SUMMARY
Serotonin (5-hydroxytryptamine, 5-HT) is an ancient and conserved neuromodulator of energy balance. Despite its importance, the neural circuits and molecular mechanisms underlying 5-HT-mediated control of body fat remain poorly understood. Here we decipher the serotonergic neural circuit for body fat loss in C. elegans and show that the effects of 5-HT require signaling from octopamine, the invertebrate analog of adrenaline, to sustain body fat loss. Our results provide a potential molecular explanation for the long-observed potent effects of combined serotonergic and adrenergic weight loss drugs. In metabolic tissues we find that the conserved regulatory adipocyte triglyceride lipase ATGL-1 drives serotonergic fat loss. We show that the serotonergic chloride channel MOD-1 relays a long-range endocrine signal via C. elegans body cavity neurons to control distal ATGL-1 function, via the nuclear receptor NHR-76. Our findings establish a conserved neuroendocrine axis operated by neural serotonergic and adrenergic-like signaling, to regulate body fat.
INTRODUCTION
The central nervous system is a major regulator of systemic fat loss and energy balance. Across different species, genes functioning solely in the central nervous system have been shown to control body fat and energy expenditure, independent of effects on food intake (Greer et al., 2008; Kong et al., 2012; Liu et al., 2012; Lu et al., 2011). Identifying regulators of neural pathways that alter body fat without a multitude of other physiological effects have been difficult to disentangle in mammalian systems. Thus, it has been challenging to address the fundamental question of whether systemic changes in body fat result from long-range endocrine signals communicated directly by the nervous system.
The neuromodulator serotonin (5-hydroxytryptamine, 5-HT) is a conserved regulator of energy balance, and 5-HT signaling serves as an important paradigm for the study of neural regulators of body fat. In mice, loss of the 5HT2c receptor expressed in the central nervous system leads to adult-onset obesity (Nonogaki et al., 2003). In humans, increased neural serotonergic signaling via pharmacological intervention decreases body fat and increases energy expenditure in obese subjects (Chan et al., 2013; Wise, 1992). Interestingly however, combined neural serotonergic and adrenergic stimulation has more potent effects on fat loss compared to 5-HT-based treatments alone (Fanghanel et al., 2000). The mechanisms underlying this effect remain poorly understood. In the nematode C. elegans, 5-HT controls many food-related behaviors and physiological outputs including body fat and energy expenditure (Srinivasan et al., 2008; Sze et al., 2000). 5-HT is synthesized by the conserved rate-limiting enzyme tryptophan hydroxylase (tph-1), which is expressed in only a few neurons and, unlike mammals, is not found in the gut or other fat storage tissues (Gershon, 2013; Yadav et al., 2008). In the ADF chemosensory neuron pair that receives and transduces environmental signals, 5-HT synthesis is finely-tuned to food availability: short-term food-deprivation decreases tph-1 expression in the ADF neurons, whereas animals re-introduced to food restore tph-1 expression back to that of well-fed animals (Cunningham et al., 2012). Genes regulating many aspects of 5-HT-mediated behavior and physiology have been identified, allowing the dissection of genetic pathways that control various 5-HT-regulated behaviors (Chase and Koelle, 2007). In previous work we showed that the potent effects of 5-HT signaling on body fat are independent of its other physiological effects including food intake, locomotion, reproduction and stress response (Srinivasan et al., 2008). Loss of 5-HT production leads to increased body fat despite reduced food intake, whereas pharmacologically-induced 5-HT signaling stimulates fat loss and energy expenditure despite increased food intake. Thus, 5-HT-mediated control of body fat is genetically dissociable from food intake. Three G protein-coupled receptors (GPCRs) coordinately control 5-HT-mediated food intake, and are required in distinct sensory and pharyngeal neural circuits to control different aspects of 5-HT-mediated feeding behavior (Cunningham et al., 2012; Song and Avery, 2012). In contrast, a single 5-HT-gated chloride channel called MOD-1 controls fat loss, without altering 5-HT-mediated effects on food intake. 5-HT signaling also requires key fat oxidation genes in metabolic tissues to promote fat loss via increased energy expenditure (Srinivasan et al., 2008).
Despite the importance of 5-HT signaling in the control of body fat, many questions remain. First, the neural mechanisms governing 5-HT synthesis and signaling with respect to fat loss have not been studied, and the extent to which 5-HT functions in concert with other neuromodulators remains unknown. Second, the site of MOD-1 action is not known. Defining the site of MOD-1 action will answer the critical question of whether 5-HT itself functions as a long-range neuroendocrine signal, or whether 5-HT signaling in the nervous system leads to the release of downstream effectors which in turn act as neuroendocrine signals. Third, in metabolic tissues where fat stores are mobilized, the intracellular regulatory pathways that must be activated to stimulate 5-HT-mediated fat oxidation remain unknown. The C. elegans model system is well-suited to address questions in neuroendocrine biology. In recent years, studies of C. elegans fat regulatory pathways have revealed extensive conservation of function between organisms as diverse as mammals and nematodes (Jones et al., 2009; Mak, 2012; Narasimhan et al., 2011; O'Rourke et al., 2013; Perez and Van Gilst, 2008; Walker et al., 2011) suggesting conserved regulatory principles of energy regulation across metazoans.
In the present study, we begin by describing the neural circuit underlying 5-HT-mediated fat loss in C. elegans. We show that 5-HT-mediated fat loss requires octopamine (Oct, the invertebrate analog of adrenaline) via the biosynthetic enzyme tyramine beta-hydroxylase (tbh-1), and that Oct signaling in turn modulates the expression of the 5-HT biosynthesis enzyme tryptophan hydroxylase (tph-1), and that combined 5-HT and Oct administration leads to a synergistic loss of body fat. Thus 5-HT and Oct function together in a positive regulatory loop to sustain a neural signal for body fat loss. We define the site of mod-1 function to a unique pair of C. elegans neurons called the URX neurons that have access to the circulatory fluid. The anatomy of the URX ‘body cavity’ neurons allows for a functional basis for studies of molecular communication between the nervous system and metabolic tissues. In the intestine where body fat is stored, we found that the conserved regulatory adipocyte triglyceride lipase atgl-1 drives serotonin-dependent fat loss. Finally, we show that the serotonergic neural circuit relays a long-range instructive signal via the URX neurons to control intestinal atgl-1 transcriptional levels, and that this signaling pathway requires an intestinal nuclear receptor called nhr-76. Together, our studies describe a serotonergic and adrenergic-like neuroendocrine signaling pathway that controls body fat in C. elegans.
RESULTS
5-HT signaling requires neural Oct to elicit fat loss
To expand our knowledge of 5-HT-mediated fat loss, we began by testing the roles of several candidate genes. We focused on the major biogenic amine signaling pathways because they have been implicated in modulating 5-HT signaling in metazoans (Nonogaki, 2000; Yadav et al., 2009). In our candidate screen, we observed that tyramine beta-hydroxylase tbh-1(n3247) mutants suppress the fat loss elicited by exogenous 5-HT. As observed previously, 5-HT-treated wild-type animals have approximately 40% of the fat of vehicle-treated animals whereas tbh-1 mutants retain ~ 60% of the fat of vehicle-treated animals (Figures 1A, B and Table S1), as judged by Oil Red O staining, and by quantitation of biochemically extracted triglycerides (Figure S1A). Vehicle-treated tbh-1 mutants do not show a discernable difference in body fat compared to wild-type animals (Figure 1A, B). TBH-1 is a rate-limiting enzyme that controls Oct biosynthesis in C. elegans, thus tbh-1 mutants lack Oct (Alkema et al., 2005). Oct is a catecholamine neurotransmitter that is thought to function as a “fight-or-flight” stress hormone in insects, and is generally regarded as the invertebrate counterpart to adrenaline (Roeder, 2005). In C. elegans, Oct and its precursor Tyramine (Tyr) regulate locomotion (Alkema et al., 2005; Maguire et al., 2011) and feeding behavior (Greer et al., 2008) in response to stress signals, however the role of these neurotransmitters in regulating C. elegans body fat remains unclear. We found that wild-type animals treated with exogenous Oct had approximately 50% less body fat than vehicle-treated controls (Figure 1C, D and Figure S1A), whereas animals treated with the same dose of the closely-related Tyr, did not. Thus, octopaminergic/adrenergic-like treatment decreases body fat in C. elegans.
