Abstract
Background
Short-term (<1 year) calorie restriction (CR) has been reported to decrease physical activity and metabolic rate in humans and non-human primate models; however, studies examining the very long-term (>10 year) effect of CR on these parameters are lacking.
Objective
The objective of this study was to examine metabolic and behavioral adaptations to long-term CR longitudinally in rhesus macaques.
Design
Eighteen (10 male, 8 female) control (C) and 24 (14 male, 10 female) age matched CR rhesus monkeys between 19.6 and 31.9 years old were examined after 13 and 18 years of moderate adult-onset CR. Energy expenditure (EE) was examined by doubly labeled water (DLW; TEE) and respiratory chamber (24hrEE). Physical activity was assessed both by metabolic equivalent (MET) in a respiratory chamber and by an accelerometer. Metabolic cost of movements during 24h were also calculated. Age and fat-free mass were included as covariates.
Results
Adjusted total and 24hr EE were not different between C and CR. Sleeping metabolic rate was significantly lower, and physical activity level was higher in CR than in C independent from the CR-induced changes in body composition. The duration of physical activity above 1.6 METs was significantly higher in CR than in C, and CR had significantly higher accelerometer activity counts than C. Metabolic cost of movements during 24h were significantly lower in CR than in C. The accelerometer activity counts were significantly decreased after seven years in C animals, but not in CR animals.
Conclusions
The results suggest that long-term CR decreases basal metabolic rate, but maintains higher physical activity with lower metabolic cost of movements compared with C.
Keywords: caloric restriction, energy expenditure, physical activity, energy cost of movement, rhesus monkey, adaptation
1.0 Introduction
The concept of delaying the morbidities of aging through caloric restriction (CR) can be traced back at least three centuries to, Kaibara Ekiken (1630–1714) who wrote at the age of 83 years in his Yōjōkun (The Book of Life-nourishing Principles) that one way to remain healthy and increase longevity is to stop eating when the stomach is less than full (Kaibara and Translated by Wilson 2009 (Originally written in 1712)). Far more recently, careful laboratory studies of CR without malnutrition have shown that CR does indeed extend the maximal life span in multiple short-lived species (Anderson et al. 2009). In nonhuman primates, CR has been shown to reduce or delay the onset of diverse age-related diseases and disorders such as diabetes (Gresl et al. 2001), sarcopenia (Colman et al. 2008), immune senescence (Messaoudi et al. 2006), hypertension, cancer, bone demineralization, and brain atrophy (Colman and Anderson 2011; Colman et al. 2009). The study of rhesus monkeys at the University of Wisconsin, begun in 1989, also demonstrated a reduction in age-related mortality in CR animals (Colman and Anderson 2011; Colman et al. 2009), although an effect on longevity was not found in a second study performed at the National Institute of Aging (Mattison et al. 2012).
In rhesus monkeys CR is associated with an initial weight loss, but body weight plateaus indicating that caloric balance is reestablished during the CR intervention (Colman et al. 2008). The metabolic transition is characterized by a decrease in energy expenditure (Ramsey et al. 2000a), presumably to match the reduction in energy intake. Randomized controlled trials for CR have shown that much of the adaptation is driven by a reduction in body size, but reductions in energy expenditure that cannot be explained simply by the smaller body size have been reported (DeLany et al. 1999; Ramsey et al. 1997; Weed et al. 1997), although findings are inconsistent (Kemnitz et al. 1993; Moscrip et al. 2000; Ramsey et al. 1997; Weed et al. 1997).
Daily energy expenditure has three major components: resting metabolic rate, the thermic effect of food, and the energy expenditure of physical activity. Most studies to date have focused on resting metabolic rate and total energy expenditure. Physical activity has also been studied and earlier reviews of the subject concluded that CR did not alter physical activity in macaques (Heilbronn and Ravussin 2003; Ingram et al. 2001; Roth et al. 2002). In rodent studies, however, differing results have been reported; specifically, it has been found that wheel-running activity of rats was generally reduced in CR groups early in life, but CR resulted in higher activity levels later in life when control groups began to exhibit a marked age related decline in activity (Goodrick et al. 1983).
The objective of this current study was to determine the metabolic and behavioral adaptations to long-term diet restriction in rhesus monkeys. Longitudinal changes in energy expenditure including the metabolic cost of movement, and duration and intensity of physical activity during 24h were determined in monkeys enrolled in the on-going University of Wisconsin CR and Aging study (Kemnitz et al. 1993; Ramsey et al. 2000a). The youngest animals in this longitudinal study were 19 years of age at the last assessment time-point for this analysis, an age considered past the age of sarcopenia onset, which has been clinically assessed to be 14–16 years of age in rhesus monkeys at the Wisconsin National Primate Research Center (WNPRC) (Colman et al. 2005).
2.0 Materials and Methods
2.1 Animals
The CR study at WNPRC has been previously described (Kemnitz et al. 1993; Ramsey et al. 1997). Briefly, the study includes three groups of adult rhesus monkeys (Macaca mulatta of Indian derivation); 30 male monkeys entered in the study in 1989 (Group 1), and 30 females (Group 2) and an additional 16 males (Group 3) were introduced in 1994. All groups averaged ~10 years of age at the onset of CR. Within each group animals were stratified by body weight and randomly assigned to either the control (C) or CR group. For this study, energy expenditure data were collected at two time-points, 1999–2000 and 2007–2008, along with additional analysis of activity at interim time-points. At the end of the 2008 data collection period, 18 of 38 animals in C group and 24 of 38 animals in CR group were still alive, and it was these 42 surviving animals that were included in this analysis at both time-points so that within-animal changes could be evaluated. Mean age was 24.6 ± 2.8 years old and intervention duration was over 18 yr for Group 1 and over 13 yr for Group 2 and 3. The median life span of control rhesus monkeys at WNPRC is 27 years and the maximum life span is 40 years (Colman and Anderson 2011).
The animals were housed in individual cages to minimize aggressive encounters and to control and quantify food intake. All animals had extensive visual and auditory contact with other study animals. The animals were allowed continuous access to water and the rooms were maintained at 21–26°C with ~50–65% relative humidity. Artificial room lighting was automatically controlled to provide 12-h light and dark periods. The protocol was approved by the Animal Care and Use Committee of the Graduate School of the University of Wisconsin-Madison, an AAALAC-accredited program.
2.2 Diet
At the outset of the study, ad libitum food intake was assessed to define an individual baseline intake for each animal. Thereafter, the C monkeys were allowed ad libitum access to food for 6–8 h per day, while CR monkeys underwent individualized calorie restriction of ~30% from the baseline assessment amount. The C monkeys were fed a defined, pelleted diet (no. 85387, Teklad Co., Madison, WI) containing 15% lactalbumin, 10% corn oil, and approximately 65% carbohydrate, as previously described (Ramsey et al. 2000a). CR animals were fed a similar diet (no. 93131, Teklad Co., Madison, WI), except that the mineral and vitamin mix were increased by about 30% to minimize differences in mineral and vitamin intake between groups. Food was provided in the morning and removed 6–8 h later. At this time, weight of spilled food was estimated and food remaining in the trays was weighed to calculate daily food intake, and the animals were given a piece of fresh fruit.
The feeding protocol was modified slightly in the calorimetry chambers to standardize timing of intake and to insure that all animals consumed their assigned allotment (Blanc et al. 2003; Ramsey et al. 1997). A palatable sandwich (fat 49%, carbohydrate 38%, protein 13%) was provided as two meals. The first meal was at 1000 h, and the energy content of this meal was the same for all animals. The afternoon meal was provided at 1500 h, and the energy content of this meal was adjusted to conform to designated daily energy intake.
