Abstract
Flavonoid glucuronides are the main circulating metabolites of flavonoids in humans and animals. There has been a growing interest in the biological function of glucuronides. In order to differentiate biological activity and to assess efficacy it is essential to accurately determine the levels of flavonoid aglycone and metabolic conjugate in vivo. Many organs and body fluids of humans and animals exhibit β-glucuronidase against flavonoid glucuronides. Studies have shown that β-glucuronidase within the tissues hydrolyzes glucuronides to their aglycones during the tissue extraction, leading to artificially higher reported tissue levels of aglycone than actual in vivo concentrations. The aims of this study were to estimate the extent by which the aglycones were overestimated and to investigate the use of saccharo-1,4-lactone, a β-glucuronidase inhibitor, to block the ex vivo hydrolysis of flavonoid glucuronides. Our data demonstrate that in mouse liver tissues and human tumor xenografts levels of quercetin and methylated quercetin aglycones could be over-estimated by 7 fold. The inhibition of deconjugation of quercetin and baicalein glucuronides by saccharo-1,4-lactone is dose-dependent. The amount of saccharo-1,4-lactone used to produce optimal inhibition of the enzyme activity is in the range of 15 – 24 μmol per gram of liver tissue. The use of β-glucuronidase inhibitor blocks the ex vivo deconjugation resulting in an accurate estimation of tissue levels of aglycone and conjugate. Our study described here can be extended to other animal models and human studies with different types of substrates of β-glucuronidase.
Keywords: quercetin; baicalin; glucuronide conjugates; β-glucuronidase inhibitor; D-saccharic acid 1,4-lactone; in vivo
1. Introduction
Flavonoids are the most commonly distributed group of plant polyphenolic compounds. Important dietary sources of flavonoids are fruit, vegetables and other plant-derived products. Due to their multiple health benefits flavonoids have been the subject of increasing research interest [1–3]. Human intervention studies have demonstrated that flavonoid glycosides present in food are extensively altered during first-pass hepatic metabolism so that after oral administration and absorption, the resulting metabolites that reach the blood and tissues are conjugates (glucuronides and sulfates) of the flavonoids and methylated flavonoids. These conjugates are chemically different from their aglycones in regard to the size, solubility and polarity. Therefore, their physiological behavior could be different from that of aglycones. Whereas glucuronide conjugates are the most important class of Phase II xenobiotic metabolites and act in a detoxifying role, aglycones have long been considered as the more biologically active forms [4,5]. For example, quercetin is shown to be a powerful antioxidant in vitro but the antioxidant activity of quercetin conjugates is only about half that of aglycone [6,7]. Recent studies, however, show that conjugation does not always decrease the biological activities, and that glucuronide conjugates can be potent precursors of the biologically active aglycones [8–10]. In order to differentiate biological activity and assess in vivo efficacy on various diseases, it is essential to accurately quantify the level of flavonoid aglycones and metabolic conjugates in vivo.
Because of the multiple and complex glucuronide and sulfate conjugates formed in vivo and the lack of chemical standards, the total amount of glucuronide and sulfate conjugates in tissue and plasma is often measured as the amount of total aglycones detected after enzymatic treatment subtract the free aglycones measured in the absence of exogenously added enzymes (β-glucuronidase and sulfatase). Nevertheless, β-glucuronidase, an acid hydrolase enzyme, is present in many organs and body fluids of humans and animals. β-glucuronidase is localized mainly in lysosomes in humans and in both the endoplasmic reticulum and lysosomes in rodents [11], and is remarkably stable at high temperature (up to 55°C) and at a wide range of pH (4–11) [12]. It has been reported that quercetin 3-O-β-glucuronide (Q3G, Fig. 1) added to rat and pig tissues converts to quercetin aglycone during extraction [13]. Cell free extracts from human small intestine, liver and neutrophils were shown to deconjugate a mixture of five quercetin glucuronides by the endogeneous β-glucuronidase. The deconjugation activity can be completely inhibited by D-saccharic acid 1,4-lactone, a specific β-glucuronidase inhibitor [14].