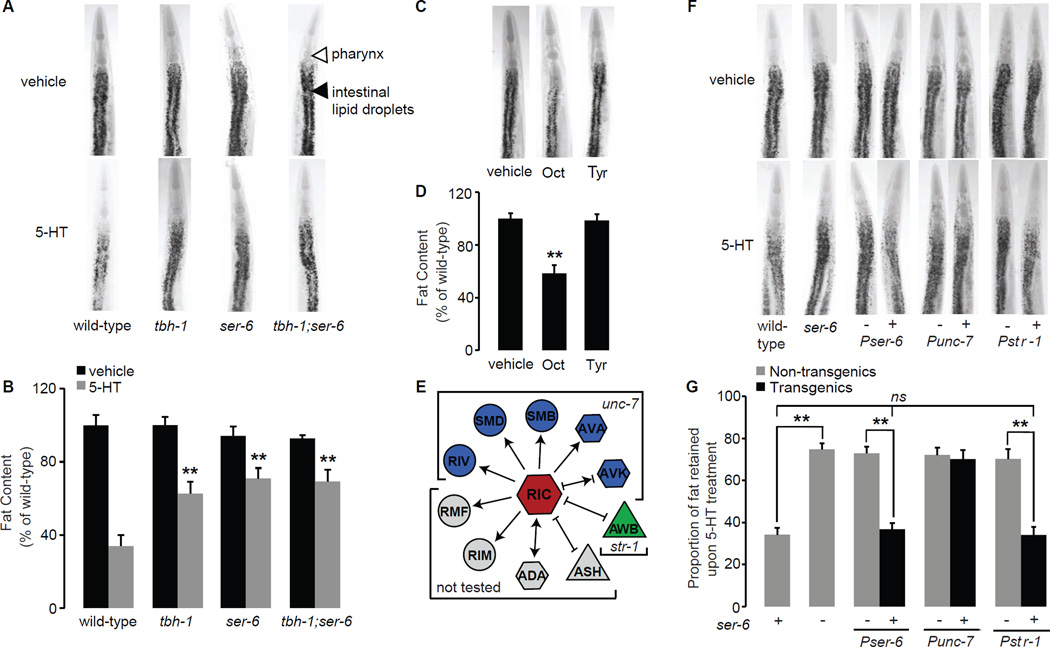
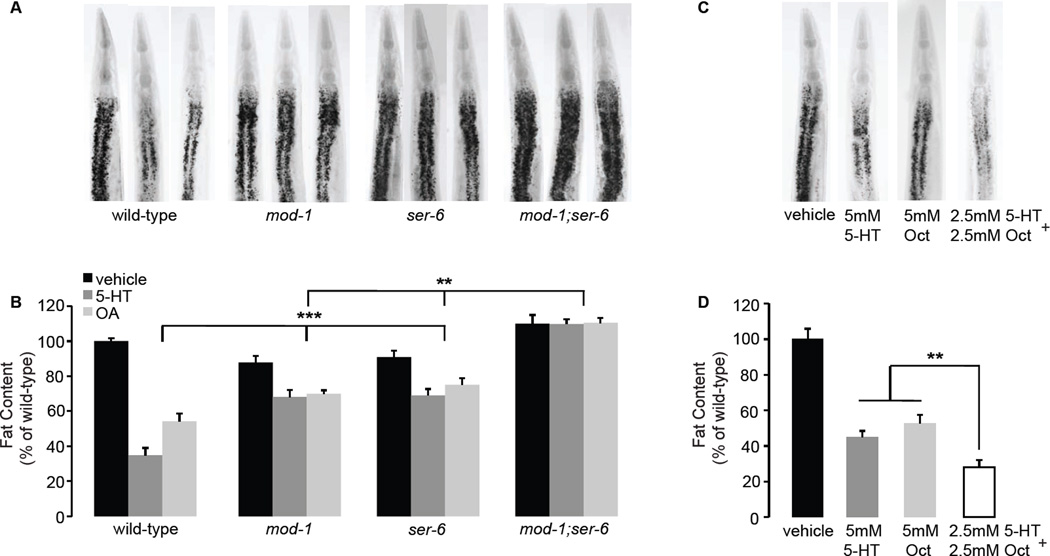
Figure 1. Oct signaling is required for 5-HT-mediated fat loss.
A. Representative images of vehicle (top row) or 5-HT (bottom row) treated animals fixed and stained with Oil Red O. Fat deposition in the intestinal cells is visible as stained lipid droplets (black arrowhead). Animals are oriented facing upwards with the pharynx (white arrowhead) at the anterior end. B. Fat content was quantified for each genotype and expressed as a percentage of wild-type animals ± S.E.M. (n=10–15). tbh-1, ser-6 single mutants and tbh-1;ser-6 double mutants retain a significantly greater proportion of fat upon 5-HT treatment compared to wild-type animals. Data are expressed as a percentage of vehicle-treated wild-type animals ± S.E.M. **, p<0.005. C. Representative images of vehicle-, Oct- or Tyr-treated animals fixed and stained with Oil Red O. D. Fat content was quantified and expressed as a percentage of wild-type vehicle-treated animals (n=10–15) ± S.E.M. **, p<0.005. E. Synaptic and gap-junction connections projecting from the RIC interneurons. The promoters used to confer expression in the relevant neurons are as marked. Arrow lines, synaptic connections; bar lines, gap junctions. F. Representative images of vehicle (top row) or 5-HT (bottom row) treated animals fixed and stained with Oil Red O. For each transgenic line bearing ser-6 expression using the indicated promoter, non-transgenic animals (−) and transgenic animals (+) are shown. Oil Red O staining intensities are given in Table S2. G. The proportion of fat retained upon 5-HT-treatment relative to vehicle treatment is shown for each genotype (n=10–15). Relative to non-transgenic controls (gray bars), ser-6 transgenic animals (black bars) bearing the ser-6 transgene under the control of the endogenous ser-6 and the heterologous str-1 promoters restore 5-HT-mediated fat loss. Data are expressed as a percentage of vehicle-treated wild-type animals ± S.E.M. **, p<0.005; ns, not significant. See also Figures S1 and Tables S1 and S2.
We reasoned that if 5-HT-mediated fat loss requires intact Oct signaling, a downstream octopaminergic G protein coupled receptor (GPCR) must also be required. The C. elegans genome contains three octopaminergic and three closely-related tyraminergic GPCRs. We examined loss-of-function mutants for each of the six GPCRs and found that only loss of the ser-6 GPCR suppressed the fat loss induced by 5-HT (Figure 1A, B and Figure S1B, C), recapitulating previous studies (Srinivasan et al., 2008). ser-6(tm2146) mutants treated with 5-HT have a nearly two-fold-greater proportional increase in body fat (70–75%) compared to wild-type animals (35–40%; Figure 1A, B and Table S1). tbh-1;ser-6 double mutants resemble tbh-1 and ser-6 single mutants and retain body fat upon 5-HT treatment to the same extent as either single mutant alone, providing in vivo evidence that tbh-1 and ser-6 function in a linear pathway (Figure 1A, B). Our results show that 5-HT-induced fat loss requires neural Oct synthesis via tbh-1 and signaling via the ser-6 octopaminergic receptor.
The octopaminergic receptor SER-6 functions in AWB chemosensory neurons to control 5-HT-mediated fat loss
Since ser-6 signaling is required for 5-HT-mediated fat loss, we wanted to determine its site of action. ser-6 has been characterized as a selective Oct receptor (Mills et al., 2012), and tbh-1, the enzyme that synthesizes Oct, is expressed in a single pair of interneurons called RIC (Alkema et al., 2005). Therefore, we examined the C. elegans wiring diagram (White, 1986) for neurons that receive direct synaptic input from RIC (Figure 1E). A survey of the published literature and examination of our ser-6-expressing transgenic lines showed that it is expressed across many C. elegans neurons, including both sensory and interneurons (Mills et al., 2012). Introduction of the ser-6 transgene under the control of its own promoter fully restored the ability of 5-HT to elicit fat loss in ser-6(+) transgenic animals but not in ser-6(−) non-transgenic controls (Figure 1F, G and Table S2). In ser-6 mutants we used the unc-7 promoter to reconstitute ser-6 function, because of its broad expression in neurons that receive direct synaptic input from RIC (Starich et al., 2009). However, in ser-6(+) transgenic animals, ser-6 expression under the control of the unc-7 promoter did not rescue the suppression of fat loss seen in non-transgenic controls (Figure 1F, G). A closer examination of our ser-6(+) transgenic lines revealed strong expression of ser-6 in the AWB sensory neurons (Figure S1 D–F). We restored ser-6 expression to ser-6 mutants solely in the AWB sensory neurons using the str-1 promoter (Troemel et al., 1997). We found that 5-HT treatment elicits fat loss in str-1::ser-6(+) transgenic animals to a similar extent as that seen with the endogenous ser-6 promoter and wild-type animals (Figure 1F, G). Thus, ser-6 function in AWB olfactory neurons fully rescues 5-HT-mediated fat loss. Additionally, we wanted to test whether ser-6 modifies another aspect of AWB function. AWB olfactory neurons control the behavioral avoidance of a volatile repellent 2-nonanone, and we found that ser-6 mutants were defective in 2-nonanone avoidance (Figure S2A), thus corroborating an independent functional role for ser-6 in AWB neurons.
