Abstract
Background
Few studies have explored how noise might contribute to social health inequalities, and even fewer have considered infant mortality or its risk factors as the health event of interest.
In this paper, we investigate the impact of neighbourhood characteristics - both socio-economic status and ambient noise levels - on the spatial distribution of infant mortality in the Lyon metropolitan area, in France.
Methods
All infant deaths (n = 715) occurring between 2000 and 2009 were geocoded at census block level. Each census block was assigned multi-component socio-economic characteristics and Lden levels, which measure exposure to noise. Using a spatial–scan statistic, we examined whether there were significant clusters of high risk of infant mortality according to neighbourhood characteristics.
Results
Our results highlight the fact that infant mortality is non-randomly distributed spatially, with clusters of high risk in the south-east of the Lyon metropolitan area (RR = 1.44; p = 0.09). After adjustments for socio-economic characteristics and noise levels, this cluster disappears or shifts according to in line with different scenarios, suggesting that noise and socio-economic characteristics can partially explain the spatial distribution of infant mortality.
Conclusion
Our findings show that noise does have an impact on the spatial distribution of mortality after adjustments for socio-economic characteristics. A link between noise and infant mortality seems plausible in view of the three hypothetical, non-exclusive, pathways we propose in our conceptual framework: (i) a psychological pathway, (ii) a physiological disruption process and (iii) an unhealthy behaviours pathway. The lack of studies makes it is difficult to compare our findings with others. They require further research for confirmation and interpretation.
Keywords: Noise exposure, Neighbourhood deprivation, Infant mortality, Spatial analysis
Background
Over the past 20 years, the literature has confirmed that, in developed countries, the leading causes of neonatal morbidity and mortality are related to various adverse pregnancy outcomes such as pre-term birth (PTB) [1-5], congenital malformation [6,7], low birth weight (LBW) [8] and intrauterine growth retardation (IUGR) [8,9]. Moreover, socio-epidemiological research has documented a social gradient in stillbirth and infant mortality, [10-14] despite long term improvements in mortality rates [13-15]. It is well established that both infant mortality and its risk factors are more common among women of low socio-economic status [16,17]. A wide literature describes various deprivation measures related to infant mortality and its determinants as well as to composite indices [16,18-20] or proxy variables of socio-economic characteristics such as income [21-24], education level [21,23,25], unemployment [25,26], occupation category [25], percentage of persons below the poverty level [25] or renting their house [27], percentage of immigrants [22]. In addition to socio-economic and demographic characteristics, environmental factors have been also reported to influence neonatal and infant mortality [28-35].
In order to further explain these health inequalities, researchers on infant mortality and its determinants have advanced the hypothesis that differential environmental exposures might add to role of social determinants [28-35]: “deprived populations are more likely to be exposed to a higher number of environmental nuisances or to a higher level of environmental exposure such as ambient air pollution” [28,31,32,35-37]. In other words, socially disadvantaged inhabitants and ethnic minority populations are more likely to live near traffic or industrial activity than better-off residents. Some authors concluded that the area level effect of air pollution modifies the socio-economic patterns of pre-term birth [28,31,34,35], low birth weight [31], and infant mortality [32,33]. Overall, these studies show that exposure to ambient air particulates yields (i) a three-fold increase in risk of pre-term birth for an increase in PM10 in low-income groups [28], (ii) a significant increase in the risk of all-cause mortality only among infants with low and medium SES [32].
The vast majority of these studies considered air pollution to be the principal environmental nuisance. Few studies have explored how noise might contribute to social health inequalities, and even fewer have considered infant mortality or its risk factors as the health event of interest. Noise exposure is also unevenly distributed across socio-economic groups [38,39] in such a way that exposure to traffic-related noise is particularly high for low socio-economic groups [38] and disadvantaged neighbourhoods [39]. Moreover, noise is increasingly considered to be an important public health problem [40,41]. Residential noise represents a major environmental nuisance affecting a wide population. In the European Union, the World Health Organization (WHO) has estimated that during the daytime, approximately 40% of the population is exposed to residential noise levels in excess of 55 dB (A) while more than 30% is also exposed to the same noise levels at night [41]. Authors have suggested that long-term exposure to excessive noise affects health and well-being through sleep disturbance [42], psychological stress [43], cardiovascular disease [44,45] as well as adverse pregnancy outcomes such as low birth weight [46]. Thus, the consideration of noise as an environmental hazard may improve our understanding of the mechanisms underlying social health inequalities.
In this context, the aim of the present study is to investigate whether spatial clustering of infant mortality exists, as well as whether adjusting for noise exposure and/or socio-economic deprivation can explain any clustering measured at French census block level, in the Lyon metropolitan area. The results of our spatial investigation will be put into the perspective of a theoretical framework of different possible and plausible pathways that may relate adverse pregnancy outcomes to neighbourhood characteristics.
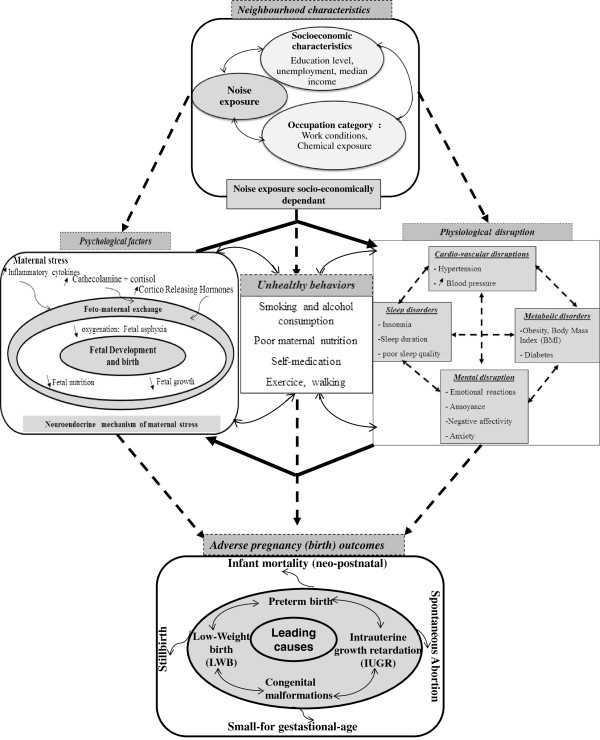
Having reviewed the epidemiological and experimental research, we constructed a theoretical model of the underlying mechanisms in which chronic exposure to noise might related to various adverse pregnancy outcomes (Figure 1). This theoretical model highlights three main potential pathways: (i) psychosocial factors as a plausible biologic pathway, based mainly on the role of stress generated by deprived socio-economic status or by exposure to occupational and/or neighbourhood noise; (ii) physiological disruption factors as a hypothetical pathway, based on many physiological disorders related to adverse pregnancy outcomes and generated by the socio-economic characteristics or occupational noise; and (iii) health behavioural disorders related to both socio-economic characteristics and exposure to noise.
Figure 1.
A conceptual model to explain the overlapping relationship between neighborhood characteristics (including exposure to noise and socioeconomic status) and adverse pregnancy outcomes, including neonatal mortality.
The paper is structured as follows. Firstly, we elaborate a theoretical model (Figure 1) that aims to explain the mechanisms through which neighbourhood characteristics may relate to adverse pregnancy outcomes. Secondly, we describe the data and the spatial analysis strategy we used. Finally, our findings are discussed using a theoretical model which we briefly introduce below, and elaborate upon in the discussion section.
Because of limited literature linking infant death to noise, our model includes several adverse pregnancy outcomes, rather than confining itself to infant mortality: pre-term birth, low birth weight and Intrauterine Growth Restriction, which can be viewed as determinants of infant mortality [1,5].
Methods
Study setting and statistical units
The setting of our study is the Lyon metropolitan area, an urban area of 515.96 km2 located in the Rhône-Alpes region of central-eastern France, with a population of 1,249,216 inhabitants in 1999. Analysis was conducted at French census block level (called IRIS by INSEE, the French National Statistics Institute). Our study area was sub-divided into 510 census blocks/IRIS, each having 2,000 inhabitants on average.