2.3 Doubly Labeled Water procedures
Total energy expenditure (TEE) was determined during a 5-d period by the two-point DLW methodology described previously (Blanc et al. 2003). After anesthesia (ketamine HCl, 15 mg/kg im), a baseline blood sample was collected, and a premixed 1.51 g/kg estimated total body water (TBW) dose of DLW was given intravenously. The dose was composed of 0.3 g/kg estimated TBW of 94% H2 18O (Rotem Industries Ltd., Beer Sheva, Israel) and 0.17 g/kg estimated TBW of 99.9% 2H2O(Cambridge Isotope Laboratories, Andover, MA) and was diluted with 3% NaCl to physiological osmolarity. Blood samples were collected at 2 hr and 1 and 5 d after dosing. Immediately after collection, blood was centrifuged for 10 min for serum separation. Serum was stored at −20°C in cryogenically stable tubes until analysis by isotope ratio mass spectrometry.
Water from serum samples was extracted by centrifugation (4°C, 1 h, RCF 12,000×g) on regenerated cellulose filters (YM-50, Centricon, Bedford, MA). Deuterium and oxygen-18 isotopic enrichments were determined as previously described (Blanc et al. 2003). CO2 production was calculated according to the equation of Schoeller et al. (1986):
rCO2(mol/d) = N/2.078(1.007ko−1.041kd) −0.0266N(1.007ko−1.041kd)
where N represents the average isotope dilution space of deuterium and 18O calculated from Coward et al. by the plateau method using the 2-h post-dose sample and corrected for isotope exchange by the factors 1.041 and 1.007, respectively. The observed isotope dilution space ratio was 1.041 ± 0.009 (mean ± SD). ko and kd represent the isotope elimination rates calculated by linear regression of the natural logarithm of isotope enrichment as a function of elapsed time from approximately one day after the dose (day 1 samples). TEE was calculated using the modified Weir equation and a food quotient of 0.93, which was estimated from the animal’s diet. Day 1 samples were chosen for calculation of ko and kd to avoid the potential artifacts (hypometabolism, hypoactivity, etc.) introduced in the TEE estimates by anesthesia.
2.4 Calorimetry
Twenty-four hour energy expenditure (24hEE) was measured in a standard cage enclosed within a transparent metabolic chamber with dimensions of 75 cm wide × 75 cm deep × 80 cm high as previously described (Raman et al. 2007a; Raman et al. 2007b). Chamber temperature was maintained at 21°C to maintain the animals at thermoneutrality. The animals were housed in these chambers 1 day before the start of measurements for acclimatization. Respiratory gas exchange was measured for2 consecutive days. The chamber was located in a room where other animals were housed to provide a familiar social environment.
Filtered air was drawn into the chamber, and the flow rate, temperature, and humidity were measured. A portion of the exhaust air from the chamber was dried and analyzed for oxygen (S-3AO2 analyzer; Ametek, Pittsburgh, PA) and CO2 (CD-3 CO2 analyzer; Ametek) contents. The 24hrEE was calculated on the basis of the oxygen consumption and CO2 release rates using the modified Weir equation. Ethanol was burned from a lamp and the %recovery of O2 and CO2 was used to calibrate the chambers. Night EE (NEE) was defined as the EE during the light-off period of 6PM to 6AM. Sleeping metabolic rate (SMR) was defined as the lowest continuous 3 h period recorded during the night with confirmation of no physical activity by activity count. Physical activity level (PAL) was calculated both by TEE/SMR and 24hr EE/SMR. Metabolic equivalent intensity (MET) were calculated every 5 min as times of SMR.
2.5 Accelerometer
Physical activity data were collected using a commercial accelerometer (Actiwatch AW-64, Resprionics/Mini-Mitter, Bend, OR) (Zhdanova et al. 2002). Prior to using the accelerometer-based system, physical activity (i.e. movement) was recorded for each animal over a one week period by motion detectors. We fully validated the accelerometer data against the motion detector data before switching methodologies. During this validation there was no indication of a differential response to the collars by the two diet groups. The accelerometer was attached on a collar fastened around the animal’s neck. Annual accelerometry measures were taken over a 3–4 week period each year. The first ~5 days of activity were considered to be the adaptation period and those data were not included in the analysis. For accelerometry measures during the 2007–8 metabolic rate assessment periods, collars were attached a minimum of 5 days prior to animals being placed in the respiratory chambers. The accelerometer sampled activity counts every 1 min and these measurements were averaged for every hour, day (0600–1800 h), night (1800–0600 h), morning (0600–1200 h), afternoon (1200–1800 h), and 24hr.
Accelerometer data were collected for all CR animals but only 13 of 18 C animals because at the time of measurement the collars were not well tolerated by 5 of the C animals.
2. 6 Metabolic cost for movements
Gross metabolic rate was divided by body mass (BM), BM0.67, and SMR to obtain the array of normalized gross metabolic rate that have been published by others (Blanc et al. 2003; Kleiber 1947; White and Seymour 2005). The relationship between metabolic rate and activity count is approximately linear over a broad range of activity counts (Puyau et al. 2002; Puyau et al. 2004; Yamada et al. 2009), and cost of activity (COA) can be calculated as the slope of the regression of metabolic rate (the dependent variable) on activity counts because the regressions of activity counts on metabolic rates return significant intercepts (Rezende et al. 2006). We plotted metabolic rates against activity counts for every hour in the respiratory chamber to estimate COA for each individual. Using least squares linear regressions, we estimated the slope (i.e., incremental COA) and intercept for each individual. The sum of the so-called postural and thermic effect of food (TEF) costs was calculated as the difference between SMR and the zero-activity count intercept of the activity count versus gross metabolic rate regression (Rezende et al. 2006; Taylor et al. 1982; Taylor et al. 1970). This is equal to the zero-activity intercept of the activity count versus net metabolic rate regression. The slopes (COA) were calculated with gross metabolic rate and gross metabolic rate divided by BM, BM0.67, and SMR.
2. 7 Body composition analysis
Dual-energy X-ray absorptiometry (DXA) (Model DPX-L, GE/Lunar Corp., Madison, WI) was used to assess lean tissue mass of the total body and limbs and to estimate skeletal muscle mass. Following an overnight fast, animals were sedated with ketamine HCl (10 mg/kg, IM) and weighed. Animals were further administered a mixture of ketamine HCl and xylazine (7 mg/kg, 0.6 mg/kg xylazine, respectively, IM) for additional muscle relaxation and anesthesia maintenance. Upon scan completion, yohimbine (0.06 mg/kg, IV) was given to reverse xylazine. Approximate scan time was 20 minutes per animal.
Total body scans were acquired with the animal in the supine position and analyzed using Lunar pediatric software (version 1.5e for acquisition, version 4.0a for analysis) as previously described (Blanc et al. 2005; Colman et al. 2008). Analyses were conducted on the total body and regions of interest (arms, legs). For total body composition, fat free mass (FFM) and fat mass (FM) were obtained. Regions of interest were defined based upon bony landmarks (i.e., upper and lower legs were divided at the interface of the femur and tibia). Appendicular skeletal muscle mass (ASM) was determined by summing the lean tissue mass from the arms and legs (Colman et al. 2008). DXA coefficients of variation ([mean/standard deviation]*100) for sites evaluated in this study were as follows: total body lean mass 0.8%, ASM 1.6%, lean mass of legs 1.9%, lean mass of upper legs 3.7%, lean mass of lower legs 4.3%, and lean mass of arms 2.2% (Colman et al. 2008).
2. 8 Statistical analysis
All analyses were performed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL). Results were presented as the mean ± standard deviation (SD). The group differences in physical and metabolic characteristics were tested by repeated-measures three-way analysis of covariance (ANCOVA) with groups (C vs. CR) and sex (male vs. female) as between-subject-factors; time (1999–2000 vs. 2007–8) as a within-subject factor; and age as a covariate in the model, because the animals of all three groups varied in age. For the metabolic characteristics, the ANCOVA were examined also with age and FFM as covariates. The group differences of the duration of metabolic intensity in the respiratory chamber were tested by repeated three-way ANCOVA with groups (C vs. CR) as a between subject-factor; intensity (1.2–1.4, 1.4–1.6, 1.6–1.8, 1.8–2.0 vs. 2.0 METs and over) and time (1999–2000 vs. 2007–8) as within-subject factors; and age as a covariate in the model. For the variables that did not have normal distribution, ANCOVA was applied for the data after logarithmic transformation when appropriate. For all of the analyses, an alpha of 0.05 was used to denote statistical significance.