The ser-6 octopaminergic receptor would also be expected to suppress the observed Oct-induced fat loss. Accordingly, we found that ser-6 mutants retain nearly 80% of their body fat upon Oct treatment (Figure 2A, B), compared to ~50% retained by wild-type animals. Because our ser-6 transgenic rescue experiments indicated a role for ser-6 in AWB neurons, we tested whether loss of AWB neurons also modified Oct-induced fat loss. We generated a transgenic line expressing tetanus toxin under the control of the AWB-specific str-1 promoter. Transgenic animals in which synaptic signaling from AWB neurons was abolished, suppressed Oct-induced fat loss to the same extent as ser-6 mutants (Figure 2C, D). We recapitulated this result in the C. elegans lineage mutant lim-4 which lacks AWB neurons (Sagasti et al., 1999) (Figure S2B, C). AWB neurons receive direct synaptic input from RIC neurons where Oct is synthesized. Together, our results show that Oct synthesis via tbh-1 in RIC neurons leads to ser-6-mediated signaling in AWB neurons, and that this activation is required to mediate both serotonergic and octopaminergic fat loss.
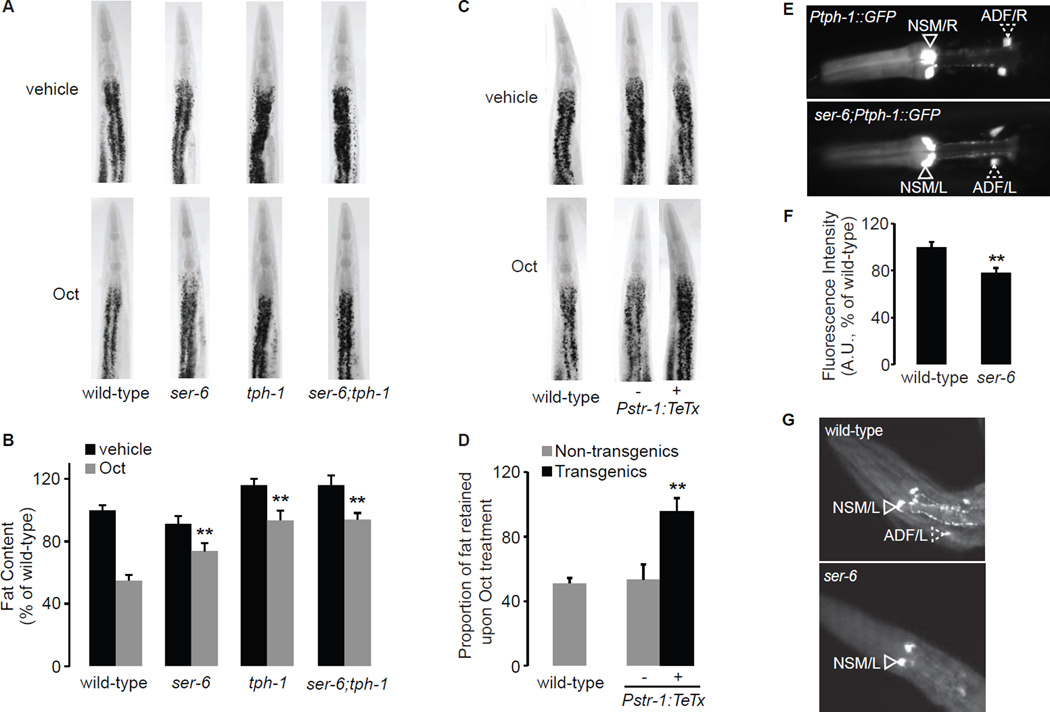
Figure 2. The octopaminergic ser-6 GPCR in AWB neurons controls serotonergic functions in ADF neurons.
A. ser-6, tph-1 single mutants and ser-6;tph-1 double mutants retain a significantly greater proportion of fat upon Oct treatment compared to wild-type animals. B. Fat content was quantified for each genotype and expressed as a percentage of wild-type animals ± S.E.M. (n=10–15). **, p<0.005. The proportion of fat retained upon 5-HT administration for each genotype is given in Table S1. C. Representative images of vehicle (top row) or Oct (bottom row) treated animals fixed and stained with Oil Red O. Genotypes are as indicated in the figure. For the indicated transgenic line bearing tetanus toxin using the str-1 promoter, non-transgenic animals (−) and transgenic animals (+) are shown. D. The proportion of fat retained upon Oct treatment relative to vehicle treatment is shown for transgenic and non-transgenic animals, ± S.E.M. (n=10–15). **, p<0.005. E. Representative images of wild-type (upper panel) and ser-6(tm2146) (lower panel) animals expressing GFP under the control of the tph-1 promoter. tph-1 expression is visible in the NSM (open arrowheads) and in the ADF (dashed arrowheads) neurons. In ser-6 mutants, tph-1 expression in ADF chemosensory neurons is decreased relative to wild-type animals. The anterior end of the animals is oriented towards the left. F. The fluorescence intensity of tph-1 expression in ADF neurons is quantified and expressed relative to wild-type animals, ± S.E.M. (n=30–40). **, p<0.05. G. Anti-5-HT immunostaining shows that 5-HT production from ADF chemosensory neurons (dashed arrowheads) is diminished in ser-6 mutants (lower panel) relative to wild-type (upper panel). See also Figure S2 and Table S1.
Oct signaling via SER-6 in AWB olfactory neurons maintains 5-HT production
How might Oct regulate 5-HT-mediated fat loss? We noted that one of the strongest synaptic outputs of the AWB olfactory neurons in which ser-6 expression regulates 5-HT-mediated fat loss, is to the ADF chemosensory neurons (White, 1986) (www.wormweb.org). The ADF neurons are best-known for their role in controlling 5-HT-mediated behaviors via modulation of the rate-limiting enzyme for 5-HT synthesis, tryptophan hydroxylase (tph-1, not to be confused with tbh-1). This prompted us to test whether Oct signaling via ser-6 modifies the 5-HT pathway by altering some aspect of tph-1 function. We crossed ser-6(tm2146) mutants into a tph-1::GFP reporter transgenic line and measured tph-1 expression in ADF neurons. We found that relative to wild-type animals, loss of ser-6 decreases tph-1 expression in ADF neurons by approximately 25% (Figure 2E, F). Interestingly, anti-5-HT immunostaining revealed that ser-6 mutants lack 5-HT in ADF neurons (Figure 2G). We note that lim-4 mutants which lack AWB neurons (Sagasti et al., 1999), are reported to have decreased tph-1 expression and 5-HT production in ADF neurons (Zheng et al., 2005). Thus, ser-6 function in AWB neurons is required to maintain 5-HT synthesis and production in ADF neurons.
A prediction that arises from our observation is that the fat loss induced by Oct should, at least in part, be dependent on intact 5-HT signaling. We treated tph-1(mg280) mutants with exogenous Oct and observed an approximately 80% retention of body fat compared to wild-type Oct-treated animals (~50%, Figure 2A, B). tph-1 mutants have increased body fat relative to wild-type animals, however the proportional suppression of Oct-induced fat loss is the same as in the ser-6 mutants (~80%, see Table S1). The body fat of vehicle-treated ser-6;tph-1 double mutants resemble tph-1 single mutants, and suppress Oct-induced fat loss to the same extent as either single mutant alone (Figure 2A, B). These results suggest that tph-1 functions downstream of ser-6 in a linear pathway to suppress Oct-induced fat loss. Thus, we find that just as 5-HT signaling requires Oct production via tbh-1 (Figure 1), Oct signaling in turn requires 5-HT production via tph-1 (Figure 2).
