Abstract
Recurrent hypoglycemia (RH) is the major complication of intensive insulin treatment for diabetes mellitus. Of particular concern is the perceived potential for long-term impact of RH on cognition. Because diabetic patients have been reported to have deficits in mental flexibility and judgment, both generally considered to be mediated predominantly by the prefrontal cortex, the purpose of the present study was to determine whether RH would affect prefrontal cortex function. Medial prefrontal cortex (mPFC)-mediated set-shifting ability was tested in male Sprague-Dawley rats using a maze-based, food-reward Set-Shift task analogous to the Wisconsin card-sorting task. The performance measure was the number of trials to criterion on both day 1 (initial rule-learning) and day 2 (set-shifting in response to a changed contingency). In vivo microdialysis was used to measure mPFC extracellular glucose, lactate, pyruvate, glutamate, and dopamine. Post-mortem measures within the mPFC included glucose transporter 3 (GluT3) and c-Fos. RH animals had enhanced performance on day 1, consistent with previous work that showed RH to improve subsequent hippocampal function when euglycemic. The key finding of the present work is that RH led to impaired set-shifting performance on day 2, suggesting impairment in e.g. mental flexibility. Consistent with this finding, RH animals show decreased mPFC glycolysis on day 2 compared to controls. Our data show that RH can lead to subsequent impaired judgment, accompanied by reduced prefrontal cortex function. The findings suggest a potential underlying mechanism for the impaired judgment seen in diabetic patients.
Keywords: Dopamine, glucose, medial prefrontal cortex, set-shifting ability, recurrent hypoglycemia
INTRODUCTION
Intensive insulin therapy to prevent hyperglycemia is the current recommended gold standard for treatment of type 1 diabetes (T1DM), based on the results of the landmark Diabetes Control and Complications Trial and other studies; insulin treatment is also a common therapy for type 2 diabetic patients [1–3]. One major drawback of intensive insulin therapy is the increased risk of recurrent hypoglycemia (RH) [4–6]. RH leads to breakdown of autonomic counter-regulatory responses (commonly termed hypoglycemia-associated autonomic failure, or HAAF) and subsequent hypoglycemia unawareness [7, 8]. Patients often have significant fear of the long-term neural and cognitive consequences of potential interruptions in glucose supply to the brain which reduces compliance with therapy [7–9]. The long term cognitive and neural consequences of RH remain uncertain, not least because of the difficulty in human studies of accurately parsing the effects of RH from such confounds as duration of diabetes, age of onset, hyperglycemic neuropathies, and so on [10–13].
The first studies of RH in animal models, focusing on the impact of RH on hippocampal function, showed that RH can indeed affect not only subsequent cognitive performance but also, for example, brain metabolism and synaptic plasticity [14, 15, reviewed in 16]. Specifically, it has been previously shown that spatial memory performance was enhanced by prior RH if tested at euglycemia, but impaired if tested during further hypoglycemia; a matching pattern of changes was seen in hippocampal glucose transport and metabolism, and in synaptic plasticity [14]. The finding that c-Fos activity, a marker of neural activation, is increased by acute hypoglycemia but diminishes subsequent to RH, further suggests that RH affects subsequent neural function [17].
Clinically, a history of RH and impaired awareness of hypoglycemia is associated with impaired judgment and decision-making, domains mediated in large part by the prefrontal cortex [18, 19]. When a cohort of T1DM patients was asked to estimate their blood glucose levels and then asked whether they would drive, 38% decided to drive despite blood glucose levels below 40 mg/dl [20]. A second study found that 43% of T1DM patients with impaired awareness of hypoglycemia decided to drive despite hypoglycemia [21]. Judgment and decision-making are both regulated by executive processes that appear to be primarily localized to the medial prefrontal cortex (mPFC) [18, 19, 22–24]. Manipulations of receptors for dopamine, a primary neurotransmitter within the mPFC, modulate mental flexibility [25–28]; however, neither mPFC function nor dopaminergic activity have been examined as potential mechanistic links between RH and impaired decision-making.
