Abstract
Age and APOE are the most robust risk factors for dementia and cognitive decline, but the underlying neurobiology remains unclear. We examined the extent to which the hallmark pathologies of Alzheimer’s disease, Lewy body disease, and cerebrovascular diseases account for the association of age and APOE with decline in episodic memory versus non-episodic cognitive abilities. Up to 20 waves of longitudinal cognitive data were collected from 858 autopsied participants in two ongoing clinical-pathologic cohort studies of aging. Neuropathologic examinations quantified measures of beta amyloid plaque (Aβ), mesial temporal and neocortical neurofibrillary tangles, macro- and microinfarcts, and neocortical Lewy bodies. Random coefficient models estimated person-specific slopes of decline in episodic memory and non-episodic cognition. Path analysis examined the relation of age, APOE and the six pathologic indices to the slopes of cognitive decline. The effect of age on decline in episodic memory was mediated by Aβ, mesial temporal and neocortical tau tangles, as well as macroscopic infarcts; age on decline in non-episodic cognition was mediated by Aβ, neocortical tangles, and macroscopic infarcts. The effect of APOE on decline in episodic memory was mediated by Aβ, mesial temporal and neocortical tangles, as well as neocortical Lewy bodies; APOE on non-episodic cognition was mediated by Aβ, neocortical tangles and neocortical Lewy bodies. There were no direct effects of age and APOE on decline after accounting for these pathologic pathways.
Keywords: Age, APOE, Neuropathologies, Alzheimer’s disease, Cognitive decline, Path analysis
INTRODUCTION
Loss of cognition and the development of mild cognitive impairment (MCI) and dementia in late life are associated with multiple age-related neuropathologies including Alzheimer’s disease (AD, i.e., beta amyloid plaque (Aβ) deposition and PHF-tau immunoreactive neuronal neurofibrillary tangle (tau tangle) formation), cerebrovascular disease (i.e. macroscopic and microscopic infarcts) and neocortical Lewy body disease (i.e. alpha-synuclein immunoreactive neocortical Lewy bodies (LB)) (Medical Research Council Cognitive Function and Aging Study 2001, Sonnen et al 2007, Launer et al 2008, Troncoso et al 2008, Nelson et al 2010, Wilson et al 2012, Royall and Palmer 2012). A general consensus on neurodegeneration in AD places tau tangle formation downstream of Aβ deposition (Sperling et al 2011, Jack et al 2011). This hypothesis is supported by the fact that mutations in all three causal genes of familial AD (i.e. APP, PS1 and PS2) lead to the accumulation of Aβ and tau tangles, whereas mutations in the tau gene do not result in Aβ accumulation (Hardy and Selkoe 2002, Hutton et al 1998). Clinical-pathologic studies have also shown that the association of Aβ with cognition is mediated by tau tangle pathology (Bennett et al 2004, Roberson et al 2007). The recent revision of neuropathologic criteria for AD requires Aβ deposition (Hyman et al 2012), which differs from a traditional view that mesial temporal tangles, even in the absence of amyloid, is the earliest manifestation of AD (Hyman et al 1984, Braak and Braak 1995). Thus, it has been argued that tau tangles, frequently seen in the mesial temporal lobe in the absence of Aβ, may represent a separate age-related pathologic process (Yamada 2003, Jellinger and Attems 2007, Nelson et al 2009). Further, while there is some evidence of an association between amyloid and cerebrovascular dysfunction (Han et al 2008) and colocalization of tau and alpha-synuclein (Ishizawa et al 2003), most clinical pathologic studies suggest infarcts and LB pathologies have relatively independent effects on cognitive impairment and dementia (Medical Research Council Cognitive Function and Aging Study 2001, Sonnen et al 2007, Launer et al 2008, Troncoso et al 2008).
Older age and Apolipoprotein E (APOE) are the two most robust risk factors for late life cognitive decline, MCI and dementia (Evans et al 1989, Karlamangla et al 2009, Lipnicki et al 2013, Corder et al 1993, Dubé 2013). However, the neurobiologic pathways linking age and APOE with cognition have not been fully elucidated. It is well known that AD, CVD, and LB pathologies accumulate with age. Further, APOE is a risk factor for the pathologies of all three diseases but the relationships are more complex. APOE is strongly related to amyloid deposition and slightly less so with tau tangle pathology (Mortimer et al 2009, Morris et al 2010). We are not aware of prior studies that have disentangled the effect of APOE on mesial temporal versus neocortical tangles. On the other hand, APOE is only weakly associated with infarcts and LB (Schneider et al 2005, Tsuang et al 2013).