Figure 1.

Chemical structure of quercetin (A), isorhamnetin (B), quercetin 3-O-β-D-glucuronide (C), baicalein (D), oroxylin A (E), baicalein-7-O- β-D-glucuronide (F) and D-saccharic acid 1,4-lactone (G).
During the course of our studies on the distribution of quercetin and baicalin (5, 6, 7-trihydetroxyflavone-7-β-D-glucuronide, or baicalein-7-O-glucuronide, BG, Fig. 1) and their metabolites in mouse tissues and human tumor xenografts, we found a large percentage of aglycones present in various organ tissues and tumors but a limited amount present in plasma [15,16]. These data are consistent with literature on the flavonoid aglycones found in tissues from humans, pigs and rats administered orally with quercetin or baicalin diet [13,17]. In addition, we found that about 28% of BG added to the liver homogenate converted to its aglycone form immediately after its addition while 60 % was deconjugated after 30 min incubation. These experimental data and studies provided clear evidence that endogenous β-glucuronidase in the tissues of humans and animals (pig, rat and mouse) hydrolyzes flavonoid glucuronides to their aglycones during the tissue extraction, leading to the artificially higher reported tissue levels of aglycones (or lower levels of glucuronides) than the actual in vivo concentrations. The aims of this study were to estimate the extent by which the aglycones were overestimated and to investigate the use of D-saccharic acid 1, 4-lactone (SL, Figure 1) to block the ex vivo hydrolysis of flavonoid glucuronides.
2. Materials and Methods
2.1. Materials
Quercetin (>98%), isorhamnetin (>95.0%) baicalein (>98%), β-glucuronidase (from E. coli, recombinant) and β-glucuronidase/sulfatase (from Helix pomatia) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Baicalein-7-O-glucuronide (80%) and quercetin 3-O-β-D-glucuronide were purchased from Chromadex (Irvine, CA, USA). Oroxylin A (99%) was purchased from Nanjing Selang Medical Technology Co. Ltd., China. D-saccharic acid 1,4-lactone (SL, 98% purity) was purchased from MP Biomedicals (Solon, OH, USA). Internal standard 3, 3′, 4′-trihydroxyflavone was purchased from Indofine (Hillsborough, NJ, USA). All solvents used were of HPLC grade (Fisher Scientific, Fair Lawn, NJ, USA).
2.2. Animal tissues
The pancreatic xenograft mouse model fed with standard diet (AIN-93G, Dyets, Bethlehem, PA, USA) and standard diet supplemented with 1% or 5% quercetin, or 1% Scutellaria baicalensis Georgi (SB) extract containing 20.6% (wt/wt) baicalin has been reported previously [18,19]. In both studies, orthotopic tumor xenografts in nude mice (Charles River Laboratories, San Diego, CA, USA) were established by implantation of a small piece of subcutaneously grown xenografts of human pancreatic cancer cells (MiaPaCa-2). All animal studies were approved by the Chancellor’s Animal Research Committee of the University of California, Los Angeles. Unused tissues and tumors from these studies were stored in a −78°C freezer and used in this study.
2.3. Inhibition of ex vivo deglucuronidation of Q3G and BG by SL in tissue homogenates
Stock solution of SL (100 or 200 mM) was prepared in Milli Q H2O. Known concentrations of SL in homogenizing buffer (200μl, 50 mM potassium phosphate, pH 7.4) containing 1% ascorbic acid were added to 0.05 g frozen mouse liver. All tissues were homogenized in ice-water using a tissue grinder. Glucuronide (Q3G or BG, 46 nmol/g tissue) was then added, vortex mixed and the resulting homogenate was transferred to a clean tube. The tissue grinder was quickly rinsed with 100μl of buffer which was combined with homogenate. The homogenate was then incubated in a 37°C water bath for 30 min, followed by the addition of 600 μl of acetone (for quercetin metabolites) or MeOH (for baicalin metabolites) and internal standard (IS). The resulting mixture was vortexed for 2 min and then centrifuged at 13,500×g for 10 min. Supernatant was separated and the residue was extracted one more time with the same amount of solvent. The combined supernatant was dried completely in a SpeedVac at room temperature and the residue was reconstituted in 200 μl of acetone/water or methanol/water (7:3) and analyzed by HPLC as indicated below.