We examined the impact of recurrent hypoglycemia on mPFC-mediated behaviors. We used a rodent cognitive task that relies on ability to perform cognitive shifts and is analogous to human Wisconsin card-sorting test [29, 30]. The Set-Shift task engages higher-order functions such as, attentional processes, working memory, and decision-making ability and is detailed in the Methods section [22, 23, 31]. In addition to behavioral measures, we used in vivo microdialysis of the mPFC before, during, and after behavioral testing as our previous studies of RH suggested that in addition to cognitive changes, we might see alterations in neural metabolism during task performance. Post mortem, we additionally measured within mPFC protein expression of c-Fos and neuronal glucose transporter 3 (GluT3).
MATERIALS AND METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committee and were in accordance with NIH mandated principles of laboratory animal care. During one week of acclimatization, animals were housed in pairs in a temperature-and light-controlled room (12-h light, 12-h dark; lights on at 08:00 AM) with food and water available ad libitum. After this, from the 3rd day of handling and during the habituation and testing phase, animals were singly housed and placed on a restricted diet of 18g of rat chow per day per rat, with ad libitum access to water. Food restriction was aimed at reducing them to 80% of their free-feeding bodyweight and increasing motivation to perform food-reward tasks. A total of 40 adult male Sprague-Dawley rats (6–8 animals/group; 275–300 g from Charles River, Wilmington, MA) were used for these experiments. A separate cohort of animals (n=6) was used for blood glucose measurements to confirm the level of hypoglycemia produced by insulin injections.
Apparatus
The Set-Shift maze was a rotating 4-arm plus maze with a food well (1.9 cm in diameter and 0.63cm deep) at the end of each arm; arms were 14cm wide × 40.6cm long × 20.3cm high and constructed from painted Plexiglas. The food well was sufficiently deep to hide the 45mg food pellet (Dustless Precision Pellets, purified formula, 45mg; BioServ, Frenchtown, NJ) from the view of the rat. Maze arms varied along each of two stimulus dimensions (brightness and texture), giving arms that were light-smooth, light-rough, dark-smooth, and dark-rough. There was also a holding chamber, 35.6cm × 35.6cm × 35.6cm, constructed of Plexiglas, placed adjacent to the maze that allowed the animals to be placed in it as the maze was being rotated in between trials.
For microdialysis, the table which held the maze and the holding chamber also held an infusion pump (CMA Microdialysis) and a two-channel swivel (Instech Solomon).
Handling
Animals were extensively handled for a week prior to starting habituation, with the contact time increasing to a minimum of 10 min/animal/day by the end of the week. Food reward pellets were given to the animals in their home cages, after handling, to familiarize them with their taste.
Set-Shift Maze Habituation
Set-Shift Maze Habituation followed the protocol of Stefani and colleagues for habituating the animals to the Set-Shift maze and for behavioral testing [29, 30, 32]. Briefly, during the open-arm habituation, which lasted for 5 days, animals were familiarized to all 4-arms of the maze and learned to eat reward pellets from the food wells. During closed arm habituation of 5 days animals were placed in the maze when in a T-shape configuration: one arm was blocked at a time and the rat placed in the opposite arm, hence being placed into the stem of a T-configuration. Rats were then allowed to explore whichever arm they chose to enter after leaving the stem, including eating the food reward if it was a pseudorandomly baited arm. Between trials, animals were placed in the holding chamber or held in hands. Inter-trial interval (ITI) was about 22 sec.
Surgery
Following closed-arm habituation microdialysis probes (CMA12, CMA/Microdialysis) were stereotaxically implanted in the mPFC of the animals anesthetized with 2% isoflurane-oxygen mix (4.2mm anterior to bregma, 0.6mm lateral, and 2.1mm ventral from dura), using standard aseptic surgical technique [33]. Animals were allowed to recover and handled for a week post-surgery.
RH Induction
RH was induced, in animals randomly assigned to that condition, by i.p. insulin (Humulin, Eli Lilly) administration on each of three consecutive days (doses of 10, 8, and 6 IU/kg respectively), following an established protocol [14]. RH-treated animals were tested on the two days immediately following RH, with normal glycemic levels at the time of testing.
Insulin doses were adjusted to take account of increased sensitivity to repeated insulin treatments, based on previous work and their effect was confirmed in a separate cohort of animals that were not included in the set-shift protocol [14, 15]. Briefly, handled and habituated animals were treated according to the RH protocol and 1 hour post-insulin injection, tail-vein blood was sampled to measure glucose levels using a blood glucose meter (One Touch Ultra Mini).
