Abstract
The cross-talk between insulin and angiotensin II signalling pathways plays a significant role in the co-occurrence of diabetes and hypertension. We developed a mathematical model of the system of interactions among the biomolecules that are involved in the cross-talk between the insulin and angiotensin II signalling pathways. We have identified several feedback structures that regulate the dynamic behavior of the individual signalling pathways and their interactions. Different scenarios are simulated and dominant steady-state, dynamic and stability characteristics are revealed. The proposed mechanistic model describes how angiotensin II inhibits the actions of insulin and impairs the insulin-mediated vasodilation. The model also predicts that poor glycaemic control induced by diabetes contributes to hypertension by activating the renin angiotensin aystem.
Introduction
Diabetes, hypertension and cancer affect a great fraction of people worldwide. Clinical and pharmacological data suggest that these diseases are related [1]–[8]. This study analyzes the structure and the dynamics of the complex cellular networks associated with the cross-talk between diabetes and hypertension.
Biological actions are carried out through complex dynamic interactions of many cellular agents. Insulin stimulates multiple signalling pathways to regulate glucose homeostasis, vascular tone, and cell growth. Deregulation of processes downstream of insulin may result in diseases such as diabetes, hypertension and cancer [9]. AKT (also known as protein kinase B) is an important molecule in the insulin signalling pathway. AKT is activated through a PI3K dependent mechanism and promotes glucose uptake by translocating GLUT-4 to the cell surface [9]–[14]. Activated AKT drives cell proliferation [12] and also enhances vasodilation by stimulating NO production [15], [16]. Insulin resistance can develop through impairments in signalling events involved in activation of AKT. Angiotensin II (Ang II) contributes to the pathogenesis of insulin resistance by inducing inhibition of key signalling elements of insulin AKT pathway [15]. At the same time, diabetes induced hyperglycemia may lead to hypertension by activating Ang II. The renin angiotensin system (RAS) is found to be activated in hypertension and Ang II is an essential vasoconstrictor hormone in RAS [17]. Altogether, these findings suggest that the cross-talk between insulin AKT and Ang II signalling pathways plays a significant role in the co-occurrence of diabetes and hypertension.
This study aims to provide a better understanding of operating principles of processes such as glucose uptake, cell proliferation, and blood pressure control by developing mathematical models of interactions at the system level. System behavior is analyzed within the context of signalling pathways and feedback regulation. We show that complex signalling pathways that govern the cross-talk between hypertension and diabetes are regulated by feedback structures that are organized in hierarchical fashion. Using the dynamic models we develop, we simulate different scenarios to elucidate the functions of these feedback structures. While doing so, dominant steady-state and dynamic characteristics that determine the normal and diseased states are revealed.
Models
Before we present the mathematical model and the results, it is important to describe the methodology first. Signalling pathway structures are constructed based on established literature knowledge. Known regulatory relations and interactions between biomolecules are integrated to the model systematically. Wherever it is necessary and makes sense, molecular interactions are lumped together to reduce the complexity of the cellular network. Feedback loops are identified as well. Finally, the chemical reaction network of the biomolecules is modelled using mass-action kinetics and conservation laws. The resulting model represents a nonlinear dynamical system, which is in the form of ordinary differential equations. In the following, the physical description of the pathways of interest is given before the analytical model is presented.
Insulin AKT signalling pathway and renin angiotensin systems are standard pathways. It is also well-known that these pathways interact with each other. However, studies reported in the literature do not present the available knowledge in a coherent picture that is amenable to modeling and analysis. We have carefully selected the most relevant cross-talk interactions that are reported in the literature and presented in pathway databases such as KEGG [18]. We have constructed a pathway structure, which is able to reproduce known physiological behaviors. In the sequel, we explain the employed interactions in detail and illustrate the pathway structure upon which the mathematical modeling and subsequent analysis is based.
Insulin AKT Signalling Pathway
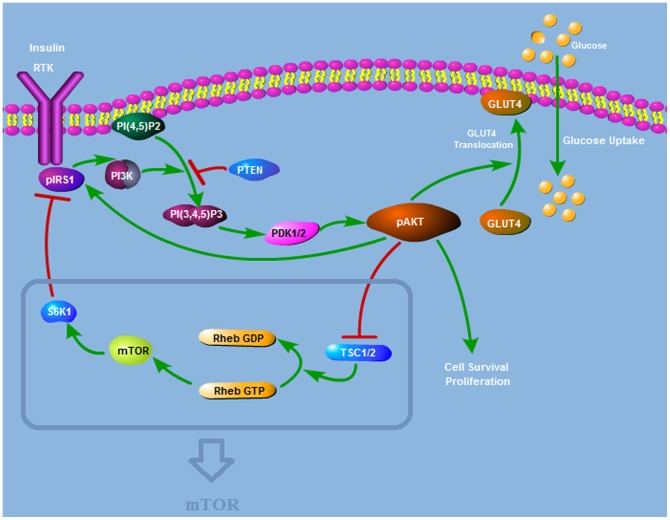
The pathway is illustrated in Figure 1. In all the pathway diagrams to follow, nodes represent the molecules and links or edges denote the interactions between these nodes. Directed edges with arrows stand for activation and edges with hammer heads represent inhibition. AKT is activated by the events downstream of insulin and its activation is essential for insulin mediated glucose transport. This activation is mediated by PI3K, and mTOR is linked to the pathway to make it sensitive to nutrients.
Figure 1. Insulin AKT Signalling Pathway Structure.
AKT is activated by PDK1 and PDK2 downstream of insulin. Activated AKT (pAKT) enables the translocation of glucose transporter-4 (GLUT-4). pAKT activates mTOR by phosphorylating the tuberous sclerosis complex (TSC). mTOR activates S6K which phosphorylates and inhibits IRS1.
This pathway can be retrieved from KEGG PATHWAY database. The pathway data has been verified with different sources and simplified for the sake of focusing on the essential paths that give rise to the biological phenomena under study.
Growth factors like insulin, insulin-like growth factor IGF-I, and IGF-II activate insulin/IGF-I receptor tyrosine kinases (RTKs) on the cell surface. Activated RTKs autophosphorylate and create phosphotyrosine binding sites for the insulin receptor substrate (IRS). IRSs are phosphorylated by insulin/IGF-I RTKs on tyrosine residues. Phosphorylated IRSs act as binding sites for proteins containing src homology 2 domains, including the p85 regulatory subunit of class I phosphoinositide 3-kinase (PI3K) [13]. PI3Ks generate PIP3 from PIP2. Subsequently, PIP3 recruits AKT and 3-phosphoinositide-dependent kinase 1 (PDK1) by binding to their PH domain. AKT is activated by phosphorylation on Thr308 and Ser473 via PDK1 and PDK2, respectively [11]. PP2A dephosphorylates AKT on both Thr-308 and Ser473 sites. PHLPPs also dephosphorylate AKT. The lipid protein phosphatase PTEN negatively regulates AKT activation by converting PI3K-generated PIP3 into PIP2, thus blocking AKT phosphorylation on both Thr308 and Ser473 by PDK1 and PDK2, respectively [12].
Plasma glucose homeostasis is maintained during feeding and fasting by regulating the absorption from the intestine, the storage and the release by the liver and the availability for cell uptake via insulin, which is produced in the pancreas, and metabolism by the cells [9]. Activated AKT (pAKT) enables the translocation of glucose transporter-4 (GLUT-4) from cytosol to the plasma membrane, thus glucose is taken into the cell [10], [14]. By stimulating the recruitment of GLUT-4 to the cell surface, pAKT plays a key role in the most significant metabolic action of insulin, which is the glucose uptake.
IRS1 is phosphorylated on serine residues by the activated AKT. This phosphorylation protects IRS1 from the action of protein-tyrosine phosphatases (PTPases) and prevents its dephosphorylation [19]. PTPases dephosphorylate the insulin receptor. PTP1B is found to be upregulated in insulin resistant cells [20]. pAKT negatively regulates PTP1B and prevents dephosphorylation of IRS1 by PTP1B [21]. As a result, AKT positively regulates IRS1 function since IRS1 maintains its tyrosine phosphorylated active conformation.
Nutrient availability is sensed [22], [23] and regulatory signals are transmitted through mTOR (mammalian Target of Rapamycin). pAKT activates mTOR by phosphorylating the tuberous sclerosis complex (TSC), heterodimer of hamartin (TSC1) and tuberin (TSC2). The TSC1/2 complex controls the balance between two forms of a small GTPase called Rheb. Rheb-GDP is the inactive form whereas Rheb-GTP directly activates mTOR. AKT-dependent phosphorylation of TSC2 inactivates the TSC complex and thus the conversion to the inactive Rheb-GDP form is inhibited [24]. Since active Rheb-GTP form is favored, mTOR becomes activated. mTOR activates S6K which phosphorylates and inhibits IRS1 [25]–[27].