2.4. Inhibition of ex vivo deglucuronidation by SL in liver tissues and tumors from mouse administered with quercetin and SB
To the liver tissues and tumor samples, an aliquot of known concentration of SL mixed with 600 μl of buffer (pH 7.4) was added to 0.15 g of frozen tissue which was homogenized in a tissue grinder. The combined homogenate and rinse (300 μl buffer) was then transferred to a tube and divided equally into 3 parts by weight. For tumor samples, 0.05 g frozen tissue was homogenized in 200 μl buffer. Sample was added IS and then extracted with 800 μl of MeOH or acetone twice. Products were extracted and analyzed by HPLC as described above.
2.5. Analysis of flavonoids and glucuronides by high-pressure liquid chromatography (HPLC)
Quantification of flavonoids and glucuronide conjugates was performed with a Luna C18 column (150 × 4.6 mm, 3 μm, Phenomenex, Torrance, CA, USA) on an Agilent 1100 HPLC system (Santa Clara, CA, USA) comprised of an autosampler and a quaternary pump coupled to a photodiode array detector. The mobile phase consisted of a binary gradient of 0.1% (v/v) ortho-phosphoric acid in water (eluent A) and acetonitrile (eluent B) as previously described [16,18]. Quercetin and metabolites were detected by UV absorbance at a wavelength of 258 nm and baicalin and metabolites were detected at 278 nm. Data were analyzed with Hewlett Packard Chemstation® software. Concentrations were determined by calculating the peak area ratio of the glucuronide to the IS. The specificity was evaluated by analyzing the chromatograms of blank liver samples from mice for possible interferences at the retention time of Q3G, quercetin, BG, baicalien and the IS. The limit of detection in tissues was defined as the lowest concentration resulting in a signal-to-noise ratio of 3:1. Quality control (QC) was performed by daily injection of standards and by analyzing tissue samples spiked with three concentrations of quercetin for accuracy, precision and recovery. The intra-day and inter-day precision and accuracy were determined by replicative analysis of three QC samples at concentrations of 2.0, 10.0 and 25.0 nmol/g liver for quercetin on the same day and on four consecutive validation days, respectively. The extraction recovery was determined by comparing the ratio of the analyte peak areas of the extracted QC samples with the standard solutions of the same concentration.
2.6. Statistical analysis
Descriptive statistics, such as mean and SD, were used to summarize the results. Data were analyzed by paired student t-test. Statistical significance was defined by a p-value of 0.05.
3. Results
3.1. Ex vivo deglucuronidation of Q3G and BG by the endogenous β-glucuronidase
We first examined ex vivo deconjugation of Q3G in the suspension of blank mouse liver. Q3G at a concentration of 46 nmol/g tissue was added to liver tissues. Samples were collected every 10 min at 0, 10, 20 and 30 time points after incubation. Q3G and its deconjugation products were extracted and analyzed by HPLC immediately. At 10 min, less than 15% of the Q3G was detected. Q3G was completely degraded within 20 min. At the same time quercetin and detectable amounts of methylated quercetin (isorhamnetin) and quercetin sulfate were found. These data demonstrated the ability of liver tissue to deconjugate quercetin glucuronide under ex vivo condition. We also added the same concentration of Q3G into the Sprague Dawley rat liver and found that the degradation rate of Q3G in the rat liver was similar (data not shown).
