Summary
DR3 (TNFRSF25) is a member of the tumor necrosis factor receptor (TNFR) superfamily expressed primarily on lymphocytes and is a receptor for the TNF family cytokine TL1A (TNFSF15). DR3 costimulates T cell activation, but it is unique among these receptors in that it signals through an intracytoplasmic death domain and the adapter protein TRADD. TL1A costimulates T cells to produce a wide variety of cytokines and can promote expansion of activated and regulatory T cells in vivo. Studies in mice deficient in DR3 or TL1A or in animals treated with antibodies that block the activity of TL1A have revealed a specific role for DR3 in enhancing effector T cell proliferation at the site of tissue inflammation in autoimmune disease models. DR3 appears to be required in autoimmune disease models dependent on a variety of different T cell subsets and also invariant natural killer T (iNKT) cells. Chronic expression of TL1A induces a distinct Interleukin-13 dependent pathology in the small intestine marked by goblet cell hyperplasia and other features associated with allergic and anti-parasitic responses. These studies suggest that TL1A may be a viable target for therapies designed to inhibit the T-cell dependent component of diverse autoimmune diseases.
Keywords: TL1A, DR3, TNF-family cytokines, autoimmune disease, inflammatory bowel disease, allergic disease, asthma
A large subset of TNF family receptors including DR3, HVEM, OX40, 4-1BB, TNFR2 and CD27 function by costimulating T cells to promote more efficient immune responses (1, 2). DR3 (TNFRSF25), a TNFR family receptor expressed primarily in lymphocytes, is the closest homolog of TNFR1 in the human and mouse genomes and is unique among these receptors in having an intra-cytoplasmic ‘death domain’. DR3 signals through the adapter protein TRADD, which in turn recruits TRAF proteins, resulting in signaling more like the other T cell costimulators which activate NF-κB and MAPK signaling. Despite these similarities in signaling, experiments in mouse models have shown that DR3 plays a unique role in immune responses, acting later during T cell activation and expansion than the other members of the TNF-receptor superfamily. The ligand for DR3 is the TNF family member TL1A (TNFSF15), whose expression is induced by pro-inflammatory stimuli. TL1A-DR3 interactions promote effector T cell proliferation at the site of inflammation and in draining lymph nodes. Blockade of TL1A-DR3 interactions strikingly reduces pathology in a number of animal models in which autoreactive T cells play a role. However, TL1A blockade or genetic deficiency of TL1A or DR3 has remarkably little effect on primary or secondary systemic T cell response to immunogens. The highly restricted and transient expression of TL1A in response to inflammatory stimuli likely limits the role of DR3 in vivo to the inflammatory environment in autoimmune or infected tissue.
Characterization of the TNF receptor DR3 and its ligand TL1A
DR3 was initially cloned by a number of research groups through homology with TNFR1, the prototypical member of the TNFR superfamily, and initially was given a number of other names (LARD, TRAMP, APO-3, WSL-1) (3–7). It remains the receptor with the highest homology to TNFR1 in the mouse and human genomes. Like TNFR1, DR3 has four cysteine-repeat domains (CRD) in its extracellular domain, and although the crystal structure of DR3 has not been solved, structural modeling predicts a similar structure to TNFR1 in which primary contacts with its ligand TL1A are in the 2nd and 3rd CRD (8). DR3, like TNFR1, contains a death domain that signals through the adapter protein TRADD (4, 9). TRADD contains a TRAF-biding motif in its N-terminal region that allows recruitment of a complex containing TRAF2 and RIP, which coordinately activate NF-κB and MAP kinase signaling. Apoptosis is only triggered by DR3 when NF-κB is inhibited (10). Presumably, as has been found for TNFR1, under NF-κB conditions, TRADD-containing complexes initially assembled at the DR3 death domain recruit FADD and Caspase-8 into a secondary death-inducing signaling complex (11). Despite these similarities in signaling between DR3 and other TNF-family receptors, as will be discussed below, studies in animal models have revealed a unique role for TL1A-DR3 interactions in effector T cell expansion and regulatory T cell (Treg) homeostasis, as well as in allergic immune responses in the lung and gastrointestinal tract. This specificity is likely achieved because of highly restricted expression of TL1A and a unique propensity of TL1A signaling to enhance IL-13-dependent immune responses in the mucosal environment.