Cross-talk Between Angiotensin II and Insulin Signalling Pathways
Renin is secreted from the kidney and it cleaves angiotensinogen, which is synthesized in the liver, to produce angiotensin I. Then angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II [28]. Angiotensin II is the active peptide in RAS which plays an important role in controlling blood pressure. It can directly elevate the arterial pressure by rapid vasoconstriction in many areas of the body [17]. Another aspect of the regulation of blood pressure is related to the ability of angiotensin II to affect sodium and extracellular fluid homeostasis by the stimulation of aldosterone production. Aldosterone increases reabsorption of ions and water in the kidney, increasing blood volume and, therefore, increasing blood pressure.
Insulin can mediate vasodilation through AKT activation and the consequential NO production. Vasodilator effects of insulin are mediated by the signalling pathway involving IRS-1/PI-3 kinase/AKT/eNOS that leads to increased NO production by endothelium [16]. AKT leads to phosphorylation of eNOS on serine 1177. eNOS, endothelial nitric oxide synthase, catalyzes the production of nitric oxide from L-arginine.
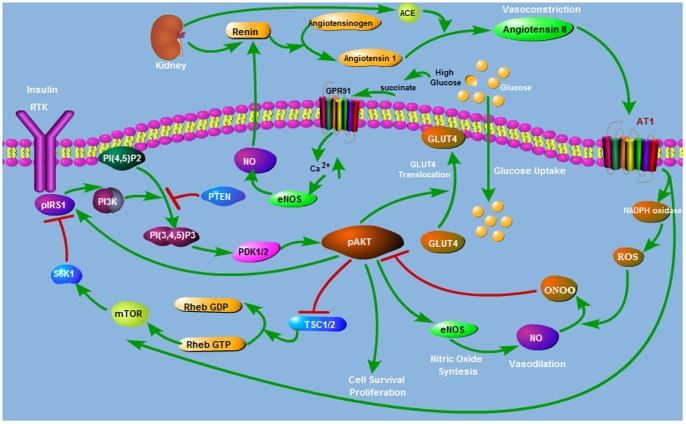
Ang II impairs insulin signalling pathway through several mechanisms as shown in Figure 2. Ang II is known to generate ONOO-[29]–[33]. Ang II stimulates production of reactive oxygen species (ROS) such as superoxide O2- through activation of NAD(P)H oxidase [34]–[36]. When both O2- and NO are synthesized, they will react spontaneously to form peroxynitrite (ONOO-) [14]. ONOO- nitrates AKT and prevents its phosphorylation on Ser473 and Thr308 and thus inhibits its catalytic activity [37]. Inhibitory S-Nitrosation of AKT in response to ONOO is shown in [38], [39]. Thus, Ang II would affect insulin-stimulated production of nitric oxide negatively since it leads to inhibition of AKT activation [15]. In addition, Ang II inhibits insulin metabolic signalling and promotes insulin resistance through activation of the (mTOR)/S6 kinase 1 (S6K1) mediated IRS-1 serine phosphorylation [40].
Figure 2. Interaction Between AKT and Ang II Pathways.
Renin Angiotensin System (RAS) interacts with insulin signalling through several mechanisms.
NO regulates renin synthesis and has stimulatory effect on renin secretion and Ang II activation through a cGMP mediated mechanism by inhibiting cAMP degradation [41].
Recently, it is proposed that there exists a direct link between high glucose levels and renin activation mediated by the G-protein-coupled receptor GPR91 and succinate [42]. High glucose levels lead to accumulation of succinate which activates GPR91. Through the activation of GPR91 endothelial cytosolic calcium is increased causing production of prostaglandins and NO. Prostaglandins and NO activate renin release from the juxtaglomerular granular cells (JGG) [41]. Glomerular hyperfiltration and JGG renin activation are observed in diabetes. Since prostaglandins and NO are vasodilator agents that cause relaxation of the afferent arteriole, they can also explain the development of hyperfiltration [42].
Embedded Regulatory Feedback Structures
Frequently biological networks consist of several feedback loops that are coupled with each other. Complex systems such as biological systems perform their functions through coordinated action of several feedback mechanisms. In [43] it is demonstrated that coupled feedback loops enforce actions which are not possible by single feedback loops. It is shown that coupled positive feedbacks can amplify signals and induce bistability; coupled negative feedbacks maintain homeostasis; coupled positive and negative feedbacks strengthen regulation capacity for better responses regarding time and magnitude. In [43], [44] it is argued that signalling networks consist of coupled feedback loops to enhance robustness of the system. In this paper, nested feedback loops are studied to investigate their role in regulatory mechanisms such as glucose uptake and vasodilation-vasoconstriction balance. Next, we elucidate the feedback loops present in the insulin signalling and angiotensin II pathways.
Figure 3 shows a simplified version of the insulin AKT signalling pathway after lumping some of the molecular interactions that are explicitly shown in Figure 1. This is similar to the AKT pathway structure given in [45]. The simplified pathway consists of one positive and one negative feedback loop, which are explained next.
Figure 3. Insulin AKT Signalling Feedback Loops.
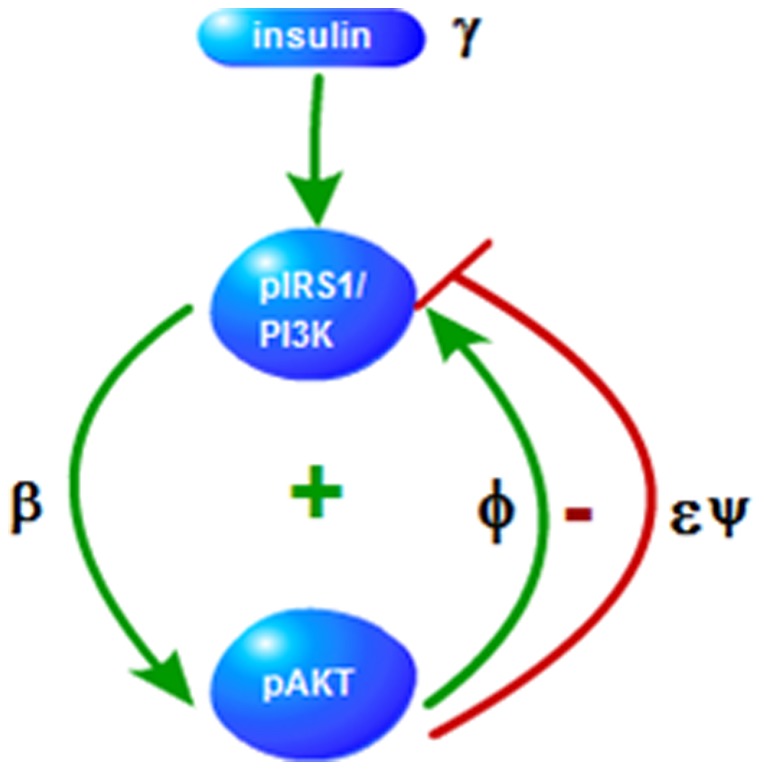
Signs + and − indicate positive and negative feedback loops, respectively. Parameters (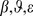 ) on the edges denote the strengths of the edges.
) on the edges denote the strengths of the edges. 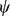 is the nutrient level sensed my mTOR.
is the nutrient level sensed my mTOR.
pIRS1→pAKT→pIRS1 positive feedback loop
pAKT is activated downstream of insulin. In turn, pAKT positively regulates pIRS1 function since it helps IRS1 maintain its tyrosine phosphorylated active conformation [19] and forms a positive feedback loop. Glucose uptake should be sensitive to insulin and this positive feedback regulation ensures the required bistable response [46]. pAKT should switch between high and low values according to the cellular and extracellular conditions since glucose uptake, translocation of GLUT4 to the plasma membrane, is an all or none process [46].
Second property associated with AKT is cell proliferation. Cell proliferation operates also in all or none manner which are controlled by insulin. Insulin resistant systems that cannot switch between on-state and off-state lead to diseases. Systems with persistently low pAKT values are associated with type II diabetes whereas systems with persistently high pAKT values are associated with cancer [45].
pIRS1→pAKT→pIRS1 negative feedback loop
pAKT also forms a negative feedback loop by inhibiting pIRS1 through activation of mTOR (see Figure 1). Coupling of the negative feedback with the positive feedback enables the fine adjustment of the response dose and provides a stable regulatory response to nutrient level and helps maintain glucose homeostasis. mTOR is involved in many signalling pathways and especially plays significant role in cell growth, proliferation, survival and protein synthesis. mTOR pathway is found to be activated in cancer and drugs that inhibit mTOR are used for anticancer therapy [47], [48]. As opposed to its cancer promoting effects via other signalling pathways, in this particular system, mTOR acts to reduce cancer. Through inhibition of pIRS1, mTOR decreases the level of pAKT which is one of the agents responsible for cell proliferation. In other words, inhibition of mTOR may reduce cancer through other mechanisms which are not considered in this study, but does drive cancer progression through elevating pAKT levels and driving uncontrolled cell proliferation. This can explain the findings that mTOR inhibition for cancer treatment being less effective than expected [49]. In summary, the relative strengths of the pIRS1 positive and negative feedback loops determine the phenotypes of the system such as normal, diabetes, or cancer.