3.2. Dose effect of SL on the deglucuronidation of Q3G and BG
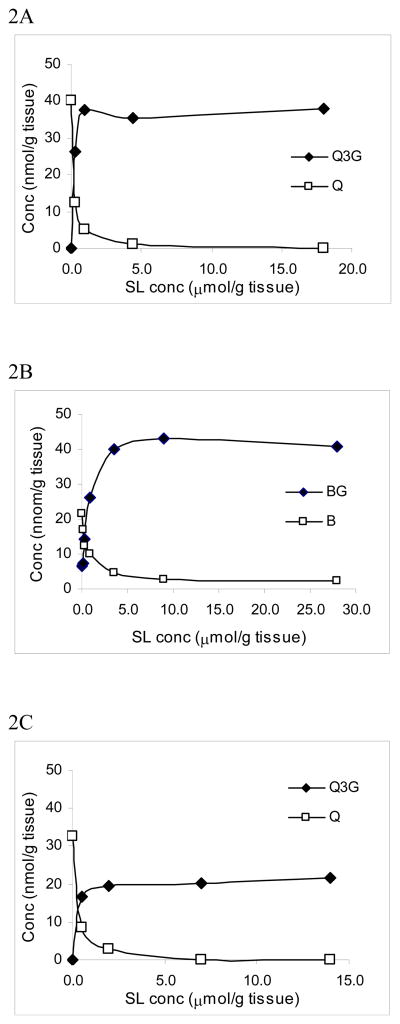
We then evaluated the effect of increasing concentration of SL on the deconjugation of Q3G and BG. A known amount of SL was added into blank liver, followed by the addition of 46 nmol/g tissue of Q3G or BG. As shown in Fig, 2A–B, there is a concentration-dependent decrease in the deconjugation of Q3G (Fig. 2A) and BG (Fig. 2B) with an increase in SL. Q3G deconjugated more rapidly than BG but the initial degradation of BG (in absence of SL) was fast. The deconjugation of Q3G and BG by the endogenous β-glucuronidase was blocked completely when SL concentration reached 4 μmol/g tissue for Q3G and 8 μmol/g tissue for BG. Incubation of liver homogenates containing the same amount of Q3G at pH 5.0 resulted in a decrease in quercetin formation similarly as compared to pH 7.4, but less Q3G was recovered (Fig. 2C), indicating that Q3G converted to products other than quercetin at the specified condition.
Figure 2.
SL dose-Q3G (A) and BG (B) deconjugation curve in liver homogenates at pH 7.4. SL at indicated amount was added to the homogenates of mouse liver followed by the addition of a known concentration of Q3G or BG (46 nmol/g tissue). The mixture was incubated at 37°C for 30 min. SL dose-Q3G (C) deconjugation curve in liver homogenates at pH 5.0 with 30 min. incubation. The reactions were stopped by the addition of acetone or methanol, followed by the addition of IS and extraction as described in the section on Materials and Methods.
3.3. Inhibition of the deconjugation of quercetin glucuronides in tissues of mice administered with diets supplemented with quercetin
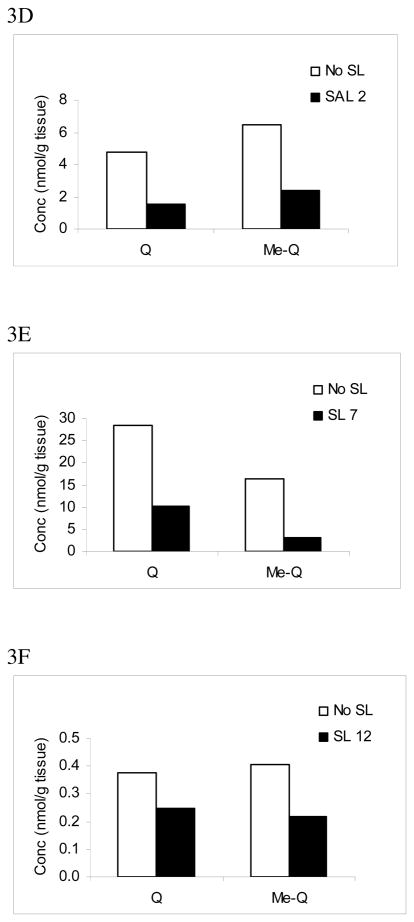
Next, we tested different amounts of SL added to the liver of mice fed with a quercetin diet. Fig. 3 shows a concentration-dependant decrease in levels of quercetin and methylated quercetin aglycones deconjugated from glucuronides with increasing SL. After adding 1 (Fig, 3A), 4 (Fig, 3B) and 15 (Fig. 3C) μmol/g of SL to liver tissue, quercetin and methyl-quercetin were found to be 36% and 50%, 28% and 33%, and 13% and 16% of their aglycones measured in the absence of SL, respectively. The optimal inhibition of β-glucuronidase activity was observed when 15 μmol/g of SL was added. At this concentration, quercetin level was 0.40 nmol/g, a 7.7-fold decrease from 3.04 nmol/g measured without adding SL. We found that SL blocked the deconjugation of quercetin glucuronides from lung tissue similarly (data not shown).