Set-Shift Task
Set-Shift testing consisted of two sessions, separated by a 24 hr interval. On Day 1 the animal learned an association between food-reward and one environmental cue (for instance, smooth texture or light color); the remaining two dimensions of arm choice (spatial relation to the start arm and whichever of texture and color is not being linked to reward) offered no information as to the correct arm choice. Learning of this relationship allowed the rat to select the correct arm in order to receive the food reward available on each trial.
On each trial, the rat was placed into a start arm chosen pseudo-randomly with the opposing arm blocked in the T-configuration. Thus, the rat had two possible choices for arms to enter on leaving the initial start arm, and after making a choice and entering the arm the rat was allowed to explore, consume a pellet if present, and then returned to the holding chamber while the maze was prepared (rotated, block moved, cleaned, re-baited as needed) for the next trial. Testing continued until criterion performance level was reached (8 consecutive correct arm choices of the baited arms). Each rat was then tested again, 24h later, with the food-reward contingency changed from a textural to a color variable or vice versa. Testing again continued until the criterion performance level was reached. Day 2 testing required the rat to shift strategy: inhibiting use of the relationship that was previously learned while acquiring and acting on a new, orthogonal contingency relationship. In addition to recording number of trials to criterion on each day, the average latency of each animal to select an arm was recorded as a measure of motor activity. After maze testing animals were sacrificed, brains removed and visually inspected for correct cannula placement. Only data from animals with correctly placed probes were analyzed.
Microdialysis Procedures
Brain extracellular fluid (ECF) samples were collected in 20-min bins from mPFC using probes with a 2mm long dialysis membrane before, during, and after the behavior testing using previously established protocols [34]. Samples were analyzed for glucose, pyruvate, lactate, and glutamate using a CMA600 analyzer. Dopamine was measured via HPLC (ESA, Acton, MA).
Immunohistochemistry (IHC)
Brains from an additional cohort of animals (n= 6–7/group) treated to induce RH and tested for behavior only (to avoid any confound from the presence of a microdialysis cannula and probe) were processed for chromogen IHC for c-Fos and GluT3 expression using an adaptation of previously published protocols specific for nuclear and membrane protein staining [17, 35, 36]. c-Fos stained nuclei were counted and mean intensity of GluT3 staining in mPFC was calculated.
Data Analysis
Behavioral data was analyzed using t-test. Biochemical data for glucose, lactate, pyruvate, glutamate, and dopamine were expressed as % of baseline and analyzed as two-way ANOVA (Treatment x Stage of testing) with Student-Newman’s-Keul’s test for post hoc comparisions. The α-level for all statistical comparisions was ≤ 0.05.
RESULTS
Efficacy of Hypoglycemia Treatment
No animal experienced coma or seizure. Baseline blood glucose before insulin administration was 137.5 ± 2.5 mg/dl. Insulin administration lowered tail-vein blood glucose to 35.33 ± 6.7 mg/dl; hence, core plasma glucose level (approximately 10 mg/dL higher than that measured from tail vein samples) was approximately 45 mg/dL, in line with our targeted hypoglycemia range [37].
Set-Shift Performance
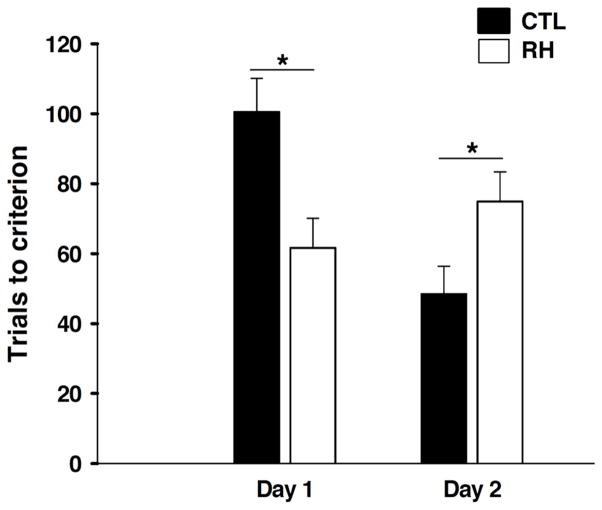
Fig. (1) shows animals’ performance on both Day 1 (spatial learning) and Day 2 (set-shifting ability) on the Set-Shift task. On Day 1, RH animals learned the task significantly faster than controls [t (14) =3.04; p<0.05], suggesting that antecedent hypoglycemia improved subsequent spatial learning. This finding is consistent with our previous work, which also showed enhanced hippocampal-dependent spatial memory following RH (when tested at euglycemia) [14, 38]. In contrast, on Day 2 RH animals required significantly more trials to reach criterion [t (14) = −2.25; p<0.05], suggesting an impairment in set-shifting performance.