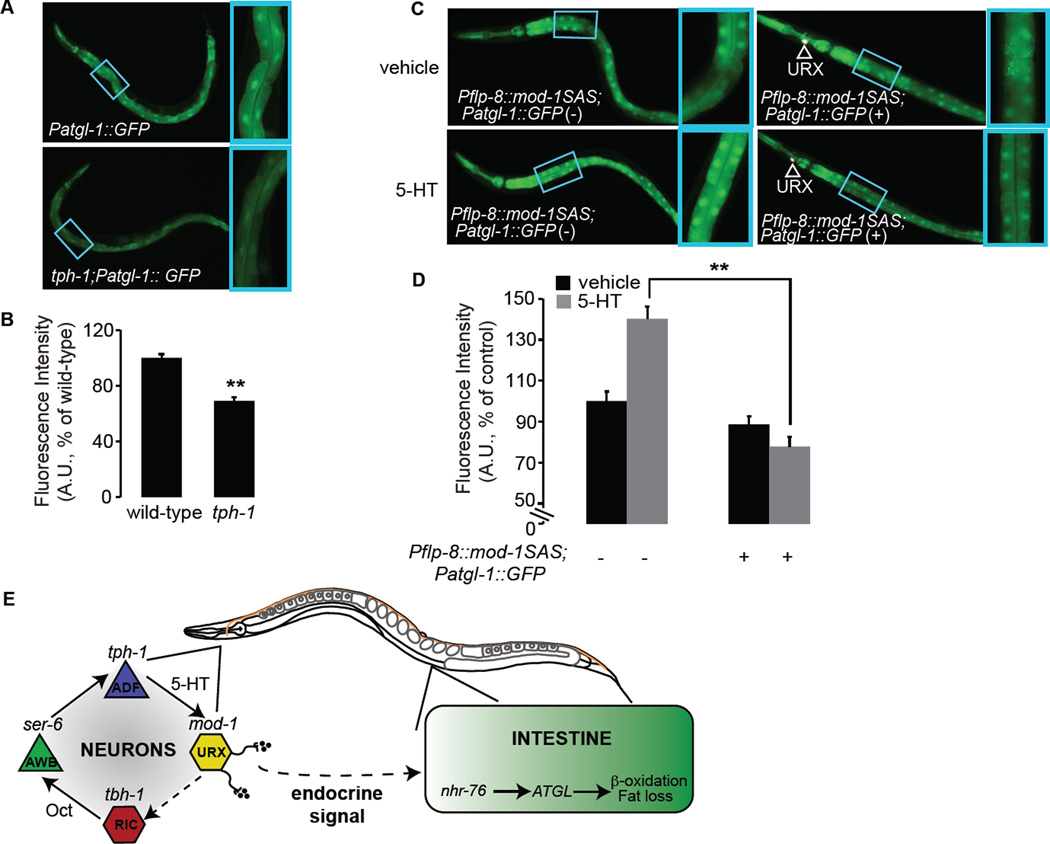
5-HT from ADF chemosensory neurons regulates fat loss
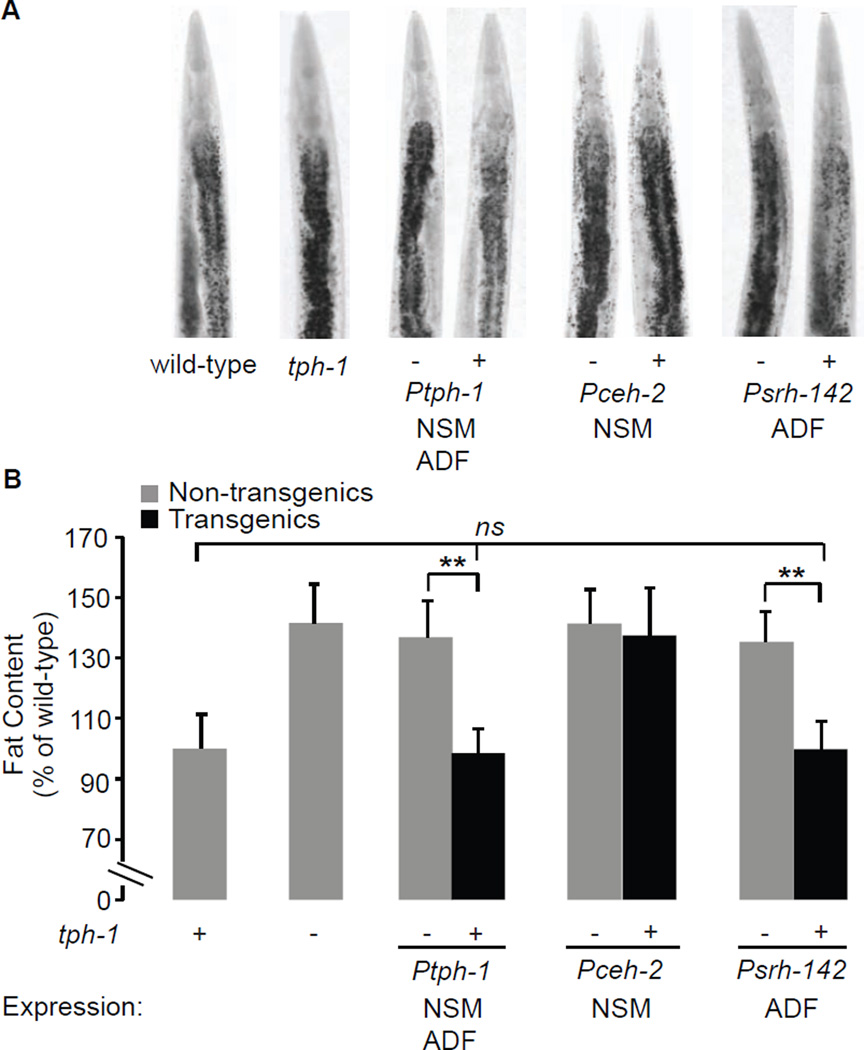
Our results indicated that the control of tph-1 expression in ADF neurons might be an important mechanism of 5-HT-mediated fat loss. Relative to wild-type animals, tph-1 mutants have increased body fat (Figure 3A, B). A similar increase in body fat has been previously noted using Sudan Black staining (Sze et al., 2000) and mass spectrometry (Ashrafi et al., 2003). tph-1 is expressed in two pairs of neurons in the head of the animal: the NSM neurosecretory motor neurons that have dendritic endings in the lumen of the pharynx (the feeding organ for C. elegans), and the ADF chemosensory neurons whose dendrites project outside the animal (White, 1986). We restored tph-1 expression to either NSM or ADF neurons, using the ceh-2 and srh-142 promoters, respectively (Zhang et al., 2005). As expected, tph-1 expression under the control of its own promoter fully restored the increased fat content of tph-1(+) transgenic animals to wild-type levels, relative to tph-1(−) non-transgenic animals. We found that transgenic rescue of tph-1 in ADF, but not NSM neurons, was sufficient to fully restore the increased fat content of tph-1 mutants, back to wild-type (Figure 3A, B). In addition to body fat, other food-related behaviors also stem from tph-1 function in ADF neurons: food intake (Cunningham et al., 2012), olfactory learning (Zhang et al., 2005) and aerotaxis (Chang et al., 2006). Because tph-1 function in ADF neurons regulates many diverse phenotypes, we propose that the control of each serotonergic ADF-mediated behavioral or metabolic outcome must either employ distinct serotonergic receptors, distinct neural circuits, or a combination of the two strategies.
Figure 3. tph-1 expression in ADF chemosensory neurons controls fat loss.
A. Representative images of animals fixed and stained with Oil Red O. For each indicated transgenic line bearing tph-1 expression using the indicated promoter, non-transgenic animals (−) and transgenic animals (+) are shown. B. Fat content was quantified for each genotype and expressed as a percentage of wild-type animals ± S.E.M. (n=10–15). Relative to non-transgenic controls (gray bars), tph-1(+) transgenic animals bearing the tph-1 transgene under the control of the endogenous tph-1 and the heterologous srh-142 promoters fully restore the increased body fat of tph-1 mutants back to wild-type. **, p<0.005; ns, not significant.