Health data
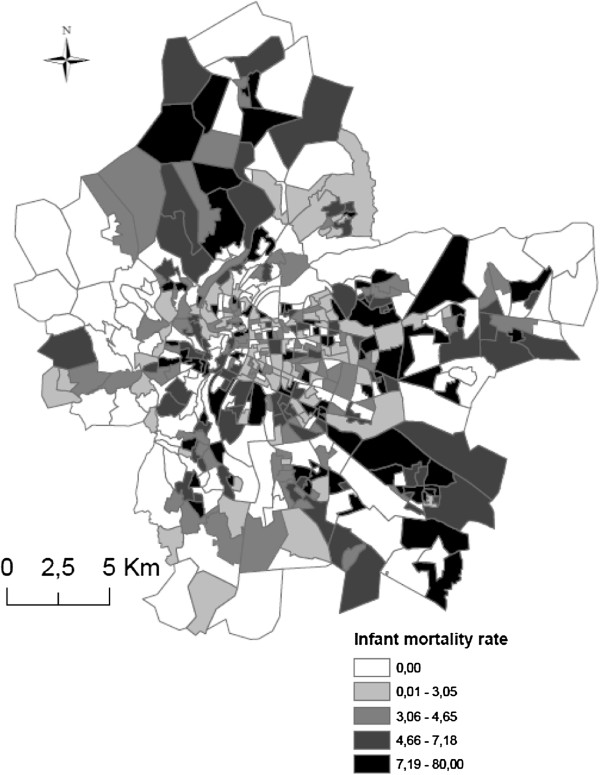
The dependant variable is infant mortality, defined as all death cases aged <1 year. Data comes from death certificates across all local municipalities. Cases were geo-coded using the parental postal address with the CAZU software from INSEE, which assigns street names and numbers to census blocks. Some cases could not be geo-coded dues to missing. The exhaustiveness of the death data is 96,5%, by comparing the total number of cases collected from the death and birth registries of the study area City Halls with the cases obtained from the National Epidemiological Center for Medical Causes of Death (CepiDc-Inserm). Overall, we collected data on 715 infant death cases in the Lyon metropolitan area between January 2000 and December 2009, including 161 census blocks having 0 deaths. The spatial display of the number of infant deaths per census block is shown in Figure 2. The CNIL (French National Commission for Digitalized Information and Liberty) gave its permission to retrieve geocode and analyze the health data.
Figure 2.
Mapping of spatial display of infant mortality cases across the Lyon metropolitan area.
Socio-economic data
Socio-economic and demographic data were obtained from INSEE (1999) at census block level (Table 1). In order to characterize the socio-economic neighbourhood level, we used: i) in a first step, a deprivation index, and ii) in a second step, an array of specific socio-economic variables (including education level, employment and occupation status, and housing characteristics). Principal-component analysis was used to create the deprivation index based on the approach described in Lalloué et al. [47].
Table 1.
Definition of the socioeconomic characteristics considered as potential predictors of the geographic distribution of infant mortality
| Data | Domain | Variables | |
|---|---|---|---|
|
Proxy socio-economic variables |
Occupation |
- Proportion of blue-collar workers in the labor force |
|
| - Proportion of managers in the labor force | |||
| Education |
- People aged 15 years or older with a higher education degree |
||
| - People aged 15 years or older with at least a lower tertiary education | |||
| - People aged 15 years or older who did not go beyond an elementary education | |||
| Immigration status |
- Proportion of foreigners in the total population |
||
| Unemployment |
- Proportion of unemployed people in the labor force |
||
| Housing condition |
- Subsidised housing among all primary residences |
||
| |
Characteristics |
Description |
Mean [95% CI] |
| Classes of deprivation | Group 1: low deprivation |
Census blocks with high median income, low proportion of households without a car, low proportion with non-owner-occupied primary residences |
30935 € [29524–32345] |
| 9.8% [8.6-11] | |||
| 31.6% [28.5-34.8] | |||
| Group 2: moderate deprivation |
Census blocks with median income average, medium proportion of households without a car, medium proportion with non-owner-occupied primary residences |
23232 € [22627–23838] |
|
| 27.4% [25.5-29.4] | |||
| 59.2% [56.7-61.7] | |||
| Group 3: high deprivation | Census blocks with low median income, high proportion of households without a car, high proportion with non-owner-occupied primary residences | 17072 € [16377–17767] |
|
| 33.2% [30.7-35.6] | |||
| 78.2% [74.7-81.7] | |||
Noise exposure data
Noise exposure modelling
In 2007, in accordance with requirements from the European Environmental Noise Directive (END, 2002/49/EC) [48], noise nuisances were measured and modelled across the Lyon metropolitan area by the urban Community of Grand Lyon using the GIpSynoise software [49].
The model used follows two stages. First, an acoustic calculation using CadnaA (Computer Aided Noise Abatement), which is a model-based computer program developed by DataKustik (Greifenberg, Germany) [50]; and second, generation of people exposure, using GipSynoise.
The calculation model (CadnaA) incorporates four specific methods recommended by END for calculating current noise levels: (i) NMPB/XPS31-133 (French national computation method – 1996) for road noise; (ii) NMPB –Fer (NF S31-133 :2011 for railway noise [51]; (iii) ISO9613 (Acoustics - Attenuation of sound during propagation outdoors-) for industrial noise; and (iv) ECAC.CEAC DOC.29 (Report on the Standard Method of Computing Noise Contours around Civil Airports) for aircraft noise. These estimations require various input data including information on road, industry, aircraft and railway characteristics, topography, meteorological factors and other various data on the phenomena of sound reflection and diffraction. Using these input data, the acoustic modelling software estimates noise levels with a spatial resolution of 10 × 10 meters at 4 metres above ground level. Based on this information, a noise value corresponding to the most exposed façade is assigned to each building. This value is called ‘building-noise-level’ and, in this work, it is a value including noise coming from all source types ((1) road traffic, (2) aircraft traffic, (3) industries and (4) railway.).
Noise exposure calculation at census block level
The metric used to characterize noise in each census block was the European standard Lden indicator (day-evening-night level) - an assessment of daily exposure over a 24 hour period; this indicator takes into account the increased sensitivity of residents to noise during the evening and night by adding an extra factor of 5 and 10 dB (A), respectively, during the corresponding periods [41].
For each census block, we assessed exposure to noise by averaging the Lden estimates of all inhabited buildings within the census block. This approach is derived from the definition of the total noise load of a population Lden,population as given in the European Environmental Agency Technical report [52]. The French Scientific and Technical Centre for Building (CSTB) has calculated a mean weighted noise level per census block, defined as:
Pop Batj.i: population size of residential building in census block/IRISi,
Pop IRISi: total population of census block/IRISi,
LDEN_AL, bat j,i: building-noise level of building j in census block/IRISi,
IRIS i: Only populated census blocks/IRIS
N bat,i: Total number of buildings in census block/IRIS i
N IRIS: Total number of census blocks/IRIS.
Analysis
Spatial methodology
To evaluate the spatial implications of adjustment for neighbourhood characteristics (noise and deprivation level) on the spatial relocation and provide an explanation of infant mortality risk across Lyon Metropolitan area, the most appropriate approach is spatial method using the spatial scan statistic [53]. We investigated presence, relocation and explanation of spatial distribution of the risk of infant mortality by cluster analysis - the spatial scan statistic implemented in the SaTScan software [54]. This approach, which is used in an increasing number of applications in the field of spatial epidemiology [55], allows exploration of the presence of high risk infant mortality clusters (named ‘most likely clusters’) and their spatial location. The number of cases in each census block is assumed to follow a Poisson distribution.
The procedure works as follows: a circle or windows of variable radius (from zero up to 50% of the population size [56]) is placed at every centroid of the census block and moves across the whole study area to compare the infant mortality rate in the windows with what would be expected under a random distribution. The identification of the most likely clusters is based on a likelihood ratio test [57] with an associated p-value obtained using Monte Carlo replications [58].
Analytical strategy and results interpretation
In this approach, the null hypothesis (H0) tested is that risk of infant mortality is the same throughout the study area; in other words, the expected infant mortality rate would be randomly distributed in space [54,56]. The alternative hypothesis (H1) is that there is an elevated risk of infant mortality within the cluster (one or several geographically close census blocks) in comparison with census blocks outside the cluster.
When the test is statistically significant, this means that the infant mortality rate is not randomly distributed in the Lyon Metropolitan area, i.e. that the identified cluster of census blocks presents a significant increase in infant mortality risk in comparison with census blocks located outside the cluster [59].
The models were adjusted on one or more co-variables, and according to the Kulldorff studies [57], several criteria were used to reject, or not, the H0 hypothesis according to the cluster’s localization and statistical significance, and the likelihood ratio value of each model:
– When, after adjustment, the most likely cluster remains in the same location, (whether or not this location is significant) and its likelihood ratio decreases, the interpretation is that the variable(s) incorporated in the model can partially explain the excess risk [56];
– when the most likely cluster shifts (changes in the location of the centroid of the cluster), this suggests that the covariate(s) in the model can explain the cluster’s excess risk [56]. In addition, another cluster is identified;
– when the most likely cluster disappears totally, it means that the adjusted infant mortality risk is distributed randomly in space.