3. 0 Results
3. 1 Dietary intake
The impact of age on calorie intake has influenced the extent of CR achieved in this study over time. Until 2001 the difference in energy intake between C and CR had been about 28–30% (Blanc et al. 2003); however, the difference in energy intake between C and CR decreased thereafter. As the study progressed, the 18 C animals ate 11% less than at the outset of the study (2.72 MJ/d at 1999 to 2.41 MJ/d at 2007, P = 0.020 by paired t-test). Energy intake did not change over the same period for the 24 CR animals (4% decrease from 2.12 MJ/d to 2.03 MJ/d, P = 0.268). Due to the age-associated decline in energy intake among C animals, the difference of energy intake between C and CR animals dropped to about 16% by the second assessment time-point in 2007–2008.
3.2 Body weight and composition
Table 1 summarizes the physical characteristics of the animals for the two times periods of study (1999–2000 and 2007–2008). The two diet treatment groups were of similar age (P = 0.425). The average body weight (BW) of all CR animals was 18 and 22% lower than that of the C animals during 1999–2000 and 2007–2008 respectively. This was reflected in a reduction in FM in all CR animals compared to C. The FFM of the CR animals was lower than the C animals by 8% during both periods, but ASM difference did not reach significance (P = 0.115). Expressed as a percentage of body weight, fat mass was 11 and 12 percentage points lower in the CR animals (P<0.001). Females had lower BW, FM, FFM, and ASM than males (P≤0.013), but did not differ in percent body fat (P = 0.703). There were significant interactions of sex vs. time in FFM and ASM. In males, average FFM and ASM were significantly decreased in 2007–2008 compared with those in 1999–2000 (P<0.001). In contrast, among females, average FFM and ASM were not significantly different between study periods (P = 0.934 and 0.128, respectively). There was no significant interaction of group vs. sex or group vs. time.
Table 1.
Physical characteristics and body composition of the subjects.
| Male | Female | P value of repeated three-way ANCOVA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement Period |
Control (n = 10) | CR (n = 14) | Difference (%) |
Control (n = 8) | CR (n = 10) | Difference (%) |
Group | Sex | Time | G×S | G×T | S×T | G×S×T | |
| Age (yr) | 1999–2000 | 17.8 ± 3.3 | 17.9 ± 3.5 | 16.6 ± 1.6 | 17.9 ± 2.0 | 0.425 | 0.512 | <0.001 | 0.521 | 0.248 | 0.902 | 0.751 | ||
| 2007–2008 | 24.8 ± 3.4 | 24.9 ± 3.5 | 23.6 ± 1.7 | 24.9 ± 2.0 | ||||||||||
| Weight (kg) | 1999–2000 | 14.13 ± 2.65 | 10.87 ± 1.84 | −23 | 9.35 ± 1.72 | 8.35 ± 1.46 | −11 | <0.001 | <0.001 | 0.986 | 0.121 | 0.448 | 0.303 | 0.219 |
| 2007–2008 | 13.11 ± 3.41 | 10.10 ± 1.11 | −23 | 9.60 ± 1.93 | 7.47 ± 0.95 | −22 | ||||||||
| FM (kg) | 1999–2000 | 4.36 ± 1.73 | 2.09 ± 0.96 | −52 | 2.78 ± 0.99 | 2.06 ± 0.87 | −26 | <0.001 | 0.013 | 0.463 | 0.053 | 0.321 | 0.850 | 0.424 |
| 2007–2008 | 4.22 ± 2.16 | 1.88 ± 0.73 | −56 | 2.83 ± 1.05 | 1.48 ± 0.69 | −48 | ||||||||
| FFM (kg) | 1999–2000 | 10.44 ± 1.21 | 9.31 ± 1.08 | −11 | 7.09 ± 1.23 | 6.64 ± 0.77 | −6 | 0.035 | <0.001 | 0.082 | 0.627 | 0.547 | 0.001 | 0.061 |
| 2007–2008 | 9.39 ± 1.52 | 8.80 ± 0.76 | −6 | 7.33 ± 1.30 | 6.47 ± 0.46 | −12 | ||||||||
| ASM (kg) | 1999–2000 | 4.38 ± 0.56 | 3.91 ± 0.53 | −11 | 2.84 ± 0.49 | 2.68 ± 0.37 | −6 | 0.115 | <0.001 | 0.317 | 0.660 | 0.242 | <0.001 | 0.044 |
| 2007–2008 | 3.61 ± 0.70 | 3.49 ± 0.41 | −3 | 2.81 ± 0.53 | 2.50 ± 0.19 | −11 | ||||||||
| Percent body fat (%) | 1999–2000 | 28.48 ± 7.35 | 17.65 ± 5.34 | −38 | 27.67 ± 7.25 | 22.88 ± 7.01 | −17 | <0.001 | 0.703 | 0.144 | 0.27 | 0.185 | 0.291 | 0.363 |
| 2007–2008 | 29.02 ± 10.82 | 17.28 ± 5.29 | −40 | 27.22 ± 7.81 | 17.98 ± 6.29 | −34 | ||||||||
Mean ± SD.
CR, calorie restriction group; FM, fat mass; FFM, fat free mass; ASM, appendicular skeletal muscle mass; G, group (C vs. CR); S, sex (male vs. female); T, time (1999–2000 vs. 2007–2008).
P values were calculated by ANOVA for age, and by ANCOVA with age as a covariate in the model for other variables.
3.3 Energy expenditure and concurrent short-term intake
As part of the study design the energy intake in the metabolic chamber was lower for CR compared with C during both assessment periods by 22 and 16%, respectively (Table 2). Energy intake during the double labeled water (DLW) periods was lower by 24% in the CR animals at the period of 1999–2000 compared with the C, but no significant difference was observed during the 2007–2008 period (6%). C monkeys ate less food under ad lib. conditions during recovery from anesthesia required for the DLW administration and body composition measures in 2007–2008, although energy intake recovered and returned to baseline within days (Supplemental Figure 1). C monkeys are about 15% less and returned to baseline with about 5 days, while CR animals displayed no dietary intake response. The chamber 24hr energy expenditure (24hrEE) and DLW total energy expenditure (TEE) were not significantly different between C and CR, even when FFM was added as a covariate (Table 2). The CR animals had significantly lower sleeping metabolic rate (SMR) than C animals during both study periods (11 and 19%, respectively), and the difference was still significant when FFM was added as covariate (P = 0.007). Physical activity levels (PAL) during DLW (PALDLW) and metabolic chamber (PALchamber) assessments were both significantly higher in the CR groups than in the C groups (P = 0.017 and 0.014 respectively).
Table 2.
Characteristics of energy metabolism of the subjects.