The pathway shown in Figure 2 includes several interactions (i.e. cross-talk) that are present between AKT and angiotensin signalling pathways. The feedback loops underlying these interactions can be organized in a hierarchy as depicted in Figure 4 and listed in Table 1. Signs + and − indicate positive and negative feedback loops, respectively. The first two loops at the top of the hierarchy are the positive and negative feedback loops of the basic AKT system and are already discussed above. Other feedback loops are introduced through the coupling with the angiotensin system and are explained next.
Figure 4. AKT-Angiotensin II Feedback Loops.

A hierarchy of feedback loops defines the interactions between the AKT and Ang II signalling pathways.Interaction parameters are labeled on the directed edges between the interacting nodes.
Table 1. Feedback Loops.
| + | pIRS1→pAKT→pIRS1 |
| _ | pIRS1→pAKT→pIRS1 |
| + | pAKT→NO→ONOO→pAKT |
| _ | NO→ANG II→ROS→NO |
| + | pAKT→NO→ANGII→ROS→ONOO→pAKT |
| _ | pIRS1→pAKT→NO→ANG II →pIRS1 |
A hierarchy of feedback loops defines the interactions between the AKT and Ang II signalling pathways.
pAKT→NO→ONOO→pAKT negative feedback loop
Vasodilator actions of insulin are mediated by nitric oxide (NO). pAKT stimulates NO production through pAKT → eNOS →NO path. NO participates in the production of peroxynitrite ONOO, which prevents phosphorylation of AKT and thus inhibits pAKT.
NO→ANGII→ROS→NO negative feedback loop
NO is converted to ONOO by the actions of ANG II. Conversion of NO to ONOO is important for preventing the accumulation of NO [50]. Thus this feedback loop helps to balance the NO level. However, when Ang II is overactive, such as in hypertension, NO will decrease through this mechanism. Since NO is a vasodilator agent, decreasing NO levels would further increase the blood pressure.
pAKT→NO→ANG II→ROS→ONOO→pAKT negative feedback loop
NO stimulates renin production and thus activates Ang II. Activation of Ang II produces reactive oxygen species ROS and ONOO which inhibits pAKT.
pIRS1→pAKT→NO→ANG II →pIRS1 negative feedback loop
Stimulation of NO production downstream of pIRS1 contributes to IRS1 inhibition by activating Ang II. Ang II promotes insulin resistance and inhibits insulin metabolic signalling by activating mTOR and thus promoting S6K1-mediated IRS-1 serine phosphorylation [40].
Figure 4 suggests that the key internal states of the system are pAKT and NO. Proper regulation of pAKT and NO by the positive and negative feedback loops is crucial in maintaining the right balance between glucose uptake and vasodilation-vasoconstriction effects.
Modeling Background
In [45] Wang developed a mathematical model for the AKT signalling pathway to investigate system-level mechanisms of cell growth and metabolism. The model centers on mTOR which senses the level of nutrients, insulin growth factor which activates the pathway, and pAKT (phosphorylated AKT) that adjusts the growth. Modeling equations are based on the phosphorylation and dephosphorylation cycle (PdPC) of AKT → pAKT:
where E1 and E2 are the enzymes. Assuming Michaelis-Menten kinetics, the following dynamical model is derived [45]:
| (1) |
| (2) |
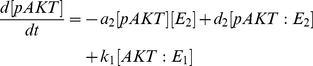 |
(3) |
| (4) |
| (5) |
with
| (6) |
| (7) |
| (8) |
| (9) |
Variable  denotes the insulin level and
denotes the insulin level and 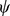 is the nutrient level. The critical parameters
is the nutrient level. The critical parameters 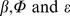 represent the strengths of the feedback loops as shown in Figure 3. The rest of the model parameters are constant physical parameters representing the reaction rates and the decay rate of pIRS1. It was shown that the steady-state solutions were determined by the insulin level,
represent the strengths of the feedback loops as shown in Figure 3. The rest of the model parameters are constant physical parameters representing the reaction rates and the decay rate of pIRS1. It was shown that the steady-state solutions were determined by the insulin level,  , relative strengths of the positive and negative feedback loops defined by
, relative strengths of the positive and negative feedback loops defined by 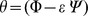 , and the Michaelis-Menten kinetic constants,
, and the Michaelis-Menten kinetic constants, 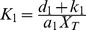 and
and 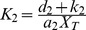 .
.
Wang studied the above model under steady-state conditions by setting equations (1) – (5) equal to zero.
Steady-state response curve between pAKT (output) and insulin (input) was calculated for different model parameter values which led to the partitioning of the parameter space into regions for the normal response and disease phenotypes including cancer and diabetes. It was shown that the normal phenotype corresponds to two steady states between which the system is able to switch. It is further shown that the switch between these steady states is possible when bistability (existence of two stable steady-states) and high sensitivity to the input insulin are present.
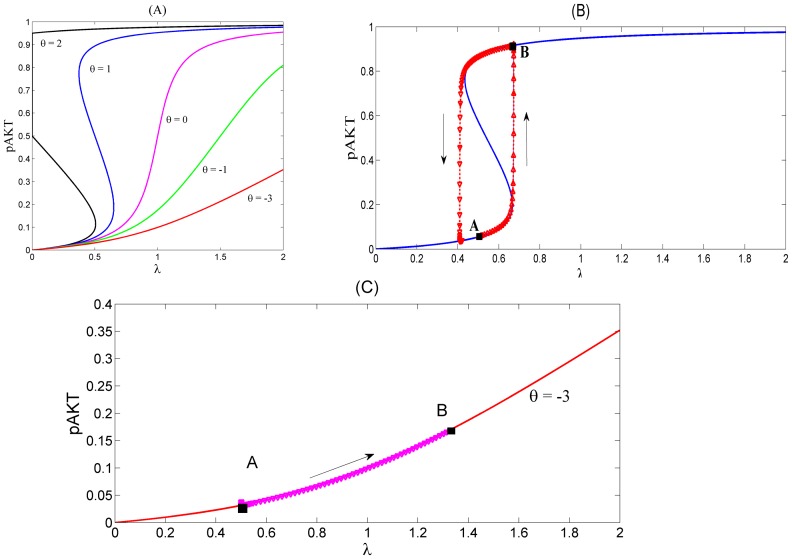
For example Figure 5 (A) shows the steady-state responses of pAKT to insulin for different values of the feedback strength parameter 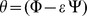 . Normal operation of the insulin signalling pathway requires the positive feedback to be greater than the negative feedback i.e.
. Normal operation of the insulin signalling pathway requires the positive feedback to be greater than the negative feedback i.e. 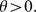 For example, when
For example, when 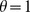 , the system is responsive to a small change in the insulin level and is able to switch both ways between high and low pAKT values as shown in Figure 5 (B). This toggle switch behavior represents bistability resulting from the existence of two stable steady states separated by an unsteady state. In Figure 5 (A) the lower and upper branches of S-shaped curves correspond to the stable steady-states with low and high activity levels, respectively (i.e. low and high pAKT). For some parameter values desired bistability is lost as shown in Figure 5 (C).
, the system is responsive to a small change in the insulin level and is able to switch both ways between high and low pAKT values as shown in Figure 5 (B). This toggle switch behavior represents bistability resulting from the existence of two stable steady states separated by an unsteady state. In Figure 5 (A) the lower and upper branches of S-shaped curves correspond to the stable steady-states with low and high activity levels, respectively (i.e. low and high pAKT). For some parameter values desired bistability is lost as shown in Figure 5 (C).
Figure 5. Response Curves of the AKT model. Insulin level is redefined as 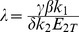 [45].
[45].
(A) Steady-state response curves of pAKT vs insulin 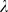 for different feedback strengths
for different feedback strengths  .
. 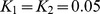 . The curves are calculated by setting equations (10) and (11) equal to zero. (B) Dynamic response curve in red superimposed on the steady-state curve shows the normal insulin cycle between states A and B.
. The curves are calculated by setting equations (10) and (11) equal to zero. (B) Dynamic response curve in red superimposed on the steady-state curve shows the normal insulin cycle between states A and B.  At State A, the cell has low nutrient level and requires glucose uptake. By stimulating insulin, the system switches to State B, where pAKT is activated and glucose is taken into the cell. Withdrawing insulin enables the switch back to low pAKT levels. (C) Bistability is lost under excess negative feedback
At State A, the cell has low nutrient level and requires glucose uptake. By stimulating insulin, the system switches to State B, where pAKT is activated and glucose is taken into the cell. Withdrawing insulin enables the switch back to low pAKT levels. (C) Bistability is lost under excess negative feedback 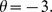 Steady-state curves in (A) are identical to those in [45]. Dynamic responses in (B) and (C) are new and they are generated form the dynamic model equations (10) and (11).