In prior studies, we reported that the association of APOE with global cognitive decline was mediated by amyloid and tangles (Yu et al 2013), and the association of APOE with perceptual speed was partially mediated by macroscopic infarcts (Li et al 2007). We also reported a two process model for AD-related pathologies such that the association of APOE with neocortical tangles and neuritic plaques represents an AD pathway, whereas a separate non-plaque age-related process leads to mesial temporal tangles (Mungas et al 2013). Here, we extend our prior work and expand this two-process model in four major ways. First, we used molecularly-specific markers of AD including Aβ load and the density of tau tangles rather than plaques and tangles with silver stain. Second, we added other common age-related pathologies to the model, including macro- and microscopic infarcts and neocortical Lewy bodies. Third, whereas our prior model used neuropathologies as the endpoint, here, we linked the neuropathologies to the downstream phenotype of cognitive decline using repeated measures of cognitive function over up to 20 years prior to death. Finally, we examined the effects of age, APOE, and neuropathologies on decline in episodic memory and decline in a composite measure of other cognitive abilities. We separated cognition into episodic memory and non-episodic abilities for several reasons. First, change in episodic memory is the clinical hallmark of AD dementia and amnestic MCI, a precursor to AD dementia. Second, APOE appears to have a relatively selective effect on change in episodic memory (Wilson et al 2002, Barral et al 2012, Wikgren et al 2012). Third, AD pathology may have a selective effect on episodic memory compared to cerebrovascular disease which may be relatively selective for measures of executive function and Lewy bodies for measures of visuospatial ability (Reed et al 2007, Johnson et al 2011, Schneider et al 2012, Yang et al 2013, Cholerton et al 2013).
METHODS
Participants
Participants came from two ongoing clinical-pathologic cohort studies of aging and AD; the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). ROS started in 1994 and enrolls older religious clergy from over 40 groups across the United States. MAP started in 1997 and enrolls older residents from retirement facilities and subsidized housing across the Chicago metropolitan area. Detailed study design and data collection procedures have been previously reported (Bennett et al 2012, Bennett et al 2012). Both studies were approved by the Institutional Review Board of Rush University Medical Center. All the participants agreed to annual clinical evaluations and brain donation at death.
Uniform ante- and postmortem data collection in ROS and MAP allows us to combine the data from both studies. At the time of these analyses, 1,131 persons with at least 2 cognitive evaluations died and were autopsied, of whom 858 (ROS=459; MAP=399) had complete data for APOE genotyping and neuropathologies. The average age at death was 88.5 years (SD=6.5, Min=65.9, Max=108.3).
Cognitive evaluations
Seventeen cognitive tests were administered to each participant every year. In order to distinguish episodic memory and other cognitive abilities and to reduce floor and ceiling effects, we created two composite scores. The composite of episodic memory consists of 7 tests, including immediate and delayed recall of story A from logical memory, immediate and delayed recall of the east Boston story, word list memory, word list recall, and word list recognition. A non-episodic composite was based on standard progressive matrices, verbal fluency, digit span forward and backward, digit ordering, symbol digit modalities, number comparison, reading test, Boston naming, and judgment of line orientation. In both cases, higher scores indicate better cognitive performance. The psychometric properties of the cognitive domains have been described previously (Wilson et al 2002).
Neuropathology assessments
Post-mortem brains were processed following a standard procedure. One hemisphere was cut coronally into 1 cm slabs and fixed in 4% paraformaldehyde. Using immunohistochemical staining and computer assisted sampling and image analysis (Bennett et al 2004), Aβ and tau tangle pathologies were assessed across 8 cortical regions including entorhinal cortex, hippocampus CA1/subiculum, superior frontal cortex (BA6/8), mid frontal cortex (BA46/9), inferior temporal cortex (BA20), angular gyrus cortex (BA39/40), cingulate cortex (BA32/33), and calcarine cortex (BA17). Overall Aβ load was derived by averaging the mean percentage area per region, across multiple regions. Similarly, mesial temporal and neocortical tangle densities were obtained by averaging PHF tau tangles across corresponding brain regions. Fixed slabs and/or pictures from both hemispheres were examined for macroscopic infarcts, followed by histological confirmation (Schneider et al 2005). Microinfarcts, defined as infarcts not seen grossly but discovered by microscopy, were identified by examining at least 9 sections stained for hematoxylin and eosin (Arvanitakis et al 2011). Only presence versus absence of chronic infarcts was considered in the analysis. Neocortical Lewy body pathology required presence of alpha-synuclein immunoreactive Lewy bodies in either midfrontal, middle temporal, or inferior parietal cortex, together with either nigral or limbic positivity (Schneider et al 2012).
Other variables
Age in years was computed from self-reported date of birth and date of death. DNA was extracted from blood, and in some cases from frozen brain tissue. APOE genotyping was done by sequencing codon 112 and codon 158 of exon 4 of the APOE gene (Boyle et al 2010). Participants with at least one copy of e4 allele were considered carriers, and the rest were considered non-carriers.