| Male | Female | P value of repeated three-way ANCOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement Period |
Control (n = 10) | CR (n = 14) | Difference (%) |
Control (n = 8) | CR (n = 10) | Difference (%) |
Covariates | Group | Sex | Time | G×S | G×T | S×T | G×S×T | |
| EIchamber (MJ/d) | 1999–2000 | 3.06 ± 0.55 | 2.31 ± 0.37 | −25 | 2.30 ± 0.78 | 1.87 ± 0.28 | −19 | Age | 0.001 | <0.001 | 0.755 | 0.656 | 0.152 | 0.684 | 0.117 |
| 2007–2008 | 2.66 ± 0.54 | 2.33 ± 0.47 | −12 | 2.10 ± 0.27 | 1.62 ± 0.21 | −23 | Age and FFM | 0.007 | 0.973 | 0.124 | 0.875 | 0.400 | 0.111 | 0.192 | |
| EIDLW (MJ/d) | 1999–2000 | 3.02 ± 0.64 | 2.31 ± 0.37 | −24 | 2.43 ± 0.82 | 1.78 ± 0.34 | −27 | Age | <0.001 | <0.001 | 0.764 | 0.973 | 0.058 | 0.526 | 0.522 |
| 2007–2008 | 2.21 ± 0.62 | 2.18 ± 0.39 | −1 | 1.91 ± 0.18 | 1.64 ± 0.27 | −14 | Age and FFM | 0.004 | 0.728 | 0.062 | 0.451 | 0.230 | 0.040 | 0.765 | |
| 24hrEEchamber (MJ/d) | 1999–2000 | 2.55 ± 0.35 | 2.35 ± 0.36 | −8 | 2.04 ± 0.26 | 2.02 ± 0.23 | −1 | Age | 0.422 | <0.001 | 0.872 | 0.190 | 0.745 | 0.123 | 0.816 |
| 2007–2008 | 2.37 ± 0.42 | 2.03 ± 0.31 | −14 | 2.18 ± 0.25 | 1.98 ± 0.19 | −9 | Age and FFM | 0.360 | 0.223 | 0.346 | 0.450 | 0.159 | 0.067 | 0.713 | |
| TEEDLW (MJ/d) | 1999–2000 | 2.63 ± 0.44 | 2.35 ± 0.59 | −11 | 1.84 ± 0.33 | 1.80 ± 0.47 | −2 | Age | 0.422 | <0.001 | 0.872 | 0.190 | 0.745 | 0.123 | 0.816 |
| 2007–2008 | 2.39 ± 0.47 | 2.19 ± 0.38 | −8 | 1.85 ± 0.26 | 1.82 ± 0.35 | −2 | Age and FFM | 0.584 | 0.832 | 0.431 | 0.402 | 0.961 | 0.996 | 0.963 | |
| SMR (MJ/d) | 1999–2000 | 1.96 ± 0.23 | 1.69 ± 0.25 | −14 | 1.49 ± 0.25 | 1.37 ± 0.25 | −8 | Age | 0.001 | <0.001 | 0.660 | 0.491 | 0.207 | 0.018 | 0.535 |
| 2007–2008 | 1.86 ± 0.40 | 1.52 ± 0.27 | −18 | 1.69 ± 0.27 | 1.39 ± 0.14 | −18 | Age and FFM | 0.007 | 0.564 | 0.540 | 0.888 | 0.304 | 0.087 | 0.494 | |
| NEE (MJ/d) | 1999–2000 | 2.13 ± 0.22 | 1.83 ± 0.26 | −14 | 1.64 ± 0.25 | 1.53 ± 0.23 | −7 | Age | 0.001 | <0.001 | 0.627 | 0.355 | 0.318 | 0.021 | 0.521 |
| 2007–2008 | 2.04 ± 0.40 | 1.70 ± 0.31 | −16 | 1.85 ± 0.25 | 1.58 ± 0.18 | −15 | Age and FFM | 0.012 | 0.357 | 0.537 | 0.708 | 0.428 | 0.099 | 0.486 | |
| PALchamber | 1999–2000 | 1.31 ± 0.21 | 1.41 ± 0.21 | +7 | 1.38 ± 0.18 | 1.54 ± 0.33 | +11 | Age | 0.014 | 0.210 | 0.567 | 0.367 | 0.685 | 0.523 | 0.964 |
| 2007–2008 | 1.29 ± 0.15 | 1.35 ± 0.14 | +5 | 1.31 ± 0.16 | 1.44 ± 0.15 | +10 | Age and FFM | 0.023 | 0.539 | 0.759 | 0.371 | 0.702 | 0.713 | 0.963 | |
| PALDLW | 1999–2000 | 1.34 ± 0.17 | 1.44 ± 0.41 | +7 | 1.25 ± 0.22 | 1.42 ± 0.64 | +14 | Age | 0.017 | 0.084 | 0.851 | 0.503 | 0.659 | 0.379 | 0.863 |
| 2007–2008 | 1.30 ± 0.14 | 1.47 ± 0.30 | +14 | 1.12 ± 0.23 | 1.32 ± 0.25 | +18 | Age and FFM | 0.012 | 0.781 | 0.338 | 0.610 | 0.944 | 0.180 | 0.993 | |
| 24hEEchamber-SMR (MJ/d) | 1999–2000 | 0.59 ± 0.36 | 0.66 ± 0.32 | +12 | 0.54 ± 0.18 | 0.65 ± 0.28 | +20 | Age | 0.148 | 0.793 | 0.261 | 0.405 | 0.547 | 0.420 | 0.723 |
| 2007–2008 | 0.51 ± 0.20 | 0.51 ± 0.18 | +0 | 0.49 ± 0.21 | 0.60 ± 0.18 | +21 | Age and FFM | 0.096 | 0.526 | 0.593 | 0.510 | 0.549 | 0.726 | 0.715 | |
| TEEDLW-SMR (MJ/d) | 1999–2000 | 0.67 ± 0.33 | 0.67 ± 0.63 | +0 | 0.35 ± 0.30 | 0.43 ± 0.54 | +24 | Age | 0.088 | 0.002 | 0.898 | 0.343 | 0.264 | 0.827 | 0.840 |
| 2007–2008 | 0.53 ± 0.23 | 0.67 ± 0.38 | +26 | 0.16 ± 0.39 | 0.43 ± 0.34 | +179 | Age and FFM | 0.030 | 0.584 | 0.267 | 0.502 | 0.517 | 0.244 | 0.676 | |
| 24hEEchamber-SMR/wt (MJ/d/kg) | 1999–2000 | 0.044 ± 0.031 | 0.061 ± 0.027 | +39 | 0.061 ± 0.028 | 0.079 ± 0.033 | +31 | Age | 0.007 | 0.017 | 0.385 | 0.289 | 0.948 | 0.681 | 0.241 |
| 2007–2008 | 0.043 ± 0.025 | 0.051 ± 0.016 | +18 | 0.052 ± 0.020 | 0.082 ± 0.030 | +59 | |||||||||
| TEEDLW-SMR/wt (MJ/d/kg) | 1999–2000 | 0.048 ± 0.024 | 0.060 ± 0.056 | +24 | 0.040 ± 0.035 | 0.045 ± 0.059 | +15 | Age | 0.014 | 0.074 | 0.534 | 0.497 | 0.108 | 0.659 | 0.410 |
| 2007–2008 | 0.043 ± 0.022 | 0.067 ± 0.039 | +57 | 0.016 ± 0.043 | 0.059 ± 0.044 | +273 | |||||||||
Mean ± SD
CR, calorie restriction group; EI, energy intake; TEE, total energy expenditure estimated by DLW method; 24hrEE, 24hr energy expenditure measured by respiratory chamber; SMR, sleeping metabolic rate; NEE, night time energy expenditure; PAL, physical activity level; G, group (C vs. CR); S, sex (male vs. female); T, time (1999–2000 vs. 2007–2008).
P values were calculated by ANCOVA with age or age and FFM as covariates in the model.
