Abstract
Purpose
To examine associations between retinal thickness and rod-mediated dark adaptation in older adults with non-exudative age-related maculopathy (ARM) or normal macular health.
Methods
A cross-sectional study was conducted with 74 adults ≥ 50 years old from the comprehensive ophthalmology and retina services of an academic eye center. ARM presence and disease severity in the enrollment eye was defined by the masked grading of stereofundus photos using the Clinical Age-Related Maculopathy (CARMS) grading system. High-definition, spectral-domain optical coherence tomography was used to estimate retinal thickness in a grid of regions in the macula. Rod-mediated dark adaptation, recovery of light sensitivity after a photo-bleach, was measured over a 20-minute period for a 500 nm target presented at 5° on the inferior vertical meridian. Main outcomes of interest were retinal thickness in the macula (μm) and parameters of rod-mediated dark adaptation (second slope, third slope, average sensitivity, final sensitivity).
Results
In non-exudative disease retinal thickness was decreased in greater disease severity; thinner retina was associated with reductions in average and final rod-mediated sensitivity even after adjustment for age and visual acuity.
Conclusions
Impairment in rod-mediated dark adaptation in non-exudative ARM is associated with macular thinning.
Keywords: age-related maculopathy, dark adaptation, rod photoreceptors, optical coherence tomography
Age-related maculopathy (ARM) is a complex, multi-factorial disease of older adults where central retinal photoreceptors are lost by an atrophic process or a neovascular event. Photoreceptor degeneration in animal models and human donor eyes with ARM is associated with dysfunction and degeneration of the retinal pigment epithelium (RPE) and Bruch’s membrane changes.[1] These processes include accumulation of extracellular material between RPE and Bruch’s membrane and RPE cell structural changes.[2, 3] Persons with greater ARM disease severity defined by fundus appearance are more likely to have greater impairments in visual function such as acuity, light sensitivity, and dark adaptation.[4, 5] Yet fundus appearance, the foundation for fundus grading systems used in clinical research, is inadequate for understanding in vivo structural alterations in the RPE, Bruch’s membrane, and photoreceptor layer since many structural changes in these tissues are largely invisible in fundus images.[2] With the development of high-definition, spectral-domain optical coherence tomography (HD SD OCT), there is now the capacity to image retinal layers in vivo including the structural hallmarks of ARM such as drusen, retinal pigment epithelial changes, and geographic atrophy.[6–8] However, there is little research on how retinal structure as revealed by HD SD OCT is quantitatively related to visual function. Visual function is the critical endpoint in most ophthalmic clinical trials (e.g., can the patient see better, can further vision loss be prevented). Information about how function changes as medical or surgical interventions impact retinal structure is critical for successful treatment development and evaluation.17
Here we report on associations between retinal thickness in the macula as measured by HD SD OCT and rod-mediated function in older adults with ARM having wide ranging non-exudative disease severity. We focus on measurements of rod photoreceptor function since histopathological and psychophysical studies have indicated an early rod vulnerability in ARM pathogenesis. This vulnerability is evident in the earliest phases of non-exudative disease, with rod loss and dysfunction being more severe than for cones in most cases evaluated.[4, 9, 10] Rod photoreceptor function in this study was evaluated using rod-mediated dark adaptometry since the parameters of dark adaptation, which is an assay for the rate of photopigment regeneration, depends on the health of the RPE/Bruch’s membrane complex.[11]
METHODS
This study was approved by the institutional review board of the University of Alabama at Birmingham and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from participants after the nature and possible consequences of the study were explained. Participants were recruited from the comprehensive ophthalmology and retina services of the University of Alabama at Birmingham. Eligibility criteria were ≥ 50 years old and presence of non-exudative ARM or normal retinal health in both eyes. ARM presence and severity were determined from grading stereoscopic color 30° fundus photographs taken with a FF450 Plus fundus camera (Carl Zeiss Meditec, Dublin CA) after dilation of the pupil to ≥ 6 mm. Photographs were evaluated by an experienced grader using the Clinical Age-Related Maculopathy Staging (CARMS) system[12] who was masked to the clinical and functional characteristics of all participants. In summary, the system has five grades ranging from 1 -- no drusen or < 10 small drusen (< 63 μm) without pigment abnormalities; 2 -- approximately ≥ 10 small drusen or < 15 intermediate drusen (≥ 63 μm but ≤ 125 μm) or pigment abnormalities associated with ARM; 3 -- approximately ≥ 15 intermediate drusen (≥ 63 μm but ≤ 125 μm) or any large drusen (≥ 125μm); 4 -- geographic atrophy (GA); and 5 -- exudative disease.