Statistical Analysis
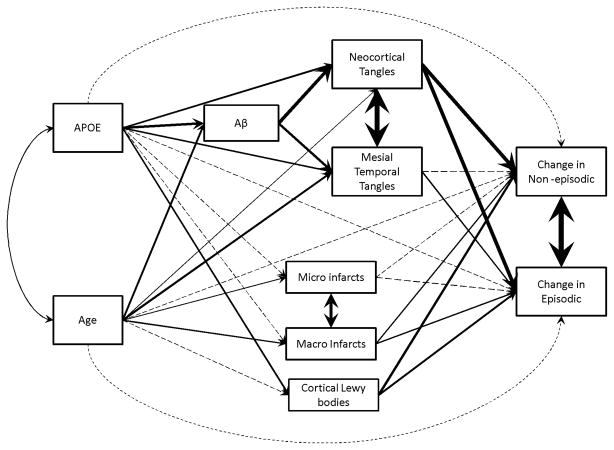
We hypothesized a structural model for the relationship of age, APOE and neuropathologies with late life cognitive decline. Figure 1 is a graphical representation of the overall model. The primary outcomes for the analyses were person-specific rates of decline (slopes) in episodic memory and non-episodic cognition, estimated from random coefficient models using all available longitudinal cognitive data. These slopes capture the between-subject deviation from the mean slope of decline. Using path analysis, we examined and compared the effect of individual contributing pathways linking age and APOE, through neuropathologies, to the slopes of episodic memory and non-episodic cognition. Goodness of fit for this structural model was assessed using comparative fit index (CFI), Tucker-Lewis index (TLI), and the root mean square error of approximation (RMSEA). The model is acceptable only if all the indices indicate goodness of fit (i.e. CFI>0.95; TLI>0.95 and RMSEA <0.05). Standardized path coefficients along individual pathways were used to capture the total direct and indirect effects of age, APOE, and neuropathologic indices on cognitive decline. We imposed a nominal threshold of p<0.05 for statistical significance. Analyses were performed using SAS/STAT software, version 9.3 [SAS Institute Inc., Cary, NC] and Mplus, Version 7.0 [Muthen & Muthen, 1998–2012].
Figure 1.
Graphical representation of the model structure. Nonsignificant coefficients were presented as dotted lines; thickness of solid lines corresponds to the relative effect size of the coefficients.
RESULTS
Participants (N=858) in the analyses were on average 88.5 years of age at the time of death (SD=6.5, Min=65.9, Max=108.3), and 224 (26.1%) were APOE e4 carriers. Over up to 19 years of follow-up, episodic memory declined a mean of 0.110 unit per year (p<0.001) and non-episodic cognition declined a mean of 0.126 (p<0.001). At autopsy, 743 (86.6%) participants were positive for Aβ, 824 (96.0%) were positive for neocortical tau tangles and 856 (99.7%) were positive for mesial temporal tau tangles. Over 13% (115 out of 858) of participants had tau tangle pathology in the absence of Aβ (including 10 participants who had only mesial temporal tangles). Approximately half of the participants had either macroscopic (36.3%) or micro (29.1%) infarcts, and 12% had neocortical Lewy bodies. Additional descriptive statistics are presented in Table 1.
Table 1.
Descriptive characteristics (N=858)
| Variable | Mean, SD, range (or # and percent) |
|---|---|
| Age at death | 88.5, 6.5, 66–108 |
| Female | 556 (64.8%) |
| Aβ load | 4.06, 4.23, 0 – 22.94 |
| Neocortical PHFtau tangle density | 2.82, 6.61, 0 – 80.10 |
| Mesial temporal PHFtau tangle density | 17.25, 15.32, 0 – 91.11 |
| Macroscopic infarcts (1+ present) | 311, 36.3% |
| Microinfarcts (1+ present) | 250, 29.1% |
| Neocortical Lewy bodies (present) | 100, 11.7% |
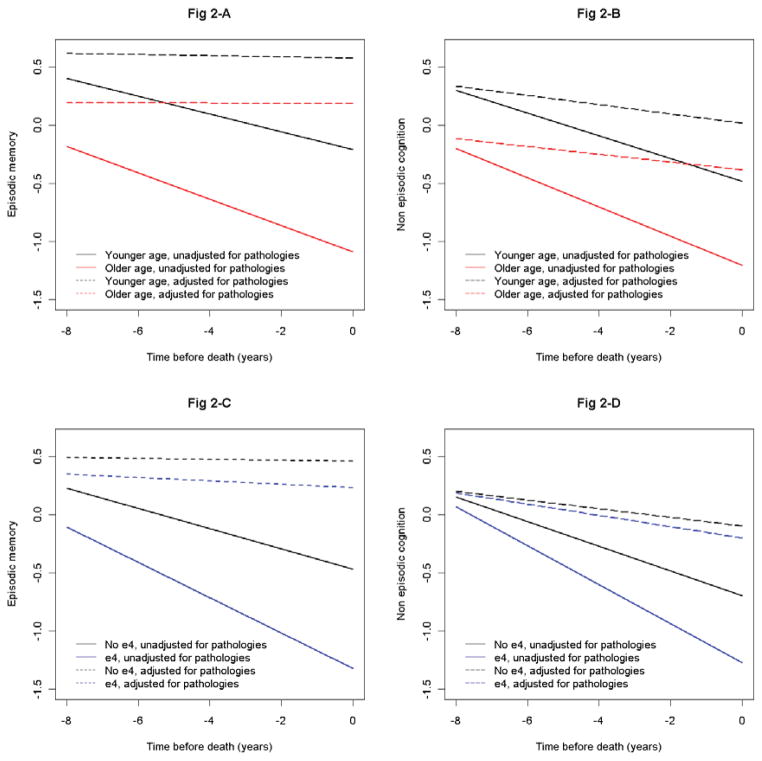
We first examined the associations of age and APOE with cognitive decline in a series of random coefficient models using longitudinal cognitive data as the outcome. Older age was associated with faster rates of decline in episodic memory (Estimate = −0.052, p<0.001) and non-episodic cognition (Estimate = −0.043, p<0.001). Similarly, the presence of e4 allele also led to faster rates of decline in episodic memory (Estimate = −0.064, p<0.001) and non-episodic cognition (Estimate = −0.061, p<0.001). On the other hand, after adjusting for Aβ, mesial temporal and neocortical tau tangles, macro- and microscopic infarcts and cortical Lewy bodies, age and APOE were no longer associated with decline in either domain (Figure 2). In addition, there was no significant decline in episodic memory when we controlled for the pathologies (Estimate = −0.004, p=0.735), and the rate of decline in non-episodic cognition was also greatly reduced (Estimate = −0.038, p=0.001).