TL1A was identified as the TNF-family ligand for DR3 in 2002 (12). TL1A structurally resembles other TNF family members (13, 14), and like most other TNF-family cytokines, it is initially expressed as a type II transmembrane protein and can be subsequently cleaved by metalloproteinases to form a soluble trimer. TL1A expression is highly regulated, being virtually undetectable in normal tissues. TL1A expression can be induced through stimulation with Toll-like Receptor (TLR) ligands such as Lipopolysaccharide (LPS) and Fc Receptor crosslinking in macrophages and dendritic cells, as well as other inflammatory cytokines such as IL-1 and TNF in endothelial cells (12, 15, 16). Induction of TL1A by these stimuli is highly transient, usually declining to baseline within 1 day of stimulation, perhaps because of multiple AU rich elements present in the TL1A mRNA 3′ untranslated region that can contribute to instability. TL1A can also be expressed in a slower and more sustained manner by T cells after TCR stimulation, possibly stabilized by autocrine stimuli through DR3 (16).
TL1A-DR3 interactions co-stimulate lymphocyte activation and cytokine production
Like other members of the TNF superfamily, TL1A enhances T cell proliferation and cytokine production of T cells activated in vitro. Proliferation is particularly enhanced in cells sub-optimally activated in the absence of CD28, and this effect is more pronounced in memory vs. naïve T cells, perhaps because of greater expression of the full-length isoform of DR3 in memory T cells (16, 17). TL1A also upregulates CD25 and CD122 (IL-2Rα and IL-2Rβ, respectively), further enhancing responsiveness to IL-2 (12). Costimulation by TL1A or agonistic anti-DR3 antibodies enhances production of a wide array of cyokines by both naïve and total T cells, including IL-2, IL-4, IL-13 and Interferon-gamma (IFNγ) (12, 16, 18–20). Although TL1A can be produced by T cells, DR3-deficient CD4+ T cells do not show a proliferation defect, implying that autocrine production of TL1A is not sufficient for T cell costimulation (16, 21). Dendritic cells are more likely the source of costimulatory TL1A, as antigen-specific DR3-deficient T cells exhibit reduced proliferation and production of IL-4 and IL-2 when activated by dendritic cells and antigen. Despite the ability of exogenous TL1A to enhance IFNγ production, secretion of IFNγ was not significantly reduced when DR3-deficient T cells were activated by antigen and antigen-presenting cells (APC) (16), indicating that the non-redundant functions of DR3 are cytokine-selective.
Less is known about the function of DR3 on CD8+ T cells. It has recently been shown that addition of soluble TL1A costimulates CD8+ T cell proliferation and IL-2 production, and administration of TL1A after antigenic peptide enhanced expansion of ovalbumin-specific TCR transgenic CD8+ T cells (OT-I cells) to levels comparable to those induced by strong stimulators of CD8+ T cell proliferation, such as polyinosinic:polycytidylic acid (poly I:C) (22). This finding may have relevance for anti-tumor responses, as TL1A-expressing tumor cells were promptly rejected in a manner dependent on CD8+ T cells (22). The non-redundant role of endogenous TL1A-DR3 interactions in CD8+ T cell responses is less clear. DR3-deficient mice mount normal responses and can contain Toxoplasma infections, a function partially dependent on CD8+ T cells, and we have observed normal primary and secondary CD8+ T cell responses to systemic influenza infection (Meylan, F, and RM Siegel, unpublished observations).
DR3 is also expressed on other types of lymphocytes besides T cells, and the effects TL1A on these cell types has recently begun to be investigated. TL1A can synergize with IL-12 and IL-18 to promote IFNγ production and cytotoxicity by natural killer (NK) cells (23, 24). DR3 is also highly expressed on NKT cells where, unlike in T cells, TL1A appears to promote a more restricted set of cytokines, enhancing IL-4 and IL-13 but not IFNγ production (19). The role of DR3 on B cells and other more recently described innate lymphocyte subsets that are specialized to secrete large quantities of cytokines without TCR-mediated recognition of antigen/MHC complexes is not known.
Effects of TL1A-DR3 interactions on T cell polarization and homeostasis
TL1A can also influence CD4+ T cell differentiation, although some of these effects can be attributed to indirect effects of the cytokines produced in response to TL1A. For example, exogenous TL1A suppresses the ability of naïve murine and human CD4+ T cells to differentiate into T-helper 17 (Th17) T cells that are the primary T cells producing IL-17 (20, 25). Th17 differentiation is known to be suppressed by IL-2 (26), and in the presence of IL-2 neutralizing antibodies, TL1A instead slightly enhances Th17 generation, showing that TL1A-mediated suppression of Th17 is likely driven through enhancement of IL-2 production (20). Support for a positive role of TL1A on Th17 differentiation in vivo comes from transgenic mice constitutively expressing TL1A, in which IL-17 expression in T cells and the small intestine is increased (27, 28). Whether endogenous TL1A-DR3 interactions promotes Th17 differentiation is less clear. DR3-deficient purified naïve T cells can differentiate normally into Th17 T cells (16), whereas ovalbumin-specific TCR transgenic CD4+ T cells (OT-II cells) primed with antigen and TL1A-deficient DC have slightly reduced Th17 differentiation (20). In experimental allergic encephalomyelitis (EAE), a mouse model of multiple sclerosis that depends on Th1 and Th17 T cells, draining lymph nodes and the central nervous system (CNS) of TL1A-deficient mice primed to develop EAE with myelin oligodendrocyte glycoprotein have slightly fewer IL-17 secreting T cells, but also fewer IFNγ secreting T cells, (16, 20). Taken together, these results support the role of dendritic-cell derived TL1A in promoting Th17 fate when IL-2 is limiting, and this does occur to some extent in vivo.