Thus, spatial analyses were performed in three stages (step by step):
(i) unadjusted analysis, to identify and localize the most likely cluster/s of high risk of mortality
(ii) adjusted analysis for noise exposure or socio-economic neighbourhood (deprivation index and the set of socio-economic variables)
(iii) adjusted analysis for noise and socio-economic characteristics at neighbourhood level (including interaction between the two variables)
To incorporate covariates in the model, we classified each census block as having a high, moderate or low level of socio-economic status and noise exposure. When we introduced both noise and socio-economic levels, we also included an interaction term in the model. Because the SaTScan does not allow for an interaction term to be accommodated in the model, we created several dummy variables combining the deprivation and the noise categories.
Results
Descriptive results
Figure 3A shows spatial distribution of the socio-economic deprivation index at census block level. This map highlights the fact that the wealthiest census blocks are located in the very central and peripheral parts of the study area, while the most deprived blocks are in the central-eastern and southern parts of the metropolitan area.
Figure 3.
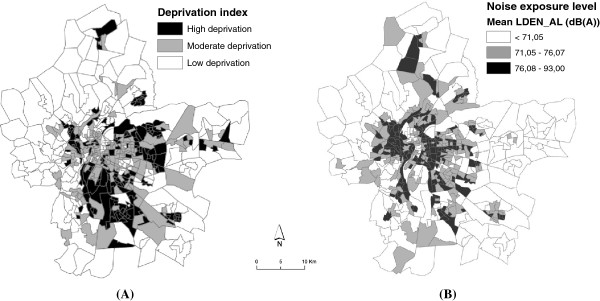
Spatial distribution of the neighborhood socioeconomic index (A) and spatial distribution of neighborhood noise exposure levels modeled across the Lyon metropolitan area (B).
Figure 3B shows spatial distribution of exposure to neighbourhood noise at census block level in the Lyon metropolitan area. We divided noise levels into tertiles, and observed that census blocks with high noise levels (>71 dB(A)) are concentrated in the central and eastern parts of the metropolitan area, whereas census blocks with the lowest levels (<71 dB(A)) are found in the ring of the city of Lyon. Typically, medium deprivation neighbourhoods had a slightly higher mean noise level. This observation was confirmed by our descriptive analysis, which shows a significant difference of exposure to neighbourhood noise between our 3 deprivation categories (mean noise level = 69.83 dB(A) versus 68. 24 dB(A) and 68.83 dB(A) for medium, low and high deprivation respectively).
Spatial results
The statistical results are summarized in Table 2 (unadjusted analysis-stage1), 3 and 4 (adjusted analysis stage 2 and 3 respectively).
Table 2.
The most likely and secondary clusters resulting from the unadjusted analysis –stage1
| Confounders | Radius (meter) | Census block included | Expected cases | Observed cases | RR a | LLr b | P-value | |
|---|---|---|---|---|---|---|---|---|
|
Most likely cluster |
None |
6966.29 |
64 |
116.35 |
156 |
1.44 |
7.52 |
0.09 |
| Secondary cluster | None | 0 | 1 | 1 | 7 | 3.80 | 4.16 | 0.86 |
a LLr: log likelihood ratio.
b RR: Relative Risks.
Stage 1-Identification of high risk infant mortality clusters
Figures 4A and B reveal the location of the most likely cluster and of a small secondary one, respectively. The most likely cluster, in the south-eastern Lyon metropolitan area, has one risk that is 1.44 greater than in the rest of the study area (p-value = 0.09, borderline significant). This cluster comprises 64 census blocks and hosts around 24, 076 inhabitants. The small cluster identified in the immediate northern part of the metropolitan area (RR = 3.8) was not statistically significant (p-value = 0.86) (Table 2).
Figure 4.
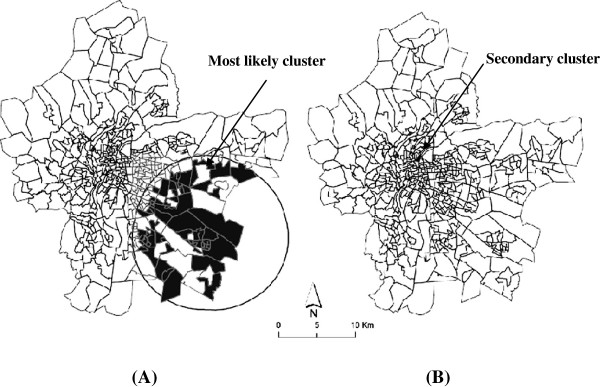
Mapping of the most likely cluster (A) and secondary cluster (B) of infant mortality. Legend: Dark area identify census block included in the most likely cluster. This means that the centroid for these blocks falls within the cluster.
Stage 2 results
Adjusted scan statistical analysis is detailed below according to the variables for which the model was adjusted.
Noise exposure and spatial distribution of infant mortality
After adjusting on noise, we found the same most likely cluster (p = 0.10, RR = 7.14), with a slightly lower likelihood value, which decreased from 7.525 to 7.142 (Table 3). We can conclude that noise alone does not explain the excess of infant mortality risk observed in the south-eastern part of the Lyon metropolitan area.
Table 3.
The most likely clusters resulting from the adjusted analysis –stage2
| Confounders | Radius (meter) | Censusblock included | Expected cases | Observed cases | RR a | LLr b | P-value | |
|---|---|---|---|---|---|---|---|---|
|
Noise exposure |
|
|
|
|
|
|
|
|
|
Mean Lden level |
6966.29 |
64 |
117.00 |
156 |
1.43 |
7.14 |
0.10 |
|
|
Socioeconomic characteristics |
|
|
|
|
|
|
|
|
|
Unemployment |
6966.29 |
64 |
159.00 |
156 |
1.40 |
6.36 |
0.32 |
|
|
Immigration status |
6966.29 |
64 |
121.00 |
156 |
1.37 |
5.52 |
0.50 |
|
|
Housing conditions |
2795.19 |
8 |
13.00 |
29 |
2.25 |
7.17 |
0.10 |
|
|
% blue-collar workers |
2795.19 |
8 |
15.00 |
29 |
1.91 |
4.77 |
0.60 |
|
|
Education |
2795.19 |
8 |
15.00 |
29 |
1.94 |
5.02 |
0.70 |
|
| Neighborhood deprivation | 2795.19 | 8 | 14.70 | 29 | 1.98 | 5.58 | 0.36 | |
a LLr: log likelihood ratio.
b RR: Relative Risks.
Unemployment, immigrant status and spatial distribution of infant mortality
Contrariwise, when adjusted on socio-economic variables, such as unemployment levels (proportion of unemployed people) or immigrant status (proportion of foreigners in the total population), the most likely cluster remained the same size (Figure 4A), with a relatively larger decrease in the likelihood ratio (the value decreases from 7.14 to 6.36 and 5.52 respectively) (Table 3).
The cluster was no longer significant (p = 0.3 and 0.5 respectively), indicating that unemployment and immigration status could explain a relatively large amount of the excess of infant mortality observed in the south-eastern area, in comparison with noise alone.
Occupation, housing conditions, education, neighbourhood deprivation level and spatial distribution of infant mortality
Adjusting on housing conditions (characterized by the variable “proportion of subsidised housing among all primary residences”), occupation (% blue-collar workers) or education level (% people over 15 years having a higher-education degree;% people over 15 years with at least a lower tertiary education;% people over 15 years who did not go beyond an elementary education) resulted in the most likely cluster being smaller in size, though located in the same zone (Figure 4A). These clusters where the excess risk of infant mortality span from 1.91 to 2.25 (Table 3, details not shown for all variables) consist of 8 census blocks with about 2,890 inhabitants.
The centroid of the cluster shifted (see Figure 5A) and the likelihood ratio decreased from 7.52 to, successively, 7.17, 4.77, 5.58 and 5.02 (Table 3) when the model included housing, occupation, deprivation or the education neighbourhood levels, respectively , indicating that these socio-economic characteristics explain a great part of the excess of infant mortality observed in the unadjusted analysis. The excess risk remaining to be explained becomes not significant (Table 3). The same result was obtained (Figure 5A) when more than one dimension of socio-economic neighbourhood characteristics (such as, for example, housing and education or housing and occupation) was used in the adjusted model (data not shown).
Figure 5.
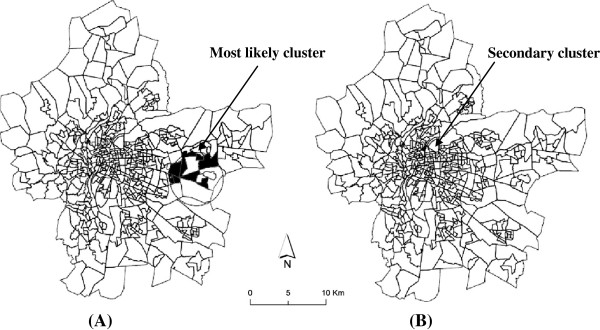
Spatial shift the most likely cluster (A) and secondary cluster (B) of infant mortality after adjustment. Legend: Dark area identify census block included in the most likely cluster. This means that the centroid for these blocks falls within the cluster.