Fig. 1.
Behavioral performance on the Set-Shift task on day 1 and day 2 of testing in control animals (CTL) and in animals induced with recurrent bouts of hypoglycemia (RH) on each of preceding 3 days of testing. A previous history of recurrent hypoglycemia aided acquisition learning on day 1, however impaired mental flexibility, tested on day 2 in RH group. Data are means + SEM. Asterisks indicate significantly different behavioral performance between the treatment groups (p<0.05).
In a separate experiment, we tested whether the effects of RH would interact with glycemic state at the time of testing. Animals being made hypoglycemic on both test days either as their first experience of hypoglycemia (Hypo group) or following previous RH (RH-Hypo group). The rationale for these groups was our previous finding of a significant interaction between RH and acute glycemic state in modulating some cognitive and metabolic processes, including spatial working memory [14, 38]. Not surprisingly, acute hypoglycemia has been shown to impair performance on a variety of cognitive measures [39–45]. However, on this food-rewarded task, both acutely hypoglycemic groups had performance comparable to that of euglycemic control animals, and this appeared to be due at least in part to a confounding effect of hunger on motivation and task performance, so these data were not analyzed further (data not shown).
Latency
RH and control animals did not differ in average latency to enter choice arms, a proxy measure to control for any differences in motor activity or motivation, on either day of testing (data not shown).
mPFC Neurochemistry
Glucose (Fig. 2)
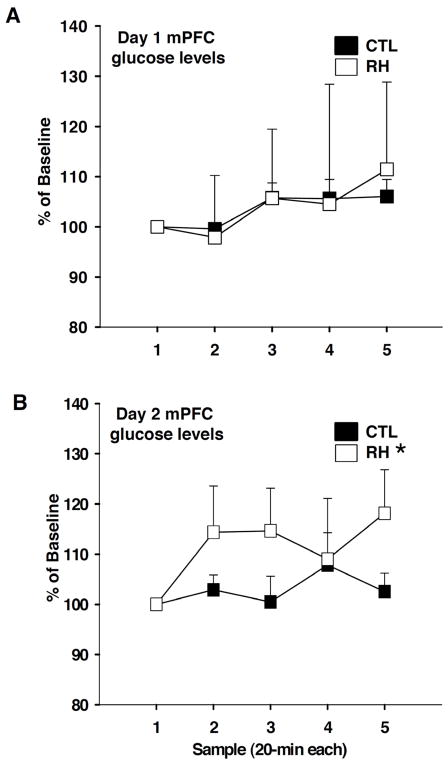
Fig. 2.
Medial prefrontal cortex (mPFC) extracellular fluid (ECF) glucose levels, measured before, during, and after Set-Shift testing of control animals (CTL) and animals with prior history of recurrent hypoglycemia (RH) on day 1 (A) and day 2 (B) of the behavior task. ECF samples were collected in 20 min bins. Data are expressed as percentage of baseline + SEM. Asterisks indicate significantly different group findings (p<0.05).
There were no group differences in the mPFC ECF glucose levels on day 1 of testing. However, on day 2 of testing, mPFC glucose levels of RH animals were elevated compared to controls (F(1, 69) = 6.21, p<0.05).