Persons were excluded if the medical record or general health interview indicated glaucoma, optic neuropathy, or other vision-impaired conditions besides ARM; neurological diseases (Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), or history of stroke; diabetes, and/or they were unable to cooperate with the testing protocol. Eyes that received a fundus grade of 5 (exudative disease) were excluded since the focus of the study is on non-exudative disease.
Best-corrected distance visual acuity was measured using the Early Treatment of Diabetic Retinopathy Study chart expressed as logarithm of the minimum angle of resolution (logMAR). The eye with better acuity was the enrollment eye. Dark adaptation for the enrollment eye was measured psychophysically using the AdaptDx (Apeliotus Technologies, Atlanta, GA), a computer-automated dark adaptometer described previously.[13] Before testing, the eye was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride so that a pupil diameter of ≥ 6 mm was achieved. Trial lenses were added for the 30-cm viewing distance if needed to correct for optical blur. The fellow eye was occluded with an opaque patch. The participant placed the head in the forehead-chinrest. An infrared camera positioned behind the fixation light displayed the eye on a monitor viewed by the examiner, who facilitated the positioning of the participant’s test eye to the red fixation light using a reticule displayed on the eye’s image. The procedure began with a photo-bleach exposure to an intense flash (0.25 ms duration, 6.38 log scot Td second−1 intensity; equivalent ~82% bleach[14]) while the participant was focused on the fixation light. This bleach was sufficiently intense to generate impaired dark adaptation parameters in early ARM patients using a 20-minute duration test protocol.[13] The photobleach flash, subtending 4°, was centered at 5° on the inferior vertical meridian, which was also the test target’s position for measuring light sensitivity. Threshold measurement for a 1.7° diameter, 500 nm circular target began 15 seconds after bleach offset. During threshold measurement, the participant was instructed to always maintain fixation on the red fixation light and to press a response button when a flashing target first became visible within the bleached area. Threshold was estimated using a three-down/one-up modified staircase estimate procedure described previously.[13] Threshold measurement continued at 30 second intervals for 20 minutes. Log thresholds were expressed as sensitivity in decibel units as a function of time from bleach offset.
Parameters of rod-mediated dark adaptation were based on the thresholds measured during the protocol. Data were fit with a biological model of rod-mediated dark adaptation[11] using a non-linear regression model composed of three linear components described in detail previously.[15] The first component represents the rapid conemediated recovery of sensitivity, and the second and third components represent the second and third components of rod-mediated recovery, respectively. The breaks between the components were included as parameters in the model and solved for in an iterative fashion. Four rod-mediated variables of dark adaptation were used in analyses. Two variables consisted of the slopes of the second and third linear components, representing the rates of sensitivity recovery during dark adaptation; these rates are largely dictated by the rate of rhodopsin regeneration.[16–18] Two additional measures were a variable called final threshold defined as the last threshold measured during the 20-minute test period, and a variable called mean threshold defined as the average of all thresholds measured after 300 seconds had passed in the protocol. This cut-point was selected since the rod-cone break had occurred for all participants by this time point and thresholds were thus rod-mediated.