At this stage, occupation, housing conditions, education, and the neighbourhood deprivation level reduce the LLr to a larger degree than unemployment or the immigrant status; these variables explain a relatively large amount of the excess of infant mortality observed in the south-eastern area in comparison with unemployment or immigrant status.
Stage 3 results: noise, neighbourhood socio-economic characteristics and spatial distribution of infant mortality
After adjustment for both noise and socio-economic characteristics, in stage 3 of the analysis, the most likely cluster observed in the unadjusted analysis (Figure 4A) now became limited to 8 census blocks (Figure 5A) (with P value from 0.18 to 0.78 and RR from 1.94 to 2.2, see Table 4), yet in the same general location but with the centroid of cluster shifted; also, the likelihood ratio decreased and infant mortality rates did not vary significantly, irrespective of the combination of variables included in the model (Table 4).
Table 4.
The most likely clusters resulting from adjusted analysis –stage 3
| Confounders | Radius (meter) | Census block included | Expected cases | Observed cases | RR a | LLr b | p Value |
|---|---|---|---|---|---|---|---|
|
Noise and unemployment |
2795.19 |
8 |
15.00 |
29 |
1.97 |
5.26 |
0.49 |
|
Noise and immigration status |
2795.19 |
8 |
15.60 |
29 |
1.90 |
4.75 |
0.78 |
|
Noise and housing conditions |
2795.19 |
8 |
13.40 |
29 |
2.21 |
6.92 |
0.18 |
| Noise and neighborhood deprivation | 2795.19 | 8 | 15.30 | 29 | 1.94 | 5.03 | 0.60 |
a LLr: log likelihood ratio.
b RR: Relative Risks.
In our study, the significant finding is that while SES had some impact on the LLR of the most likely cluster alone (as shown in Table 3), further adjustment for noise (in Table 4) reduces the LLr to a larger degree than with socio-economic level alone; and this is also larger than the effect of controlling for noise alone.
Discussion
Our analysis reveals a spatial aggregation of infant mortality located in the south-east of the Lyon metropolitan area. This means that infant mortality rates are not randomly distributed across the study area. After controlling neighbourhood characteristics, the cluster of high risk disappears or shifts according to different scenarios combining socio-economic characteristics and noise neighbourhood levels. Our findings suggest that these factors can partially explain the excess risk of infant mortality. According to our conceptual model (Figure 1), several pathways may explain these associations.
Noise, socio-economic characteristics and infant mortality
Our study shows that noise contributes to the explanation of spatial distribution of infant mortality beyond SES characteristics, and only after first controlling for SES. This leads us to suspect an effect of chronic exposure to noise. Few studies have explored the relationship between neighbourhood noise and infant mortality – making it difficult to compare our findings with those of others. Our observation is however consistent with previous works which explored occupational and/or aircraft noise and adverse pregnancy outcomes such as perinatal mortality [60], spontaneous abortions [61], pre-term birth [62,63] and low birth weight [64,65] - some of these associations being more pronounced after adjustment on socio-economic condition [64]. These findings were not reported in other papers [66,67]. Noise alone does not seem to be an abortion trigger, yet when combined with occupational characteristics and socio-economic category, Nurminen and Kupra found a more than two-fold excess risk of threatened abortion [68,69].
More recently, both animal and human studies have become more consistent concerning pregnancy outcomes. The Committee on understanding pre-term birth and assuring healthy outcomes [70] provided the theoretical basis for understanding the mechanisms whereby noise could affect pregnancy outcomes. These different pathways were detailed in our conceptual model (see Figure 1), which shows that both neighbourhood socio-economic (described in section above) and noise levels are separately related to adverse pregnancy outcomes by several pathways.
The principal pathway by which noise affects adverse pregnancy outcomes operates through psychosocial factors that elicit stress. Human and animal studies demonstrate that subjects exposed to stressors during pregnancy experience a greater risk of spontaneous abortion and of low birth weight [71-74]; also, an increase in stress hormone levels (cathecolamine, cortisol) was found in several studies in workers exposed to noise [74-78]. Such findings were used to explain the results of ecological studies on residents living near airports [46,65,79-81].
A second pathway by which noise could alter pregnancy outcomes and result in pre-term birth [82-84] or foetal growth restriction [85] is physiological disruption, a term that refers mainly to sleep disorders such as insomnia, shorter sleep duration and poor sleep quality. Laboratory experiments and epidemiology surveys were two major approaches employed to assess the effect of noise on sleep (see reviews [42,86,87]). Overall, these studies report that sleep disturbance is significantly more frequent in urban populations exposed to traffic noise above 65 Leq dB (A) [88,89] and among populations living near airports [90]. The prevalence of insomnia was found to be higher among inhabitants living closest to busy highways [91]. While experimental studies suggest a hypothetical physiological mechanism for the effect of noise on sleep (see review by Perron et al., 2012) [42], the mechanism underlying the association between sleep and adverse pregnancy outcomes remains unclear. Okun et al. in 2009 proposed a conceptual model involving oxidative stress, endothelial dysfunction and inflammation [92].
Furthermore, the relevant finding emerging from our conceptual model shows an overlap of pathways between neighbourhood characteristics. These suggest that noise effects may add to socio-economic factors on adverse pregnancy outcomes according to two main hypotheses (described below): psychological factors as a biologic plausible pathway and physiological disruption as a hypothetical pathway.
Psychological factors as a plausible biologic pathway
The role played by stress generated by deprived socio-economic status [93] or occupational and/or neighbourhood noise [46] is well documented. However, despite substantial literature linking psychological factors to adverse birth outcomes, little research has examined potential biological mechanisms to explain these associations [94-96]. Through neuroendocrine and immune mechanisms, the pathway proposed in our model is that chronic stress triggers a series of biological events, through activation of the central autonomic nervous system [97]. As shown in the first part of our model, maternal stress has been implicated in the production of catecholamine, cortisol [46] and inflammatory cytokines [74] in which were found to be increased in both mother and foetus. The release of catecholamine may alter foeto-maternal exchanges by increasing uterine contractions, blood pressure, vasoconstriction of placental vessels and reducing uterine blood flow [46,68,98,99]. In turn, limited foeto-maternal exchanges may affect foetal nutrition and/or oxygenation, and subsequently foetal growth. Therefore, exposure to noise may result in foetal asphyxia [72,100] and elicit both pre-term birth [101,102] and foetal growth restriction [96].
More recent research suggests that the Cortico-Releasing Hormone (CRH) stimulates prostaglandin and oxytocin, the mediators of uterine contraction; therefore it can cause pre-term labour [96,103]. Yet, overall, the biologic pathway underlying stress-induced adverse pregnancy outcomes remains poorly understood.
Physiological disruptions as a hypothetical pathway
The second part of our theoretical model posits that socio-economic characteristics may be related to many physiological disorders such as cardiovascular [104], metabolic [105,106] and mental disruption [105,107] which are interlinked [108-110], and in turn related to adverse outcomes [111-113]. In this framework, we suggest that annoyance caused by neighbourhood or occupational noise may induce or enhance such disorders specifically cardiovascular [44,45], sleep [42,86] and mental [114,115]. Therefore, as described in the last part of our model, two pathways link these physiological disorders stemming from socio-economic deprivation and/or exposure to noise, infant mortality and its determinants, such as low birth weight and pre-term birth.
Some authors suggest that each of these physiological disruptions – such as cardiovascular conditions [111], obesity [112,116], insomnia [117], and mental disorders [113] is directly related to adverse pregnancy outcomes. As described in our model, unhealthy behaviours relating to socio-economic characteristics, noise exposure and the physiological disorders mentioned above may affect adverse birth.
Social inequalities regarding infant mortality
The relationship seen between the neighbourhood socio-economic level and infant mortality confirms previous works regarding occupation [14], unemployment [118], education [14] or neighbourhood deprivation level [16], and pregnancy outcomes (infant mortality, stillbirth, low birth weight or pre-term birth) [17]. Advanced educational achievement enhances acquisition of - and compliance with - healthy practices and recourse to health services [119,120]. Besides educational achievement, other socio-economic characteristics have been related to infant mortality and various adverse pregnancy outcomes via a range of pathways (see conceptual model in Figure 1). Below, we emphasize three specific pathways. Psychological factors such as neighbourhood safety [121], stressful life events [93] or lack of social support, cohesive social networks and reciprocal exchanges between residents [122], may impact birth outcomes. Numerous studies have shown that chronic stressors are embedded within and accrued from the environment of women of low socio-economic status [118,123] and/or living in deprived neighbourhoods, who experience more stressful life events during pregnancy than other women [124].