Figure 2.
Fig 2-A and B. Association of age with declines in episodic memory and non-episodic cognition.
Fig 2-C and D: Association of APOE with the declines in episodic memory and non-episodic cognition.
We next applied the path analysis to examine the extent to which how the effects of age and APOE on cognitive decline are mediated by individual common neuropathologies (Figure 1). Our model fit the data well (CFI = 0.995, TLI = 0.982, RMSEA = 0.027 (95% CI = 0 – 0.047)). Individual standardized path coefficients and corresponding p-values were shown in Table 2. Further in Figure 1, nonsignificant coefficients were presented as dotted lines and thickness of solid lines corresponds to the relative effect size of the coefficients.
Table 2.
Model path coefficients
| Effects | Std Coeff | P-value |
|---|---|---|
| Aβ (regressed on) | ||
| Age | 0.223 | <0.001 |
| APOE | 0.278 | <0.001 |
| Mesial temporal tangles (regressed on) | ||
| Age | 0.246 | <0.001 |
| APOE | 0.179 | <0.001 |
| Aβ | 0.280 | <0.001 |
| Neocortical tangles (regressed on) | ||
| Age | 0.054 | 0.030 |
| APOE | 0.190 | <0.001 |
| Aβ | 0.409 | <0.001 |
| Macroscopic infarcts (regressed on) | ||
| Age | 0.176 | <0.001 |
| APOE | 0.067 | 0.156 |
| Microinfarcts (regressed on) | ||
| Age | 0.112 | 0.013 |
| APOE | 0.021 | 0.675 |
| Neocortical Lewy bodies (regressed on) | ||
| Age | 0.077 | 0.243 |
| APOE | 0.195 | 0.001 |
| Episodic decline (regressed on) | ||
| Age | −0.034 | 0.296 |
| APOE | −0.019 | 0.544 |
| Mesial temporal tangles | −0.172 | <0.001 |
| Neocortical tangles | −0.418 | <0.001 |
| Macroscopic infarcts | −0.160 | 0.001 |
| Microinfarcts | −0.027 | 0.544 |
| Neocortical Lewy bodies | −0.228 | <0.001 |
| Non-episodic decline (regressed on) | ||
| Age | −0.032 | 0.318 |
| APOE | 0.009 | 0.791 |
| Mesial temporal tangles | −0.061 | 0.112 |
| Neocortical tangles | −0.437 | <0.001 |
| Macroscopic infarcts | −0.146 | <0.001 |
| Microinfarcts | −0.048 | 0.298 |
| Neocortical Lewy bodies | −0.268 | <0.001 |
Std Coeff: standardized coefficient
Neuropathologies on cognitive decline
We examined the relation of the six pathologies to cognitive decline. Neocortical tau tangle pathology, macroscopic infarcts and neocortical Lewy bodies were associated with decline in both episodic memory and non-episodic cognition, while mesial temporal tau tangle pathology was only associated with decline in episodic memory. The effect of Aβ on cognitive decline was largely mediated by neocortical tau tangle pathology. On decline in episodic memory, 78% of the Aβ effect was mediated by neocortical tau tangles (Estimate = −0.171, p<0.001), and 22% through mesial temporal tau tangles (Estimate = −0.048, p<0.001). On decline in non-episodic cognition, virtually all of the effect of Aβ was mediated by neocortical tau tangles (Estimate = −0.179, p<0.001), and the indirect effect through mesial temporal tau tangles was not significant (Estimate = −0.017, p=0.121).
Age, APOE on tau tangle pathology
We also examined the relation of age and APOE to tau tangle pathology. In doing so, we allowed in the model links from age and APOE to tau tangle pathology with and without Aβ. Both direct and indirect (through Aβ) effects from age and APOE to tau tangles were significant (Table 3), suggesting separate pathways that link age and APOE to tau tangle pathology. The total estimated effect of age on neocortical tau tangles was 0.145 (p<0.001), of which more than 60% went through Aβ (Estimate=0.091, p<0.001). In contrast, approximately 80% of age effect on mesial temporal tau tangles was attributable to a direct effect (Estimate = 0.246, p<0.001), and only 20% worked through Aβ (Estimate = 0.062, p<0.001). This result supports a non-amyloid based process that involves the formation of mesial temporal tau tangles, primarily driven by age. The estimated effect of APOE on neocortical tau tangles was 0.304 (p<0.001), of which 37.5% (Estimate = 0.114, p<0.001) was an indirect effect through Aβ. Thirty percent of the total effect of APOE on mesial temporal tau tangles was mediated by Aβ.