Interleukin-2 is also critical for generation and homeostasis of regulatory T cells, and it is thus not surprising that TL1A affects these processes. A percentage of naïve T cells can be differentiated into FoxP3 expressing ‘inducible’ Treg in the presence of TGF-β, and this process is inhibited by TL1A when IL-2 is not limiting. However, when exogenous IL-2 is not added, TL1A does not suppress, and can even enhance Treg development, perhaps because of the positive role that IL-2 plays in iTreg generation (25, 26) (A Richard and F Meylan, unpublished observations). Administration of the agonistic anti-DR3 antibody 4C12 was found to selectively promote Treg expansion in vivo in a manner dependent on IL-2, while not affecting other T cell subsets, and allergic lung inflammation can be suppressed if induced at the peak of Treg expansion (29). Transgenic constitutive expression of TL1A chronically expands Treg, although to a lesser extent than the 4C12 agonistic DR3 antibody (27, 28). This may result from affinity or stability differences between the antibody and the authentic ligand (30). Functionally, Treg exhibit decreased ability to suppress proliferation of conventional T cells in the presence of exogenous or transgene-derived TL1A, but mixing experiments showed that much of this effect is due to costimulation of responder cells by TL1A, which counteracts the suppressive effect of Treg (27, 28). As discussed above, the net effect of TL1A expression by tumor cells is to enhance tumor rejection(22), rather than inhibiting tumor rejection which would be expected if TL1A activated Treg. Taken together, these results suggest that the effects of TL1A on Treg are highly dependent on the context of the immune response that is being modulated.
TL1A-DR3 interactions in autoimmune disease and animal models
Increased levels of TL1A have been found at the site of inflammation in rheumatoid arthritis and inflammatory bowel disease. Major efforts have been made over the last few years to understand the physiological and pathological role of TL1A in mouse models of disease to determine the mechanism and utility of TL1A blockade and anticipate possible deleterious effects of blocking TL1A-DR3 interactions for therapeutic purposes. A remarkably wide variety of T-cell dependent autoimmune disease models have been found to be dependent on TL1A-DR3 interactions. An especially critical role for TL1A in expanding effector T cells at the site of inflammation, as well as in allergic disease in the lung and gastrointestinal tract, has emerged from these studies. In EAE, two independent studies have shown the importance of TL1A-DR3 interactions in the development of neuroinflammation and paralysis (16, 20). TL1A- and DR3-deficient mice had lower clinical disease scores, and reduced numbers of T cells in the CNS. Cell transfer studies established that expression of DR3 on T cells and TL1A on non-T cells is critical for EAE.
Elevated levels of TL1A are detectable in the synovial fluid and serum from patients with rheumatoid arthritis (31, 32), and a role for TL1A-DR3 interactions has been found in both the collagen-induced arthritis (CIA) and antigen-induced arthritis (AIA) models of human rheumatoid arthritis. Daily injection of TL1A during the course of CIA increased the severity and speed of arthritis development, and histological examination of the joints revealed cartilage damage, bone destruction and deformation, and increased cellular infiltrates (33). TL1A treatment also increased anti-collagen antibody production. Blocking TL1A-DR3 interactions with an antagonistic anti-TL1A antibody before disease development in the CIA model effectively reduced swelling, synovial leukocyte infiltration and bony erosions (34). In the AIA model, DR3-deficient mice displayed similar joint swelling at the peak of the response, but resolution was much faster than in wild-type mice, and pathological features of chronic arthritis in this model were significantly reduced. In addition, co-administration of TL1A during the course of AIA exacerbated the severity of bone destruction in a dose-dependent fashion while being ineffective on DR3-deficient mice (34). Data in this study also suggested an independent role for TL1A in promoting osteoclast development during inflammatory arthritis. Numbers of osteoclasts were reduced in the areas adjacent to pannus formation but not at the femoral head in the DR3-deficient mice. The reduction in osteoclast numbers was not due to impaired recruitment of macrophage osteoclast precursors to the joints, suggesting that TL1A may influence osteoclast differentiation from macrophage precursors. These data implicate TL1A as an important cytokine in driving the inflammatory features as well as bone erosions in the CIA autoimmune joint disease model. Correspondingly, TNF also has a dual role in promoting inflammation and synovial erosions in CIA and in rheumatoid arthritis, but mouse models have shown that TNF acts principally through TNFR1 on innate immune and non-hematopoietic cells to promote these pathological features. The lymphocyte-restricted nature of DR3 would suggest that TL1A acts mainly through modulating T and B cells in arthritis models, which would make the effects of TL1A non-overlapping and potentially synergistic with TNF, but future studies are needed to test this hypothesis.