Occupational conditions may also influence infant mortality and their determinants [125] through physical fatigue, work duration and intensity, and anxiety or stress which are often associated with such conditions [126].
Unhealthy behaviours such as smoking [127] or poor maternal nutrition [128], especially around the time of conception, are known to increase the risk of adverse birth outcomes. Further, some studies suggest that, as a consequence of unequal spatial distribution of various services, [129] pregnant women living in poor or deprived neighbourhoods may have fewer choices and less access to healthy food than their higher-income counterparts [130-132].
Our approach, which uses ecological data, has several limitations. One is the non-inclusion of gender in the analysis, which is known to be a risk factor for infant mortality [133]. Also, for statistical power considerations, we included all mortality cases of infants less than 1 year in our analysis. Focusing on perinatal or neonatal mortality in further studies on larger populations is recommended, and might produce clearer results. Because baby girls have a lower risk of infant and neonatal mortality [134,135], and because the birth gender ratio is uneven across the area census blocks spatial confounding by sex may have partially masked the significance of location of the most likely cluster in analysis.
A second limitation is the absence of individual data such as maternal age, marital status, previous births, parental occupation, and smoking habits. However, in our study we chose a fine geographical resolution scale (IRIS) that is designed by the national statistical institute to be as homogeneous as possible in terms of population size and socio-economic characteristics. The homogeneity of the census blocks ensures minimization of ecological bias, and results stemming from this spatial level lend themselves to analyses that are considered to be close to what can be observed at individual level [136,137]. In other words, the finer the geographical unit, the more homogeneous the population features tend to be, so that the analysis approximates residents’ individual characteristics and environmental exposure patterns. In spite of this, some degree of misclassification inevitably exists in terms of both individual characteristics and environmental exposures, and these results in associations being biased towards the null.
The third limitation concerns the noise data used in the analysis. The modelling technique calls for information on topography, land use, road traffic, population, etc. that may be collected at different periods, leading to uncertainty in matching all the data. Another source of uncertainty is the population calculation for each residential building (Pop Batj.i) which is computed in proportion to the volume of each building within each census block. For instance, each inhabited building with a height lower than 4 metres is considered to be 4 metres high, according to the methodology used to predict noise levels using the GIpSynoise software [138]. However, compared to crude average, our noise exposure indicator was built to obtain a noise value weighted by the resident population in each building for each census block. It allows the accommodation of census blocks in which a few people are exposed to high noise levels, and larger groups to lower noise levels.
Also, the absence of noise data in the Lyon metropolitan area for each year forced us to use one measure over the 9 years of our study period. No other noise data is available prior to the implementation of the European Environmental Noise Directive. The only available data, therefore, concerns the year 2009. However, according to a report from the Lyon acoustic observatory about the noise evolution between 2008 and 2011, nuisance levels have varied from 0 to 2 dB(A) at most, over this period (i.e. a variation of 2.97% of the exposure level, on average) [139]. Therefore, although we cannot evaluate how noise changes might have influenced our results, our hypothesis is that the variation described by the acoustic observatory is unlikely to alter the contrasts between spatial units and thus would not significantly modify our results. Further, the exposure computation procedure assigns to all residents of a building the value of the loudest face. This tends to over-estimate average exposure levels, but we see no reason why this misclassification should be differential across census blocks. The effect of such random misclassification is to bias the measures of association towards the null. A final limitation of our work is the absence of consideration of air pollution in the analysis. The relationship between noise and the spatial display of infant mortality suggested by our results remains disputable, since noise may be a proxy for other environmental nuisances associated with traffic or industry. Now noise and some air pollutants are strongly correlated. We explored the impact of air pollution in another study [140] conducted in the Lyon metropolitan area, whose results suggest that the socio-economic status and exposure to NO2 partially explain the spatial pattern. This means that spatial variation may be due to insufficient adjustment for other risk factors not taken into account in the model, which might explain this remaining heterogeneity in the distribution of infant mortality. In the present paper, we do not take account of noise and air pollution in the same model. They are correlated, with Pearson’s correlation R = 0.57. If we were to use noise exposure and an NO2 together in the same model, we would be faced with collinearity, problems in controlling confounding and the possibility of over- or under-fitting of the nuisance environmental variables. To our knowledge, there is no way that this collinearity can be accommodated in the model.
To our knowledge, such a work had never been performed to explore the effects of neighbourhood noise on the risk of infant mortality. Most related studies examined other adverse pregnancy outcomes (pre-term birth, low birth weight). The spatial analysis we chose in order to explore this relationship has been described in only few papers [53,55]. Further, the conceptual framework we present in this paper attempts to integrate the many theoretical pathways and hypotheses that are discussed in the literature to relate neighbourhood characteristics to adverse birth outcomes.
Conclusion
Our findings suggest that there is an association between noise levels, the neighbourhood socio-economic profile and the spatial distribution of infant mortality. However, the relationship between noise and infant mortality is complex and the association we found requires further research for confirmation and interpretation. The conceptual model exposed in the discussion section offers opportunities for new investigations on a topic that has been little explored to date.
Abbreviations
PM: Particulate matter; CO: Carbon monoxide; NO2: Nitrogen dioxide; PCB: Polychlorinated biphenyls; IRIS: Ilots Regroupés pour l’Information Statistique; INSEE: the French National Statistics Institute; dB: Lden, day-evening-night level; PTB: Pre-term birth; LBW: Low birth weight; IUGR: Intrauterine growth retardation; LLr: Log likelihood ratio; RR: Relative risks.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WK performed the spatial analysis, produced the map, conceptual model, drafted the article, and conducted the literature review. C.MP has collected health data, geocoded the cases to IRIS level, and contributed to interpretation of results. BL implemented the statistical models, provided methodological rigor and contributed to the drafting of article and its finalisation. CR and JD have implemented models to assess noise exposure, provided methodological rigor and contributed to the drafting of the article, and its finalisation. DZN, Head of the Environmental and Occupational Health Department at the EHESP and co-principal investigator of the Equit’Area Project, was responsible for quality assurance and rigor in the data analysis, reviewed the drafts of the article and contributed to finalize it. SD, principal investigator of the Equit’Area project studying the role of environmental exposure on health inequalities, monitored the general work, helped with the analysis and interpretation of the results and contributed to draft and finalized the paper. All authors read and approved the final manuscript.
Contributor Information
Wahida Kihal-Talantikite, Email: wahida.kihal@ehesp.fr.
Cindy M Padilla, Email: cindy.padilla@ehesp.fr.
Benoit Lalloue, Email: Benoit.lalloue@ehsp.fr.
Christophe Rougier, Email: christophe.rougier@cstb.fr.
Jérôme Defrance, Email: jerome.defrance@cstb.fr.
Denis Zmirou-Navier, Email: Denis.zmirou@inserm.fr.
Séverine Deguen, Email: Severine.deguen@ehesp.fr.
Acknowledgements
This project is funded by the French National Research Agency (ANR, contract-2010-PRSP-002-01).
This work was supported by the French Environment and Energy Management Agency (ADEME) through a doctoral grant for Cindy. M Padilla.
The authors thank all of the organizations that kindly provided the data used in these analyses: especially the Scientific and Technical Centre for Building (CSTB) and the Grand Lyon metropolitan authority.
We thank Michael Osei MIREKU for his idiomatic editing of the paper.
We thank Pr Clive E Sabel and Pr Martin Kulldorff for their valuable advice and guidance in this work.