Figure 3.
Comparison of quercetin and methylated quercetin levels in liver (A–C) and tumors (D–F) from mice administered with a diet containing quercetin in absence or presence of SL. Samples were homogenized, extracted and analyzed by HPLC as described in the section on Material and Methods. Values are means SD of three measurements for liver tissues (*, p < 0.05; **, p < 0.01). Q: quercetin; Me-Q: methylated quercetin; SL (μmol/g tissue): D-saccharic acid 1,4-lactone.
In tumor samples, we tested SL concentrations at 2 (Fig. 3D), 7 (Fig. 3E), 12 (Fig. 3F) μmol/g tumor. Optimal inhibition of β-glucuronidase activity was observed when 7 μmol/g of SL (equivalent to 1.17 mM) was added, in which the deconjugation of quercetin and methyl quercetin glucuronides was reduced by 65% and 80%, respectively. The highest concentration of SL at 12 μmol/g resulted in less inhibition (34% for quercetin and 44% for methylated quercetin). Of note, because of the limited availability of tumor samples, we were not able to determine our data in triplicate.
3.4. Inhibition of deconjugation of BG in tissues of mice administered with diet supplemented with SB extract
We also tested different amounts of SL added to the liver of mice fed with a diet containing BG. Fig. 4A presents a concentration-dependent decrease in levels of baicalein and methylated baicalein (oroxylin A) aglycones in mouse liver samples. SL at 24 μmol/g (or 4.0 mM) produced the maximum inhibition of the deconjugation of baicalin by 20-fold and methylated BG by 2.7-fold. In tumor samples, the concentration of SL at 120 μmol/g produced maximal effect on the inhibition of deconjugation of BG but higher concentration of SL (240 μmol/g) was necessary to further inhibit the deglucuronidation of methylated BG (Fig. 4B).
Figure 4.
Comparison of baicalein and methylated baicalein levels in liver (A) and tumors (B) from mice administered with a diet containing BG in absence or presence of SL. Samples were homogenized, extracted and analyzed by HPLC as described in section on Material and Methods. Values are means SD of three measurements for liver tissues (*, p < 0.05; **, p < 0.01). B: baicalein; Me-B: methylated baicalein; SL (μmol/g tissue): D-saccharic acid 1,4-lactone.
3.5. Method Validation
The HPLC method was evaluated through intra-day and inter-day analysis for precision and accuracy. The accuracy and precision of the method were assessed by determining quality control samples using 4 replicated preparations of blank liver samples at three concentration levels (0.2, 2 and 8 μg/mL) of quercetin. The accuracy of this method is 97.5, 102.2 and 107.5% at low, intermediate and high concentration, respectively. The precision indicates that all coefficients of variation (CVs) were below 10.0. Table 1 summarizes the intra- and inter-day precision and accuracy for quercetin evaluated by assaying the quality control samples of mouse liver as a representative example. These results demonstrated that the values were within the acceptable range and the method was accurate and precise.
Table 1.