Steady-state curves in (A) are identical to those in [45]. Dynamic responses in (B) and (C) are new and they are generated form the dynamic model equations (10) and (11).
In this paper we first extend the AKT model to include the cross-talk with the angiotensin signalling pathway. Next a similar parametric sensitivity analysis is performed to describe the effect of the cross-talk interaction parameters on both the steady-state and dynamic response characteristics of the system.
Development of the New Dynamic Model
Since we focus on the relationship between diabetes and hypertension in this study, we have extended Wang's AKT model by including the interactions with the angiotensin signalling pathway. Using this new model, bistability and parameter sensitivity analysis is carried out to shed light on the key interactions and feedback loops that govern the development of diabetes and hypertension.
In order to facilitate the dynamic analysis, first we reduce the five-state dynamic model (1) – (5) to a two-state reduced-order model (see File S1 for the derivation):
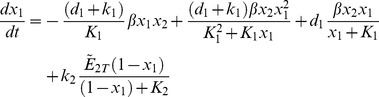 |
(10) |
| (11) |
where the normalized states are:
and the parameters are:
The above model is next modified by introducing the additional mass balances and interactions for the species NO, ANG II, ROS and ONOO which are involved in the cross-talk between AKT and angiotensin pathways (see Fig. 2). The new model is given by (see File S2 for derivation):
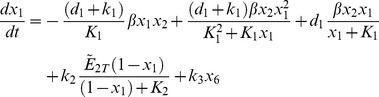 |
(12) |
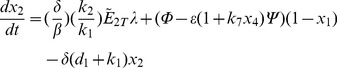 |
(13) |
| (14) |
| (15) |
| (16) |
| (17) |
where:
| (18) |
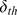 is a threshold value for [pAKT].
is a threshold value for [pAKT].
and the states are:
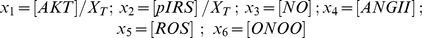
New model parameters 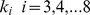 represent the strengths of the interactions between different states or nodes involved in the cross-talk and are labeled on the corresponding edges in Figure 4.
represent the strengths of the interactions between different states or nodes involved in the cross-talk and are labeled on the corresponding edges in Figure 4.
When bistability is lost and the level of pAKT stays persistently low, the glucose cannot be taken into the cell and high levels of glucose remain in blood. High glucose levels lead to GPR91 mediated activation of NO
[41]. This hyperglycemia effect is expressed by the third term 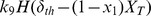 in the mass balance of NO given by equation (14).
in the mass balance of NO given by equation (14). 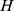 is a step function defined by (18). When
is a step function defined by (18). When 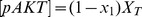 is below the threshold value of
is below the threshold value of 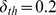 and hyperglycemia occurs,
and hyperglycemia occurs, 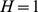 ; otherwise,
; otherwise, 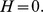 In equation (14) parameter
In equation (14) parameter 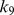 represents the rate constant for the production of NO during hyperglycemia. Parameters
represents the rate constant for the production of NO during hyperglycemia. Parameters 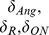 and
and 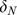 are the decay constants of ANG II, ROS, ONOO and NO, respectively.
are the decay constants of ANG II, ROS, ONOO and NO, respectively.
The above cross-talk model is reproduced using the modeling tool CellDesigner and extracted in SBML (Systems Biology Markup Language) format (http://sbml.org/). SBML file is provided as a file S3.
Results and Discussion
Analysis of the AKT Model Without the Cross-talk
In order to establish a basis of comparison, we first present the dynamic performance of the AKT system without any of its interactions with the angiotensin signalling pathway. In this case, model equations reduce to (10) and (11). In [45] the same AKT system is studied but only steady-state response curves are provided. Here, in addition to the steady-state behavior, we present dynamic simulation results as well. For this purpose dynamic differential equations (10) and (11) are simulated with the following values of the model parameters:
Biological systems possess a certain degree of robustness meaning that some desired properties hold against uncertainty such as variations in the kinetic parameters [51]. Due to lack of reliable data, true values for most of the above parameters are not available. However, bistability is known to be a robust property of the system that is preserved for a wide range of parameter values. The above chosen values represent one such set although they may not be the real values. Therefore chosen parameter values are biologically relevant as they characterize qualitatively the typical normal response behavior observed in real systems. In Figure 5 (A) we see that when the critical parameter  is chosen equal to 1, bistability exists. The dynamic response of the model for this normal phenotype is shown in Figure 5 (B). The dynamic trajectory shows how the system is able to switch between:
is chosen equal to 1, bistability exists. The dynamic response of the model for this normal phenotype is shown in Figure 5 (B). The dynamic trajectory shows how the system is able to switch between:
Steady State A:
and
Steady State B:
At State A, the cell has low nutrient level and requires glucose uptake. By stimulating insulin, the system switches to State B, where pAKT is activated and glucose is taken into the cell. Withdrawing insulin enables the switch back to low pAKT levels. Due to high sensitivity established by the positive and negative feedback actions, the required range of insulin change is quite narrow.
When  falls to negative values, e.g.
falls to negative values, e.g. 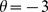 , the system remains in the low pAKT state even if large quantities of insulin are supplied as shown in Figure 5 (C). Dynamic model's behavior confirms the steady-state predictions reported in [45] which indicates the loss of bistability for
, the system remains in the low pAKT state even if large quantities of insulin are supplied as shown in Figure 5 (C). Dynamic model's behavior confirms the steady-state predictions reported in [45] which indicates the loss of bistability for 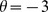 . This resulting state is defined as the insulin resistant state where the glucose uptake is impaired due to persistently low pAKT activity. Disturbances that strengthen the negative feedback can cause the diabetes progression. For example mTOR is known to phosphorylate S6K which inhibits the function of IRS1 (see Figure 1). This is represented in the model by the negative feedback strength
. This resulting state is defined as the insulin resistant state where the glucose uptake is impaired due to persistently low pAKT activity. Disturbances that strengthen the negative feedback can cause the diabetes progression. For example mTOR is known to phosphorylate S6K which inhibits the function of IRS1 (see Figure 1). This is represented in the model by the negative feedback strength 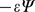 . If mTOR activity becomes unregulated (e.g. due to prolonged exposure to insulin and genetic loss of tuberin TSC2 which down regulates mTOR), negative feedback dominates the positive feedback and
. If mTOR activity becomes unregulated (e.g. due to prolonged exposure to insulin and genetic loss of tuberin TSC2 which down regulates mTOR), negative feedback dominates the positive feedback and 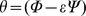 becomes negative and insulin resistance emerges. High blood glucose levels can eventually develop into type II diabetes. Also, due to less activated AKT, cells become more susceptible to apostosis.
becomes negative and insulin resistance emerges. High blood glucose levels can eventually develop into type II diabetes. Also, due to less activated AKT, cells become more susceptible to apostosis.
Coupling of positive and negative feedbacks plays a significant role in the physiology of the system. Positive and negative feedback strengths should be balanced for a normal healthy operation. When positive feedback strength is increased to a higher level than its normal value or when the negative feedback is decreased,  increases and the response curve becomes a one way switch, e.g. curve
increases and the response curve becomes a one way switch, e.g. curve 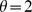 in Figure 5 (A). The system can switch from low pAKT to high pAKT level; however, once it reaches the high pAKT state, it stays there even if all insulin is removed. Persistently high pAKT levels drive uncontrolled cell proliferation, defined as cancer state.
in Figure 5 (A). The system can switch from low pAKT to high pAKT level; however, once it reaches the high pAKT state, it stays there even if all insulin is removed. Persistently high pAKT levels drive uncontrolled cell proliferation, defined as cancer state.
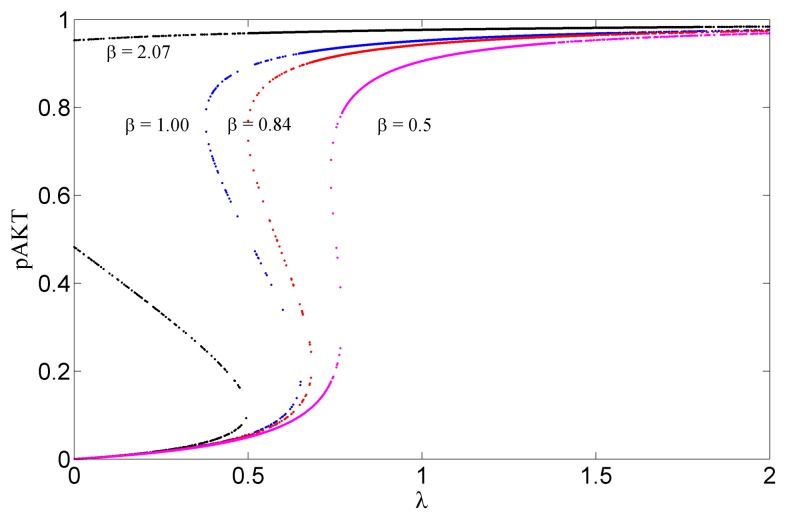
The lipid protein phosphatase PTEN negatively regulates AKT activation and acts as tumor suppressor [52]. Mutations in PTEN gene result in various cancers. In our model the feedback strength 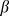 can be used to simulate the decrease/loss of PTEN function. Figure 6 shows the bifurcation diagrams for
can be used to simulate the decrease/loss of PTEN function. Figure 6 shows the bifurcation diagrams for 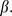 The system exhibits bistability for values of
The system exhibits bistability for values of 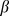 between 0.84 and 2.07. Large values of
between 0.84 and 2.07. Large values of 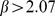 would correspond to the decreased function of PTEN. In this case pAKT stays at a high level once it is activated. This results in elevated mTOR signalling which can lead to preferential growth of tumor cells.
would correspond to the decreased function of PTEN. In this case pAKT stays at a high level once it is activated. This results in elevated mTOR signalling which can lead to preferential growth of tumor cells.