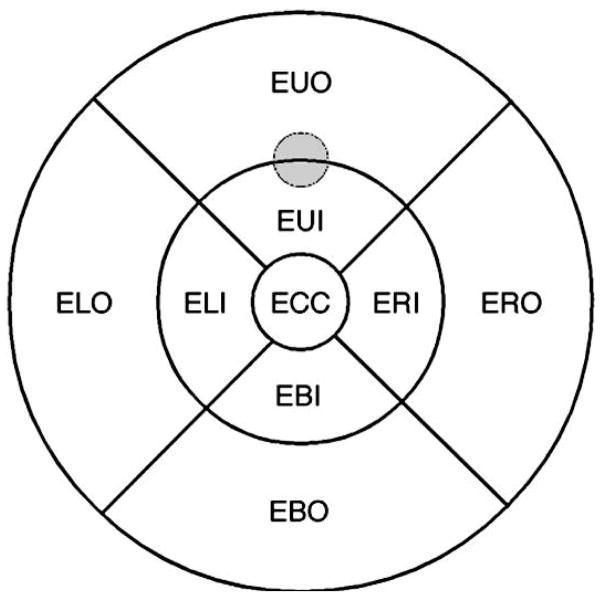
HD SD OCT was performed on the macula of the dilated eye using Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA), which uses a super-luminescent diode (840 nm) to obtain 27,000 optical coherence A-scans per second. The axial and transverse resolutions are 5 μm and 15 μm. Retinal thickness was determined by an automated algorithm that defines the reflectance border of the retinal surface and the retinal pigment epithelium. The macular cube (512 × 128) protocol provided estimates of retinal thickness (μm) for a grid of regions covering a 10.4° radius area centered on the fovea (Figure 1). The test target from the psychophysical protocol was on the border of the EUI and EUO regions of the OCT grid. To examine the association between rod-mediated dark adaptation parameters and retinal thickness, the average retinal thickness in the EUI and EUO region combined was used in the analysis.
Figure 1.
Grid of regions, centered on the fovea, assessed by the Cirrus HD OCT macular cube protocol, which provides an estimate of retinal thickness (μm) in each region. The grid covers a 6-mm diameter of retina. The gray circle indicates the location of the test target during dark adaptation testing.
Statistical analysis
Rod-mediated dark adaptation and OCT retinal thickness for the CARMS groups were compared using analysis of variance. Spearman’s correlation coefficient quantified the association between dark adaptation parameters and the retinal thickness variable. Association between acuity and retinal thickness in the central region (ECC) was also evaluated. Associations were adjusted for age since age is related to retinal thickness,[19] dark adaptation,[20] and acuity.[21] P-values of ≤0.05 (two-sided) were considered statistically significant.
RESULTS
Results are reported for all participants who met eligibility criteria (N = 74). The average age was 77 years (53 to 95), and most participants were white of non-Hispanic origin. (Table 1) Visual acuity ranged from 20/16 to 20/632, with 89.2% of the sample having 20/60 or better. The sample was approximately evenly distributed among CARMS categories 1 to 4 (17–20 participants in each category). Twelve of 19 participants with geographic atrophy (GA) used a preferred retinal locus outside the fovea during visual acuity measurement, dark adaptation and OCT measurement since the fovea fell within GA.
Table 1.