Summary of precision and accuracy from quality control sample of mouse liver extract of quercetin (n=4 days, 4 replicates)
| Spiked (nmol/g tissue) | Measured (nmol/g tissue) | Accuracy (%) | Precision (%) | |
|---|---|---|---|---|
| Inter-run | Intra-run | |||
| 2.00 | 1.95 | 97.5 | 4.1 | 1.9 |
| 10.0 | 10.22 | 102.2 | 2.6 | 1.0 |
| 25.0 | 26.87 | 107.5 | 1.9 | 2.2 |
4. Discussion
Flavonoid glucuronides and sulfates are the main circulating metabolites of flavonoids in human and animals after ingestion of a flavonoid rich diet. Quercetin aglycone found in the plasma of human and animals constitutes a very small portion of the total amount of quercetin (aglycones + conjugates) present [4,18,20]. Tissues are exposed to flavonoids via the blood, which is the only route through which dietary flavonoids can reach tissues and their cells except the cells lining the intestinal tract. Therefore, it is conceivable that aglycones should exist in small amount as well in these tissues. Several studies have reported high proportions of aglycones from organ tissues in various animal models [13,15,20,21]. These levels of aglycones were likely overestimated, which can be attributed to the presence of endogenous β-glucuronidase that deconjugates the glucuronide metabolites during sample preparation. We reported earlier that BG added to homogenates of several organ tissues (small intestine, colon, liver, lung, kidney, prostate and pancreas) and tumors of mice converted to its aglycone rapidly under ex vivo condition. However, the lung and kidney tissues from treated mice had the highest portion of aglycones (91% and 90% of the total concentration, respectively), but blank tissue suspensions with added BG converted only 16% (lung) and 24% (kidney). These data are in agreement with the published data on quercetin by Bieger and colleagues who found no correlation between the proportion of aglycones and the specific β-glucuronidase activity in the various tissues [20].
SL is the most commonly used in vitro β-glucuronidase inhibitor. It has been used frequently to inhibit β-glucuronidase for estimating the glucuronide formation rates by the uridine diphosphoglucuronosyl tranferases (UGTs) [22,23], and, to a lesser extent, to block the β-glucuronidase activity in quantifying tissue flavonoids and their conjugates. In this study, we have demonstrated that the inhibition of β-glucuronidase activity by SL is dose-dependent in liver and tumor samples. SL at concentrations ranging from 15 to 24 μmol/g tissue (equivalent to 2.5 to 4.0 mM) completely inhibited the deglucuronidation of selected flavonoids in mouse liver. These concentrations are generally in line with published data where 1 – 4 mM concentration of SL was used to inhibit endogenous β-glucuronidase activity [9,14,24]. Higher than optimal concentration of SL may result in reduced inhibition. For different flavonoid glucuronides, the amount of SL needed to produce optimum inhibition varies. We and others [17] also show that the conversion of glucuronides is pH- and temperature-dependent. Therefore, acidic conditions and incubation should be avoided when enzymatic treatment is not carried out.
O’Leary and colleagues reported that levels of the aglycone within tissues depend on the substrate specificity of the deconjugating enzyme β-glucuronidase towards circulating flavonoid glucuronides [14]. Some studies show that although deconjugation can potentially occur in vivo to produce aglycones, it occurs only at certain sites [4,25]. β-glucuronidase activity is high in liver and is increased in certain sites or under physiological conditions, such as inflammation [26], carcinogenic tissue [21] and tumor [27,28]. Yuan et al. reported that β-glucuronidase activity in tumor xenografts is approximately 8 times higher than that of liver [29]. Our current study demonstrated that higher concentrations of SL may be needed in tumor samples in order to achieve the optimal inhibition of deconjugation. Because of the large individual variation in activity and expression of β-glucuronidase in tissues and blood [13,14,30], the heterogeneity of the enzyme activity expressed in tumors [31] and the difference in catalytic efficiency of β-glucuronidase toward structurally dissimilar flavonoid glucuronides, the optimal dose of SL needs to be tested in different tissues.