3. 4 Activity
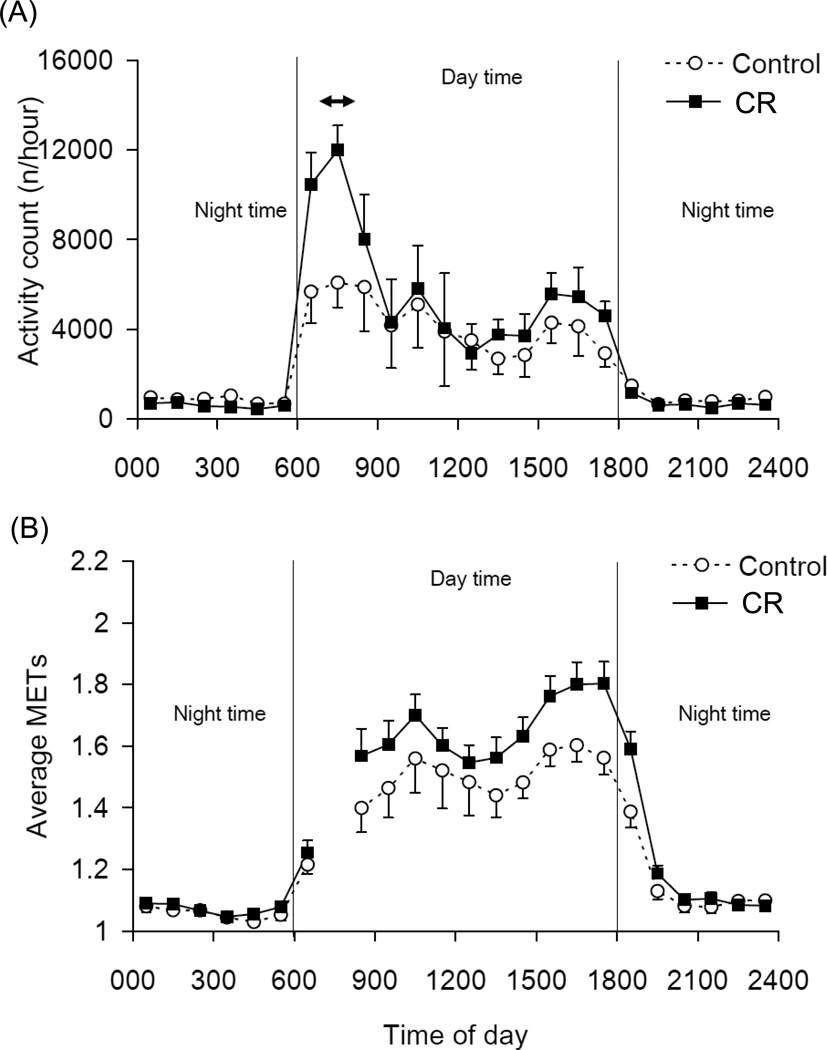
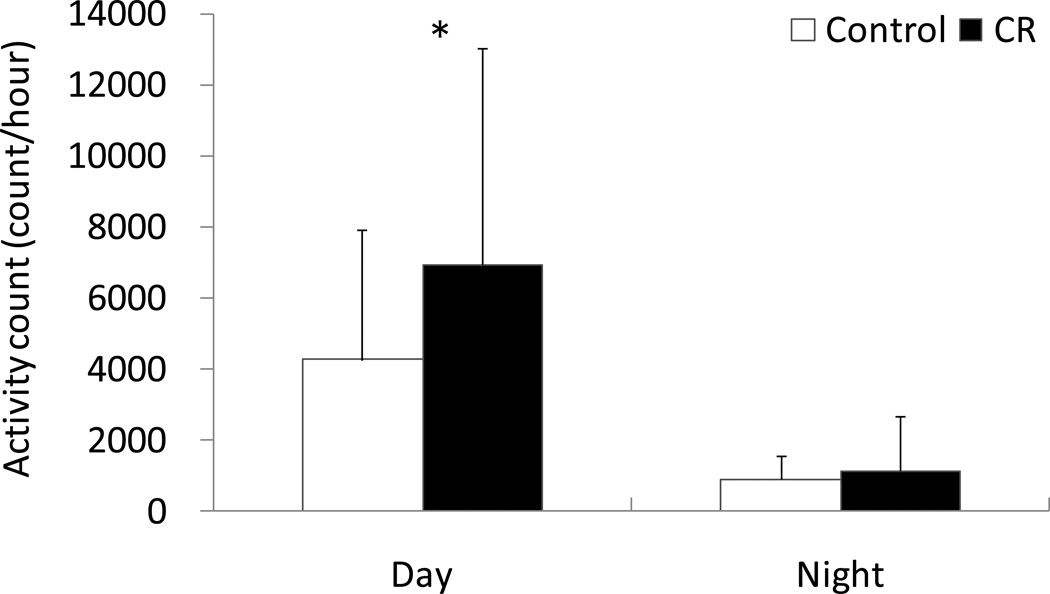
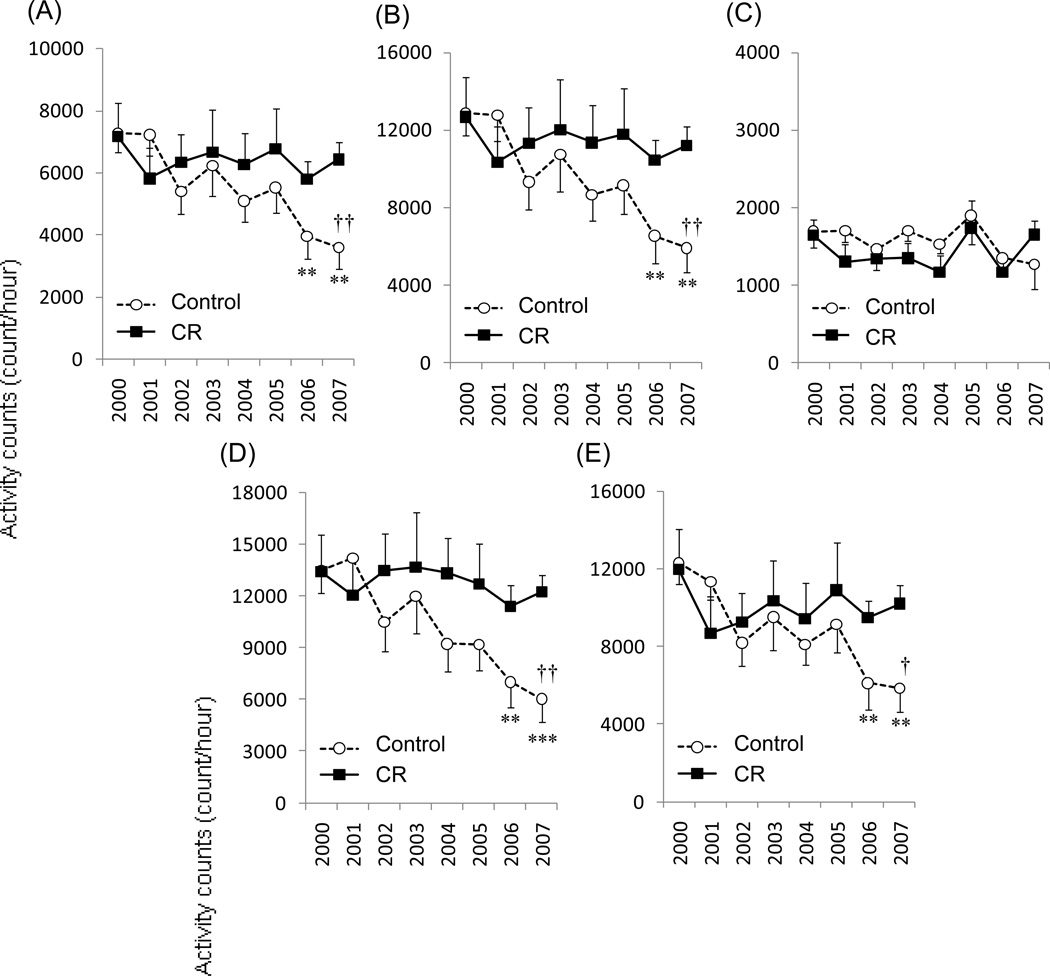
The average time-series activity and associated metabolic equivalent intensity (MET) measures for C and CR animals during the respiratory chamber assessments in 2007–2008 are shown in Figure 1. The CR animals displayed greater accelerometer activity counts and higher MET during the day than the C animals. There was no difference in accelerometer activity frequencies between C and CR during the night (P = 0.407) (Figure 2). Figure 3 shows the longitudinal change of accelerometer activity counts in home cages measured each year between 2000 and 2007. Significant interactions of time × group were observed in 24hr, day time, morning, and afternoon activity counts, but not in night activity counts. The activity counts were significantly decreased with time in C animals, but not in CR animals.
Figure 1.
Time-series of the activity count (A) and metabolic equivalent intensities (METs) (B) for C and CR animals in the metabolic chamber. The animals were taken out of the chamber between 7:00–8:00 (↔) for recalibration of the equipment (2007–2008). Data are shown as Mean ± SEM.
Figure 2.
Activity counts during the 12h of light (day) and dark (night) for C and CR animals. Although there was no significant difference between C and CR during the night, a significant difference (* P<0.05) was observed during the day (2007–8). Data are shown as Mean ± SD.
Figure 3.