Pyruvate (Fig. 3)
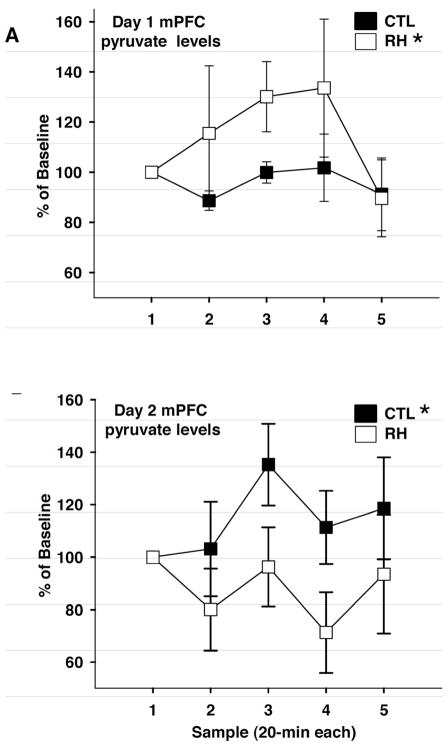
Fig. 3.
Medial prefrontal cortex (mPFC) extracellular fluid (ECF) pyruvate levels, measured before, during, and after Set-Shift testing of control animals (CTL) and animals with prior history of recurrent hypoglycemia (RH) on day 1 (A) and day 2 (B) of the behavior task. ECF samples were collected in 20 min bins. Data are expressed as percentage of baseline + SEM. Asterisks indicate significantly different group findings (p<0.05).
In contrast to glucose, ECF pyruvate levels on day 1 of testing were significantly higher in RH animals than in controls (F(1, 53) = 4.17, p<0.05), while on day 2, RH animals had significantly lower mPFC ECF pyruvate (F(1, 54) = 6.16, p<0.05).
Lactate, Glutamate, and Dopamine
ECF lactate and glutamate levels did not vary between the treatment groups on either day of testing. Surprisingly, we saw no changes in mPFC ECF dopamine levels across treatment groups or across stages of testing on either day (data for lactate, glutamate, and dopamine not shown).
Protein Expression in mPFC
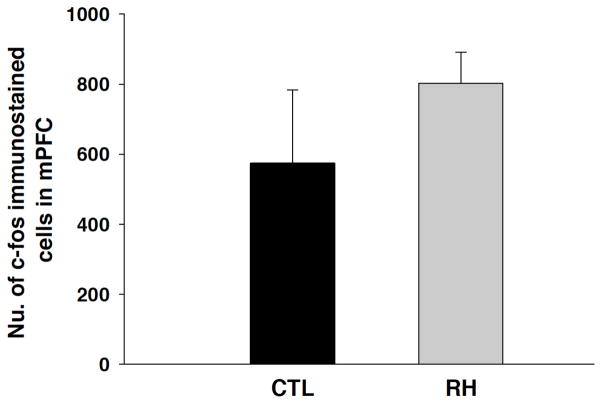
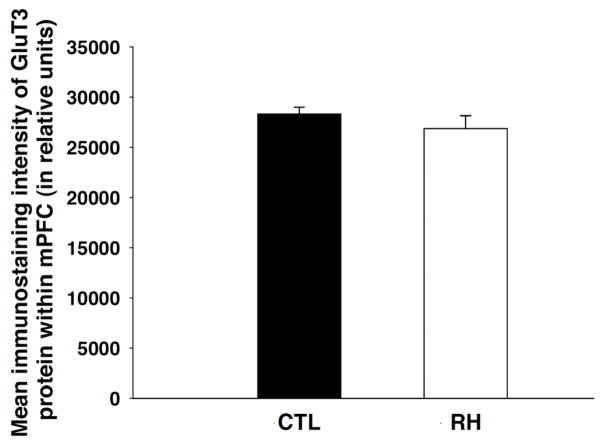
Prior history of RH but subsequent euglycemia (at the time of testing) did not affect c-Fos expression within the mPFC. Immunolabeling for c-Fos in RH group was comparable to the controls (Fig. 4A). We also saw no difference in mPFC GluT3 expression between groups (Fig. 5A). Representative immunostaining images for c-Fos and GluT3 are depicted in Fig. (4B) and Fig. (5B), respectively.
Fig. 4.
Fig. (4A). The mean (+ SEM) number of stained nuclei for c-Fos protein within the medial prefrontal cortex (mPFC) at the end of Set-Shift testing in control animals (CTL) and in animals induced with recurrent bouts of hypoglycemia on each of preceding 3 days of testing (RH). No significant differences were observed in the number of c-fos stained cells between the treatment groups.