References
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep. 2007;12:1–32. [PubMed] [Google Scholar]
- Kramer MS, Demissie K, Yang H, Platt RW, Sauvé R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;12:843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- Leem JH, Ha EH, Lim M, Kim K, Hong YC, Lee BE. Birth defects monitoring systems utilizing public and private medical resources in Incheon. Kor J Obstet Gynecol. 2002;12:1145–1154. [Google Scholar]
- Reagan PB, Salsberry PJ. Race and ethnic differences in determinants of preterm birth in the USA: broadening the social context. Soc Sci Med. 2005;12:2217–2228. doi: 10.1016/j.socscimed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;12:1–32. [PubMed] [Google Scholar]
- Kurinczuk JJ, Hollowell J, Boyd PA, Oakley L, Brocklehurst P, Gray R. Inequalities in infant mortality project briefing paper 4. The contribution of congenital anomalies to infant mortality. Oxford: National Perinatal Epidemiology Unit; 2010. [Google Scholar]
- Linhart Y, Bashiri A, Maymon E, Shoham-Vardi I, Furman B, Vardi H, Mazor M. Congenital anomalies are an independent risk factor for neonatal morbidity and perinatal mortality in preterm birth. Eur J Obstet Gynecol Reprod Biol. 2000;12:43–49. doi: 10.1016/S0301-2115(99)00196-7. [DOI] [PubMed] [Google Scholar]
- Vorherr H. Factors influencing fetal growth. Am J Obstet Gynecol. 1982;12:577–588. doi: 10.1016/0002-9378(82)90765-7. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;12:502–511. [PubMed] [Google Scholar]
- Chalmers I. Short, black, Baird, himsworth, and social class differences in fetal and neonatal mortality rates. Br Med J (Clin Res Ed) 1985;12:231–233. doi: 10.1136/bmj.291.6490.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch BK. Early origins of the gradient: the relationship between socioeconomic status and infant mortality in the United States. Demography. 2003;12:675–699. doi: 10.1353/dem.2003.0033. [DOI] [PubMed] [Google Scholar]
- Olsen O, Madsen M. Effects of maternal education on infant mortality and stillbirths in Denmark. Scand J Public Health. 1999;12:128–136. [PubMed] [Google Scholar]
- Singh GK, Kogan MD. Persistent socioeconomic disparities in infant, neonatal, and postneonatal mortality rates in the United States, 1969–2001. Pediatrics. 2007;12:e928–939. doi: 10.1542/peds.2005-2181. [DOI] [PubMed] [Google Scholar]
- Borrell C, Cirera E, Ricart M, Pasarín MI, Salvador J. Social inequalities in perinatal mortality in a Southern European city. Eur J Epidemiol. 2003;12:5–13. doi: 10.1023/a:1022524914396. [DOI] [PubMed] [Google Scholar]
- Jorgensen T, Mortensen LH, Andersen A-MN. Social inequality in fetal and perinatal mortality in the Nordic countries. Scand J Public Health. 2008;12:635–649. doi: 10.1177/1403494808089653. [DOI] [PubMed] [Google Scholar]
- Calling S, Li X, Sundquist J, Sundquist K. Socioeconomic inequalities and infant mortality of 46,470 preterm infants born in Sweden between 1992 and 2006. Paediatr Perinat Epidemiol. 2011;12:357–365. doi: 10.1111/j.1365-3016.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- Janevic T, Stein CR, Savitz DA, Kaufman JS, Mason SM, Herring AH. Neighborhood deprivation and adverse birth outcomes among diverse ethnic groups. Ann Epidemiol. 2010;12:445–451. doi: 10.1016/j.annepidem.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C, Messer LC, Kaufman JS, Laraia BA. Neighborhood deprivation and preterm birth among non-Hispanic black and white women in eight geographic areas in the United States. Am J Epidemiol. 2008;12:155–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- Messer LC, Kaufman JS, Dole N, Savitz DA, Laraia BA. Neighborhood crime, deprivation, and preterm birth. Ann Epidemiol. 2006;12:455–462. doi: 10.1016/j.annepidem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Piotrowski ZH, Pickett KE. Neighborhood economic disadvantage, violent crime, group density, and pregnancy outcomes in a diverse, urban population. Soc Sci Med. 2007;12:2440–2457. doi: 10.1016/j.socscimed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- O’Campo P, Xue X, Wang MC, Caughy M. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. Am J Public Health. 1997;12:1113–1118. doi: 10.2105/AJPH.87.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger N, Giraud J, Daniel M. The joint influence of area income, income inequality, and immigrant density on adverse birth outcomes: a population-based study. BMC Public Health. 2009;12:237. doi: 10.1186/1471-2458-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z-C, Wilkins R, Kramer MS. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;12:1415–1420. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JS, Dole N, Savitz DA, Herring AH. Modeling community-level effects on preterm birth. Ann Epidemiol. 2003;12:377–384. doi: 10.1016/S1047-2797(02)00480-5. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Ahern JE, Selvin S, Abrams B. Neighborhood socioeconomic status, maternal race and preterm delivery: a case–control study. Ann Epidemiol. 2002;12:410–418. doi: 10.1016/S1047-2797(01)00249-6. [DOI] [PubMed] [Google Scholar]
- Pattenden S, Dolk H, Vrijheid M. Inequalities in low birth weight: parental social class, area deprivation, and “lone mother” status. J Epidemiol Community Health. 1999;12:355–358. doi: 10.1136/jech.53.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM. Neighborhood social environments and the distribution of low birthweight in Chicago. Am J Public Health. 1997;12:597–603. doi: 10.2105/AJPH.87.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi O, Kim H, Ha E. Does area level socioeconomic status modify the effects of PM(10) on preterm delivery? Environ Res. 2010;12:55–61. doi: 10.1016/j.envres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Genereux M, Auger N, Goneau M, Daniel M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J Epidemiol Community Health. 2008;12:695–700. doi: 10.1136/jech.2007.066167. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Aguilera I, Ballester F, Basterrechea M, Esplugues A, Guxens M, Larrañaga M, Lertxundi A, Mendez M, Murcia M, Marina LS, Villanueva CM, Sunyer J. Socioeconomic status and exposure to multiple environmental pollutants during pregnancy: evidence for environmental inequity? J Epidemiol Community Health. 2012;12:106–113. doi: 10.1136/jech.2010.117408. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Ghaji N, Mbah AK, Alio AP, August EM, Boubakari I. Particulate pollutants and racial/ethnic disparity in feto-infant morbidity outcomes. Matern Child Health J. 2012;12:1679–1687. doi: 10.1007/s10995-011-0868-8. [DOI] [PubMed] [Google Scholar]
- Carbajal-Arroyo L, Miranda-Soberanis V, Medina-Ramón M, Rojas-Bracho L, Tzintzun G, Solís-Gutiérrez P, Méndez-Ramírez I, Hurtado-Díaz M, Schwartz J, Romieu I. Effect of PM(10) and O(3) on infant mortality among residents in the Mexico City metropolitan area: a case-crossover analysis, 1997–2005. J Epidemiol Community Health. 2011;12:715–721. doi: 10.1136/jech.2009.101212. [DOI] [PubMed] [Google Scholar]
- Romieu I, Ramírez-Aguilar M, Moreno-Macias H, Barraza-Villarreal A, Miller P, Hernández-Cadena L, Carbajal-Arroyo LA, Hernandez-Avila M. Infant mortality and air pollution: modifying effect by social class. J Occup Environ Med. 2004;12:1210–1216. [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;12:942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce NA, Hoggatt KJ, Wilhelm M, Ritz B. Preterm birth: the interaction of traffic-related air pollution with economic hardship in Los Angeles neighborhoods. Am J Epidemiol. 2005;12:140–148. doi: 10.1093/aje/kwi173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;12:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, Wilkinson P, Fletcher T, Cifuentes L, Schwartz J. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;12:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braubach M, Fairburn J. Social inequities in environmental risks associated with housing and residential location--a review of evidence. Eur J Public Health. 2010;12:36–42. doi: 10.1093/eurpub/ckp221. [DOI] [PubMed] [Google Scholar]
- Havard S, Reich BJ, Bean K, Chaix B. Social inequalities in residential exposure to road traffic noise: an environmental justice analysis based on the RECORD cohort study. Occup Environ Med. 2011;12:366–374. doi: 10.1136/oem.2010.060640. [DOI] [PubMed] [Google Scholar]
- Passchier-Vermeer W, Passchier WF. Noise exposure and public health. Environ Health Perspect. 2000;12(Suppl 1):123–131. doi: 10.1289/ehp.00108s1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for Community Noise. Geneva: WHO; 1999. http://www.who.int/docstore/peh/noise/guidelines2.html. [Google Scholar]
- Perron S, Tétreault L-F, King N, Plante C, Smargiassi A. Review of the effect of aircraft noise on sleep disturbance in adults. Noise Health. 2012;12:58–67. doi: 10.4103/1463-1741.95133. [DOI] [PubMed] [Google Scholar]
- Ising H, Braun C. Acute and chronic endocrine effects of noise: review of the research conducted at the institute for water, soil and Air hygiene. Noise Health. 2000;12:7–24. [PubMed] [Google Scholar]
- Babisch W. Traffic noise and cardiovascular disease: epidemiological review and synthesis. Noise and Health. 2000;12:9. [PubMed] [Google Scholar]
- Babisch W. Road traffic noise and cardiovascular risk. Noise and Health. 2008;12:27. doi: 10.4103/1463-1741.39005. [DOI] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Denham M, Ravenscroft J. Effects of pollution on human growth and development: an introduction. J Physiol Anthropol. 2006;12:103–112. doi: 10.2114/jpa2.25.103. [DOI] [PubMed] [Google Scholar]
- Lalloué B, Monnez JM, Padilla C, Kihal W, Le Meur N, Zmirou-Navier D, Deguen S. A statistical procedure to create a neighborhood socioeconomic index for health inequalities analysis. Int J Equity Health. 2013;12:21. doi: 10.1186/1475-9276-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. Directive 2002/49/EC of the European Parliament and of the Council of 25, June 2002 relating to the assessment and management of environmental noise. Off J Eur Communities. 2002;12:12–25. [Google Scholar]
- Bloquet S, Faiget L, Aujard C, Vallet J, Prastracos P, Vincent B. GIpSynoise: A GIS Tool Adapted to the European Directive on Assessment and Management of Environmental Noise 2002/49/CE. Rio de Janeiro, Brazil: The 34th International Congress and Exhibition on Noise Control Engineering; 2005. (Federação Iberoamericana de Acústica, I-INCE International Institute of Noise Control Engineering: Inter-Noise 2005). [Google Scholar]
- CadnaA-“Software Program for Noise Prediction”. Germany: DataKustik GmbH; http://www.datakustik.com. [Google Scholar]
- Defrance J, Gabillet Y, Van Maercke D, Dine C, Gautier P-E. A New French Method for Railway Noise Prediction. Nice, France: Proceedings of the 29th international congress on noise control engineering; 2000. p. 6. (Internoise 2000). [Google Scholar]
- Good Practice Guide on Noise Exposure and Potential Health Effects, EEA Technical Report No 11/2010. Copenhagen; 2010. ISSN 1725–2237. [Google Scholar]
- Sabel CE, Wilson JG, Kingham S, Tisch C, Epton M. Spatial implications of covariate adjustment on patterns of risk: respiratory hospital admissions in Christchurch, New Zealand. Soc Sci Med. 2007;12:43–59. doi: 10.1016/j.socscimed.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. Information management services, Inc: SaTScan version 6.0: software for the spatial, temporal, and space-time scan statistics. 2009. http://www.satscan.org.