The longitudinal change of accelerometer daily activity counts in home cages measured each year between 1999 and 2008 for 24hr (A), day time (B), night (C), morning (D), and afternoon (E) activity counts. Except for night-time, a significant time × group interaction was observed. Activity counts were significantly decreased with time in C animals, but not in CR animals. ** P<0.01, *** P<0.001; significantly lower than 2000 period. † P<0.05, †† P<0.01; significantly lower than CR group.
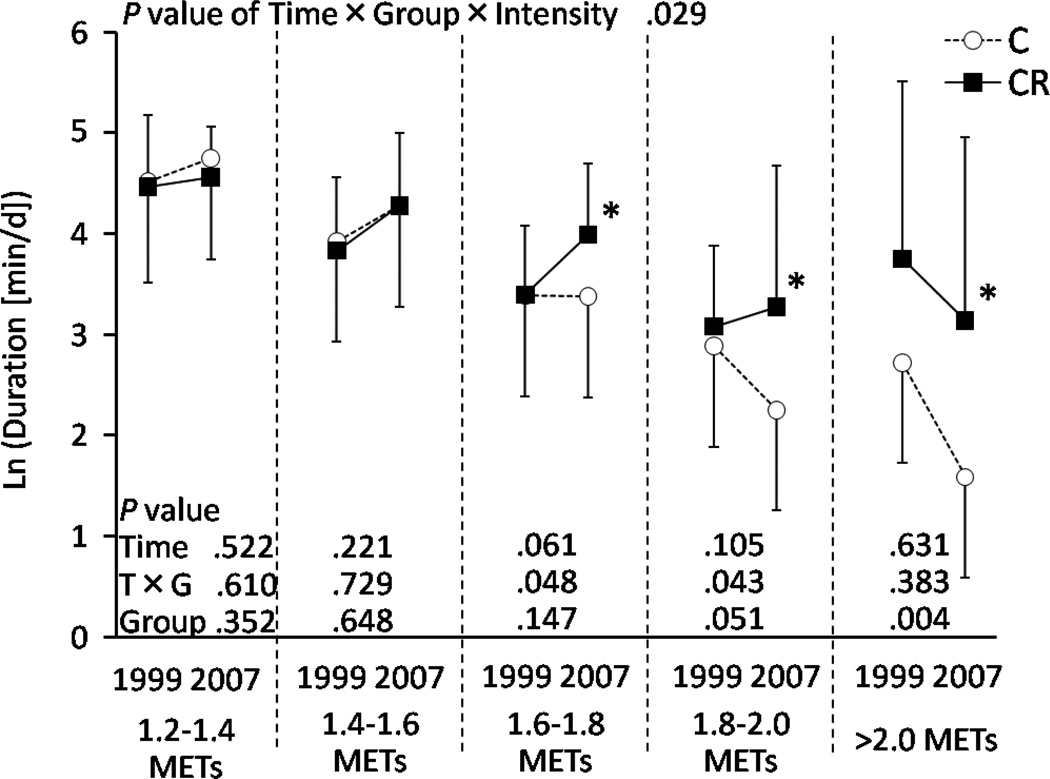
Figure 4 shows the duration of physical activity categorized by metabolic intensity (EE/SMR) in the respiratory chamber during both assessment periods. A significant time × group × intensity interaction was observed (P = 0.029) indicating that the duration of each intensity changed between the two assessment periods, but in a different manner for C and CR animals. Thus, we further analyzed each intensity category separately. The results of repeated-measures two-way ANCOVA and post-hoc analysis are shown in Figure 4. The CR animals had significantly longer (~2 times longer) duration of the activities at 1.6METs and above than the C animals for the period of 2007–2008.
Figure 4.
Duration of physical activity in the respiratory chamber for the range of metabolic equivalent intensities. Logarithmic transformations were applied because the data could not be regarded as a normal distribution. * Significantly higher than C group (P<0.05).
3. 5 Metabolic costs for movements
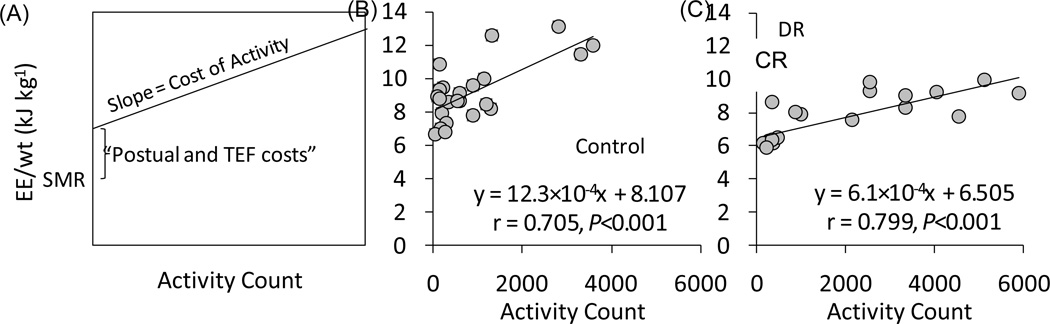
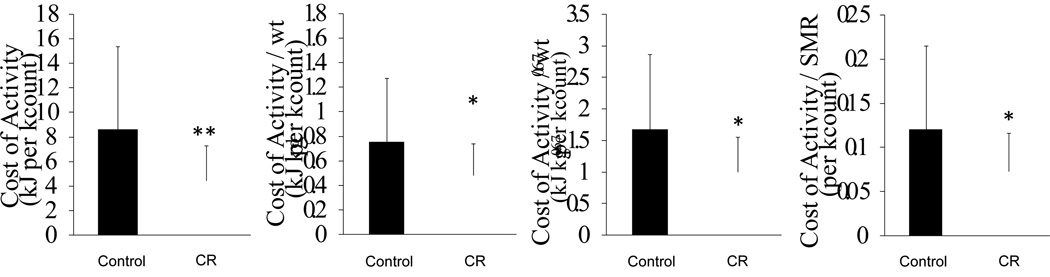
Figure 5 shows the relationship between energy expenditure and activity count for every hour in the respiratory chamber of a representative C monkey and a representative CR monkey. The mean correlation coefficients were 0.689 (25% and 75% quartiles by value of r; 0.640 and 0.705, P < 0.001) for the C group and 0.650 (0.527 and 0.773, P < 0.001) for CR group. The energy cost of activity (COA) which was calculated from the slope of energy expenditure per kg for each hour vs. activity count for that hour was different for C and CR animals. Figure 6 shows the unadjusted COAs of C and CR animals shown as gross metabolic and adjusted COA where gross metabolic rate was divided by either BM, BM0.67, or SMR. All four expressions of COA were significantly lower in CR compared with the C group (P < 0.05) by ANCOVA with age and sex as a covariate in the model. The slope, which reflects COA, was independent mathematically from SMR and intercept. The intercept of the regression analysis, which reflects the sedentary energy expenditure during the day but also includes themic effect of food (TEF) and postural costs, was not significantly different between two diet groups. When the intercepts were adjusted for BM, BM0.67, and SMR (P < 0.05), significant differences were observed in the postural and TEF cost by ANCOVA with age and sex as a covariate in the model.
Figure 5.
Summary calculation of incremental cost of activity using regression as shown for energy expenditure per kg against activity count based on 1-hour averages obtained in the metabolic chamber (A). Cost of activity (COA) was determined as the slope of the linear regression. Postural and TEF costs were estimated as the difference between the y-intercept of the metabolic rate on activity counts least squares linear regression and SMR. (B) The data show a representative C monkey and a representative CR monkey.
Figure 6.
Cost of activity (COA) unadjusted or adjusted for body weight during assessments in the metabolic chamber. The CR monkeys had significantly lower unadjusted COA and COA adjusted by body mass (BM), BM0.67, and SMR than C monkeys. Data are shown as Mean ± SD.