Table 3.
Effect partition of age and APOE on tangles pathologies
| Mesial temporal tangles | Neocortical tangles | |||
|---|---|---|---|---|
| Pathway | Std Coeff | p-value | Std Coeff | p-value |
| APOE→Aβ→tangles | 0.078 | <0.001 | 0.114 | <0.001 |
| APOE→tangles | 0.179 | <0.001 | 0.190 | <0.001 |
| age→Aβ→tangles | 0.062 | <0.001 | 0.091 | <0.001 |
| age→tangles | 0.246 | <0.001 | 0.054 | 0.030 |
Std Coeff: standardized coefficient
Age, APOE, and neuropathologies on cognitive decline
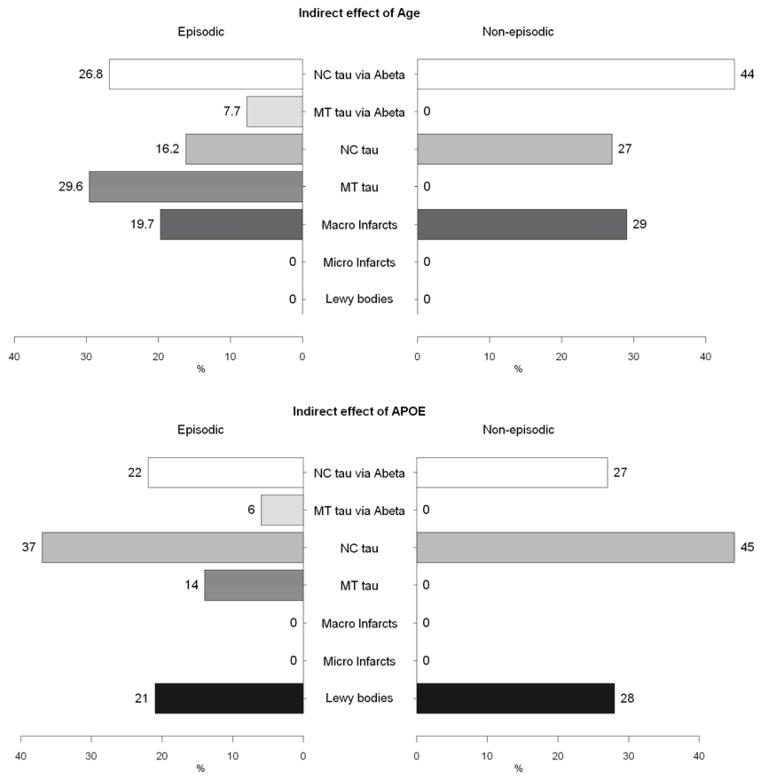
Finally, we examined the product of path coefficients along each pathway, which allows us to assess the relative contributions of the individual pathways that link age and APOE with cognitive decline (Table 4 and Figure 3). A substantial proportion of the age effect on decline in episodic memory worked through mesial temporal tau tangles (direct effect 30%, and indirect effect through Aβ 8%). In addition, a comparable proportion was attributable to Aβ and neocortical tau tangles (43%), and macroscopic infarcts explained the remaining 20%. On decline in non-episodic cognition, the effect through mesial temporal tau tangles was attenuated and no longer significant. As a result, the effect of age on decline in non-episodic cognition was shared by Aβ and neocortical tau tangle pathology (71%), as well as macroscopic infarcts (29%).
Table 4.
Effect partition of age and APOE on declines in cognition*
| Episodic | Non-episodic | |||
|---|---|---|---|---|
| Pathway | Std Coeff | p-value | Std Coeff | p-value |
| Age→Decline | −0.034 | 0.296 | −0.032 | 0.318 |
| Age→Neocortical Lewy bodies→Decline | −0.018 | 0.251 | −0.021 | 0.248 |
| Age→Microinfarcts→Decline | −0.003 | 0.567 | −0.005 | 0.361 |
| Age→Macroscopic infarcts→Decline | −0.028 | 0.013 | −0.026 | 0.015 |
| Age→Mesial temporal tangles→Decline | −0.042 | <0.001 | −0.015 | 0.130 |
| Age→Neocortical tangles→Decline | −0.023 | 0.031 | −0.024 | 0.029 |
| Age→Aβ→Mesial temporal tangles→Decline | −0.011 | <0.001 | −0.004 | 0.128 |
| Age→Aβ→Neocortical tangles→Decline | −0.038 | <0.001 | −0.040 | <0.001 |
| APOE→Decline | −0.019 | 0.544 | 0.009 | 0.791 |
| APOE→Neocortical Lewy bodies→Decline | −0.045 | 0.015 | −0.052 | 0.009 |
| APOE→ Microinfarcts→Decline | −0.001 | 0.741 | −0.001 | 0.704 |
| APOE→ Macroscopic infarcts→Decline | −0.011 | 0.205 | −0.010 | 0.202 |
| APOE→ Mesial temporal tangles→Decline | −0.031 | <0.001 | −0.011 | 0.132 |
| APOE→ Neocortical tangles→Decline | −0.079 | <0.001 | −0.083 | <0.001 |
| APOE→Aβ→Mesial temporal tangles→Decline | −0.013 | <0.001 | −0.005 | 0.126 |
| APOE→Aβ→Neocortical tangles→Decline | −0.048 | <0.001 | −0.050 | <0.001 |
Nonsignificant path coefficients were set to zero in estimating the percent contribution
Std Coeff: standardized coefficient
Figure 3.