Figure 6. Steady State Response Curves for Different 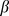 values.
values.
Bistability exists for values of 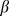 between 0.84 and 2.07. Larger values of
between 0.84 and 2.07. Larger values of 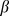 would correspond to the decreased function of PTEN. In this case pAKT stays at a high level once it is activated. This leads to elevated mTOR signalling and tumor growth.
would correspond to the decreased function of PTEN. In this case pAKT stays at a high level once it is activated. This leads to elevated mTOR signalling and tumor growth.
mTOR plays an important role in cell growth and proliferation. mTOR is a known target for the anticancer drug ramapycin [47], [48]. Inhibition of mTOR increases the pAKT production since 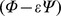 increases i.e. negative feedback effect of mTOR decreases. This reverses the insulin resistance. This is consistent with the literature, where it is stated that ramapycin reverses the insulin resistance [53]. mTOR inhibition by drugs lessens the effects leading to cancer through other pathways. However, as far as the insulin signalling pathway is concerned, mTOR inhibition weakens the mTOR mediated negative feedback to pIRS1, and account for persistent pAKT activation, driving uncontrolled cell proliferation. This can help explain why anticancer drugs that target mTOR inhibition are less successful than expected [49].
increases i.e. negative feedback effect of mTOR decreases. This reverses the insulin resistance. This is consistent with the literature, where it is stated that ramapycin reverses the insulin resistance [53]. mTOR inhibition by drugs lessens the effects leading to cancer through other pathways. However, as far as the insulin signalling pathway is concerned, mTOR inhibition weakens the mTOR mediated negative feedback to pIRS1, and account for persistent pAKT activation, driving uncontrolled cell proliferation. This can help explain why anticancer drugs that target mTOR inhibition are less successful than expected [49].
Analysis of the AKT-NO Model
After analyzing the basic AKT system presented above, we next analyze the contributions of different regulatory feedback structures to the cross-talk between the AKT and Ang II signalling pathways. For this purpose, we simulate the dynamic models we have developed earlier. We are not able to compare our simulation results with other studies since there is no similar mathematical model for the cross-talk in the literature. However, we do provide a discussion of our model predictions in light of experimental observations reported in the literature. It is shown that the model is able to reproduce the known physiological behaviors.
The first cross-talk model includes the additional interaction of pAKT with NO. Thus, only
pAKT→NO→ONOO→pAKT negative feedback loop is added to the base AKT model. Interaction parameters 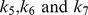 are set equal to zero. The resulting model consists of equations (12), (13), (14) and (17). Reactive oxygen species (ROS) enters the system as an independent stimulus (see Figure 4). Through the pathway pAKT→NO→ONOO→pAKT, pAKT enables NO production; NO and ROS react to form ONOO and ONOO in turn directly inhibits pAKT.
are set equal to zero. The resulting model consists of equations (12), (13), (14) and (17). Reactive oxygen species (ROS) enters the system as an independent stimulus (see Figure 4). Through the pathway pAKT→NO→ONOO→pAKT, pAKT enables NO production; NO and ROS react to form ONOO and ONOO in turn directly inhibits pAKT.
Five new parameters need to be specified in this case: 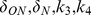 and
and 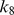 . The following values were assigned to these parameters:
. The following values were assigned to these parameters:
.
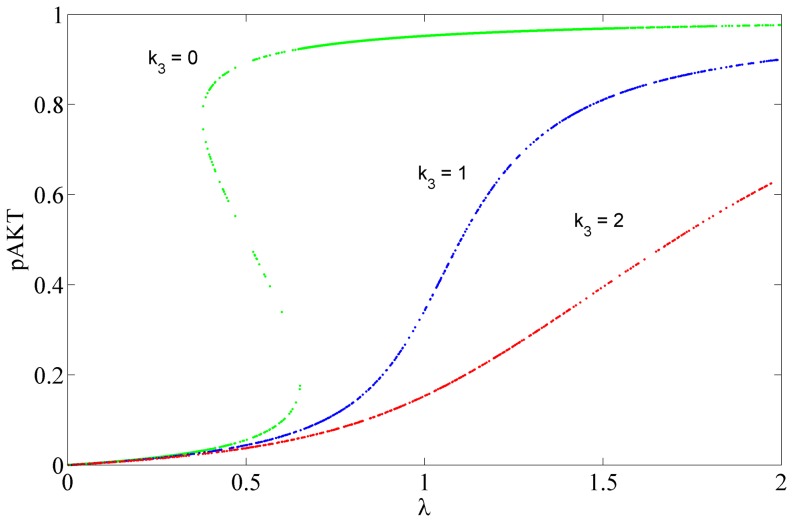
These nominal values were chosen because they preserve the bistability of the AKT signalling pathway and qualitatively produce the normal insulin cycle behavior. Parameter 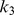 represents the strength of inhibition of pAKT by ONOO as expressed by the last term in equation (12). Next this parameter was perturbed from its nominal value to analyze the effect of ONOO inhibition on the insulin pathway. In Figure 7 steady-state response curves for pAKT are plotted for different values of
represents the strength of inhibition of pAKT by ONOO as expressed by the last term in equation (12). Next this parameter was perturbed from its nominal value to analyze the effect of ONOO inhibition on the insulin pathway. In Figure 7 steady-state response curves for pAKT are plotted for different values of 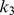 . It is shown that as
. It is shown that as 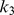 increases, bistability disappears and sensitivity to insulin decreases. This is in line with the fact that ONOO prevents AKT phosphorylation and inhibits its catalytic activity which promotes insulin resistance [37]. pAKT is positively influenced by insulin and PI3K as shown in Figure 4 by the edge that has strength β. At the same time, it is negatively influenced by ONOO as shown by the edge that has strength
increases, bistability disappears and sensitivity to insulin decreases. This is in line with the fact that ONOO prevents AKT phosphorylation and inhibits its catalytic activity which promotes insulin resistance [37]. pAKT is positively influenced by insulin and PI3K as shown in Figure 4 by the edge that has strength β. At the same time, it is negatively influenced by ONOO as shown by the edge that has strength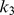 . Thus, insulin sensitivity is adversely affected if inhibition by ONOO dominates the upregulation of pAKT by PI3K.
. Thus, insulin sensitivity is adversely affected if inhibition by ONOO dominates the upregulation of pAKT by PI3K.
Figure 7. Steady State Response Curves for Different Levels of Inhibition by ONOO.
The strength of inhibition is represented by the parameter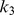 . Increasing
. Increasing 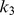 or inhibition reduces the sensitivity to insulin.
or inhibition reduces the sensitivity to insulin.