Data from genome-wide association studies and animal models support a role for TL1A in the pathogenesis of human inflammatory bowel disease (IBD), particularly Crohn’s disease. Multiple genome-wide association studies have linked polymorphisms in the TNFSF15 locus encoding TL1A to genetic susceptibility to Crohn’s disease (35, 36). Functional analysis of Crohn’s disease patients harboring a susceptibility haplotype at the TNFSF15 locus found increased surface expression and secretion of TL1A in response to Fc receptor crosslinking (37). Immunochemical analysis of inflamed intestinal tissue from IBD patients and the SAMP-1/Yit and TNFΔARE spontaneous mouse models of IBD revealed increased TL1A and DR3 expression (17, 38). Studies in mouse models of colitis have shown a powerful effect of TL1A blockade particularly in T-cell mediated intestinal inflammation. In acute Dextran Sulfate Sodium (DSS) colitis, a model more dependent on innate immunity and integrity of tissue repair than T cells, DR3-deficient mice had a small reduction in weight loss, and TL1A blockade in already established DSS colitis reduced pathology and the generation of Th1 and Th17 T cells (28, 39). In acute colitis induced by administration of the hapten 2,4,6 –trinitro benzene sulfonic acid (TNBS), which depends more highly on Th1 T cells and IFNγ, TL1A blockade almost completely blocked weight loss and histological evidence of inflammation (28). As in rheumatoid arthritis, TNF blockade is an effective therapy for Crohn’s disease but likely works by modulating innate immune responses. These studies suggest a role for blocking TL1A to target pathological T cell responses in IBD.
TL1A-DR3 interactions in allergic asthma
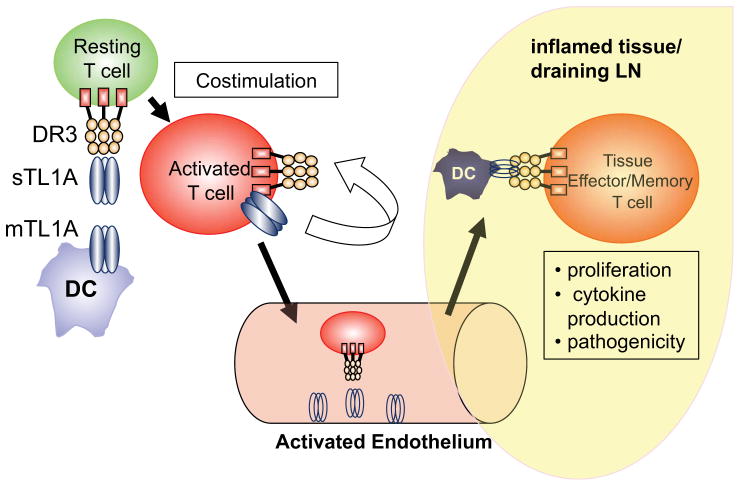
TL1A-DR3 interactions appear are important in the effector phase of allergic responses in the lung that model human asthma. DR3-deficient mice were found to be resistant to the ovalbumin lung hypersensitivity pneumonitis model of asthma, which is dependent on Th2 T cells and IL-13 (16). Lung pathology, eosinophilia in the BAL, and levels of IL-5 and IL-13 in the lung were significantly reduced in DR3-deficient mice, while production of these cytokines in response to ovalbumin in the spleen were preserved. Transfer experiments confirmed that DR3 on T cells was required for allergic responses, and tracking of transferred T cells revealed a severe defect in the numbers and proliferation of antigen-specific T cells lacking DR3 in the bronchial lymph nodes after ovalbumin challenge (16). Transgenic mice expressing a dominant-negative DR3 isoform in T cells exhibited similar resistance to this Ova-induced lung hypersensitivity, and blockade of TL1A-DR3 interactions with an antagonistic anti-TL1A antibody as late as one day before ovalbumin aerosol challenge resulted in reduced lung pathology, inflammatory cells in the BAL, and levels of the Th2 cytokines IL-13 and IL-5 (19). Taken together, these studies provide strong evidence that DR3 is required on effector T cells for their expansion and production of pathogenic cytokines after rechallenge with antigen in this mouse model of asthma and more generally in autoimmune disease (Figure 1).