Fig. (4B). A representative medial prefrontal cortex (mPFC) c-Fos immunostaining from CTL and RH animals. Bar = 100μm.
Fig. 5.
Fig. (5A). The mean (+ SEM) staining intensity of glucose transporter 3 (GluT3) immunoreactivity (quantified by thresholded pixels) within the medial prefrontal cortex (mPFC) at the end of Set-Shift testing in control animals (CTL) and in animals induced with recurrent bouts of hypoglycemia on each of preceding 3 days of testing (RH). No significant differences were observed in the mean staining intensity of GluT3 between the treatment groups.
Fig. (5B). A representative medial prefrontal cortex (mPFC) GluT3 immunostaining from CTL and RH animals. Bar = 10μm.
DISCUSSION
These data, taken together with previous studies of RH and hippocampal function, present a more complete picture of the neurobehavioral effects of RH. Findings from our studies were consistent with clinical observations. Thus, prior history of hypoglycemia impaired subsequent executive function as demonstrated by impaired set-shifting performance when the rule was changed. However, prior history of hypoglycemia may in fact enhance initial acquisition learning. As the animals learned a contingency rule (day one of testing), animals with a history of RH had increased ECF pyruvate during testing without any difference in ECF glucose, and showed superior learning. This suggests that, again consistent with previous studies brain glucose supply and metabolism may be enhanced as an adaptive response to RH and hence underlie improved cognitive performance [14, 15]. In contrast, on day two of testing, when the contingency rule was altered and demand on the mPFC would have been expected to be highest, RH animals had higher mPFC ECF glucose and lower pyruvate levels. These data suggest that less glucose was being metabolized within the mPFC, which may impair behavior. This explanation is also consistent with the idea that altered glucose metabolism may underlie some of cognitive effects of RH. Conversely, post-mortem mPFC c-Fos expression was not significantly altered by RH. This finding suggests that at the point at which performance criterion was reached, total mPFC activity may have been similar across groups, but that the RH animals achieved that state more slowly (requiring more trials), once again indicative of a lower level of metabolism. Given that we saw no alteration in mPFC GluT3 following RH, glucose supply to neurons is unlikely to be significantly different between groups. This suggests that the behavioral impairment observed in our study may be due to changes in glucose demand rather than being affected by glucose delivery to the mPFC. It is known that in response to pathophysiological metabolic changes, free radicals like NO can regulate GluT-3-mediated glucose uptake and trigger glycolysis independent of changes in membrane-protein expression [46]. Although this possibility was not tested in the present study, the fact that we observed significant group differences supports the suggestion that the impact of RH on glucose metabolism is sufficient to overcome such compensatory mechanisms. However, an effect of RH to increase other glucose transporters (e.g. GluT1 at the blood-brain barrier) cannot be ruled out, and studies have reported upregulation in whole-brain or cortical GluT1 expression consequent to chronic hypoglycemia [47, 48]. Overall, the elevated mPFC metabolic activity observed on Day 1 suggests that the requirement for learning of an association between a particular maze attribute and reward places significant load on the mPFC, consistent with literature findings [22, 23, 31, 49, 50].
The results are consistent with, but significantly extend, past findings. McNay & Sherwin showed, using an identical short-term RH model, that both, performance on a hippocampally-mediated spatial task and hippocampal metabolism were enhanced following RH when tested at euglycemia but impaired when measured during subsequent hypoglycemia [14]. Importantly, the effects of RH were identical in diabetic and control animals at behavioral, neural, and metabolic levels, as well as with subsequent long-term studies of RH in rodents, strongly supporting the use (as here) of non-diabetic animals in the study of RH in order to avoid any potentially confounding comorbidities of diabetes such as neuropathy, vision impairment, and so on [15]. The present results from Day 1 are highly consistent with the euglycemic portion of those findings; the key finding from the present experiment is that when the task demands are altered to require cognitive shifts and inhibition of prior learning, antecedent RH impairs, rather than enhances, performance, even at euglycemia. Thus, it appears that the impact of RH on subsequent cognitive and neural performance varies by brain region and the details of cognitive demand.