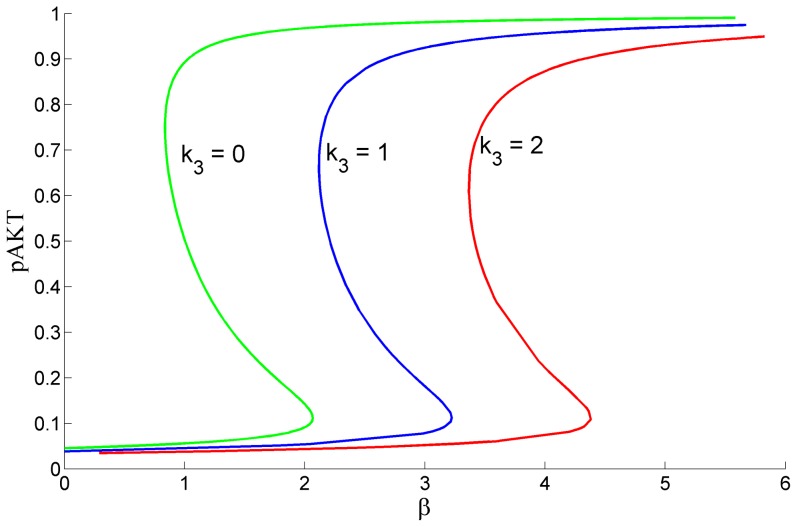
Angeli et al [54] has presented a simple graphical method to detect bistability for biological positive feedback systems. Here we have applied this method to identify the range of values for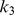 and β that guarantee bistability. The bistability limits for different
and β that guarantee bistability. The bistability limits for different 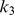 values are displayed in Figure 8. As k3 increases, bistability is lost unless β increases to compensate the inhibitory action of ONOO by activating pAKT more. For example, when
values are displayed in Figure 8. As k3 increases, bistability is lost unless β increases to compensate the inhibitory action of ONOO by activating pAKT more. For example, when 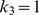 , for β<2.07 there exists only 1 solution at a low pAKT level and the system is not bistable. However, for a slightly higher value
, for β<2.07 there exists only 1 solution at a low pAKT level and the system is not bistable. However, for a slightly higher value 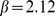 , the system will recover the desired bistability. That is, changing the strength of the negative feedback to pAKT changes the limits of β that produce bistability. This suggests that for systems where there is a disturbance that enhances insulin mediated activation of pAKT, inhibition of pAKT by the negative ONOO feedback loop would recover the system back to bistability and prevent persistent activated pAKT state (cancer). Conversely, if β values are normal, increase of this negative feedback causes the system to lose its bistability characteristics. The system may reside in a low pAKT state and thus develop insulin resistance.
, the system will recover the desired bistability. That is, changing the strength of the negative feedback to pAKT changes the limits of β that produce bistability. This suggests that for systems where there is a disturbance that enhances insulin mediated activation of pAKT, inhibition of pAKT by the negative ONOO feedback loop would recover the system back to bistability and prevent persistent activated pAKT state (cancer). Conversely, if β values are normal, increase of this negative feedback causes the system to lose its bistability characteristics. The system may reside in a low pAKT state and thus develop insulin resistance.
Figure 8. Bifurcation Diagram Showing Bistability Limits for β for Different k3 Values when λ = 0.5.
As k3 increases, bistability is lost unless β increases to compensate the inhibitory action of ONOO by activating pAKT more.
Analysis of the ANGII-AKT Model
AngII-AKT Model is the full model that includes all the interactions between insulin and Ang II signalling pathways. Therefore interaction parameters 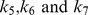 are no longer set to zero, and the model consists of all the equations (12) – (17).
are no longer set to zero, and the model consists of all the equations (12) – (17).
The base scenario represents the normal phenotype where the bistability of the insulin signalling pathway is preserved in the presence of the cross-talk with the ANGII signalling system. In addition to the AKT system's parameter values chosen earlier, we have assigned the following values to the newly introduced parameters:
These values can be considered biologically relevant since the normal insulin cycle of the best case is maintained in the presence of the cross-talk as shown next.
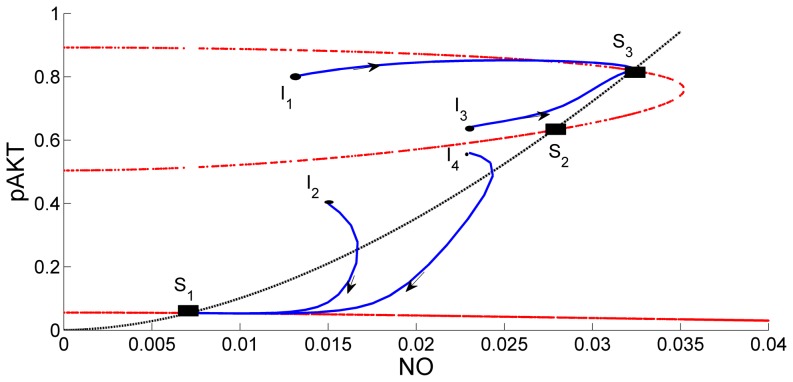
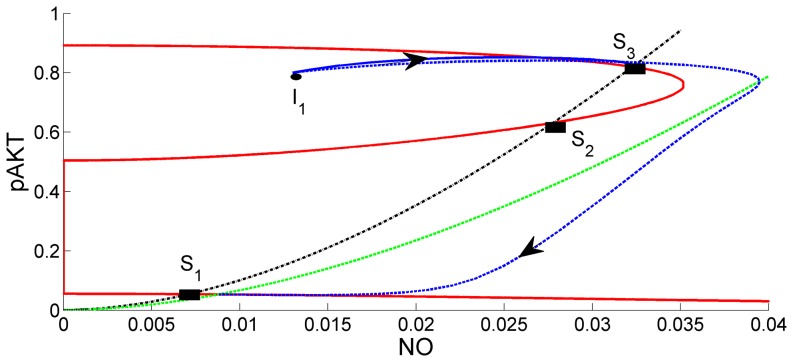
In Figure 9 the intersections of the red curves and black curve give the steady state solutions for the selected parameter values. At these points the mass balance for NO (black curve) and mass balance for pAKT (red curve) are jointly satisfied. The curves intersect at three steady states: S1 (low pAKT), S2 and S3 (high pAKT). Dynamic simulations are carried out starting from initial points, I1, I2, I3, and I4. Steady State S3 is reached when the dynamic simulation starts from nearby initial conditions I1 and I3. Steady State S1 is reached when the dynamic simulation starts from nearby initial conditions I2 and I4. Steady states S1 and S3 are stable, whereas S2 is unstable. Steady-state solutions for different insulin levels λ are plotted in Figure 10. In addition to the known bistability characteristic of pAKT in the insulin pathway[45], our model predicts a similar behavior for NO since NO production depends on activation of pAKT. In conclusion both NO and pAKT are found to exhibit the desired bistability and switching dynamics behavior.
Figure 9. Steady State Solutions and Trajectories Predicted by ANGII-AKT Model.
S1, S2 and S3 denote the three steady-states when insulin level λ = 0.5. The steady-states are at the intersection of red curves and black curve. Solid blue lines are the trajectories that start from different initial conditions I1, I2, I3 and I4. NO and pAKT both exhibit the desired bistability and switching dynamics behavior.
Figure 10. State Solution Curves for the Base Scenario Showing Joint Bistability for Various Insulin Levels.
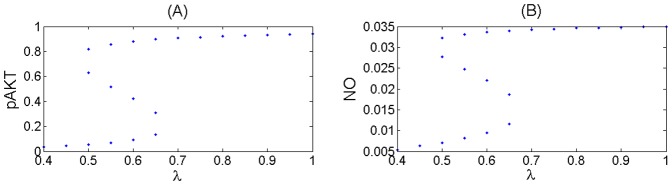
(A) for pAKT and (B) for NO.
Ang II inhibits the insulin metabolic signalling
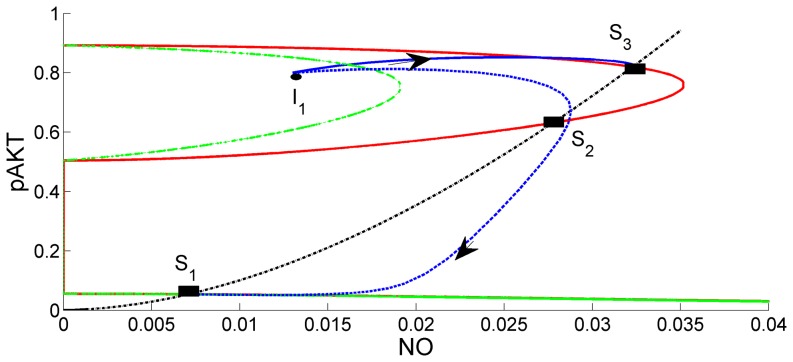
It is known that angiotensin II inhibits the insulin metabolic signalling by promoting S6K1-mediated IRS-1 serine phosphorylation [40]. In order to simulate this negative effect, feedback parameter k7 is increased from 0.1 to 100 in the model. Such a large perturbation is reasonable since abnormal behavior and diseases are expected to develop due to large perturbations. As the effect of Ang II is increased by increasing k7, pAKT decreases (original red curve in Figure 11 shifts to the left as green) and the system loses bistability. A single steady-state S1 emerges where both pAKT and NO are low. The trajectories starting from different initial conditions converge to this steady state. This is shown for one initial condition I1 in Figure 11. As a result of low levels of pAKT and NO at the single steady-state S1, both insulin sensitivity and vasodilation are impaired. Thus, the model correctly predicts angiotensin II inhibition of the insulin pathway and associated insulin resistance [40].
Figure 11. Comparison of Base Case (k7 = 0.1) with the Case k7 = 100.
Red curves are the steady-state curves for the base case (k7 = 0.1). Green curves are the same curves when k7 = 100. Intersections of red and green curves with the black define the steady-states for the two cases. Solid blue curve is the state trajectory for the base case. Dashed blue curve is the state trajectory for the case when k7 = 100. As the effect of Ang II is increased by increasing k7, the system loses bistability. Trajectories converge to the single steady-state S1 at low levels of pAKT and NO.