- Bambhroliya AB, Burau KD, Sexton K. Spatial analysis of county-level breast cancer mortality in Texas. J Environ Public Health. 2012;12:8. doi: 10.1155/2012/959343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulldorff M, Feuer EJ, Miller BA, Freedma LS. Breast cancer clusters in the Northeast United States: a geographic analysis. Am J Epidemiol. 1997;12:161–170. doi: 10.1093/oxfordjournals.aje.a009247. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. A spatial scan statistic. Comm Stat Theory Methods. 1997;12:1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- Dwass M. Modified randomization tests for nonparametric hypotheses. Ann Math Statist. 1957;12:181–187. doi: 10.1214/aoms/1177707045. [DOI] [Google Scholar]
- Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;12:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- McDonald AD, Armstrong B, Cherry NM, Delorme C, Diodati-Nolin A, McDonald JC, Robert D. Spontaneous abortion and occupation. J Occup Med. 1986;12:1232–1238. [PubMed] [Google Scholar]
- McDonald AD, McDonald JC, Armstrong B, Cherry NM, Côté R, Lavoie J, Nolin AD, Robert D. Fetal death and work in pregnancy. Br J Ind Med. 1988;12:148–157. doi: 10.1136/oem.45.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen-Sorri AL, Sorri M, Anttonen HP, Tuimala R, Läärä E. Occupational noise exposure during pregnancy: a case control study. Int Arch Occup Environ Health. 1988;12:279–283. doi: 10.1007/BF00378474. [DOI] [PubMed] [Google Scholar]
- Mamelle N, Laumon B, Lazar P. Prematurity and occupational activity during pregnancy. Am J Epidemiol. 1984;12:309–322. doi: 10.1093/oxfordjournals.aje.a113750. [DOI] [PubMed] [Google Scholar]
- Hartikainen AL, Sorri M, Anttonen H, Tuimala R, Läärä E. Effect of occupational noise on the course and outcome of pregnancy. Scand J Work Environ Health. 1994;12:444–450. doi: 10.5271/sjweh.1376. [DOI] [PubMed] [Google Scholar]
- Knipschild P, Meijer H, Sallé H. Aircraft noise and birth weight. Int Arch Occup Environ Health. 1981;12:131–136. doi: 10.1007/BF00378433. [DOI] [PubMed] [Google Scholar]
- Wu TN, Chen LJ, Lai JS, Ko GN, Shen CY, Chang PY. Prospective study of noise exposure during pregnancy on birth weight. Am J Epidemiol. 1996;12:792–796. doi: 10.1093/oxfordjournals.aje.a008817. [DOI] [PubMed] [Google Scholar]
- Peoples-Sheps MD, Siegel E, Suchindran CM, Origasa H, Ware A, Barakat A. Characteristics of maternal employment during pregnancy: effects on low birthweight. Am J Public Health. 1991;12:1007–1012. doi: 10.2105/AJPH.81.8.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T. Female noise exposure, shift work, and reproduction. J Occup Environ Med. 1995;12:945–950. doi: 10.1097/00043764-199508000-00010. [DOI] [PubMed] [Google Scholar]
- Nurminen T, Kurppa K. Occupational noise exposure and course of pregnancy. Scand J Work Environ Health. 1989;12:117–124. doi: 10.5271/sjweh.1873. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Butler AS. Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. Institute of Medicine. Washington DC: National Academies Press; 2007. Preterm birth: causes, consequences and prevention. ISBN 13: 978-0-309-10159-2. [Google Scholar]
- De Catanzaro D, Macniven E. Psychogenic pregnancy disruptions in mammals. Neurosci Biobehav Rev. 1992;12:43–53. doi: 10.1016/S0149-7634(05)80050-8. [DOI] [PubMed] [Google Scholar]
- Myers RE. Production of fetal asphyxia by maternal psychological stress. Pavlov J Biol Sci. 1977;12:51–62. doi: 10.1007/BF03001799. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, de Medina PG R, Huizink AC, Van den Bergh BRH, Buitelaar JK, Visser GHA. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;12:3–14. doi: 10.1016/S0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- Christian LM. Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev. 2012;12:350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo A, Nguyen AL, Jonai H, Matsuda S, Villanueva MB, Sotoyama M, Nguyen TC, Le VT, Hoang MH, Nguyen DT, Nguyen S. Effects of earplugs on catecholamine and cortisol excretion in noise-exposed textile workers. Ind Health. 1996;12:279–286. doi: 10.2486/indhealth.34.279. [DOI] [PubMed] [Google Scholar]
- Ising H, Babisch W, Kruppa B. Noise-induced endocrine effects and cardiovascular risk. Noise Health. 1999;12:37–48. [PubMed] [Google Scholar]
- Melamed S, Bruhis S. The effects of chronic industrial noise exposure on urinary cortisol, fatigue and irritability: a controlled field experiment. J Occup Environ Med. 1996;12:252–256. doi: 10.1097/00043764-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Rojas-González L, Martínez-Leal R, Paz-Araviche V, Chacín-Almarza B, Corzo-Alvarez G, Sanabria-Vera C, Montiel-López M. [Serum cortisol levels in pre and post journal labor and non auditory manifestations in noise exposed workers of a brewer industry] Invest Clin. 2004;12:297–307. [PubMed] [Google Scholar]
- Ando Y, Hattori H. Effects of noise on human placental lactogen (hpl) levels in maternal plasma. BJOG. 1977;12:115–118. doi: 10.1111/j.1471-0528.1977.tb12536.x. [DOI] [PubMed] [Google Scholar]
- Ando Y, Nakane Y, Egawa J. Effects of aircraft noise on the mental work of pupils. J Sound Vib. 1975;12:683–691. doi: 10.1016/0022-460X(75)90228-X. [DOI] [Google Scholar]
- Rehm S, Jansen G. Aircraft noise and premature birth. J Sound Vib. 1978;12:133–135. doi: 10.1016/0022-460X(78)90490-X. [DOI] [Google Scholar]
- Okun ML, Schetter CD, Glynn LM. Poor sleep quality is associated with preterm birth. Sleep. 2011;12:1493–1498. doi: 10.5665/sleep.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Luther JF, Wisniewski SR, Sit D, Prairie BA, Wisner KL. Disturbed sleep, a novel risk factor for preterm birth? J Womens Health (Larchmt) 2012;12:54–60. doi: 10.1089/jwh.2010.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange LB, Parker KP, Moore ML, Strickland OL, Bliwise DL. Disturbed sleep and preterm birth: a potential relationship? Clin Exp Obstet Gynecol. 2009;12:166–168. [PubMed] [Google Scholar]
- Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, Chatzi L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;12:738–744. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- Pirrera S, De Valck E, Cluydts R. Nocturnal road traffic noise: a review on its assessment and consequences on sleep and health. Environ Int. 2010;12:492–498. doi: 10.1016/j.envint.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Kawada T. Noise and health–sleep disturbance in adults. J Occup Health. 2011;12:413–416. doi: 10.1539/joh.11-0071-RA. [DOI] [PubMed] [Google Scholar]
- Jakovljevic B, Belojević G, Paunović K, Stojanov V. Road traffic noise and sleep disturbances in an urban population: cross-sectional study. Croat Med J. 2006;12:125–133. [PMC free article] [PubMed] [Google Scholar]
- Kawada T. Effects of traffic noise on sleep: a review. Nihon Eiseigaku Zasshi. 1995;12:932–938. doi: 10.1265/jjh.50.932. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Fidell S, Pearsons K, Campbell KC, Keith SE. Review of field studies of aircraft noise-induced sleep disturbance. J Acoust Soc Am. 2007;12:32–41. doi: 10.1121/1.2400613. [DOI] [PubMed] [Google Scholar]
- Kageyama T, Kabuto M, Nitta H, Kurokawa Y, Taira K, Suzuki S, Takemoto T. A population study on risk factors for insomnia among adult Japanese women: a possible effect of road traffic volume. Sleep. 1997;12:963–971. [PubMed] [Google Scholar]
- Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes a hypothesis. Obstet Gynecol Surv. 2009;12:273–280. doi: 10.1097/OGX.0b013e318195160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders AEB, Grobman WA, Amsden LB, Holl JL. Chronic stress and low birth weight neonates in a low-income population of women. Obstet Gynecol. 2007;12:331–338. doi: 10.1097/01.AOG.0000250535.97920.b5. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;12:333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;12:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;12:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;12:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Myers RE, Aldrich TE, Easterly CE. Effects of noise and electromagnetic fields on reproductive outcomes. Environ Health Perspect. 1989;12:193–200. doi: 10.1289/ehp.8981193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima HO, Yeh MN, James LS. Reduced uterine blood flow and fetal hypoxemia with acute maternal stress: experimental observation in the pregnant baboon. Am J Obstet Gynecol. 1979;12:270–275. doi: 10.1016/s0002-9378(16)33032-0. [DOI] [PubMed] [Google Scholar]