Demographic, visual acuity and ARM characteristics of the sample (N = 74)
| Characteristic | |
|---|---|
| Age, years, mean (SD) | 77 (8) |
| Gender, n (%) | |
| Female | 36 (48.7) |
| Male | 48 (51.3) |
| Race, n (%) | |
| White | 71 (96.0) |
| African American | 3 (4.0) |
| Visual acuity, enrollment eye, n (%) | |
| 20/20 or better | 12 (16.2) |
| Worse than 20/20 to 20/30 | 29 (39.2) |
| Worse than 20/30 to 20/40 | 19 (25.7) |
| Worse than 20/40 to 20/60 | 6 (8.1) |
| Worse than 20/60 to 20/100 | 3 (4.1) |
| Worse than 20/100 to 20/200 | 1 (1.3) |
| Worse than 20/200 | 4 (5.4) |
| CARMS grade, enrollment eye, n (%) | |
| 1 | 17 (23.0) |
| 2 | 18 (24.3) |
| 3 | 20 (27.0) |
| 4 | 19 (25.7) |
Average visual acuity was worse with greater disease severity (Table 2), but this trend is largely due to the sharp decline in acuity in those with GA (CARMS 4), as compared to acuity in the CARMS 1–3 groups. CARMS grade also had a significant impact on dark adaptation parameters, with participants with greater disease severity exhibiting more impairment in these parameters. The second and third slopes were lower (less steep) and average and final light sensitivity decreased with increasing disease severity (Table 2). Of the 19 participants with GA (CARMS 4), for 12 of them the dark adaptation test target was positioned over an area of the macula that did not have GA and for 7 of them it was positioned over an area of the macula that had GA. One would expect that dark adaptation parameters might be more severely impaired when the target was over GA since most photoreceptors would be seriously degenerated or dead. The rate of dark adaptation (second and third slopes) was not different between the two groups, however for those participants with the test target positioned amidst GA, their final and average rod-mediated sensitivity was significantly depressed (20.2 and 17.3 respectively) compared to those where the test target was positioned away from the GA (35.0 and 31.2 ), p <.01.
Table 2.
Visual acuity and rod-mediated dark adaptation parameters for enrollment eye stratified by disease severity (N = 74)
| CARMS 1 | CARMS 2 | CARMS 3 | CARMS 4 | p-value 1 | CARMS 4 with test target over non-GA area n = 12 | p-value 2 | CARMS 4 with test target over GA area n = 7 | p-value 3 | |
|---|---|---|---|---|---|---|---|---|---|
| n =17 | n = 18 | n = 20 | n = 19 | ||||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (D) | M (SD) | ||||
| Visual acuity (logMAR) | .15 (.09) | .08 (.11) | .17 (.18) | .54 (.51) | < .0001 | .44 (.45) | .0006 | .72 (.59) | <.0001 |
| Rod-mediated dark adaptation parameters | |||||||||
| Second slope | .0244 (.0380) | .0247 (.0166) | .0062 (.0095) | .0102 (.0158) | .0198 | .0114 (.0168) | .0359 | .0083 (.0150) | .0359 |
| Third slope | .0172 (.0090) | .0133 (.0074) | .0102 (.0115) | .0052 (.0075) | .0020 | .0070 (.0074) | .0280 | .0023 (.0071) | .0064 |
| Final threshold (dB of sensitivity) | 49.5 (8.4) | 45.4 (10.4) | 34.2 (7.7) | 29.6 (12.3) | < .0001 | 35.0 (10.6) | < .0001 | 20.3 (9.6) | < .0001 |
| Mean threshold (dB of sensitivity) | 42.6 (5.7) | 39.2 (8.5) | 30.6 (5.0) | 26.1 (10.3) | < .0001 | 31.2 (8.0) | < .0001 | 17.3 (7.6) | < .0001 |
P-value for comparison among CARMS 1, 2, 3, and 4 groups.
P-value for comparison among CARMS 1, 2, 3 and 4 groups, but in the CARMS 4 group, only those participants where the test target was over a non-GA area were included in the analysis.
P-value for comparison among CARMS 1, 2, 3, and 4 groups but in the CARMS 4 group only those subjects where the test target was over a GA area were included in the analysis.
For most rod-mediated parameters, their average values moved in a more impaired direction as disease severity increased, including CARMS 4 being worse than CARMS 3. We wondered if this was largely attributable to the participants in CARMS 4 with the test target directly over an area of GA. The p-value for differences in the rod-mediated parameters across all CARMS categories is significant regardless of whether the CARMS 4 group is defined as those having the test target over GA versus not over GA (Table 2, right four columns). However, the CARMS 4 values for those where the test target was not over the GA area are are not different from values for CARMS 3 participants (p > .25 for all rod-mediated parameters).