Figure 1. TL1A-DR3 interactions are critical for effector CD4+T cells at the site of inflammation.
TL1A is produced by dendritic cells as a type II transmembrane protein (mTL1A) and then cleaved by metalloproteinases to form soluble TL1A (sTL1A). TL1A costimulates T cell activation and production of cytokines in vitro. However, TL1A-DR3 interactions are apparently not required for T cell priming in vivo. Activated T cells also produce mTL1A but the significance of autocrine TL1A is not known. In the inflamed tissue, endothelial cells and Dendritic Cells activated by inflammatory stimuli produce TL1A and provide costimulation for effector and memory T cells entering the tissue, which is required for efficient expansion and cytokine production by these T cells, leading to greater pathogenicity in animal models of disease.
Although DR3 is expressed on both resting and activated T cells, its function appears to be dispensable for systemic priming against antigen in these studies. The relative redundancy of DR3 in T cell priming may stem from complementation by other costimulatory receptors or alternative splicing that increases the amount of full-length DR3 in memory vs. naïve T cells (3, 17). Another factor may be that the expression of TL1A is restricted to an inflammatory environment where it is induced by stimulation through TLR’s and Fc receptors. In any case, these studies place DR3 as a regulator of effector T cell responses distal to the activities of other TNF-family receptors that costimulate earlier phases of T cell activation and differentiation. For example, OX40-deficient mice are similarly resistant to ovalbumin-induced hypersensitivity pneumonitis but have impaired systemic Th2 responses to antigen (40).
Like DR3-deficient mice, Jα18-deficient mice that lack invariant NKT cells are resistant to airway inflammation in the Ova-induced asthma model (41). Transfer of wild-type but not DR3-dominant negative NKT cells restored airway inflammation in Jα18-deficient mice (19). In DR3-deficient mice, NKT cells were reduced in the lung after rechallenge with Ova (16). These studies suggest that NKT cells responding to TL1A expand and secrete cytokines such as IL-13 that act on mucous-producing and smooth muscle cells in the lung, promoting pathology in this model of asthma. As NKT cells have also been implicated in human asthma (42), the role of TL1A in this aspect of asthma pathogenesis in humans will be of interest.
Sustained TL1A expression costimulates T cells in vivo and results in small intestinal pathology dependent on IL-13
Recent studies with mice constitutively expressing TL1A have uncovered a unique role for TL1A in promoting small intestinal inflammation that resembles allergic and anti-parasitic responses. Our group and two others independently generated mice expressing TL1A in the T cell and dendritic cell or myeloid compartments (27, 28, 43). Mice from all of these lines developed age-dependent increases in the percentage of activated T cells, memory T cells and Tregs, confirming the in vivo role of TL1A as a T cell costimulator. Interestingly NKT cells including invariant NKT cells were specifically depleted from the periphery of TL1A transgenic mice (27, 28), suggesting that constitutive exposure to TL1A may induce apoptosis in NKT cells, perhaps through over-stimulation.
Strikingly, all lines of TL1A transgenic mice uniformly developed small intestinal inflammation centered on the ileum, with kinetics and severity roughly proportional to the degree of transgene expression (27, 28, 43). This pathology developed earlier and became more severe in mice constitutively expressing TL1A in the T cell compartment. Levels of TL1A in these mice are comparable to the peak of expression in stimulated myeloid cells. Although intestinal sections from mice with the highest levels of TL1A expression had histological evidence of inflammation, the pathology differed from that seen in inflammatory bowel disease, with an absence of crypt abscesses or granulomas seen in Crohn’s disease. Instead, the histology of the ileum and, to a lesser extent, other areas of the small intestine was marked by goblet cell and paneth cell hyperplasia, appearance of mast cells in the submucosa, and thickening of the smooth muscle layer accompanied by fibrosis. As a result of these hyperplastic changes, the small intestine became thickened and lengthened by up to 5 cm compared to wild-type controls, a very different picture than many other forms of experimental inflammatory bowel disease that include marked shortening of the affected section of gastrointestinal tract. Between two and five percent of the TL1A transgenic mice produced by Shih et al. also developed extra-intestinal manifestations including ulcerative skin lesions and joint swelling, perhaps due to environmental antigens particular to these mouse colonies or differences in transgene expression due to the use of different promoters for TL1A by this group (lck-CD2 rather than CD2 for T cells, and FMS rather than CD11c for myeloid cells) (43).