5. Conclusion
Taken together, our results show that mouse liver and xenograft tumors exhibited β-glucuronidase activity. Levels of quercetin aglycone can be overestimated as much as 7-fold in liver tissues. This occurred during the extraction of flavonoids and metabolic conjugates from tissues expressing β-glucuronidase enzyme that hydrolyzes glucuronides to their parent aglycones. The use of β-glucuronidase inhibitor SL blocks the ex vivo deglucuronidation resulting in an accurate quantification of tissue aglycones and glucuronide conjugates. In view of the extent of ex vivo deconjugation, routine inclusion of SL may be necessary. Additional data from other types of glucuronide substrates in different animal models and in humans are warranted but our study described here can be extended to these studies. Clarifying the mechanism of action in vivo exerted by specific flavonoids or their structurally modified conjugates will advance our understanding of diet- and plant-derived flavonoids in relation to chemoprevention and other disease prevention.
Highlights.
Many organs of animals exhibit β-glucuronidase against flavonoid glucuronides.
Quercetin in mouse liver was overestimated by 7 fold due to tissue β-glucuronidase.
Saccharo-1,4-lactone inhibits the ex vivo deconjugation dose-dependently.
Saccharo-1,4-lactone used is in the range of 15 to 24 μmol/g liver tissue.
Acknowledgments
This work was supported by the National Institutes of Health (P01AT003960) and the Hirshberg Foundation for Pancreatic Cancer Research. We thank Ms. Jennifer B. Carney for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. 2009;15:1072–1084. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as Promising Lead Compounds in Type 2 Diabetes Mellitus: Molecules of Interest and Structure-Activity Relationship. Curr Med Chem. 2011 doi: 10.2174/092986711795933777. [DOI] [PubMed] [Google Scholar]
- 4.Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Kawai Y, Saito S, Nishikawa T, Ishisaka A, Murota K, Terao J. Different profiles of quercetin metabolites in rat plasma: comparison of two administration methods. Biosci Biotechnol Biochem. 2009;73:517–523. doi: 10.1271/bbb.80516. [DOI] [PubMed] [Google Scholar]
- 6.Rice-Evans C, Miller N. Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukot Essent Fatty Acids. 1997;57:499–505. doi: 10.1016/s0952-3278(97)90435-x. [DOI] [PubMed] [Google Scholar]
- 7.Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29:1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 8.Beekmann K, Actis-Goretta L, Van Bladeren PJ, Dionisi F, Destaillats F, Rietjens IM. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012;3:1008–1018. doi: 10.1039/c2fo30065f. [DOI] [PubMed] [Google Scholar]
- 9.Galindo P, Rodriguez-Gomez I, Gonzalez-Manzano S, Duenas M, Jimenez R, Menendez C, Vargas F, Tamargo J, Santos-Buelga C, Perez-Vizcaino F, Duarte J. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS One. 2012;7:e32673. doi: 10.1371/journal.pone.0032673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terao J, Murota K, Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- 11.Sperker B, Backman JT, Kroemer HK. The role of beta-glucuronidase in drug disposition and drug targeting in humans. Clin Pharmacokinet. 1997;33:18–31. doi: 10.2165/00003088-199733010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lin CW, Orcutt ML, Fishman WH. Purification and characterization of mouse kidney beta-glucuronidase. J Biol Chem. 1975;250:4737–4743. [PubMed] [Google Scholar]