The 5-HT-gated chloride channel MOD-1 functions in URX body cavity neurons to promote fat loss
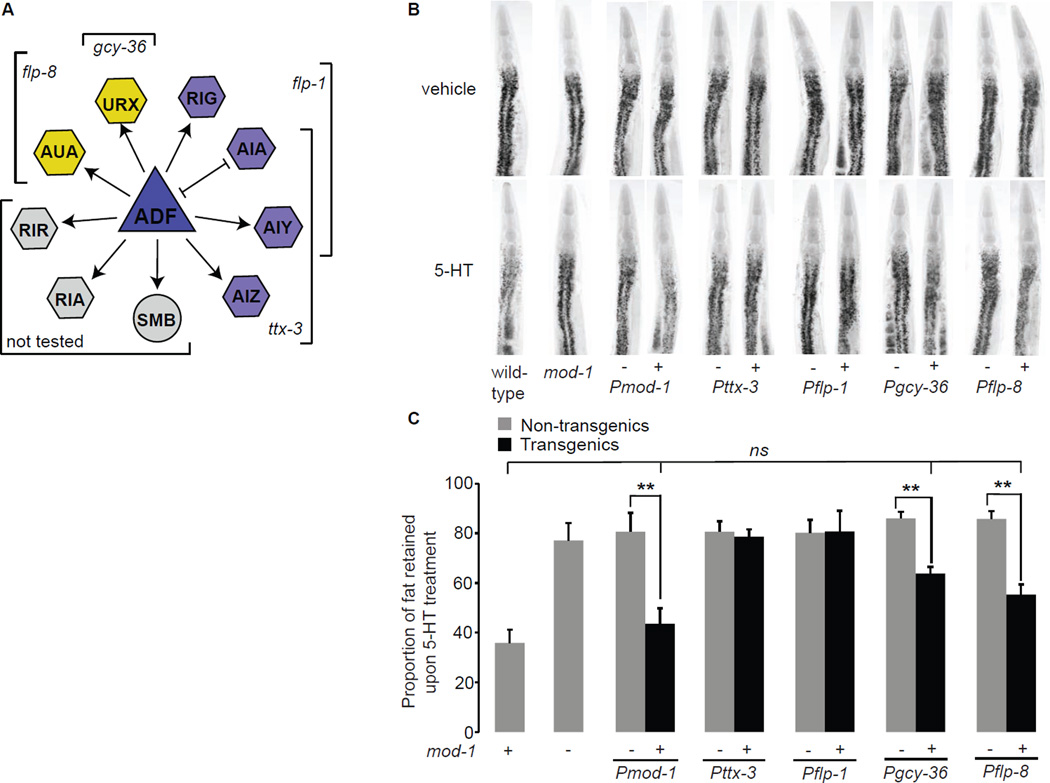
Previous studies have shown that food intake and fat content are regulated by distinct neurally-expressed serotonergic receptors: three GPCRs (ser-1, ser-5 and ser-7) regulate 5-HT-mediated increase in food intake whereas the 5-HT-gated chloride channel mod-1 controls fat loss (Cunningham et al., 2012; Srinivasan et al., 2008). MOD-1 is activated by direct binding of 5-HT which functions as its only known ligand (Ranganathan et al., 2000; Ringstad et al., 2009). We wanted to identify the neurons in which mod-1 functions to control 5-HT-mediated fat loss. 5-HT-treated mod-1(ok103) mutants have a two-fold-greater proportional increase in body fat (75–80%) compared to vehicle-treated controls (Figure 4B, C and Table S1), thus mod-1 mutants suppress 5-HT-induced fat loss, as described previously (Srinivasan et al., 2008). Introduction of the mod-1 transgene under the control of its own promoter fully restored the ability of 5-HT to elicit fat loss in mod-1(+) transgenic animals but not in mod-1(−) non-transgenic controls (Figure 4B, C and Table S3). Next, we expressed the mod-1 transgene in the major class of interneurons onto which ADF synapses: the AIA, AIY and AIZ neurons (Figure 4A). These neurons regulate forward and backward locomotion and chemosensation in response to a variety of stimuli (Gray et al., 2005). In addition, all known functions of mod-1 have been associated with expression in the AIY and AIZ neurons (Ha et al., 2010; Harris et al., 2009; Zhang et al., 2005). To our surprise, mod-1 expression in the AIA, AIY and AIZ neurons using the canonical ttx-3 promoter did not rescue the suppression of 5-HT-mediated fat loss in mod-1 mutants (Figure 4B, C). We verified these results by generating independent transgenic lines bearing the mod-1 transgene under the control of the flp-1 promoter which allows expression in the RIG, AIA and AIY neurons and obtained similar results. Thus, the control of 5-HT-mediated fat loss by mod-1 must employ a distinct set of neurons from those used in other behaviors.
Figure 4. The mod-1 chloride channel functions in URX body cavity neurons to control 5-HT-mediated fat loss.
A. Synaptic and gap-junction connections projecting from the ADF chemosensory neurons are shown. The promoters used to confer expression in the relevant neurons are as marked. Arrow lines, synaptic connections; bar lines, gap junctions. B. Representative images of vehicle (top row) or 5-HT (bottom row) animals fixed and stained with Oil Red O. For each transgenic line bearing mod-1 expression using the indicated promoter, non-transgenic animals (−) and transgenic animals (+) are shown. C. The proportion of fat retained upon 5-HT treatment relative to vehicle treatment is shown for each genotype (n=10–15). Relative to non-transgenic controls (gray bars), mod-1 transgenic animals (black bars) bearing the mod-1 transgene under the control of the endogenous mod-1 and the heterologous gcy-36 and flp-8 promoters restore 5-HT-mediated fat loss. Data are expressed as a percentage of vehicle-treated wild-type animals ± S.E.M. **, p<0.005; ns, not significant. See also Figure S3 and Table S3.
Visual inspection of the C. elegans wiring diagram revealed that ADF neurons synapse onto the URX pair of “body cavity” neurons (www.wormweb.org). These neurons are best-known for their role in oxygen sensation (Gray et al., 2004) and more recently, for the control of body size (Mok et al., 2011). The URX neurons receive synaptic input from many sensory neurons and importantly, are anatomically positioned to access the coelomic fluid that functions as the circulatory system for C. elegans (White, 1986). We restored mod-1 expression in URX neurons using two well-established promoters: the gcy-36 promoter that also confers expression in the AQR and PQR body cavity neurons and the flp-8 promoter which is expressed strongly in the URX neurons and sporadically in the AUA interneurons (Cheung et al., 2005; Gray et al., 2004) (Figure 4A). In both sets of transgenic animals in which mod-1 expression had been restored to URX neurons, we observed rescue of 5-HT-induced fat loss (Figure 4B, C). To verify that mod-1 is endogenously expressed in URX neurons, we re-examined transgenic lines in which mod-1 is controlled by its own promoter. We observed expression of mod-1 in neurons consistent with the position and structural anatomy of URX neurons (Figure S3). Thus, our results show that the 5-HT-gated chloride channel mod-1 functions in the URX body cavity neurons to control 5-HT-mediated fat loss.
Synergistic effects of combined 5-HT and Oct administration
Thus far, our studies of the serotonergic neural circuit predict a linear pathway that connects the synthesis of Oct in RIC interneurons and the ser-6 octopaminergic GPCR in AWB olfactory neurons, to the synthesis of 5-HT in ADF chemosensory neurons and mod-1 function in the URX body cavity neurons. However, multiple lines of evidence indicate a positive regulatory relationship between 5-HT and Oct signaling. First, Oct pathway genes tbh-1 and ser-6 regulate 5-HT-mediated fat loss (Figure 1) and 5-HT pathway genes tph-1 and mod-1 regulate Oct mediated fat loss (Figures 2 and 5A, B). Second, ser-6/AWB neurons regulate 5-HT production in ADF neurons (Figure 2), which control serotonergic fat loss (Figure 3). Third, we found that upon either 5-HT or Oct administration mod-1;ser-6 double mutants have a greater proportion of body fat (100% compared to ~75%) than either single mutant alone (Figure 5A, B and Table S1). Thus, whereas mod-1 and ser-6 single mutants function as partial suppressors of 5-HT-and Oct-induced fat loss and vehicle-treated mutant animals have no overt change in body fat, mod-1;ser-6 double mutants fully suppress the fat loss induced by either 5-HT or Oct and vehicle-treated animals have an approximate 10% increase in body fat (Figure 5A, B). Finally, we found that wild-type animals simultaneously administered low doses (2.5mM) of 5-HT and Oct show a greater decrease in body fat compared to double the dose (5mM) of either 5-HT or Oct alone (Figure 5C, D). Interestingly, the URX neurons in which mod-1 regulates serotonergic fat loss also form a direct synaptic connection to the RIC neurons in which the enzyme TBH-1 synthesizes Oct. Together, our analysis describes an integrated neurotransmitter circuit in which 5-HT and Oct pathways in the nervous system regulate one another in a positive regulatory loop and coordinately control distal body fat loss. Given the unique anatomy of URX neurons, our results provide a structural and functional basis for communication between the nervous system and the metabolic tissues where body fat is stored.
Figure 5. Synergistic effects of combined 5-HT and Oct treatment.
A, C. Representative images of vehicle (left panels), 5-HT (center panels) and Oct (right panels) treated animals fixed and stained with Oil Red O. B, D. Fat content was quantified for each genotype and expressed as a percentage of wild-type animals ± S.E.M. (n=10–15). Upon either 5-HT (dark gray bars) or Oct (light gray bars) administration, mod-1;ser-6 double mutants retain a greater proportion of fat than either mod-1 or ser-6 mutants alone. Combined 5-HT and Oct treatment was administered at half the dose (2.5mM each; white bar). Data are expressed as a percentage of vehicle-treated wild-type animals ± S.E.M. ***, p<0.005, **, p<0.05. See also Table S1.