Many of the features of TL1A-driven intestinal pathology are similar to those seen in the response to parasite infections, and IL-13 is known to be a key mediator of the intestinal anti-parasite response. Consistent with this, IL-13 and IL-5 mRNA were found to be markedly elevated in the small intestine and mesenteric lymph nodes of TL1A transgenic mice, with mild elevation of IL-17 but no increases in IFNγ (27, 28). Blockade of IL-13 but not IL-17 reduced the small intestinal pathology in TL1A transgenic mice, (28) and crossing TL1ATg mice to mice lacking IL13Rα1, the active receptor for IL-13, ameliorated the goblet cell hyperplasia and small bowel wall thickening in TL1A transgenic mice (F Meylan and RM Siegel, unpublished observations), confirming the importance of IL-13 in the pathogenesis of this aspect of TL1A-driven pathology.
Interestingly, despite the abundant evidence of T cell activation in TL1A transgenic mice, T cells may only indirectly induce the IL-13 dependent gastrointestinal pathology that ensues. In transgenic mice expressing the highest levels of TL1A, increased percentages of T cells could be found in the lamina propria of the small intestine, and the percentage of T cells expressing chemokine receptors such as CCR9 that mark gut-circulating T cells were increased in mesenteric lymph nodes (28). To investigate the contribution of activated T cells to the pathological effects of constitutively expressed TL1A, we crossed transgenic mice expressing TL1A on T cells to OT-II TCR transgenic mice on a RAG1−/− background, which fixes the CD4+ T cell repertoire to essentially monoclonal reactivity against ovalbumin and limits TCR stimulation through endogenous or environmental antigens (28). T cells in these mice were uniformly naïve, supporting the role of TL1A as a T cell costimulator that cannot function without antigenic activation signals through the TCR. Histological evidence of intestinal inflammation was reduced in RAG1 −/− OT-II TCR TL1A transgenic mice, but IL-13 expression, goblet cell hyperplasia and thickening of the muscularis layer was preserved (28). Thus it appears that T cells are not the only source of IL-13 that drives intestinal pathology in TL1A transgenic mice. Likewise, when T cells from the mesenteric lymph node or intestinal lamina propria of TL1A transgenic mice were restimulated in vitro, IL-13 production was increased, but the increases were not much greater than the small increases in frequencies of other cytokines seen in the various strains of TL1A transgenic mice. T cell production of IL-13 did not appear to account for the large increases in IL-13 mRNA detected in the small intestine, mesenteric lymph nodes and serum of these mice.
These observations support distinct roles for acutely vs. chronically expressed TL1A (Figure 2). When TL1A is transiently expressed, T cell costimulation through DR3 enhances the production of a variety of cytokines including IL-2, TNFα, IFNγ, IL-17, and IL-4. These cytokines may be responsible for the DR3-dependent expansion of effector T cells in the target tissues of mice with experimental autoimmune disease. However, chronically produced TL1A, acting most likely through a cell type other than a classical CD4+ T cell, stimulates the production of IL-13 to produce the ‘allergic’ type features of intestinal pathology seen in TL1A transgenic mice. Why TL1A induces these features in the small intestine but not the lungs or large intestine is not resolved, but it may be due to the microbial environment of the small intestine or the presence of higher levels of the decoy receptor IL13Rα2 in the colon and lung that dampen the effects of IL-13 in these tissues (44, 45). Higher levels of TL1A drive the additional inflammatory features seen in CD2-TL1A transgenic mice which are dependent on T cell activation. What cell type(s) produce IL-13 in response to TL1A, and when the IL-13-dependent response is triggered by endogenous TL1A in normal and pathological immune responses are important questions for future study.
Figure 2. Distinct effects of transient vs. sustained TL1A expression.
Transient expression of TL1A is induced in innate immune cells by microbial and endogenous stimuli including Toll-like Receptor (TLR) ligands and Fc-Receptor (FcR) crosslinking. Similar stimuli provoke transient TNF production. TNF feeds back on these cells through TNFR1 to enhance cellular activation and cytokine production. Transiently expressed TL1A costimulates T cells through DR3 to produce the indicated cytokines, which enhance T cell expansion in inflamed tissues and also further activate antigen-presenting cells in the tissue, completing a positive feedback loop. Chronic TL1A expression promotes the production of IL-13 by cells other than a conventional T cells. IL-13 drives the indicated features of small intestinal pathology. TL1A also costimulates T cells, which alters T cell homeostasis and promotes cellular inflammation in the intestinal lamina propria. (DC: dendritic cells, EC: endothelial cells)
Looking forward: What is TL1A for, and what is T1A blockade good for?