4. 0 Discussion
The present study provides the most comprehensive analysis of energy expenditure in nonhuman primates under control and CR conditions conducted to date. To circumvent the prior limitation of possible night-time wakefulness, we refined our proxy resting metabolic rate measurement by using a sleeping metabolic rate (lowest 3 h night time energy expenditure) in place of a 12 h night-time metabolic rate, and confirmed our previous report of reduced resting metabolic rate among CR animals (Blanc et al. 2003). This metabolic rate remained significantly lower after adjustment for body size suggesting that CR animals are metabolically distinct from the C animals. In the previous study, we could not explain why TEE only tended to be lower in CR than C, despite the significantly lower resting metabolic rate. The current study provides us with an explanation: the old CR animals perform more movements than the C animals and although these movements are associated with a lower energy cost compared to C, the increase in movement results in increased TEE although unadjusted TEEDLW still tended to be lower in CR.
Although both this study and our previous study found the SMR was reduced in CR, we believe the use of 3 h SMR in this study is a superior proxy measure of resting metabolic rate under near basal conditions. As shown in Figure 2B, the energy expenditure was still high after turning off the lights at 6 PM. The animals do move during some periods of the night and this may explain some of the variation in energy expenditure across the night-time hours, and thus why night EE has not always been found to be different between our CR and C animals. For example, a significant decrease in mass-adjusted night EE was reported after 30 months of CR in these animals (Ramsey et al. 2000a). Subsequent measures a decade later detected only a trend in the night EE between C and CR at 9 to 15 years of CR after adjustment by FFM (P=0.06) (Raman et al. 2007b). A possible explanation for the lower SMR in CR animals reported here could be a difference in thyroid hormone levels that have been shown to be lower in rhesus monkeys on CR (DeLany et al. 1999). Although we did not find that TEE was significantly lower in CR animals compared to C animals, it did tend to be lower. An independent long-term primate CR study reported that CR caused a significant and sustained reduction in TEE when corrected for lean body mass (DeLany et al. 1999). It should be mentioned that the former study applied a weight-clamping procedure that may have had a different impact on body composition compared to the CR regimen used in the current study.
Recent human studies on the metabolic and behavioral effects of CR used both 24h room calorimetry and DLW to measure energy expenditure (Heilbronn et al. 2006; Redman et al. 2009), but at this time, the data is limited to only 3 and 6 months of CR intervention. During these early phases of CR, significant decreases in SMR, 24h energy expenditure and free-living TEE were detected in human CR subjects and this difference remains significant after adjusting for body composition. In contrast to our long-term findings, a reduction in physical activity has been reported for humans on short-term CR. In the human study metabolic rate measures were conducted during the period of CR induced weight loss. In this way, measures of energy expenditure could have been influenced by the energy imbalance that occurs before weight plateaus during long-term CR.
Behavioral changes in physical activity and the metabolic costs of physical activity have a greater impact on TEE in human studies compared to non-human primate studies because physical activity is a larger component of TEE in free-living adults. Assuming that the thermic effect of meals (TEM) contributes 10% of TEE, and that PAL values average about 1.65, physical activity energy expenditure (PAEE) is calculated to comprise about one-third of TEE in healthy free-living subjects (Prentice et al. 1996; Westerterp 2008). Moreover this fraction does not change much with age, although TEE and PAEE both decrease in terms of MJ/d (Blanc et al. 2004). In the current non-human primate study, however, the PAL was about 1.3 meaning that PAEE is much smaller; contributing only about one-fifth of TEE in C animals and thus our primate model differs slightly from the human. This of course is likely to have been influenced by restrictions on physical activity imposed by the monkeys being housed in cages. Interestingly, among humans housed in our indirect calorimetry chamber having 3.5 m2 of floor space and without instructions to exercise, PAL average about 1.4, which is more similar to our non-human primate value than free-living humans.
Even though there was no overall change in PAEE observed in the monkeys during the chamber measure, breaking down the PAEE by intensity did find interactions between diet group, time (year of study), and intensity (Figure 2). Intensities between 1.6 and 2.0 METS or multiples of SMR decreased between 1990–2000 and 2007–2008 in the C animals but not the CR animals. This is consistent with a delay in age-related decreases in vigor in the CR animals. Parallel studies of skeletal muscle mass and fiber morphology in the same cohort have shown that CR delays the onset of sarcopenia, at 2007–2008 the C animals had lost 43% of their upper leg muscle mass whereas CR animals had lost 27% (McKiernan et al. 2012)
In non-human primates, older adult C animals spend less time in locomotion, and there is a marked decline in vertical movement by older monkeys (Ramsey et al. 2000b). In humans, older subjects also spend significantly less time at moderate or high intensity activities than young subjects and spend significantly more time at low intensity activities (Blanc et al. 2004; Harris et al. 2007a; Meijer et al. 2001). Despite this, elderly individuals consume more metabolic energy during walking than young adults at same speeds (Dean et al. 2007; Harris et al. 2007b; Jones et al. 2009; Mian et al. 2006; Ortega and Farley 2007). Harris et al. (2007b) suggested that the elderly individuals may have a biological drive to be less active than the young because of their higher energetic cost of movements.
The adaptive changes in energy expenditure reported in the present study may be explained by changes in metabolism. In humans undergoing weight loss, the energy cost of activities has been reported to be lower than for weight stable individuals and shown to be due to an increase in skeletal muscle work efficiency with energy restriction (Rosenbaum and Leibel 2010). Metabonomic analysis of serum collected from the monkeys in this study reveals that CR has a significant impact on circulating metabolites, including changes in key lipoproteins and metabolites associated with energy metabolism (Rezzi et al. 2009). These findings are consistent with the concept that CR induces a reprogramming of metabolism (Anderson and Weindruch 2010). The impact of age on the serum metabolite profile was attenuated by CR, and distinct age-dependent metabolic trajectories were identified in CR animals that correlate with insulin sensitivity and diminished adiposity. Taken together these findings suggest that changes in metabolism induced by CR are linked to the improved energy cost of movement identified in this study.
The metabolic intensity (EE/SMR) calculated from the respiratory chamber data depends on SMR. These are ratios and the denominator does influence the data analysis. However, the COA calculated from the accelerometer and respiratory chamber data is independent of SMR. We plotted metabolic rates against activity counts for every hour in the respiratory chamber to estimate COA for each individual. Using least squares linear regressions, we estimated the slope (i.e., incremental COA) and intercept for each individual animal. The zero-activity count intercept was calculated from the activity count versus gross metabolic rate. In this way the intercept includes SMR, postural effects, and TEF. With this analysis, the measured SMR was not used in the calculations. The slope (COA) is mathematically independent from SMR (Puyau et al. 2002; Puyau et al. 2004; Rezende et al. 2006). The COA is statistically lower in CR group than in C group whether unadjusted or adjusting with BM, BM0.67, or SMR. This results suggest that not only SMR but also COA is decreased, and thus, higher physical activity can be achieved by long-term CR despite of decreasing energy intake.
An additional finding of this study is that measures of TEE in the metabolic chamber are equivalent to those measured by DLW in the animal’s home cage. This reduces concern over a potential artifact in energy expenditure measures performed in a cage other than the animal’s home cage. Non-human primates have been shown to be sensitive to changes in their facilities and handling and thus there was some concern on our part that TEE measures in the chamber might be artificially increased by the stress of being outside their home cage. It was also noted that the stress of anesthesia or perhaps handling associated with the annual exam and start of the IV injection of the DLW dose may have had an effect on animal behavior. We observed a slight decrease in food intake in the C animals following this procedure. This was not noted in the CR animals and we speculate that indicates a stronger food drive in the CR animals.