C. elegans ATGL regulates 5-HT-mediated lipolysis in metabolic tissues
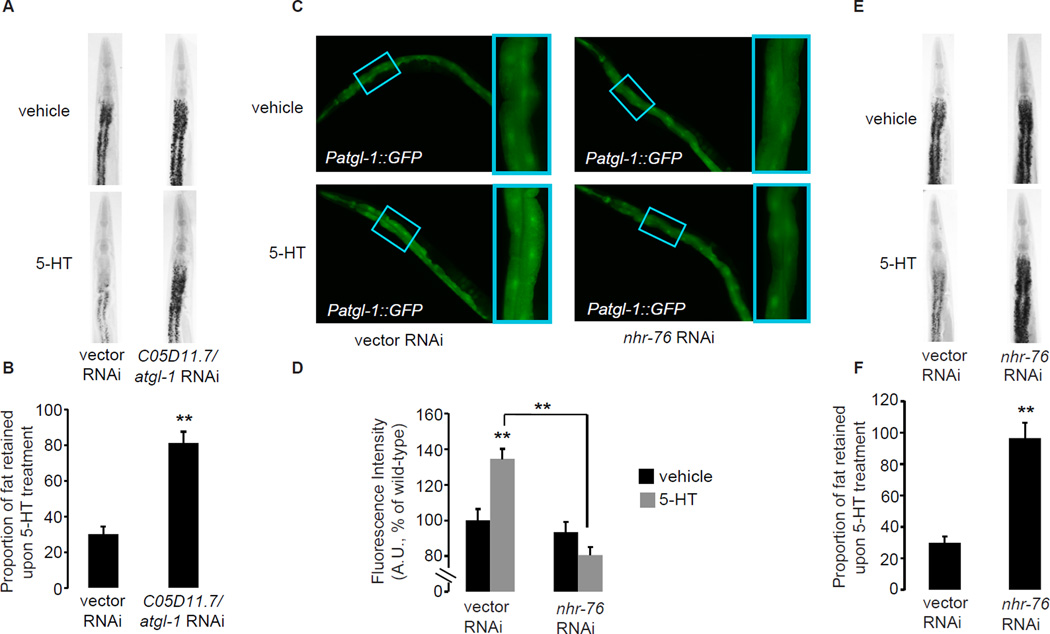
Lipases are a family of enzymes that are rate-limiting for lipid hydrolysis and the generation of free fatty acids targeted for oxidation and the production of energy in metabolic tissues. Because previous studies had shown that the 5-HT signaling pathway stimulates lipid hydrolysis and increased energy expenditure (Srinivasan et al., 2008), we reasoned that one or more lipases may function as upstream regulators that generate free fatty acids from stored lipids. The C. elegans genome contains ~100 genes containing the conserved “lipase domain” that includes the Ser-His-Asp/Glu catalytic triad characteristic of this family of enzymes. We screened all the genes for which RNAi clones were available from the C. elegans genome-wide libraries to identify the lipase/s essential for 5-HT-mediated fat loss. We observed the strongest effect upon depletion of C05D11.7/atgl-1, the sole C. elegans ortholog of Adipocyte Triglyceride Lipase (ATGL). As expected, 5-HT-treated wild-type animals have approximately 35–40% of the fat of untreated animals, whereas atgl-1-inactivated animals retain nearly all of the fat compared to untreated animals (Figure 6A, B), suggesting that loss of atgl-1 leads to near-complete suppression of 5-HT-mediated fat loss. Other well-conserved C. elegans lipase orthologs including hormone-sensitive lipase, diacylglycerol lipase and gastric lipase were not essential for 5-HT-mediated fat loss (not shown). In mammals, ATGL is expressed in several tissues in which fat is mobilized upon energy demand, and is the rate-limiting enzyme for triglyceride hydrolysis (Zimmermann et al., 2004). As expected for a lipase that controls body fat loss, we observed strong atgl-1 expression in the intestine, the major body fat depot in C. elegans (Figure 6C). Interestingly, upon addition of exogenous 5-HT, we observed an approximately 40% induction of GFP expression in the intestine (Figure 6C, D), suggesting that the atgl-1 promoter is transcriptionally induced by 5-HT. To corroborate our experiments using the atgl-1 reporter line, we conducted qPCR studies and found an approximately 1.5 fold increase in atgl-1 expression upon 5-HT treatment (Figure S4A). Oct treatment alone did not induce GFP expression in the atgl-1 reporter line (Figure S4B).
Figure 6. The conserved triglyceride lipase atgl-1 and the nuclear receptor nhr-76 control 5-HT-mediated fat loss in the intestine.
A, E. Representative images of vehicle (top row) or 5-HT (bottom row) animals fixed and stained with Oil Red O. B, F. The proportion of fat retained upon 5-HT treatment relative to vehicle treatment is shown for each condition (n=10–15). Data are expressed as a percentage of vehicle-treated wild-type animals ± S.E.M. **, p<0.005. C. Images of wild-type animals bearing an integrated atgl-1::GFP transgene exposed to vehicle (upper left panel) or 5-HT (lower left panel), compared to nhr-76-inactivated animals exposed to vehicle (upper right panel) or 5-HT (lower right panel). Inset, magnified view of region (third and fourth pair of intestinal cells) used for quantification. D. The fluorescence intensity of atgl-1 expression is quantified (see panel C inset) and expressed relative to control-treated animals ± S.E.M. (n=20–30). Black bars, vehicle treatment; gray bars, 5-HT treatment and **, p<0.005. See also Figure S4 and Table S1.
The nuclear receptor NHR-76 regulates 5-HT-mediated induction of ATGL
In vitro studies have identified transcriptional regulators of mammalian ATGL expression in cell culture (Chakrabarti et al., 2011; Kershaw et al., 2007), however the regulatory control of ATGL transcription in vivo remains poorly characterized. We sought to identify transcriptional regulators of atgl-1 expression, by taking advantage of the transparent body of C. elegans in which expression level changes in the atgl-1 GFP reporter can be clearly observed in living animals. In C. elegans the nuclear hormone receptor (NHR) family consists of ~280 members, the majority of which are expressed in metabolic tissues (Arda et al., 2010). Because nuclear hormone receptors often have endocrine roles in metabolism, and evidence in the literature suggests that nuclear receptors regulate ATGL in vitro (Kershaw et al., 2007), we screened this family of transcriptional regulators for suppression of the 5-HT-induced increase in atgl-1 transcription. We identified nhr-76 as the sole hit from our RNAi-based nuclear receptor screen: nhr-76 knockdown abrogated the increase in atgl-1 expression seen in 5-HT-treated animals (Figure 6C, D). We also found that nhr-76 is essential for 5-HT-mediated fat loss (Figure 6E, F). 5-HT-treated animals in which nhr-76 was inactivated retained a significant proportion of their body fat compared to vehicle-treated animals (Figure 6E, F and Table S1). Finally, to determine the site of nhr-76 action we generated a reporter line in which a 7kb upstream promoter element was fused to GFP, and found that nhr-76 is expressed in the C. elegans intestine, where atgl-1 is also expressed (Figure S4C). Thus, we observe that nhr-76 functions as an activator of 5-HT-mediated atgl-1 transcription in the C. elegans intestine to control body fat.
Neural 5-HT relays an instructive endocrine signal to control ATGL transcription
Thus far, our genetic studies of the serotonergic fat loss pathway describe the nhr-76 and atgl-1-mediated regulation of fat loss in the intestine, and an upstream neural circuit that implicates a role for mod-1 in the URX neurons in releasing signals that are relayed to the intestine. To explicitly test this hypothesis, we conducted three experiments. First, we tested whether loss of 5-HT modified atgl-1 expression. We crossed tph-1(mg280) mutants into the atgl-1 reporter line and found that atgl-1 expression was decreased to approximately 65% of wild-type (Figure 7A, B). Our data show that atgl-1 expression levels and the concomitant changes in body fat are responsive both to increased 5-HT signaling as well as to loss of 5-HT. Thus, 5-HT functions as an instructive neural regulator of body fat via modulation of atgl-1.
Figure 7. Neural 5-HT signaling relays an instructive signal to the intestine to control atgl-1 expression.
A. Images of animals bearing the atgl-1::GFP transgene in the wild-type (upper panel) or tph-1 (lower panel) backgrounds. Inset, magnified view of third and fourth pair of intestinal cells. Loss of neural 5-HT signaling via abrogation of tph-1 significantly decreases atgl-1 expression in the intestine. B, D. Fluorescence intensity of atgl-1 expression in the third and fourth intestinal cells is quantified and expressed relative to wild-type animals ± S.E.M. (n=20). **, p<0.005. C. Images of atgl-1::GFP animals bearing anti-sense mediated inactivation of mod-1 in the URX neurons. In non-transgenic animals, relative to vehicle treatment (upper left panel) 5-HT treatment induces an increase in atgl-1::GFP expression (lower left panel). In contrast, in transgenic animals in which mod-1 is inactivated in the URX neurons (white arrowheads), 5-HT treatment no longer elicits an increase in atgl-1 expression (lower right panel), and resembles vehicle treatment (upper right panel). Inset, magnified view of third and fourth pair of intestinal cells. E. Schematic depiction of the serotonergic neuroendocrine pathway that controls body fat. The described neural circuit for fat loss integrates Oct/adrenaline-like signaling with 5-HT production and function. 5-HT production in the ADF chemosensory neurons activates the serotonergic chloride channel MOD-1 in the URX body cavity neurons to elicit fat loss. 5-HT signaling relays a neuroendocrine instructive signal from the nervous system to the distal intestine via unknown neuroendocrine effectors. In intestinal cells where body fat is stored, activation of the neural circuit leads to increased expression of the conserved lipase atgl-1 via the nuclear receptor nhr-76, which in turn liberates triglycerides and elicits fat loss. See also Figure S5.