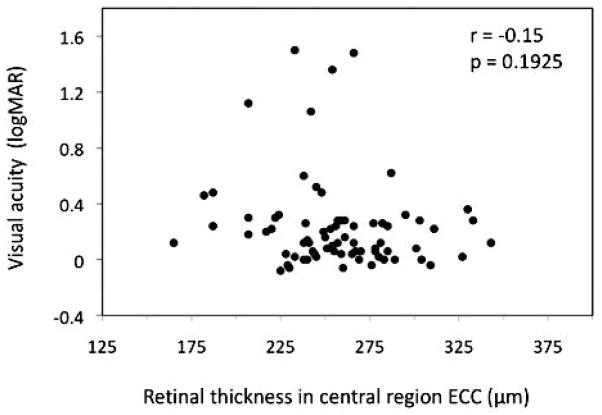
Table 3 provides retinal thickness (μm) as measured by HD SD OCT stratified by CARMS grade, presented for each region. Retinal thickness on the inner rings is decreased as a function of disease severity for CARMS grades 1 to 4. Thickness for CARMS grades 2 and 3 in the areas on the inner ring are very similar. The trend toward decreasing retinal thickness with increasing disease severity is not present on the outer ring regions except for region EUO. Visual acuity was not related to retinal thickness in the region in which it was measured, ECC, (r = −0.15, p = .19; age-adjusted r = −0.19, p = .13) (Figure 2).
Table 3.
OCT retinal thickness parameters for enrollment eye stratified by disease severity (N = 74)
| Retinal thickness of region, μm | CARMS classification | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p-value | |
| Mean (standard deviation) | |||||
| ECC | 276.4 (30.3) | 263.4 (23.4) | 260.4 (37.5) | 228.4 (29.8) | .0001 |
| Inner ring | |||||
| EUI | 319.3 (23.2) | 303 (19.2) | 309.5 (26.8) | 270.0 (27.2) | <.0001 |
| ERI | 314.9 (23.2) | 304.2 (20 7) | 309.5 (26.6) | 258.3 (33.2) | <.0001 |
| EBI | 317.6 (22.5) | 303.7 (20.2) | 307.9 (23.3) | 258.5 (32.1) | <.0001 |
| ELI | 319.0 (24.4) | 303.3 (18.9) | 305.4 (26.1) | 274.8 (30.1) | <.0001 |
| Outer ring | |||||
| EUO | 270.7 (17.8) | 262.4 (15.4) | 273.8 (19.1) | 254.5 (23.7) | .0131 |
| ERO | 269.4 (21.2) | 266.9 (20.5) | 276.1 (23.2) | 259.4 (23.4) | .1399 |
| EBO | 263.2 (16.2) | 258.7 (17.7) | 267.7 (19.9) | 258.3 (19.4) | .3465 |
| ELO | 275.9 (24.8) | 264.3 (21.4) | 275.2 (21.6) | 265.3 (28.8) | .3135 |
Figure 2.
Scatterplot illustrates the relationship between visual acuity (logMAR) and retinal thickness (μm).