The RH-induced behavioral impairment on the Set-Shift task could not be explained by an alteration in mPFC dopamine release. Dopaminergic neurotransmission is known to play an important role in mediating mental flexibility [26, 51–53]; mPFC dopamine release is not critical for rule acquisition on day 1 of testing but is more important for shifting strategies [22, 30]. However, as we did not find a significant RH treatment effect on mPFC dopamine on either days of testing, our data suggest that some other neurochemical system may underlie the effects of RH on set-shift performance, rather than dopamine. Consistent with this, one previous study has suggested that dopamine release (albeit under different conditions) is unaffected by alterations in brain glucose metabolism that persist for up to 80 minutes [54].
Our data show that a history of prior hypoglycemia may lead to impaired set-shifting ability, which is a measure of decision-making ability, consistent with clinical reports of impaired mental flexibility in patients with diabetes. Moreover, the behavioral impairment is associated with reduced mPFC glucose metabolism subsequent to RH, despite systemic euglycemia when tested. These data have important implications for diabetics undergoing insulin replacement therapy: especially when taken together with previous findings, they strongly suggest that mental function is altered following even short-term, moderate RH. Further research may be necessary to develop treatment regimens which reduce the risk of RH and at the same time achieve optimal glycemic control.
Acknowledgments
This work was supported by NIH 1072696 to ECM. We also would like to thank Dr. Mark Stefani for his generous assistance with the Set-Shift task and the maze construction. Also, many thanks to the undergraduates who were involved in processing of the tissue for immunohistochemistry.
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Spain M, Edlund BJ. Introducing insulin into diabetes management: transition strategies for older adults. J Gerontol Nurs. 2011;37(4):10–5. doi: 10.3928/00989134-20110309-01. [DOI] [PubMed] [Google Scholar]
- 2.Mudaliar S, Edelman SV. Insulin therapy in type 2 diabetes. Endocrinol Metab Clin North Am. 2001;30(4):935–82. doi: 10.1016/s0889-8529(05)70222-x. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Tamborlane WV, Ahern J. Implications and results of the diabetes control and complications trial. Pediatr Clin North Am. 1997;44(2):285–300. doi: 10.1016/s0031-3955(05)70477-6. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications Trial. Diabetes. 1997;46(2):271–86. [PubMed] [Google Scholar]
- 6.The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90(4):450–9. [PubMed] [Google Scholar]
- 7.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91(3):819–28. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care. 2009;32(7):1196–8. doi: 10.2337/dc08-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15(1):42–6. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Fruehwald-Schultes B, Born J, Kern W, Peters A, Fehm HL. Adaptation of cognitive function to hypoglycemia in healthy men. Diabetes Care. 2000;23(8):1059–66. doi: 10.2337/diacare.23.8.1059. [DOI] [PubMed] [Google Scholar]
- 11.Langan SJ, Deary IJ, Hepburn DA, Frier BM. Cumulative cognitive impairment following recurrent severe hypoglycaemia in adult patients with insulin-treated diabetes mellitus. Diabetologia. 1991;34(5):337–44. doi: 10.1007/BF00405006. [DOI] [PubMed] [Google Scholar]
- 12.Wredling R, Levander S, Adamson U, Lins PE. Permanent neuropsychological impairment after recurrent episodes of severe hypoglycaemia in man. Diabetologia. 1990;33(3):152–7. doi: 10.1007/BF00404042. [DOI] [PubMed] [Google Scholar]
- 13.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45(4):262–8. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 14.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53(2):418–25. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- 15.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55(4):1088–95. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 16.McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav. 2010;100(3):234–8. doi: 10.1016/j.physbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjape SA, Briski KP. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience. 2005;130(4):957–70. doi: 10.1016/j.neuroscience.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 19.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19(11):4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke WL, Cox DJ, Gonder-Frederick LA, Kovatchev B. Hypoglycemia and the decision to drive a motor vehicle by persons with diabetes. JAMA. 1999;282(8):750–4. doi: 10.1001/jama.282.8.750. [DOI] [PubMed] [Google Scholar]
- 21.Stork AD, van Haeften TW, Veneman TF. The decision not to drive during hypoglycemia in patients with type 1 and type 2 diabetes according to hypoglycemia awareness. Diabetes Care. 2007;30(11):2822–6. doi: 10.2337/dc06-1544. [DOI] [PubMed] [Google Scholar]