The past few years have seen rapid progress in research into the function of TL1A and DR3 in normal and pathological immune responses. However there are still many unanswered questions about the biology of this ligand-receptor pair. Remarkably, we have a much better understanding of the pathological role of TL1A in autoimmune diseases than its physiological function in immune responses and host defense. From an evolutionary point of view, the diversification of TNF family cytokines and their receptors into a large superfamily of distinct genes is thought to allow each cytokine to function in a particular niche in host defense against specific pathogens. Genetic deficiency or and blockade of individual members of the TNF cytokine family tend to produce very restricted immune deficiencies, such as the well-known example of susceptibility to mycobacterial disease from TNF blockade. Beyond traditional roles in host defense, TL1A and DR3 may also play a role in modulating sterile inflammation, such as its recently described role in the promotion of foamy macrophages involved in the pathogenesis of atherosclerosis (46, 47). DR3 may have functions outside the immune system, suggested by recently described defects in cortical motor neuron innervation that result in gait and coordination defects in ageing DR3-deficient mice (48).
Which pathogens TL1A has evolved to counter is not yet clear. Thus far, relatively few infectious pathogens have been tested in DR3 or TL1A-deficient mice or in the animals treated with reagents that block TL1A-DR3 interactions. After infection with the intracellular parasite Toxoplasma gondii, which is lethal in the absence of Th1 or IFNγ and then requires effective T cell immunity to sequester surviving parasites in brain cysts during chronic infection, DR3-deficient mice survived at rates similar to controls and formed normal numbers of brain cysts (16). A number of ongoing studies are seeking to determine what non-redundant role DR3 plays in other models of infectious disease. As TNF enhances immune responses to mycobacterial infections through activation of macrophages and other innate immune cells, the role TL1A plays in mycobacterial infection, and whether it may perform an analogous role of boosting adaptive immunity to mycobactera, is of interest. Knowing the physiological role of TL1A in immunity will also be important to predict possible deleterious consequences of therapeutics that block TL1A-DR3 interactions if they are used in clinical practice. The success of TL1A blockade in mouse models of multiple sclerosis, inflammatory bowel disease, and rheumatoid arthritis has spurred efforts towards that goal.
Acknowledgments
This work was supported by funding from the Intramural Research Program, NIAMS, NIH, and a fellowship grant to F Meylan from the Crohn’s and Colitis Foundation of America. The authors would like to thank Vera Siegel for editing of the manuscript.
References
- 1.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003 Aug;3(8):609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 2.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Screaton GR, Xu XN, Olsen AL, Cowper AE, Tan R, McMichael AJ, et al. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci U S A. 1997;94(9):4615–9. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnaiyan AM, O’Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274(5289):990–2. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer JL, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6(1):79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 6.Kitson J, Raven T, Jiang YP, Goeddel DV, Giles KM, Pun KT, et al. A death-domain-containing receptor that mediates apoptosis. Nature. 1996 Nov 28;384(6607):372–5. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 7.Marsters SA, Sheridan JP, Donahue CJ, Pitti RM, Gray CL, Goddard AD, et al. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol. 1996 Dec 1;6(12):1669–76. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 8.Borysenko C, Furey W, Blair H. Comparative modeling of TNFRSF25 (DR3) predicts receptor destabilization by a mutation linked to rheumatoid arthritis. Biochemical and Biophysical Research Communications. 2005 Mar 18;328(3):794–9. doi: 10.1016/j.bbrc.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Pobezinskaya YL, Choksi S, Morgan MJ, Cao X, Liu ZG. The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling. J Immunol. 2011 May 1;186(9):5212–6. doi: 10.4049/jimmunol.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003 Oct 3;278(40):39251–8. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]
- 11.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003 Jul 25;114(2):181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 12.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002 Mar;16(3):479–92. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Zhang L. Identification of naturally secreted soluble form of TL1A, a TNF-like cytokine. J Immunol Methods. 2005 Mar;298(1–2):1–8. doi: 10.1016/j.jim.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Jin T, Guo F, Kim S, Howard A, Zhang YZ. X-ray crystal structure of TNF ligand family member TL1A at 2.1A. Biochem Biophys Res Commun. 2007 Dec 7;364(1):1–6. doi: 10.1016/j.bbrc.2007.09.097. [DOI] [PubMed] [Google Scholar]