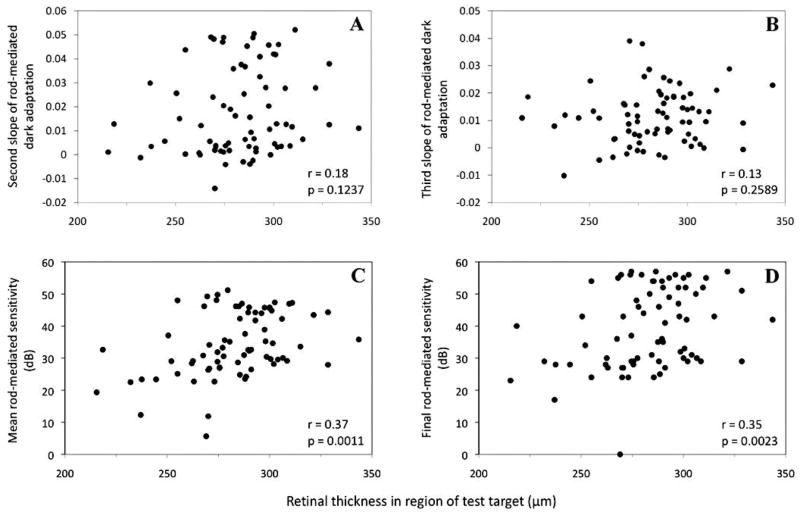
Figure 3 portrays the relationship between each rod-mediated dark adaptation parameter and retinal thickness in the region of the test target. Thinner retinal thickness was associated with lower average rod-mediated sensitivity (Panel C), r = .37, p = .0011 and final rod-mediated sensitivity (Panel D), r = .35, p = .0023 (age-adjusted average sensitivity, r = .30, p = .0093 and final sensitivity, r = .29, p < .0126; acuity-adjusted average sensitivity, r = .27, p = .0239 and final sensitivity, r = .27, p < .0235). Although associations between retinal thickness and second and third parameters of dark adaptation were in the direction of lower slopes (slower rates) corresponding to thinner retinal thickness (Panels A and B), these associations were not statistically significant.
Figure 3.
Scatterplots illustrate the relationship between the four parameters of dark adaptation and retinal thickness (μm). Panel A: Second slope of rod-mediated dark adaptation. Panel B: Third slope of rod-mediated dark adaptation. Panel C: Average rod-mediated sensitivity. Panel D: Final rod-mediated sensitivity.
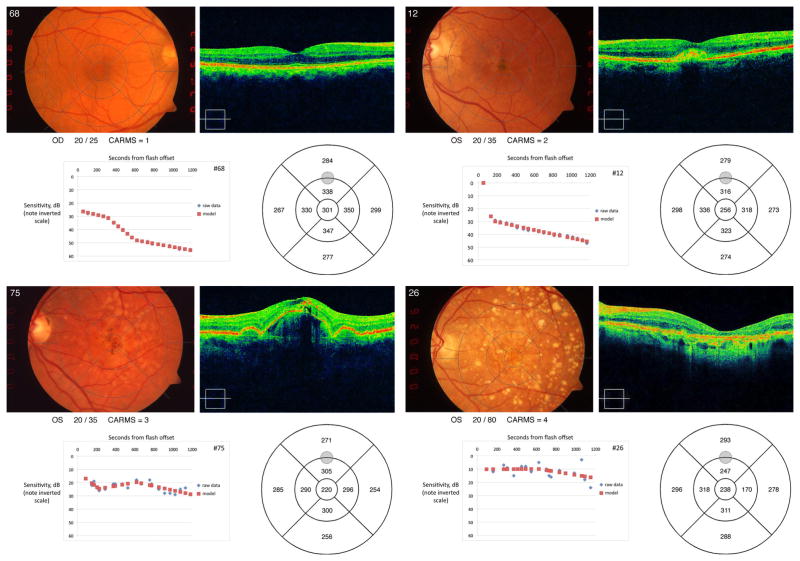
Figure 4 provides example results from four participants with different CARMS grades (1 – 4, normal macular health to geographic atrophy). With increasing non-exudative ARM disease severity, retinal thickness in the region of the test target decreases and the dark adaptation function tends to be flatter and higher on the ordinate, signifying poorer sensitivity.
Figure 4.
The panels contain examples of results from four participants representing each level of non-exudative disease severity. Each panel contains a participant’s fundus photo, visual acuity, CARMs grade, HD SC OCT results, dark adaptation function, and retinal thickness estimates in each grid region. Panel A: CARMS 1. Panel B: CARMS 2. Panel C: CARMS 3. Panel D: CARMS 4.