Second, we wished to ascertain whether the loss of mod-1 specifically from the URX neurons modified atgl-1 transcription. Using antisense technology, we generated transgenic animals in which mod-1 sense and antisense (SAS) expression were under the control of the flp-8 promoter, in atgl-1::GFP reporter animals. Thus, in the mod-1 SAS(+) animals, mod-1 expression is abrogated specifically in the URX neurons. We measured atgl-1::GFP expression intensity in the C. elegans intestine in transgenic and non-transgenic animals treated with vehicle or 5-HT. As expected, 5-HT treatment increased atgl-1 expression in mod-1 SAS(−) non-transgenic animals by approximately 45%. Transgenic animals bearing the mod-1 SAS(+) constructs in the URX neurons fully blocked the 5-HT-mediated increase in atgl-1 expression (Figure 7C, D). Upon 5-HT treatment, the inactivation of nhr-76 did not further decrease atgl-1 expression in mod-1 SAS(+) transgenic animals (Figure S5A). Finally, we examined body fat content of animals in which mod-1 was inactivated in URX neurons and found that 5-HT-mediated fat loss was suppressed by approximately 50% compared to wild-type animals (Figure S5B, C). Our results show that 5-HT signaling via mod-1 in the URX neurons relays an instructive endocrine signal that is received by the intestinal tissue to control atgl-1 expression and body fat via nhr-76 (Figure 7E).
DISCUSSION
In this report we describe a C. elegans neuroendocrine signaling pathway that originates in the nervous system, and operates via body cavity neurons to regulate body fat in C. elegans. We describe the neural circuit for 5-HT-mediated fat loss and show that 5-HT signaling requires Oct synthesis via tbh-1 in the RIC neurons, and that Oct administration itself is capable of eliciting body fat loss. Oct-induced fat loss in turn, requires 5-HT synthesis via tph-1. Combined serotonergic and octopaminergic signaling has a synergistic effect on body fat loss. Thus, integrated 5-HT and Oct signaling in the nervous system function in a positive regulatory loop to promote distal fat loss. Downstream of Oct production, the octopaminergic ser-6 GPCR in the AWB sensory neurons is required for 5-HT-mediated fat loss. Previous studies have shown that the RIC and AWB neurons, two major components of the serotonergic fat loss neural circuit, are both sites of integration of sensory cues. RIC neurons integrate long-term food-deprivation and stress signals via the TGF-beta receptor daf-1 (Greer et al., 2008), which also controls fat and feeding in C. elegans. AWB neurons sense aversive olfactory cues and promote behavioral avoidance responses (Bargmann, 2006). The identification of the RIC and AWB neurons as key components of the serotonergic fat loss circuit suggests that 5-HT signaling can be fine-tuned based on information from the environment. Interestingly, we find that loss of the octopaminergic ser-6 GPCR decreases tph-1 levels and 5-HT production in the ADF chemosensory neurons, but that exogenous Oct administration does not alter tph-1 expression, suggesting that Oct signaling acts as a permissive, rather than instructive signal to promote 5-HT signaling in the nervous system. The invertebrate Oct and the vertebrate adrenaline have many functional similarities in regulating organismal stress responses and in promoting fat loss, and differ in their chemical structure only by a single hydroxyl group. Recent studies in mice have also shown that brain-derived 5-HT regulates sympathetic tone (Yadav et al., 2009). We contemplate whether the necessity of Oct in promoting 5-HT signaling may underlie the widelynoted potent fat loss effects of the combined adrenergic and serotonergic drugs sibutramine and fen-phen (now off the market), in humans (Cuellar et al., 2000; Fanghanel et al., 2000; Talbot et al., 2010; Wong et al., 2012).
Our neural circuit analyses show that 5-HT synthesis in ADF chemosensory neurons regulates fat loss. Of the neurons in which 5-HT is synthesized, only the ADF chemosensory neurons have ciliated endings that are exposed to the environment. 5-HT production in the ADF chemosensory neurons is regulated by food availability and pathogenic bacteria (Cunningham et al., 2012; Zhang et al., 2005), suggesting that the regulation of 5-HT-driven fat loss may in part, be dictated by these factors. Downstream of the ADF neurons, 5-HT signaling requires the function of the mod-1 5-HT-gated channel in the URX body cavity neurons. The URX(L/R) neuron pair along with the AQR and PQR body cavity neurons, are best-known for their role in oxygen sensing and aerotaxis (Cheung et al., 2005; Gray et al., 2004) and more recently, in body size regulation (Mok et al., 2011). In light of our studies showing a role for the URX neurons in the control of 5-HT-mediated body fat loss and studies indicating a general role for these neurons in controlling body fat stores (our unpublished observations), they likely function in a more important capacity than previously appreciated. We propose that the body cavity neurons function as homeostatic sensors: they integrate sensory information both from external sensory circuits and from internal states, and relay signals to govern behavioral and metabolic outcome. Notably, the body cavity neurons share the unique anatomical feature of having cell bodies and neurites that are exposed to the coelomic fluid that constitutes the circulatory fluid of C. elegans (White, 1986). Because the intestine and other fat tissues are not directly innervated, our demonstration of the role of these neurons in regulating body fat allows for a neuro-anatomical basis for communication between the nervous system and metabolic tissues.
The neural circuitry underlying 5-HT-mediated fat loss suggests that rather than 5-HT itself, undiscovered downstream neuroendocrine signal(s) are relayed to peripheral metabolic tissues to control fat loss. An important step that precedes the discovery of such signaling molecules is the identification of regulatory genes that dictate fat loss in the periphery, in part to provide robust in vivo readouts of metabolic state. To this end, we use RNAi-based screening approaches and identify two regulators of serotonergic fat loss that function in distal metabolic tissues where body fat is metabolized: the conserved triglyceride lipase atgl-1, and the nuclear hormone receptor nhr-76. We show that 5-HT functions as an instructive modulator of body fat: excess 5-HT increases atgl-1 expression which decreases body fat, whereas loss of 5-HT decreases atgl-1 expression and increases body fat. We found that the 5-HT-mediated transcriptional increase of atgl-1 requires the nuclear hormone receptor nhr-76. nhr-76 is also expressed in the intestine, and our data indicate that loss of nhr-76 function abrogates 5-HT-mediated fat loss by preventing atgl-1 up-regulation. nhr-76 bears highest sequence similarity to the mammalian RXR family of nuclear receptors, well-studied for their role as heterodimeric partners in the regulation of fat metabolism (Germain et al., 2006; Mangelsdorf and Evans, 1995). Although we do not yet know whether nhr-76 forms heterodimers with other C. elegans nuclear receptors, we note that nhr-76 has sequence homology to the heterodimerization domain of the RXR receptors (greater than 65% identity). In addition, we identified two putative RXR/nhr-76 binding sites within 1.2 kb of the C. elegans atgl-1 start site. Further studies of regulation of C. elegans atgl-1 by nhr-76 may reveal new and conserved functions in fat metabolism.
Especially interesting is our observation that the inactivation of mod-1 selectively in the URX body cavity neurons prohibits the increased expression of the intestinal atgl-1 lipase and concomitant fat loss during serotonergic activation. This result provides evidence for the existence of undiscovered endocrine signals that originate in the URX neurons in response to neural 5-HT. Such signals are likely relayed via the coelomic fluid to the intestine where body fat is metabolized. Collectively, our findings establish a neuroendocrine axis in which an integrated neural 5-HT and Oct neural circuit regulates body fat via a long-range endocrine signal.
EXPERIMENTAL PROCEDURES
C. elegans culturing and strains used in the study
C. elegans were cultured as described (Brenner, 1974). N2 Bristol obtained from the Caenorhabditis Genetic Center (CGC) was used as the wild-type reference strain. The strains used in the study are: MT9455 tbh-1(n3247)X, VC224 octr-1(ok371)X, RB1631 ser-3(ok2007)I, KQ1048 ser-6(tm2146)IV, RB1690 ser-2(ok2103)X, FX1846 tyra-2(tm1846)X, VC125 tyra-3(ok325)X, SSR857 ser-6(tm2146);tbh-1(n3247), MT15434 tph-1(mg280)II, SSR861 ser-6(tm2146);tph-1(mg280), CX3937 lim-4(ky403)X, MT9668 mod-1(ok103)V, SSR346 mod-1(ok103);ser-6(tm2146), and integrated transgenic strains GR1366 tph-1::GFP, and SSR747 ser-6(tm2146);tph-1::GFP, SSR647 atgl-1::GFP, SSR781 tph-1(mg280);atgl-1::GFP. For all experiments animals were synchronized by hypochlorite treatment after which hatched L1 larvae were plated. All experiments were conducted on Day 1 adults.