Proper balance must be maintained between pAKT and NO
NO production by pAKT is enhanced by increasing the value of parameter 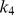 from its nominal value 0.01 to 0.015. Figure 12 shows the comparison with the base scenario. Original black curve shifts to the right (green) due to increased availability of NO. Elevated NO levels lead to activation of Ang II and production of ONOO which in turn impairs insulin signalling. In turn less NO is produced. As a result, bistability is lost and the system settles to the single steady-state S1 where both pAKT and NO are low. Our model suggests that in normal conditions (
from its nominal value 0.01 to 0.015. Figure 12 shows the comparison with the base scenario. Original black curve shifts to the right (green) due to increased availability of NO. Elevated NO levels lead to activation of Ang II and production of ONOO which in turn impairs insulin signalling. In turn less NO is produced. As a result, bistability is lost and the system settles to the single steady-state S1 where both pAKT and NO are low. Our model suggests that in normal conditions (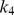 = 0.01), pIRS1-pAKT-pIRS1 and pAKT-NO-pAKT feedback loops work in coordination to maintain both insulin sensitivity and the right amount of NO by switching between S1 and S3 as necessary.
= 0.01), pIRS1-pAKT-pIRS1 and pAKT-NO-pAKT feedback loops work in coordination to maintain both insulin sensitivity and the right amount of NO by switching between S1 and S3 as necessary.
Figure 12. Comparison of Base Case (k4 = 0.01) and the Case k4 = 0.015.
Red curves are the steady-state curves for both cases. Black curve is the steady-state curve for the base case (k4 = 0.01). Green curve is the new curve when k4 = 0.015. Intersections of red curves with the black define the steady-states for the two cases. Solid blue curve is the state trajectory for the base case. Dashed blue curve is the state trajectory for the case when k4 = 0.015. NO production by pAKT is enhanced by Increasing the value of parameter 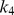 increases availability of NO and production of ONOO which impairs insulin signalling. Bistability is lost and the system settles to the single steady-state S1 where both pAKT and NO are low. In normal conditions (
increases availability of NO and production of ONOO which impairs insulin signalling. Bistability is lost and the system settles to the single steady-state S1 where both pAKT and NO are low. In normal conditions (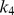 = 0.01), pIRS1-pAKT-pIRS1 and pAKT-NO-pAKT feedback loops work in coordination to maintain both insulin sensitivity and the right amount of NO by switching between S1 and S3 as necessary.
= 0.01), pIRS1-pAKT-pIRS1 and pAKT-NO-pAKT feedback loops work in coordination to maintain both insulin sensitivity and the right amount of NO by switching between S1 and S3 as necessary.
Hyperglycemia overstimulates Angiotensin II
When bistability is lost and the level of pAKT stays persistently low, the glucose cannot be taken into the cell and high levels of glucose remain in blood. High glucose levels lead to GPR91 mediated activation of NO. The increase in NO can explain the presence of glomerular hyperfiltration observed in diabetes [42]. Hyperglycemia induced high NO levels stimulate ANG II [41] which in turn decreases NO by converting it to ONOO and imparing insulin signalling. At the same time production of Ang II increases blood pressure. Thus hyperglycemia contributes to hypertension development.
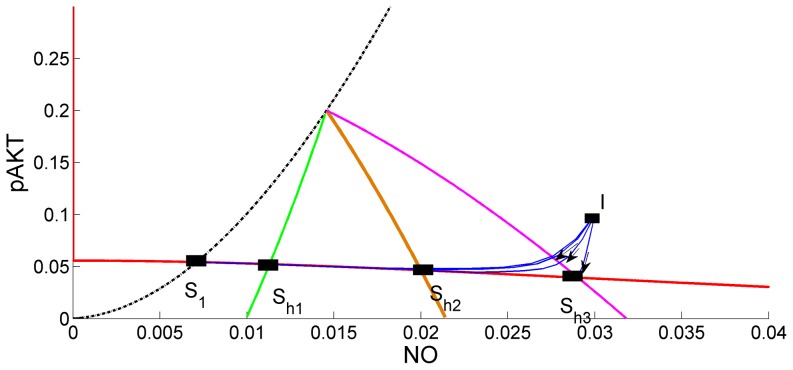
Hyperglycemia's effect can be simulated by choosing the model parameters so that bistability does not exist and by increasing the hyperglycemia parameter 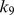 . Figure 13 shows the hyperglycemia region where pAKT stays below the threshold value 0.2. At the same time it is seen that increasing
. Figure 13 shows the hyperglycemia region where pAKT stays below the threshold value 0.2. At the same time it is seen that increasing 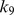 or hyperglycemia shifts the steady state to higher NO levels leading to increased production of Ang II
or hyperglycemia shifts the steady state to higher NO levels leading to increased production of Ang II
Figure 13. Effects of Hyperglycemia.
Points S1, Sh1, Sh2, Sh3 represent the steady-states. At these steady states NO levels increase as the parameter 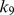 or hyperglycemia effect increases. Black, green, brown and red curves for k9 = 0, 0.005, 0.02 and 0.04, respectively. Intersections of these curves with the low-pAKT steady-state curve (red) define the steady-states for different levels of hyperglycemia. Dynamic simulations starting from initial condition I converge to different steady-states. Solid blue curves are the state trajectories for different k9 values. pAKT stays below the threshold value 0.2 and increasing
or hyperglycemia effect increases. Black, green, brown and red curves for k9 = 0, 0.005, 0.02 and 0.04, respectively. Intersections of these curves with the low-pAKT steady-state curve (red) define the steady-states for different levels of hyperglycemia. Dynamic simulations starting from initial condition I converge to different steady-states. Solid blue curves are the state trajectories for different k9 values. pAKT stays below the threshold value 0.2 and increasing 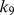 or hyperglycemia shifts the steady state to higher NO levels.
or hyperglycemia shifts the steady state to higher NO levels.
Conclusions
In this work, we have proposed a new mathematical model that is able to predict known physiological behaviors of normal and diseased states (including diabetes, hypertension) that are governed by the interaction of insulin and angiotensin II signalling pathways. This model extends the AKT signalling model of Wang [45] by including its interactions with the angiotensin signalling pathway.
The model is developed by making use of significant amount of available biological knowledge. Using this new model, bistability and parameter sensitivity analysis is carried out to reveal the interactions and feedback loops, and their parameter regions, which are significant in disease development such as cancer, diabetes, and hypertension. Simulations show that the model is able to predict the observations that many researchers have reported in the past. As such, it provides analytical insight to improve our understanding of an important biological system.
The normal regulatory response of insulin signalling is bistable so that AKT can switch between low and high levels. It is shown that the interaction parameters in the model, the balance of positive and negative feedback strengths are important in maintaining this bistability. pAKT regulates pIRS1 both positively and negatively. For a normal bistable response, the effect of positive feedback should be greater than the negative feedback. When the difference between the feedback strengths is negative, the system loses bistability and a large amount of insulin needs to be supplied to activate pAKT. In severe insulin resistant cases the system settles in low pAKT state and glucose uptake is impaired, thus characterized as diabetes. Ang II can impair insulin signalling through several mechanisms: by direct inhibition of pAKT by ONOO, by directly inhibiting pIRS1 or by increasing the negative feedback to pIRS1 via activating mTOR. Ang II is involved in both physiological and pathological blood pressure and cell proliferation respectively. Systems with overly active Ang II as a result of a disfunction (e.g diseased kidneys can stimulate Ang II) can induce diabetes through these mechanisms.
Insulin also mediates vasodilation by stimulating NO production. NO in turn activates Ang II and Ang II inhibits pIRS1 as explained earlier. The events following stimulation of NO by pIRS1, activation of Ang II by NO and inhibition of pIRS1 by Ang II forms a negative feedback loop. We also found that NO has also bistable characteristics and shows switching dynamics together with pAKT.
On the other hand, when pAKT is overly active (e.g. through enhanced activation of pAKT by pIRS1), uncontrolled cell proliferation appears and the persistent high pAKT level is characterized as cancer. In a cancer state, therapeutic actions to decrease pAKT can be taken.
Activation of pAKT is also significant for vasodilator actions of insulin as pAKT stimulates NO production. When insulin signalling is impaired, vasodilation is impaired as well. To maintain blood pressure within narrow limits NO and Ang II should be balanced. NO activates Ang II and Ang II leads to consumption of NO to produce ONOO. ONOO and Ang II in turn impair insulin signalling and lead to further decrease in NO. The effects should be balanced to keep NO and Ang II at desired levels to maintain blood pressure.
Ang II also activates pERK [37]and Ang II activated pERK inhibits pIRS by serine phosphorylation. We have modelled these additional effects as well [55] but those results are not reported here for two reasons. First, the main interactions of the Ang II-AKT pathway targeted in this paper are not affected by not considering these interactions. Second the model is kept simple in order not to complicate the analysis and obscure the physical insights.