It should be noted that TEEDLW and 24hrEEchamber was higher (~8%) in C than in CR, but the difference did not reach significance in the present study. We previously reported that 24hrEEchamber measured annually for 7yr in their younger age was significantly higher (~10%) in C than in CR (Raman et al. 2007b). That study contained 469 (67×7 time-points) measures from 34 C and 33 CR. The present study contained only 84 (42×2 time-points) measures from 18 C and 24 CR, and this lowered statistical power. In both cases, the difference in energy expenditure between groups was much smaller than that expected from the difference in energy intake. Metabolizable energy intake for the C and CR diets has been previously reported and shown to differ slightly from energy intake as measured herein (Raman et al. 2007a). Energy density of diet, urine, and feces were measured by bomb calorimetry. The metabolizable energy coefficients were 0.87±0.03 in the CR and 0.91±0.03 in C monkeys, and thus significantly lower in CR than in C monkeys (P<0.001). There was an interesting difference in eating style noted between groups. The CR animals dropped almost no small crumbs during consumption of the diet pellets, while C animals dropped crumbs equal to 6% of dietary intake. The dietary intake data herein were not corrected for crumb loss in C group because it can only be measured through labor intensive methods. Diet pellets larger than ¼ size were visually monitored and corrections made. We also reported that food moisture was higher than listed on the manufacturer’s label. Because the moisture content is probably seasonally variable and not different between diets corrections for moisture content variation were not made. Perhaps the largest single contributor to the smaller, but no longer significant, difference in TEE between C and CR was the age-associated decline in energy intake among C animals but not CR, the difference of energy intake between C and CR animals dropped to about 16% and hence the TEE necessary to be at energy balance by the second assessment time-point in 2007–2008.
5. 0 Conclusion
Short-term (<1 yr) calorie restriction (CR) lowers TEE, activity energy expenditure, and physical activity in human and non-human primates. In long-term CR, however, CR does not decrease either TEEDLW or 24hEEchamber even though SMR is lower in CR non-human primates. Furthermore, CR animals maintain a higher physical activity level than C animals, with significantly longer duration of physical activity and more frequent high intensity activities observed in CR animals. Importantly, the metabolic cost of movements is significantly lower for CR animals compared to C. No significant interactions of sex against dietary treatment were observed. These data suggest that long-term CR induces profound metabolic changes in both male and female non-human primates to sustain the ability to expend energy in movement and to improve skeletal muscle work efficiency.
Supplementary Material
Highlights.
We measured energy expenditure in rhesus monkeys in control caloric restricted after more than 10 years of study
Caloric restriction was found to reduce sleeping metabolic rate and the energy cost of movement
Caloric restriction increased physical activity as measured by movement
These differences observed after animals weight had stabilized are the opposite of those usually observed during short-term (dieting) caloric restriction
Acknowledgement
This work was supported by grants P01 AG-11915 (to R. Weindruch) and P51 RR000167 (to the Wisconsin National Primate Research Center, University of Wisconsin, Madison). This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc S, Colman R, Kemnitz J, Weindruch R, Baum S, Ramsey J, Schoeller D. Assessment of nutritional status in rhesus monkeys: comparison of dual-energy X-ray absorptiometry and stable isotope dilution. J Med Primatol. 2005;34:130–138. doi: 10.1111/j.1600-0684.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy Expenditure of Rhesus Monkeys Subjected to 11 Years of Dietary Restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- Blanc S, Schoeller DA, Bauer D, Danielson ME, Tylavsky F, Simonsick EM, Harris TB, Kritchevsky SB, Everhart JE. Energy requirements in the eighth decade of life. Am J Clin Nutr. 2004;79:303–310. doi: 10.1093/ajcn/79.2.303. [DOI] [PubMed] [Google Scholar]
- Bouten CV, Westerterp KR, Verduin M, Janssen JD. Assessment of Energy-Expenditure for Physical-Activity Using a Triaxial Accelerometer. Medicine and Science in Sports and Exercise. 1994;26:1516–1523. [PubMed] [Google Scholar]
- Colman RJ, Anderson RM. Nonhuman primate calorie restriction. Antioxid Redox Signal. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA. Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci. 1999;54:B5–B11. doi: 10.1093/gerona/54.1.b5. discussion B12-13. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp Aging Res. 1983;9:203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007a;292:E1207–E1212. doi: 10.1152/ajpendo.00509.2006. [DOI] [PubMed] [Google Scholar]
- Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007b;292:E1207–E1212. doi: 10.1152/ajpendo.00509.2006. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Chefer S, Matochik J, Moscrip TD, Weed J, Roth GS, London ED, Lane MA. Aging and caloric restriction in nonhuman primates: behavioral and in vivo brain imaging studies. Ann N Y Acad Sci. 2001;928:316–326. doi: 10.1111/j.1749-6632.2001.tb05661.x. [DOI] [PubMed] [Google Scholar]
- Jones LM, Waters DL, Legge M. Walking speed at self-selected exercise pace is lower but energy cost higher in older versus younger women. J Phys Act Health. 2009;6:327–332. doi: 10.1123/jpah.6.3.327. [DOI] [PubMed] [Google Scholar]
- Kaibara E. In: Originally written in 1712. Wilson WS, translator. Yojokun: Life Lessons from a Samurai Kodansha International; 2009. [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Kleiber M. BODY SIZE AND METABOLIC RATE. Physiol Rev. 1947;27:511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, Aiken JM, Weindruch R, Anderson RM. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Experimental Gerontology. 2012;47:229–236. doi: 10.1016/j.exger.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer EP, Goris AHC, Wouters L, Westerterp KR. Physical inactivity as a determinant of the physical activity level in the elderly. Int J Obes. 2001;25:935–939. doi: 10.1038/sj.ijo.0801644. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Ingram DK, Lane MA, Roth GS, Weed JL. Locomotor activity in female rhesus monkeys: assessment of age and calorie restriction effects. J Gerontol A Biol Sci Med Sci. 2000;55:B373–B380. doi: 10.1093/gerona/55.8.b373. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol. 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Goldberg GR, Murgatroyd PR, Cole TJ. Physical activity and obesity: problems in correcting expenditure for body size. Int J Obes Relat Metab Disord. 1996;20:688–691. [PubMed] [Google Scholar]
- Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and Calibration of Physical Activity Monitors in Children. Obesity. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36:1625–1631. [PubMed] [Google Scholar]
- Raman A, Baum ST, Colman RJ, Kemnitz JW, Weindruch R, Schoeller DA. Metabolizable energy intake during long-term calorie restriction in rhesus monkeys. Exp Gerontol. 2007a;42:988–994. doi: 10.1016/j.exger.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman A, Ramsey JJ, Kemnitz JW, Baum ST, Newton W, Colman RJ, Weindruch R, Beasley MT, Schoeller DA. Influences of calorie restriction and age on energy expenditure in the rhesus monkey. Am J Physiol Endocrinol Metab. 2007b;292:E101–E106. doi: 10.1152/ajpendo.00127.2006. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000a;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Laatsch JL, Kemnitz JW. Age and gender differences in body composition, energy expenditure, and glucoregulation of adult rhesus monkeys. J Med Primatol. 2000b;29:11–19. doi: 10.1034/j.1600-0684.2000.290102.x. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. Energy expenditure of adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol. 1997;272:E901–E907. doi: 10.1152/ajpendo.1997.272.5.E901. [DOI] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr Effects of Size, Sex, Voluntary Running Speeds on Costs of Locomotion in Lines of Laboratory Mice Selectively Bred for High Wheel running Activity. Physiological and Biochemical Zoology. 2006;79:83–99. doi: 10.1086/498187. [DOI] [PubMed] [Google Scholar]
- Rezzi S, Martin FP, Shanmuganayagam D, Colman RJ, Nicholson JK, Weindruch R. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp Gerontol. 2009;44:356–362. doi: 10.1016/j.exger.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol Regul Integr Comp Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Heglund NC, Maloiy GM. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Schmidt-Nielsen K, Raab JL. Scaling of energetic cost of running to body size in mammals. Am J Physiol. 1970;219:1104–1107. doi: 10.1152/ajplegacy.1970.219.4.1104. [DOI] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93:1039–1043. doi: 10.1016/j.physbeh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- White CR, Seymour RS. Allometric scaling of mammalian metabolism. J Exp Biol. 2005;208:1611–1619. doi: 10.1242/jeb.01501. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yokoyama K, Noriyasu R, Osaki T, Adachi T, Itoi A, Naito Y, Morimoto T, Kimura M, Oda S. Light-intensity activities are important for estimating physical activity energy expenditure using uniaxial and triaxial accelerometers. Eur J Appl Physiol. 2009;105:141–152. doi: 10.1007/s00421-008-0883-7. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.