- Myers RE. Maternal psychological stress and fetal asphyxia: a study in the monkey. Am J Obstet Gynecol. 1975;12:47. doi: 10.1016/0002-9378(75)90614-6. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;12:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Hobel CJ. Stress and preterm birth. Clin Obstet Gynecol. 2004;12:856–880. doi: 10.1097/01.grf.0000142512.38733.8c. discussion 881–882. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;12:S257–263. doi: 10.1016/S0002-9378(99)70712-X. [DOI] [PubMed] [Google Scholar]
- Brown AF, Liang L-J, Vassar SD, Stein-Merkin S, Longstreth WT Jr, Ovbiagele B, Yan T, Escarce JJ. Neighborhood disadvantage and ischemic stroke: the cardiovascular health study (CHS) Stroke. 2011;12:3363–3368. doi: 10.1161/STROKEAHA.111.622134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra M, Neudorf C, Opondo J. Health disparity by neighbourhood income. Can J Public Health. 2006;12:435–439. doi: 10.1007/BF03405223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev. 2008;12:2–20. doi: 10.1111/j.1753-4887.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Lee M-A. Neighborhood residential segregation and mental health: a multilevel analysis on Hispanic Americans in Chicago. Soc Sci Med. 2009;12:1975–1984. doi: 10.1016/j.socscimed.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American heart association. Circulation. 1999;12:1134–1146. doi: 10.1161/01.CIR.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;12:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss an update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 1997;12(113):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Samadi AR, Mayberry RM, Zaidi AA, Pleasant JC, McGhee N Jr, Rice RJ. Maternal hypertension and associated pregnancy complications among African-American and other women in the United States. Obstet Gynecol. 1996;12:557–563. doi: 10.1016/0029-7844(95)00480-7. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;12:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- Faisal-Cury A, Araya R, Zugaib M, Menezes PR. Common mental disorders during pregnancy and adverse obstetric outcomes. J Psychosom Obstet Gynaecol. 2010;12:229–235. doi: 10.3109/0167482X.2010.512404. [DOI] [PubMed] [Google Scholar]
- Stansfeld S, Haines M, Burr M, Berry B, Lercher P. A review of environmental noise and mental health. Noise and Health. 2000;12:1. [PubMed] [Google Scholar]
- Hardoy MC, Carta MG, Marci AR, Carbone F, Cadeddu M, Kovess V, Dell’Osso L, Carpiniello B. Exposure to aircraft noise and risk of psychiatric disorders: the Elmas survey–aircraft noise and psychiatric disorders. Soc Psychiatry Psychiatr Epidemiol. 2005;12:24–26. doi: 10.1007/s00127-005-0837-x. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betrán AP, Daher S, Widmer M, Dolan SM, Menon R, Bergel E, Allen T, Merialdi M. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J Matern Fetal Neonatal Med. 2009;12:957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;12:1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- Agyemang C, Vrijkotte TGM, Droomers M, van der Wal MF, Bonsel GJ, Stronks K. The effect of neighbourhood income and deprivation on pregnancy outcomes in Amsterdam, The Netherlands. J Epidemiol Community Health. 2009;12:755–760. doi: 10.1136/jech.2008.080408. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Schultz TP. Child mortality and fertility in Colombia: individual and community effects. Health Policy Educ. 1982;12:305–348. doi: 10.1016/0165-2281(82)90015-7. [DOI] [PubMed] [Google Scholar]
- Hobcraft J. Women’s education, child welfare and child survival: a review of the evidence. Health Transit Rev. 1993;12:159–175. [PubMed] [Google Scholar]
- Jaffee KD, Perloff JD. An ecological analysis of racial differences in low birthweight: implications for maternal and child health social work. Health Soc Work. 2003;12:9–22. doi: 10.1093/hsw/28.1.9. [DOI] [PubMed] [Google Scholar]
- Buka SL, Brennan RT, Rich-Edwards JW, Raudenbush SW, Earls F. Neighborhood support and the birth weight of urban infants. Am J Epidemiol. 2003;12:1–8. doi: 10.1093/aje/kwf170. [DOI] [PubMed] [Google Scholar]
- Seguin L, Potvin L, St Denis M, Loiselle J. Chronic stressors, social support, and depression during pregnancy. J Obstet Gynecol. 1995;12:583–589. doi: 10.1016/0029-7844(94)00449-N. [DOI] [PubMed] [Google Scholar]
- Rutter DR, Quine L. Inequalities in pregnancy outcome: a review of psychosocial and behavioural mediators. Soc Sci Med. 1990;12:553–568. doi: 10.1016/0277-9536(90)90154-K. [DOI] [PubMed] [Google Scholar]
- Pirastu R, Lagorio S, Miligi L, Seniori Costantini A. Health and work among women in Italy: an overview of the epidemiological literature. Eur J Epidemiol. 1999;12:51–57. doi: 10.1023/A:1007522000856. [DOI] [PubMed] [Google Scholar]
- Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am J Public Health. 2006;12:846–855. doi: 10.2105/AJPH.2004.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schempf A, Strobino D, O’Campo P. Neighborhood effects on birthweight: an exploration of psychosocial and behavioral pathways in Baltimore, 1995–1996. Soc Sci Med. 2009;12:100–110. doi: 10.1016/j.socscimed.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Robinson E, Clark PM, Becroft DMO, Glavish N, Pattison NS, Pryor JE, Thompson JMD, Wild CJ. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case–control study. Arch Dis Child Fetal Neonatal Ed. 2004;12:F431–435. doi: 10.1136/adc.2003.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;12:125–139. doi: 10.1016/S0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Jessop EG. Individual morbidity and neighbourhood deprivation in a non-metropolitan area. J Epidemiol Community Health. 1992;12:543–546. doi: 10.1136/jech.46.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway A, Macintyre S. Shopping for food in socially contrasting localities. Br Food J. 2000;12:52–59. doi: 10.1108/00070700010310632. [DOI] [Google Scholar]
- Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;12:23–29. doi: 10.1016/S0749-3797(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Rankin J, Pless-Mulloli T, Glinianaia S. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environ Res. 2007;12:400–408. doi: 10.1016/j.envres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;12:F182–185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EL, Bennett FC. Birth weight less than 800 grams: changing outcomes and influences of gender and gestation number. Pediatrics. 1990;12:27–34. [PubMed] [Google Scholar]
- Greenland S. Ecologic versus individual-level sources of bias in ecologic estimates of contextual health effects. Int J Epidemiol. 2001;12:1343–1350. doi: 10.1093/ije/30.6.1343. [DOI] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;12:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- CERTU Report. Comment réaliser les cartes de bruit stratégiques en agglomeration. Mettre en oeuvre la directive 2002/49/CE. 2006. http://www.certu.fr/
- Acoustic observatory report. Reseau permanent de mesure. http://www.acoucite.org.
- Padilla CM, Deguen S, Lalloue B, Blanchard O, Beaugard C, Troude F, Navier DZ, Vieira VM. Cluster analysis of social and environment inequalities of infant mortality. A spatial study in small areas revealed by local disease mapping in France. STOTEN. 2013;12:433–441. doi: 10.1016/j.scitotenv.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]