes of Health under award number 1S10RR027383-01. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edelman ER. Vascular tissue engineering: designer arteries. Circ Res. 1999;85:1115–7. doi: 10.1161/01.res.85.12.1115. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Lin P, Yao Q, Chen C. Development of small-diameter vascular grafts. World J Surg. 2007;31:682–9. doi: 10.1007/s00268-006-0731-z. [DOI] [PubMed] [Google Scholar]
- 3.Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74:570–81. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- 4.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 6.He H, Matsuda T. Newly designed compliant hierarchic hybrid vascular graft wrapped with microprocessed elastomeric film--II: Morphogenesis and compliance change upon implantation. Cell Transplant. 2002;11:75–87. [PubMed] [Google Scholar]
- 7.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–60. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 8.Hoerstrup SP, Cummings Mrcs I, Lachat M, Schoen FJ, Jenni R, Leschka S, et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–66. doi: 10.1161/CIRCULATIONAHA.105.001172. [DOI] [PubMed] [Google Scholar]
- 9.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Guo T, Nie C, Morris SF. Tissue-engineered blood vessel graft produced by self-derived cells and allogenic acellular matrix: a functional performance and histologic study. Ann Plast Surg. 2009;62:297–303. doi: 10.1097/SAP.0b013e318197eb19. [DOI] [PubMed] [Google Scholar]
- 11.Koch S, Flanagan TC, Sachweh JS, Tanios F, Schnoering H, Deichmann T, et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials. 2010;31:4731–9. doi: 10.1016/j.biomaterials.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Neff LP, Tillman BW, Yazdani SK, Machingal MA, Yoo JJ, Soker S, et al. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J Vasc Surg. 2011;53:426–34. doi: 10.1016/j.jvs.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 13.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–6. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 14.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 16.Wystrychowski W, Cierpka L, Zagalski K, Garrido S, Dusserre N, Radochonski S, et al. Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access. J Vasc Access. 2011;12:67–70. doi: 10.5301/jva.2011.6360. [DOI] [PubMed] [Google Scholar]
- 17.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115:536–45. doi: 10.1016/S0022-5223(98)70315-0. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 18.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soletti L, Nieponice A, Guan J, Stankus JJ, Wagner WR, Vorp DA. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863–70. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915–20. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson GN, Mirensky T, Brennan MP, Roh JD, Yi T, Wang Y, et al. Functional small-diameter human tissue-engineered arterial grafts in an immunodeficient mouse model: preliminary findings. Arch Surg. 2008;143:488–94. doi: 10.1001/archsurg.143.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248:370–7. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirensky TL, Nelson GN, Brennan MP, Roh JD, Hibino N, Yi T, et al. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. J Pediatr Surg. 2009;44:1127–32. doi: 10.1016/j.jpedsurg.2009.02.035. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31:296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Mirensky TL, Hibino N, Sawh-Martinez RF, Yi T, Villalona G, Shinoka T, et al. Tissue-engineered vascular grafts: does cell seeding matter? J Pediatr Surg. 2010;45:1299–305. doi: 10.1016/j.jpedsurg.2010.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–74. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A. 2010;16:1215–23. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235–44. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Shin’oka T, Tohyama S, Hibino N, Konuma T, Matsumura G, et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7:429–39. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 30.Zhou M, Liu Z, Liu C, Jiang X, Wei Z, Qiao W, et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res B Appl Biomater. 2012;100:111–20. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 31.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 32.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–6. 6 e1–2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012;18:1148–53. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandusky GE, Jr, Badylak SF, Morff RJ, Johnson WD, Lantz G. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. Am J Pathol. 1992;140:317–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Lepidi S, Abatangelo G, Vindigni V, Deriu GP, Zavan B, Tonello C, et al. In vivo regeneration of small-diameter (2 mm) arteries using a polymer scaffold. FASEB J. 2006;20:103–5. doi: 10.1096/fj.05-4802fje. [DOI] [PubMed] [Google Scholar]
- 36.Yokota T, Ichikawa H, Matsumiya G, Kuratani T, Sakaguchi T, Iwai S, et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J Thorac Cardiovasc Surg. 2008;136:900–7. doi: 10.1016/j.jtcvs.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 37.Zavan B, Vindigni V, Lepidi S, Iacopetti I, Avruscio G, Abatangelo G, et al. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. Faseb J. 2008;22:2853–61. doi: 10.1096/fj.08-107284. [DOI] [PubMed] [Google Scholar]
- 38.Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, et al. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. Journal of vascular surgery. 2010;51:155–64. doi: 10.1016/j.jvs.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Hashi CK, Derugin N, Janairo RR, Lee R, Schultz D, Lotz J, et al. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arterioscler Thromb Vasc Biol. 2010;30:1621–7. doi: 10.1161/ATVBAHA.110.208348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR, et al. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res A. 2011;96:436–48. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Moller M, et al. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–25. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 43.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, et al. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–81. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill MR, Duan X, Gibson GA, Watkins S, Robertson AM. A theoretical and nondestructive experimental approach for direct inclusion of measured collagen orientation and recruitment into mechanical models of the artery wall. J Biomech. 2012;45:762–71. doi: 10.1016/j.jbiomech.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Terry CM, Shiu YT, Cheung AK. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int. 2008;74:1247–61. doi: 10.1038/ki.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelson K, Schoen F. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Annals of biomedical engineering. 2006;34:1799–819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083–6. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 48.Torikai K, Ichikawa H, Hirakawa K, Matsumiya G, Kuratani T, Iwai S, et al. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg. 2008;136:37–45. e1. doi: 10.1016/j.jtcvs.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology. 2006;21:388–95. doi: 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- 50.Bull DA, Hunter GC, Holubec H, Aguirre ML, Rappaport WD, Putnam CW. Cellular origin and rate of endothelial cell coverage of PTFE grafts. J Surg Res. 1995;58:58–68. doi: 10.1006/jsre.1995.1010. [DOI] [PubMed] [Google Scholar]
- 51.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–27. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neoarteries distend at physiologic pressures. Transverse view of neoartery taken in vivo using transcutaneous ultrasound, slowed to ¼ speed. Neoartery distends with systolic blood pressure similarly to native arteries.