Investigating the regulatory and diseased mechanism at system level provides a valuable understanding of complex interacting pathways. A fine balance of interactions between the signalling agents should be maintained to avoid diseases.
Finally, due to lack of reliable data on the model parameters, future experimental measurements to estimate the key interaction parameters identified in this study will be invaluable to validate the proposed model firmly and improve our understanding of the biological network dynamics.
Supporting Information
Derivation of the two-state reduced order dynamic AKT model.
(DOCX)
Derivation of the six-state ANGII-NO model.
(DOCX)
SBML file for the model.
(XML)
Funding Statement
Koc University supported this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ando K, Fujita T (2006) Anti-diabetic effect of blockade of the renin-angiotensin system. Diabetes Obes Metab 8: 396–403. [DOI] [PubMed] [Google Scholar]
- 2. Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86: s836–842. [DOI] [PubMed] [Google Scholar]
- 3. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henriksen EJ (2007) Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 293: R974–980. [DOI] [PubMed] [Google Scholar]
- 5. Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M (2001) Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 38: 884–890. [DOI] [PubMed] [Google Scholar]
- 6. Nawano M, Anai M, Funaki M, Kobayashi H, Kanda A, et al. (1999) Imidapril, an angiotensin-converting enzyme inhibitor, improves insulin sensitivity by enhancing signal transduction via insulin receptor substrate proteins and improving vascular resistance in the Zucker fatty rat. Metabolism 48: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 7. Perkins JM, Davis SN (2008) The renin-angiotensin-aldosterone system: a pivotal role in insulin sensitivity and glycemic control. Curr Opin Endocrinol Diabetes Obes 15: 147–152. [DOI] [PubMed] [Google Scholar]
- 8. Scheen AJ (2004) Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab 30: 498–505. [DOI] [PubMed] [Google Scholar]
- 9. Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806. [DOI] [PubMed] [Google Scholar]
- 10. Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, et al. (1997) Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol 11: 1881–1890. [DOI] [PubMed] [Google Scholar]
- 11. Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88: 435–437. [DOI] [PubMed] [Google Scholar]
- 12. Liao Y, Hung MC (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2: 19–42. [PMC free article] [PubMed] [Google Scholar]
- 13. Manning BD (2004) Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167: 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sowers JR (1990) Insulin resistance and hypertension. Mol Cell Endocrinol 74: C87–89. [DOI] [PubMed] [Google Scholar]
- 15. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G (2004) Angiotensin II impairs the insulin signalling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 16. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, et al. (2000) Roles for insulin receptor, PI3-kinase, and Akt in insulin-signalling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 17. Kobori H, Nangaku M, Navar LG, Nishiyama A (2007) The Intrarenal Renin-Angiotensin System: From Physiology to the Pathobiology of Hypertension and Kidney Disease. Pharmacological Reviews 59: 251–287. [DOI] [PubMed] [Google Scholar]
- 18. Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paz K, Liu YF, Shorer H, Hemi R, LeRoith D, et al. (1999) Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem 274: 28816–28822. [DOI] [PubMed] [Google Scholar]
- 20. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, et al. (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 21. Ravichandran LV, Chen H, Li Y, Quon MJ (2001) Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol Endocrinol 15: 1768–1780. [DOI] [PubMed] [Google Scholar]
- 22. Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, et al. (1998) Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484–14494. [DOI] [PubMed] [Google Scholar]
- 23. Raught B, Gingras AC, Sonenberg N (2001) The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A 98: 7037–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winter JN, Jefferson LS, Kimball SR (2011) ERK and Akt signalling pathways function through parallel mechanisms to promote mTORC1 signalling. Am J Physiol Cell Physiol 300: C1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guertin DA, Sabatini DM (2005) An expanding role for mTOR in cancer. Trends Mol Med 11: 353–361. [DOI] [PubMed] [Google Scholar]
- 26. Nayak S, Siddiqui JK, Varner JD (2011) Modelling and analysis of an ensemble of eukaryotic translation initiation models. IET Syst Biol 5: 2. [DOI] [PubMed] [Google Scholar]
- 27. Thomas G, Hall MN (1997) TOR signalling and control of cell growth. Curr Opin Cell Biol 9: 782–787. [DOI] [PubMed] [Google Scholar]
- 28. de Kloet AD, Krause EG, Woods SC (2010) The renin angiotensin system and the metabolic syndrome. Physiol Behav 100: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ceriello A, Assaloni R, Da Ros R, Maier A, Quagliaro L, et al. (2004) Effect of irbesartan on nitrotyrosine generation in non-hypertensive diabetic patients. Diabetologia 47: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 30. Fan Q, Liao J, Kobayashi M, Yamashita M, Gu L, et al. (2004) Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant 19: 3012–3020. [DOI] [PubMed] [Google Scholar]
- 31. Guo W, Adachi T, Matsui R, Xu S, Jiang B, et al. (2003) Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 285: H1396–1403. [DOI] [PubMed] [Google Scholar]
- 32. Pueyo ME, Arnal JF, Rami J, Michel JB (1998) Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol 274: C214–220. [DOI] [PubMed] [Google Scholar]
- 33. Wattanapitayakul SK, Weinstein DM, Holycross BJ, Bauer JA (2000) Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J 14: 271–278. [DOI] [PubMed] [Google Scholar]
- 34. Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, et al. (2005) Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab 288: E353–359. [DOI] [PubMed] [Google Scholar]
- 35. Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, et al. (2006) Angiotensin II-induced NADPH oxidase activation impairs insulin signalling in skeletal muscle cells. J Biol Chem 281: 35137–35146. [DOI] [PubMed] [Google Scholar]
- 36. Wenzel P, Schulz E, Oelze M, Muller J, Schuhmacher S, et al. (2008) AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 45: 619–626. [DOI] [PubMed] [Google Scholar]
- 37. Csibi A, Communi D, Muller N, Bottari SP (2010) Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS One 5: e10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, et al. (2005) S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 54: 959–967. [DOI] [PubMed] [Google Scholar]
- 39. Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, et al. (2005) S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 280: 7511–7518. [DOI] [PubMed] [Google Scholar]
- 40. Pulakat L, Demarco VG, Whaley-Connell A, Sowers JR (2011) The Impact of Overnutrition on Insulin Metabolic Signalling in the Heart and the Kidney. Cardiorenal Med 1: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurtz A, Wagner C (1998) Role of nitric oxide in the control of renin secretion. Am J Physiol 275: F849–862. [DOI] [PubMed] [Google Scholar]
- 42. Peti-Peterdi J, Kang JJ, Toma I (2008) Activation of the renal renin-angiotensin system in diabetes—new concepts. Nephrol Dial Transplant 23: 3047–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JR, Yoon Y, Cho KH (2008) Coupled feedback loops form dynamic motifs of cellular networks. Biophys J 94: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kwon YK, Cho KH (2008) Coherent coupling of feedback loops: a design principle of cell signalling networks. Bioinformatics 24: 1926–1932. [DOI] [PubMed] [Google Scholar]
- 45. Wang G (2010) Singularity analysis of the AKT siganlling pathway reveals connections between cancer and metabolic diseases. Phys Biol 7: 046015. [DOI] [PubMed] [Google Scholar]
- 46. Giri L, Mutalik VK, Venkatesh KV (2004) A steady state analysis indicates that negative feedback regulation of PTP1B by Akt elicits bistability in insulin-stimulated GLUT4 translocation. Theor Biol Med Model 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inoki K, Corradetti MN, Guan KL (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37: 19–24. [DOI] [PubMed] [Google Scholar]
- 48. Tee AR, Blenis J (2005) mTOR, translational control and human disease. Semin Cell Dev Biol 16: 29–37. [DOI] [PubMed] [Google Scholar]
- 49. Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, et al. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118: 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stelling J, Sauer U, Szallasi Z, Doyle FJ 3rd, Doyle J (2004) Robustness of cellular functions. Cell 118: 675–685. [DOI] [PubMed] [Google Scholar]
- 52. Chu EC, Tarnawski AS (2004) PTEN regulatory functions in tumor suppression and cell biology. Medical science monitor international medical journal of experimental and clinical research 10: RA235–A241. [PubMed] [Google Scholar]
- 53. O'Reilly KE, Rojo F, She Q-B, Solit D, Mills GB, et al. (2006) mTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signalling and Activates Akt. Cancer Research 66: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Angeli D, Ferrell JE, Sontag ED (2004) Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proceedings of the National Academy of Sciences of the United States of America 101: 1822–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cizmeci D (2012) Regulatory Networks and Complex Interactions Between the Insulin and Angiotensin II Signalling Systems: Implications for Hypertension and Diabetes [MS]: Koc University. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Derivation of the two-state reduced order dynamic AKT model.
(DOCX)
Derivation of the six-state ANGII-NO model.
(DOCX)
SBML file for the model.
(XML)