RNAi
RNAi experiments were conducted as described (Srinivasan et al., 2008).
5-HT and Oct Treatment
5-HT was administered as described (Srinivasan et al., 2008). For Oct treatment, 0.2M stock Oct solution using 0.1M HCl as a vehicle was added to plates to reach the desired final concentration.
Oil Red O Staining
Oil Red O staining was conducted as described (Yen et al., 2010), with the following changes. Before the fixation step, worms were kept on ice for 10 minutes to stop pharyngeal activity. After the fixation step, worms were taken through three freeze-thaw cycles. The working solution of the Oil Red O stain (40% water:60% Oil Red O in isopropanol) was protected from light on a rotating rack for approximately 1.5–2 hours before use. In each tube 500µl Oil Red O solution was added to a 50µl suspension of fixed worms in 60% isopropanol. For all genotypes and conditions tested, approximately 2000 animals were fixed, stained and visually examined. 20–40 animals were imaged and all experiments were repeated at least three times.
Image Acquisition and Quantitation
Black-and-white and fluorescent images of Oil-Red-O-stained worms were captured using a 10X objective on a Zeiss AxioImager™ microscope. The black-and-white Oil Red O images were quantified using ImageJ software (NIH). In all cases, lipid droplet staining in the first 4 pairs of intestinal cells was quantified. Within each experiment, 10–15 animals from each condition were quantified. All reported results were consistent across biological replicates.
Anti-5-HT immunostaining
Anti-5-HT immunostaining was conducted as described (Zhang et al., 2005).
Cloning and transgenic strain construction
Promoters and genes were cloned using standard PCR techniques from N2 Bristol worm lysates, and cloned using Gateway Technology™ (Life Technologies). Promoter lengths were determined based on functional rescue and are available upon request. All rescue plasmids were generated using either polycistronic GFP or mCherry. Transgenic strains were constructed by microinjection in the C. elegans germline followed by visual selection of transgenic animals under fluorescence. For the microinjections, 10–30 ng/µl of the desired plasmid was injected with 10–20 ng/µl of an unc-122::GFP co-injection marker and 50–80 ng/µl of an empty vector to maintain a total injection mix concentration of 100ng/µl. In each case 10–20 stable transgenic lines were generated. Two lines were selected for experimentation based on consistency of expression and transmission rate.
Statistical Analyses
Data were analyzed for significance using Student’s T-test. Error bars represent Standard Error of the Mean (S.E.M.).
Supplementary Material
Research Highlights.
Integrated 5-HT and Oct signaling from neurons synergize to stimulate body fat loss
Oct, an analog of adrenaline, acts as a permissive cue to maintain 5-HT signaling
A 5-HTergic channel relays an instructive endocrine signal via body cavity neurons
The endocrine signal is relayed to the intestinal lipase ATGL to induce fat loss
ACKNOWLEDGMENTS
This work was supported by research grants to S.S. from the NIH/NIDDK (DK077427 and DK095804). We thank the Knockout Consortium at Tokyo Women’s Medical University and the Caenorhabditis Genetic Center (funded by the NIH P40OD010440), for strains. We thank Drs. Yun Zhang (Harvard University) and Sreekanth Chalasani (The Salk Institute) who provided expert technical advice for the 5-HT immunostaining and behavioral avoidance experiments, respectively. We also thank Drs. Emily Troemel (UCSD), Katja Lamia (The Scripps Research Institute) and members of the Srinivasan Lab for critical comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Arda HE, Taubert S, Macneil LT, Conine CC, Tsuda B, Gilst MV, Sequerra R, Doucette-Stamm L, Yamamoto KR, Walhout AJM. Functional modularity of nuclear hormone receptors in a Caenorhabditis elegans metabolic gene regulatory network. In Molecular Systems Biology. 2010:1–14. doi: 10.1038/msb.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J, Luo Z, Farmer SR, Kandror KV. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res. 2011;52:1693–1701. doi: 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev. 2013 doi: 10.1111/obr.12015. [DOI] [PubMed] [Google Scholar]
- Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cuellar GE, Ruiz AM, Monsalve MC, Berber A. Six-month treatment of obesity with sibutramine 15 mg; a double-blind, placebo-controlled monocenter clinical trial in a Hispanic population. Obes Res. 2000;8:71–82. doi: 10.1038/oby.2000.10. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Hua Z, Srinivasan S, Liu J, Lee BH, Edwards RH, Ashrafi K. AMP-activated kinase links serotonergic signaling to glutamate release for regulation of feeding behavior in C. elegans. Cell Metab. 2012;16:113–121. doi: 10.1016/j.cmet.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanghanel G, Cortinas L, Sanchez-Reyes L, Berber A. A clinical trial of the use of sibutramine for the treatment of patients suffering essential obesity. Int J Obes Relat Metab Disord. 2000;24:144–150. doi: 10.1038/sj.ijo.0801098. [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in endocrinology, diabetes, and obesity. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Greer ER, Perez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008;8:118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, Zhang Y. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron. 2010;68:1173–1186. doi: 10.1016/j.neuron.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, Steven R, Bamber B, Komuniecki RW. Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci. 2009;29:1446–1456. doi: 10.1523/JNEUROSCI.4585-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293:E1736–E1745. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li T, Yang D, Ma R, Moran TH, Smith WW. Synphilin-1 alters metabolic homeostasis in a novel Drosophila obesity model. Int J Obes (Lond) 2012;36:1529–1536. doi: 10.1038/ijo.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire SM, Clark CM, Nunnari J, Pirri JK, Alkema MJ. The C. elegans touch response facilitates escape from predacious fungi. Curr Biol. 2011;21:1326–1330. doi: 10.1016/j.cub.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak HY. Lipid droplets as fat storage organelles in Caenorhabditis elegans: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: from Yeast to Man. J Lipid Res. 2012;53:28–33. doi: 10.1194/jlr.R021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mills H, Wragg R, Hapiak V, Castelletto M, Zahratka J, Harris G, Summers P, Korchnak A, Law W, Bamber B, et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J. 2012;31:667–678. doi: 10.1038/emboj.2011.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok CA, Healey MP, Shekhar T, Leroux MR, Heon E, Zhen M. Mutations in a guanylate cyclase GCY-35/GCY-36 modify Bardet-Biedl syndrome-associated phenotypes in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002335. doi: 10.1371/journal.pgen.1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Bansal A, Kwon ES, Padmanabhan S, Tissenbaum HA. PDP-1 links the TGF-beta and IIS pathways to regulate longevity, development, and metabolism. PLoS Genet. 2011;7:e1001377. doi: 10.1371/journal.pgen.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- O'Rourke EJ, Kuballa P, Xavier R, Ruvkun G. omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CL, Van Gilst MR. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. In Cell Metabolism. 2008:266–274. doi: 10.1016/j.cmet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–475. doi: 10.1038/35044083. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hobert O, Troemel ER, Ruvkun G, Bargmann CI. Alternative olfactory neuron fates are specified by the LIM homeobox gene lim-4. Genes Dev. 1999;13:1794–1806. doi: 10.1101/gad.13.14.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BM, Avery L. Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J Neurosci. 2012;32:1920–1931. doi: 10.1523/JNEUROSCI.2064-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Sadegh L, Elle IC, Christensen AG, Faergeman NJ, Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metabolism. 2008;7:533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 2009;4:16. doi: 10.1186/1749-8104-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Bradley S, Clarke CP, Babalola KO, Philipp AW, Brown G, McMahon AW, Matthews JC. Brain serotonin transporter occupancy by oral sibutramine dosed to steady state: a PET study using (11)C-DASB in healthy humans. Neuropsychopharmacology. 2010;35:741–751. doi: 10.1038/npp.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. 1986;Vol 314(Issue 1165) doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wise SD. Clinical studies with fluoxetine in obesity. Am J Clin Nutr. 1992;55:181S–184S. doi: 10.1093/ajcn/55.1.181s. [DOI] [PubMed] [Google Scholar]
- Wong D, Sullivan K, Heap G. The pharmaceutical market for obesity therapies. Nat Rev Drug Discov. 2012;11:669–670. doi: 10.1038/nrd3830. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu J-H, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. Lrp5 Controls Bone Formation by Inhibiting Serotonin Synthesis in the Duodenum. In Cell. 2008:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K, Le TT, Bansal A, Narasimhan SD, Cheng JX, Tissenbaum HA. A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- Zheng X, Chung S, Tanabe T, Sze JY. Cell-type specific regulation of serotonergic identity by the C. elegans LIM-homeodomain factor LIM-4. Dev Biol. 2005;286:618–628. doi: 10.1016/j.ydbio.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. In Science. 2004:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.