- 15.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007 Apr 1;178(7):4033–8. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 16.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008 Jul 18;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, et al. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006 May 12; doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadakis KA, Zhu D, Prehn JL, Landers C, Avanesyan A, Lafkas G, et al. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005 Apr 15;174(8):4985–90. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008 May 12;205(5):1037–48. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008 May 12;205(5):1049–62. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang EC, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ. DR3 regulates negative selection during thymocyte development. Mol Cell Biol. 2001 May;21(10):3451–61. doi: 10.1128/MCB.21.10.3451-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slebioda TJ, Rowley TF, Ferdinand JR, Willoughby JE, Buchan SL, Taraban VY, et al. Triggering of TNFRSF25 promotes CD8(+) T-cell responses and anti-tumor immunity. Eur J Immunol. 2011 Jun 20; doi: 10.1002/eji.201141477. [DOI] [PubMed] [Google Scholar]
- 23.Heidemann SC, Chavez V, Landers CJ, Kucharzik T, Prehn JL, Targan SR. TL1A selectively enhances IL-12/IL-18-induced NK cell cytotoxicity against NK-resistant tumor targets. J Clin Immunol. 2010 Jul;30(4):531–8. doi: 10.1007/s10875-010-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004 Jun 1;172(11):7002–7. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 25.Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, et al. Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation. FASEB J. 2011 Jan;25(1):409–19. doi: 10.1096/fj.10-166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26(3):371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Taraban VY, Slebioda TJ, Willoughby JE, Buchan SL, James S, Sheth B, et al. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011 Mar;4(2):186–96. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meylan F, Song YJ, Fuss I, Villarreal S, Kahle E, Malm IJ, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2010 Oct; doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, et al. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010 Oct 1;120(10):3629–40. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taraban VY, Ferdinand JR, Al-Shamkhani A. Expression of TNFRSF25 on conventional T cells and Tregs. J Clin Invest. 2011 Feb 1;121(2):463–4. doi: 10.1172/JCI45832. author reply 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamias G, Siakavellas SI, Stamatelopoulos KS, Chryssochoou E, Papamichael C, Sfikakis PP. Circulating levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3 (DcR3) in rheumatoid arthritis. Clin Immunol. 2008 Nov;129(2):249–55. doi: 10.1016/j.clim.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Cassatella M, Da Silva G, Tinazzi I, Facchetti F, Scapini P, Calzetti F, et al. Soluble TNF-Like Cytokine (TL1A) Production by Immune Complexes Stimulated Monocytes in Rheumatoid Arthritis. The Journal of Immunology. 2007 Jun 1;178(11):7325. doi: 10.4049/jimmunol.178.11.7325. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Wang X, Fahmi H, Wojcik S, Fikes J, Yu Y, et al. Role of TL1A in the pathogenesis of rheumatoid arthritis. J Immunol. 2009 Oct 15;183(8):5350–7. doi: 10.4049/jimmunol.0802645. [DOI] [PubMed] [Google Scholar]
- 34.Bull MJ, Williams AS, Mecklenburgh Z, Calder CJ, Twohig JP, Elford C, et al. The Death Receptor 3-TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. J Exp Med. 2008 Oct 27;205(11):2457–64. doi: 10.1084/jem.20072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki K, McGovern D, Ragoussis J, Paolucci M, Butler H, Jewell D, et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum Mol Genet. 2005 Nov 15;14(22):3499–506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 36.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008 Aug;40(8):955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelsen KS, Thomas LS, Taylor KD, Yu QT, Mei L, Landers CJ, et al. IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS ONE. 2009;4(3):e4719. doi: 10.1371/journal.pone.0004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bamias G, Martin C, 3rd, Marini M, Hoang S, Mishina M, Ross WG, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003 Nov 1;171(9):4868–74. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 39.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008 Aug;135(2):552–67. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003 Jul 21;198(2):315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003 Aug 15;171(4):1637–41. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 42.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–92. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 43.Shih DQ, Barrett R, Zhang X, Yeager N, Koon HW, Phaosawasdi P, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS ONE. 2011;6(1):e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006 Jan 1;176(1):491–5. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng T, Liu W, Oh SY, Zhu Z, Hu B, Homer RJ, et al. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008 Jan 1;180(1):522–9. doi: 10.4049/jimmunol.180.1.522. [DOI] [PubMed] [Google Scholar]
- 46.Kang YJ, Kim WJ, Bae HU, Kim DI, Park YB, Park JE, et al. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005 Mar 7;29(5):229–35. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47.McLaren JE, Calder CJ, McSharry BP, Sexton K, Salter RC, Singh NN, et al. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010 May 15;184(10):5827–34. doi: 10.4049/jimmunol.0903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Twohig JP, Roberts MI, Gavalda N, Rees-Taylor EL, Giralt A, Adams D, et al. Age-dependent maintenance of motor control and corticostriatal innervation by death receptor 3. J Neurosci. 2010 Mar 10;30(10):3782–92. doi: 10.1523/JNEUROSCI.1928-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]