Percent contributions of indirect effect of age (upper panel) and APOE (lower panel) through common neuropathologies on declines in episodic memory and non-episodic cognition.
Only 20% of the APOE effect on episodic decline was explained by Aβ and mesial temporal tau tangle pathology, while 59% was due to the effect of neocortical tau tangle pathology, either directly or indirectly through Aβ. The presence of neocortical Lewy bodies accounted for the rest of APOE effect on decline in episodic memory (21%). On the other hand, the effect of APOE on non-episodic cognitive decline was primarily driven by Aβ and neocortical tau tangles. Forty-five percent of the total effect of APOE on decline in non-episodic cognition was explained by direct effect of neocortical tau tangles, and 27% was due to an indirect effect of neocortical tau tangles through Aβ. The rest (28%) was explained by neocortical Lewy bodies. The contribution of infarcts to the relationship of APOE on decline in both domains was minimal.
DISCUSSION
In this study, we identified multiple pathways that link age and APOE with common neuropathologies and downstream cognitive decline using clinical and pathologic data from about 900 older people who completed up to 20 years of annual cognitive evaluations. It has been shown that specific cognitive domains might be differentially associated with neuropathologic indices, e.g. memory function with AD, executive function with vascular pathology, and visuospatial construction with LBD. Overall, our data suggest that common neuropathologies are significantly associated with declines in both episodic and non-episodic cognition. This finding explains the recent observation that mixed pathologies are the most common cause of AD dementia and a common cause of both amnestic and non-amnestic MCI (Sonner et al 2007, Schneider et al 2009, White 2009). Further, there was a little to no decline in both episodic memory and non-episodic cognition after adjusting for neuropathologies, which suggests that mean age-related declines in both aspects of cognition are attributable to common neuropathologies. The effect of age was primarily due to Aβ, tangle pathology and macroscopic infarcts, while the effect of APOE on cognitive decline was primarily due to Aβ, tangle pathology and neocortical Lewy bodies. Disentangling these complex relationships among age, APOE, AD and common neuropathologic indices with late life cognitive decline offers crucial insight into the pathophysiological mechanisms that underlie cognitive aging.
A recent finding suggests dual processes for AD pathology whereby mesial temporal lobe tangle pathology represents both APOE amyloid related AD process and a separate age related process or processes (Mungas et al 2013). Mesial temporal tangles are thought to have stronger effect on episodic memory than non-episodic cognition. Our data suggest that mesial temporal tangle pathology is indeed a result of two separate processes and it is exclusively associated with decline in episodic memory. Thus, in complement to the amyloid cascade hypothesis where tangle formation only occurs downstream of amyloid, the results render further support that there may be other pathogenic processes that stimulate tau phosphorylation and subsequently result in neurofibrillary tangle degeneration. Our finding has important implications for clinical-pathologic studies. Namely, neuropathologic lesions in temporal lobe structures, particularly hippocampus, were thought to be the earliest sign of AD. However, the new pathologic criteria for AD require amyloid deposition and persons with mesial temporal lobe tangles in the absence of amyloid do not meet AD pathologic criteria. Taken together, this suggests that clinical-pathologic studies may well wish to prioritize neocortical pathology rather than the hippocampus as the latter is populated by a more heterogeneous group of tangles. Our results also showed a small direct effect of age on neocortical tau tangle pathology. Mesial temporal tangles tend to ‘spread’ into the inferior temporal lobe in aging; and such an extension might explain this small direct effect of aging on neocortical tangles. This hypothesis will be addressed in future work.
The association of macroscopic infarcts with memory impairment and executive dysfunction has been reported previously (Lim et al 2009; Carey et al 2009). In this study we confirmed that macroscopic infarcts were associated with declines in both episodic memory and non-episodic cognition. In addition, our results extended these findings and revealed that macroscopic infarcts also operate as a separate pathway that links age to late life cognitive decline.
Prior work has shown that APOE is related to cognitive impairment primarily through Aβ deposition and tangles formation. We confirmed that a large proportion of APOE effect on late life cognitive decline depends on Aβ and tau tangles, regardless of the cognitive domain studied. In part, this effect is mediated through the cascade of APOE→ Aβ → tau tangles → cognitive decline, and the rest works directly through neurofibrillary tangle pathology, primarily in the neocortical regions. Several potential mechanisms at the cellular level explain the multiple contributions of APOE to the pathogenesis of AD (Huang et al 2004). One the one hand, apoE isoforms play differential roles in regulating Amyloid β clearance such that apoE4 inhibits Aβ clearance and/or stimulates Aβ deposition (Castellano et al 2011; Arold et al 2012). Separately, apoE isoforms also differ in their effects on the phosphorylation and aggregation of tau, resulting in formation of neurofibrillary tangles. Transgenic mice models have shown that carboxyl-terminal-truncated forms of apoE induce intracellular NFT-like inclusions in neurons containing PHtau, and apoE4 isoform is more susceptible to such truncation than apoE3 (Huang et al 2001, Ljungberg et al 2002, Harris et al 2003).