- 22.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bruin JP, Feenstra MG, Broersen LM, et al. Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Prog Brain Res. 2000;126:103–13. doi: 10.1016/S0079-6123(00)26010-X. [DOI] [PubMed] [Google Scholar]
- 24.de Bruin JP, Sanchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652(2):323–33. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 25.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 26.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188(4):567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 27.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613–21. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Memory. 2002;9(1):18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119(2):420–8. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- 30.Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26(34):8810–8. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Memory. 2002;9(4):191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117(4):728–37. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates, Compact Third Edition (with CD-ROM) 3. London: Academic Press Limited; 1997. Compact. [Google Scholar]
- 34.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Memory. 2010;93(4):546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahagirdar V, Wagner CK. Ontogeny of progesterone receptor expression in the subplate of fetal and neonatal rat cortex. Cereb Cortex. 2010;20(5):1046–52. doi: 10.1093/cercor/bhp165. [DOI] [PubMed] [Google Scholar]
- 36.Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29(9):2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelkar SM, Kaklij GS. Effect of mode of blood collection procedures on blood glucose. Anal Lett. 1994;27(12):2261–5. [Google Scholar]
- 38.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–95. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 39.Pramming S, Thorsteinsson B, Theilgaard A, Pinner E, Binder C. Cognitive function during hypoglycaemia in type I diabetes mellitus. BMJ. 1986;292:647–50. doi: 10.1136/bmj.292.6521.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelligrino D, Segil LJ, Albrecht RF. Brain glucose utilization and transport and cortical function in chronic vs acute hypoglycemia. Am J Physiol. 1990;259:E729–E35. doi: 10.1152/ajpendo.1990.259.5.E729. [DOI] [PubMed] [Google Scholar]
- 41.Amiel S, Pottinger R, Archibald H, et al. Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care. 1991;14:109–18. doi: 10.2337/diacare.14.2.109. [DOI] [PubMed] [Google Scholar]
- 42.Deary I. Effects of hypglycaemia on cognitive function. In: Frier B, Fisher B, editors. Hypoglycaemia and Diabetes: Clinincal and Physiological Aspects. 1. London: Edward Arnold; 1993. pp. 80–92. [Google Scholar]
- 43.Weber A, Jacob R, Stuart P, Davis J, Sherwin R. Moderate hypoglycemia impairs the function of the inferior colliculus in the awake rat. Metabolism. 1994;43:1329–31. doi: 10.1016/0026-0495(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 44.Draelos M, Jacobson A, Weinger K, et al. Cognitive function in patients with insulin dependent diabetes mellitus during hyperglycemia and hypoglycemia. Am J Med. 1995;98:135–45. doi: 10.1016/S0002-9343(99)80397-0. [DOI] [PubMed] [Google Scholar]
- 45.Heller S, Macdonald I. The measurement of cognitive function during acute hypoglycaemia: experimental limitations and their effect on the study of hypoglycaemia unawareness. Diabet Med. 1996;13(7):634–41. doi: 10.1002/(SICI)1096-9136(199607)13:7<607::AID-DIA159>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 46.Cidad P, Almeida A, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5′-AMP-activated protein kinase. Biochem J. 2004;384(Pt 3):629–36. doi: 10.1042/BJ20040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44(12):1399–404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 48.Mastaitis JW, Wurmbach E, Cheng H, Sealfon SC, Mobbs CV. Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes. 2005;54(4):952–8. doi: 10.2337/diabetes.54.4.952. [DOI] [PubMed] [Google Scholar]
- 49.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 50.de Wit S, Kosaki Y, Balleine BW, Dickinson A. Dorsomedial prefrontal cortex resolves response conflict in rats. J Neurosci. 2006;26(19):5224–9. doi: 10.1523/JNEUROSCI.5175-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90(2):236–49. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24(2):547–53. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salamone JD. Dopamine, effort, and decision making: theoretical comment on Bardgett etal. Behav Neurosci. 2009;123(2):463–7. doi: 10.1037/a0015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bello NT, Hajnal A. Alterations in blood glucose levels under hyperinsulinemia affect accumbens dopamine. Physiol Behav. 2006;88(1–2):138–45. doi: 10.1016/j.physbeh.2006.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]