- 13.de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary KA, Day AJ, Needs PW, Sly WS, O’Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–106. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Angst E, Park JL, Moro A, Dawson DW, Reber HA, Eibl G, Hines OJ, Go VL, Lu QY. Quercetin aglycone is bioavailable in murine pancreas and pancreatic xenografts. J Agric Food Chem. 2010;58:7252–7257. doi: 10.1021/jf101192k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu QY, Zhang L, Moro A, Chen MC, Harris DM, Eibl G, Go VL. Detection of baicalin metabolites baicalein and oroxylin-a in mouse pancreas and pancreatic xenografts. Pancreas. 2012;41:571–576. doi: 10.1097/MPA.0b013e318232e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing J, Chen X, Zhong D. Stability of baicalin in biological fluids in vitro. J Pharm Biomed Anal. 2005;39:593–600. doi: 10.1016/j.jpba.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Angst E, Park JL, Moro A, Dawson DW, Reber HA, Eibl G, Hines OJ, Go VL, Lu QY. Quercetin aglycone is bioavailable in murine pancreas and pancreatic xenografts. J Agric Food Chem. 2010;58:7252–7257. doi: 10.1021/jf101192k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu QY, Zhang L, Moro A, Chen MC, Harris DM, Eibl G, Go VL. Detection of baicalin metabolites baicalein and oroxylin-a in mouse pancreas and pancreatic xenografts. Pancreas. 2012;41:571–576. doi: 10.1097/MPA.0b013e318232e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieger J, Cermak R, Blank R, de Boer VC, Hollman PC, Kamphues J, Wolffram S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J Nutr. 2008;138:1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- 21.Oi N, Hashimoto T, Kanazawa K. Metabolic conversion of dietary quercetin from its conjugate to active aglycone following the induction of hepatocarcinogenesis in fisher 344 rats. J Agric Food Chem. 2008;56:577–583. doi: 10.1021/jf072556c. [DOI] [PubMed] [Google Scholar]
- 22.Brunelle FM, Verbeeck RK. Glucuronidation of Diflunisal by Rat-Liver Microsomes - Effect of Microsomal Beta-Glucuronidase Activity. Biochemical Pharmacology. 1993;46:1953–1958. doi: 10.1016/0006-2952(93)90636-b. [DOI] [PubMed] [Google Scholar]
- 23.Oleson L, Court MH. Effect of the beta-glucuronidase inhibitor saccharolactone on glucuronidation by human tissue microsomes and recombinant UDP-glucuronosyltransferases. Journal of Pharmacy and Pharmacology. 2008;60:1175–1182. doi: 10.1211/jpp.60.9.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartholome R, Haenen G, Hollman CH, Bast A, Dagnelie PC, Roos D, Keijer J, Kroon PA, Needs PW, Arts IC. Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by beta-glucuronidase from neutrophils. Drug Metab Pharmacokinet. 2010;25:379–387. doi: 10.2133/dmpk.dmpk-10-rg-002. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 26.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–272. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 27.Yuan L, Wagatsuma C, Yoshida M, Miura T, Mukoda T, Fujii H, Sun B, Kim JH, Surh YJ. Inhibition of human breast cancer growth by GCP (genistein combined polysaccharide) in xenogeneic athymic mice: involvement of genistein biotransformation by beta-glucuronidase from tumor tissues. Mutat Res. 2003;523–524:55–62. doi: 10.1016/s0027-5107(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 28.Yue H, Yang B, Zhang H, Zhu SD, Du XJ, Feng XL, Yu Z, Xia YT, Yu JP. Clinical significance of TGF- beta1 and beta-glucuronidase synchronous detection in human pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2002;1:309–311. [PubMed] [Google Scholar]
- 29.Yuan L, Wagatsuma C, Sun B, Kim JH, Surh YJ. The role of beta-glucuronidase in induction of apoptosis by genistein combined polysaccharide (GCP) in xenogeneic mice bearing human mammary cancer cells. Ann N Y Acad Sci. 2003;1010:347–349. doi: 10.1196/annals.1299.063. [DOI] [PubMed] [Google Scholar]
- 30.Bracey LT, Paigen K. Changes in translational yield regulate tissue-specific expression of beta-glucuronidase. Proc Natl Acad Sci U S A. 1987;84:9020–9024. doi: 10.1073/pnas.84.24.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher U, Adam E, Zangemeister-Wittke U, Gossrau R. Histochemistry of therapeutically relevant enzymes in human tumours transplanted into severe combined immunodeficient (SCID) mice: nitric oxide synthase-associated diaphorase, beta-D-glucuronidase and non-specific alkaline phosphatase. Acta Histochem. 1996;98:381–387. doi: 10.1016/s0065-1281(96)80004-3. [DOI] [PubMed] [Google Scholar]