DISCUSSION
Our results suggest that in non-exudative ARM, thinning of the retina is associated with reduced rod-mediated light sensitivity, suggesting that rod-mediated light sensitivity has the potential to provide information relevant to retinal structure not revealed by the grading of fundus photographs. Our finding that retinal thinning and rod function worsen in an associated fashion during non-exudative ARM pathogenesis underscores the potential utility of structure-function assessments as endpoints in evaluating treatments to prevent or arrest progression of the disease. Since these data are cross-sectional, prospective studies are needed to determine the nature of these associations over time as non-exudative ARM develops. An interesting issue for future prospective research is to what extent rod-mediated dark adaptation and its association with retinal thickness could identify those at high risk for advanced ARM.
In our study there was no association between visual acuity and the thickness of the retinal region subserving visual acuity in persons ranging from normal macular health to different degrees of non-exudative disease. On the other hand, rod-mediated sensitivity in the macula was related to retinal thickness in the region where sensitivity was measured. These findings are consistent with histopathological and psychophysical work suggesting that rod photoreceptor degeneration and rod-mediated dysfunction are markers for early ARM pathogenesis.[9] The linear relationship between rod function and retinal thickness in non-exudative ARM, and the lack of relationship between acuity and retinal thickness, suggests that rod function in the macula may be a more sensitive indicator of disease progression in early non-exudative disease as compared to visual acuity in central vision (the typical approach for evaluating visual function in clinic and in intervention trials on ARM). Proof of concept studies and clinical trials on interventions to prevent ARM or to arrest its progression to geographic atrophy are in urgent need of validated and responsive endpoints. Thus the hypothesis that rod photoreceptor structure and function are potentially useful outcomes or endpoints is deserving of prospective evaluation with a large sample of patients.
Sunness et al. [22] have previously reported that foveal (cone-mediated) dark-adapted sensitivity is predictive of the subsequent development of advanced ARM in eyes with drusen. In our study final rod-mediated threshold during dark adaption became elevated with increased non-exudative disease severity. This raises the possibility that a dark-adapted threshold of the rod system may be a marker for which eyes are at high-risk for the later development of advanced disease, reminiscent of Sunness et al.’s finding for the cone system. If final threshold in our protocol is more predictive than the rate of dark adaptation (which is estimated based on threshold measured repeatedly over time), it could be the case that measuring a final threshold after some period of time in the dark following a photobleach would suffice as an index, in contrast to the increased patient burden of measuring threshold repeatedly over time during the adaptation process. This and related issues are being addressed in an ongoing prospective study by our group.
A strength of our study is that, to our knowledge, this is the first report using HD SD OCT to evaluate quantitative correlations between retinal structure and function in a sample of patients representing a wide range of non-exudative disease severity, from normal macular health to geographic atrophy as defined by the gold standard of fundus grading. A limitation is that it has a cross-sectional design, however, as mentioned earlier, a prospective study on this topic is underway. Another limitation is that with our methods, fixation instability cannot be ruled out, particularly for participants with seriously impaired acuity.
Recent work using spectral domain OCT indicates that reduction in macular thickness in the early stages of non-exudative ARM is partly attributable to thinning of the photoreceptor layer over drusen,[23] also in line with histopathological studies.[9, 24] Our findings that rod-mediated visual function correlates with retinal thickness in non-exudative ARM is consistent with this framework. Further analysis of our OCT images using segmentation techniques to separate retinal layers [23, 25] will allow us to specifically evaluate the connection between thinning of the photoreceptor layer and visual dysfunction. An improved understanding of structure-function relationships in ARM, particularly in its early phases, will not only shed light on underlying biological mechanisms associated with the emergence and progression of the condition, but will hasten the development of endpoints for clinical research.[26] The ARM research community is highly focused on developing treatments to prevent early ARM or arrest its progression to advanced disease. Yet without coordinated structural and functional outcomes that are sensitive and reproducible indices of early ARM disease states, these efforts could be seriously hampered.
Acknowledgments
This research was supported by National Institutes of Health grants R01-AG04212 and R21-EY14071, the EyeSight Foundation of Alabama, Research to Prevent Blindness, the Alfreda J. Schueler Trust, and the Able Trust.