Our results also reveal an intriguing finding, namely that neocortical Lewy body disease relates APOE to cognitive decline. The findings in the literature addressing this association have been mixed. Several studies reported that apoE4 increased risk of dementia due to pure synucleinopathies (Tsuang et al 2013, Kobayashi et al 2011), suggesting a separate neurodegenerative mechanism by the apoE4 isoform. Another study, however, showed no evidence of association of APOE with Lewy body counts in four brain regions of mid frontal, superior temporal, inferior parietal, and cingulate gyrus (Wider et al 2012). In a secondary analysis, we tested whether there may be additional pathways that link APOE to neocortical Lewy bodies through amyloid beta and tau tangle pathologies. The result did not support indirect effects of APOE on neocortical Lewy bodies through these pathologies, suggesting that further investigation is needed to examine the potential mechanism that links APOE to neocortical Lewy body pathology.
This study has strengths and limitations. Both the ROS and MAP cohorts had over 90% follow-up rates among survivors and over 80% autopsy rates. Up to 20 years of longitudinal cognitive data were used to measure cognitive decline and all participants underwent standard and blinded neuropathologic assessment. Thus, the potential for bias is reduced and the reliability of clinical and pathologic measures is increased. Limitations should also be noted. First, only the most common neuropathologic indices were included in the analysis. Other age-related neuropathologies such as TDP-43 (Tremblay et al 2011), hippocampal sclerosis (Corey-Bloom et al 1997, Nelson et al 2012), and white matter lesions (Inaba et al 2011) have been shown to be associated with cognitive impairment and AD. We do not yet have sufficient data to fully incorporate these disease markers in our analysis. Second, we and others have reported that the relation of neuropathology to dementia varies by age (James et al 2012, Nelson et al 2011, Saava et al 2009). It will be important to consider these complex associations in future analyses. Third, our data were collected from volunteer cohorts.
Acknowledgments
The authors thank all the participants of the Religious Order Study and the Rush Memory and Aging Project, as well as the staff at the Rush Alzheimer’s Disease Center for this work. This research was supported by National Institute on Aging grants R01AG17917, R01AG34374, R01AG15819, and P30AG10161.
Footnotes
DISCLOSURES
The authors have no conflicts to disclose that relate to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, Vinters HV, Cornwell LB, Saing T, Cole GM, Gylys KH. Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta Neuropathol. 2012;123(1):39–52. doi: 10.1007/s00401-011-0892-1. Epub 2011 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S, Bird T, Goate A, Farlow MR, Diaz-Arrastia R, Bennett DA, Graff-Radford N, Boeve BF, Sweet RA, Stern Y, Wilson RS, Foroud T, Ott J, Mayeux R National Institute on Aging Late-Onset Alzheimer’s Disease Genetics Study. Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012 May 8;78(19):1464–71. doi: 10.1212/WNL.0b013e3182553c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Cur Alzheimer Res. 2012;9:630–647. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Cur Alzheimer Res. 2012;9:648–665. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology 2010. 2010;34(1):43–9. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–78. doi: 10.1016/0197-4580(95)00021-6. discussion 8–84. [DOI] [PubMed] [Google Scholar]
- Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, Weiner MW, Chui HC. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE Isoforms differentially regulate brain amyloid-beta peptide clearance. Science Translational Medicine. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton B, Larson EB, Baker LD, Craft S, Crane PK, Millard SP, Sonnen JA, Montine TJ. Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis. 2013;36(4):699–709. doi: 10.3233/JAD-130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, Thal LJ. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48(1):154–60. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- Dubé JB, Johansen CT, Robinson JF, Lindsay J, Hachinski V, Hegele RA. Genetic determinants of “cognitive impairment, no dementia”. J Alzheimers Dis. 2013;33(3):831–40. doi: 10.3233/JAD-2012-121477. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28(50):13542–50. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, Weisgraber KH, Mucke L, Mahley RW, Huang Y. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100(19):10966–71. doi: 10.1073/pnas.1434398100. Epub 2003 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98(15):8838–43. doi: 10.1073/pnas.151254698. Epub 2001 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23(3):189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, White L, Bell C, Chen R, Petrovitch H, Launer L, Abbott RD, Ross GW, Masaki K. White matter lesions on brain magnetic resonance imaging scan and 5-year cognitive decline: the Honolulu-Asia aging study. J Am Geriatr Soc. 2011;59(8):1484–9. doi: 10.1111/j.1532-5415.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62(4):389–97. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, Shaw LM, Bernstein MA, Petersen RC, Weiner MW, Knopman DS. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68(12):1526–35. doi: 10.1001/archneurol.2011.183. Epub 2011 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307(17):1798–800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113(2):107–17. doi: 10.1007/s00401-006-0156-7. Epub 2006 Nov 7. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Galvin JE. Longitudinal changes in cognition in Parkinson’s disease with and without dementia. Dement Geriatr Cogn Disord. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170(3):331–42. doi: 10.1093/aje/kwp154. Epub 2009 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Tateno M, Park TW, Utsumi K, Sohma H, Ito YM, Kokai Y, Saito T. Apolipoprotein E4 frequencies in a Japanese population with Alzheimer’s disease and dementia with Lewy bodies. PLoS One. 2011;6(4):e18569. doi: 10.1371/journal.pone.0018569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29(10):1587–90. doi: 10.1016/j.neurobiolaging.2007.03.008. Epub 2007 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Schneider JA, Bennett DA. Estimation of the mediation effect with a binary mediator. Stat Med. 2007;26(18):3398–414. doi: 10.1002/sim.2730. [DOI] [PubMed] [Google Scholar]
- Lim C, Alexander MP. Stroke and episodic memory disorders. Neuropsychologia. 2009;47:3045–3058. doi: 10.1016/j.neuropsychologia.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lipnicki DM, Sachdev PS, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Kang K, Lux O, Mather KA, Brodaty H. Risk factors for late-life cognitive decline and variation with age and sex in the sydney memory and ageing study. PLoS One. 2013;8(6):e65841. doi: 10.1371/journal.pone.0065841. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Dayanandan R, Asuni A, Rupniak TH, Anderton BH, Lovestone S. Truncated apoE forms tangle-like structures in a neuronal cell line. Neuroreport. 2002;13(6):867–70. doi: 10.1097/00001756-200205070-00026. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA, Snowdon DA, Markesbery WR. The effect of APOE-epsilon4 on dementia is mediated by Alzheimer neuropathology. Alzheimer Dis Assoc Disord. 2009;23:152–7. doi: 10.1097/wad.0b013e318190a855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Tractenberg R, Schneider JS, Crane PK, Bennett DA. A Two-process Model for Neuropathology of Alzheimer’s Disease. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.08.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66–79. doi: 10.1111/j.1750-3639.2008.00244.x. Epub 2008 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(Pt 5):1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, Smith CD, Patel E, Markesbery WR. Brains with mesial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(7):774–84. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Reed BR, Mungas DM, Kramer JH, Ellis W, Vinters HV, Zarow C, Jagust WJ, Chui HC. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain. 2007;130(Pt 3):731–9. doi: 10.1093/brain/awl385. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer RF. Estimating the temporal evolution of Alzheimer’s disease pathology with autopsy data. J Alzheimers Dis. 2012;32(1):23–32. doi: 10.3233/JAD-2012-120430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C Medical Research Council Cognitive Function Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke. 2005;36(5):954–9. doi: 10.1161/01.STR.0000160747.27470.2a. Epub 2005 Mar 17. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135:3005–3914. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. Epub 2011 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, St-Amour I, Schneider J, Bennett DA, Calon F. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol. 2011;70(9):788–98. doi: 10.1097/NEN.0b013e31822c62cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64(2):168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, Kramer P, Woltjer R, Trojanowski JQ, Weintraub D, Chen-Plotkin AS, Irwin DJ, Rick J, Schellenberg GD, Watson GS, Kukull W, Nelson PT, Jicha GA, Neltner JH, Galasko D, Masliah E, Quinn JF, Chung KA, Yearout D, Mata IF, Wan JY, Edwards KL, Montine TJ, Zabetian CP. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–8. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18(3):713–25. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- Wider C, Ross OA, Nishioka K, Heckman MG, Vilariño-Güell C, Jasinska-Myga B, Erketin-Taner N, Rademakers R, Graff-Radford NR, Mash DC, Papapetropoulos S, Duara R, Uchikado H, Wszolek ZK, Farrer MJ, Dickson DW. An evaluation of the impact of MAPT, SNCA and APOE on the burden of Alzheimer and Lewy body pathology. J Neurol Neurosurg Psychiatry. 2012;83(4):424–429. doi: 10.1136/jnnp-2011-301413. Published online 2012 January 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Nilbrink T, Nordfjäll K, Hultdin J, Sleegers K, Van Broeckhoven C, Nyberg L, Roos G, Nilsson LG, Adolfsson R, Norrback KF. APOE ε4 is associated with longer telomeres, and longer telomeres among ε4 carriers predicts worse episodic memory. Neurobiol Aging. 2012;33(2):335–44. doi: 10.1016/j.neurobiolaging.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59(7):1154–60. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology. 2003;23(4):311–7. doi: 10.1046/j.1440-1789.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- Yang FM, Grigorenko A, Tommet D, Farias ST, Mungas D, Bennett DA, Jones RN, Crane PK. AD pathology and cerebral infarctions are associated with memory and executive functioning one and five years before death. J Clin Exp Neuropsychol. 2013;35(1):24–34. doi: 10.1080/13803395.2012.740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle P, Schneider JA, Segawa E, Wilson RS, Leurgans S, Bennett DA. APOE ε4, Alzheimer’s Disease Pathology, Cerebrovascular Disease, and Cognitive Change Over the Years Prior to Death. Psych Aging Advance online publication. 2013 doi: 10.1037/a0031642. [DOI] [PMC free article] [PubMed] [Google Scholar]