Footnotes
COMPETING INTEREST STATEMENT
Cynthia Owsley is a patent holder on the technology used to measure dark adaptation in this study.
LICENCE FOR PUBLICATION
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in BJO editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms/).
References
- 1.Marmor MF, Wolfenbuerger TJ, editors. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998. [Google Scholar]
- 2.Curcio CA, Medeiros NE, Millican CL. The Alabama age-related macular degeneration grading system for donor eyes. Invest Ophthalmol Vis Sci. 1998;39:1085–96. [PubMed] [Google Scholar]
- 3.Sarks SH. Aging and degeneration in the macular region: A clinico-pathological study. Br J Ophthalmol. 1976;60:324–41. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owsley C, McGwin G, Jackson G, et al. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114:1728–35. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Neelam K, Nolan J, Chakravarthy U, et al. Psychophysical function in age-related maculopathy. Surv Ophthalmol. 2009;54:167–210. doi: 10.1016/j.survophthal.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Erfurth U, Leitgeb RA, Michels S, et al. Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci. 2005;46:3393–402. doi: 10.1167/iovs.05-0370. [DOI] [PubMed] [Google Scholar]
- 7.Wojtkowski M, Srinivasan V, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112:1734–46. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieroni CG, Witkin AJ, Ko TH, et al. Ultrahigh resolution optical coherence tomography in non-exudative age related macular degeneration. Br J Ophthalmol. 2006;90:191–7. doi: 10.1136/bjo.2005.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–49. [PubMed] [Google Scholar]
- 10.Scholl HPN, Bellmann C, Dandekar SS, et al. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–83. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 11.Lamb TD, Pugh ENJ. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–80. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy grading system. Ophthalmology. 2006;113:260–6. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Bio Dis Infor. 2008;1:7–11. doi: 10.1007/s12177-008-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugh EN. Rhodopsin flash photolysis in man. J Physiol. 1975;248:393–412. doi: 10.1113/jphysiol.1975.sp010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGwin G, Jr, Jackson GR, Owsley C. Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput. 1999;31:712–7. doi: 10.3758/bf03200752. [DOI] [PubMed] [Google Scholar]
- 16.Dowling JE. The chemistry of visual adaptation in the rat. Nature. 1960;188:114–8. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- 17.Lamb TD. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 1980;287:349–51. doi: 10.1038/287349a0. [DOI] [PubMed] [Google Scholar]
- 18.Rushton WAH, Campbell FW, Hagins WA, et al. The bleaching and regeneration of rhodopsin in the living eye of the albino rabbit and of man. Optica Acta. 1955;1:183–90. [Google Scholar]
- 19.Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009;116:1119–24. doi: 10.1016/j.ophtha.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Res. 1999;39:3975–82. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 21.Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans. Invest Ophthalmol Vis Sci. 1997;38:557–68. [PubMed] [Google Scholar]
- 22.Sunness JS, Massof RW, Johnson MA, et al. Diminished foveal sensitivity may prdict the development of advanced age-related macular degeneration. Ophthalmology. 1989;96:375–81. doi: 10.1016/s0161-6420(89)32883-1. [DOI] [PubMed] [Google Scholar]
- 23.Schuman JS, Koreishi AF, Farsiu S, et al. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivio with spectra-domain optical coherence tomography. Ophthalmology. 2009;116:488–96. doi: 10.1016/j.ophtha.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson PT, Brown MN, Pulliam BC, et al. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005;46:4788–95. doi: 10.1167/iovs.05-0767. [DOI] [PubMed] [Google Scholar]
- 25.Chan A, Duker J, Ishikawa H, et al. Quantification of photoreceptor layer thickness in normal eyes using optical coherence tomography. Retina. 2006;26:655–60. doi: 10.1097/01.iae.0000236468.33325.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csaky KG, Richman EA, Ferris FL., III Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]