Abstract
Background
Sunitinib is a standard of care treatment in advanced clear-cell renal cell carcinoma (ccRCC). Retrospective and expanded access data suggest sunitinib has activity in advanced non-clear cell RCC (nccRCC).
Objective
To prospectively determine the clinical efficacy and safety of sunitinib in patients with advanced nccRCC.
Design, Setting, and Participants
This is a single-arm phase 2 trial with a two-stage design. Eligibility criteria included pathologically confirmed nccRCC or ccRCC with ≥ 20 percent sarcomatoid histology, performance status 0–2, measurable disease, maximum 2 prior systemic therapies, and no prior treatment with tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptors.
Intervention
Patients received sunitinib 50 mg daily on a 4-week on, 2-week off schedule.
Outcome Measurements and Statistical Analysis
Primary endpoints were objective response rate (ORR) and progression-free survival (PFS). Secondary endpoints were safety and overall survival (OS).
Results and Limitations
Fifty-seven patients were eligible [papillary (27), chromophobe (5), unclassified (8), collecting duct or medullary carcinoma (6), sarcomatoid (7), others (4)]. Median PFS for 55 evaluable patients was 2.7 months [95% CI: 1.4, 5.4]. Two patients with chromophobe and one patient with unclassified histology had a confirmed partial response (5% ORR). Median PFS for patients with papillary histology was 1.6 months (95% CI: 1.4, 5.4). Median PFS for patients with chromophobe histology was 12.7 months (95% CI: 8.5, NA). Median OS for all patients was 16.8 months (95% CI: 10.7, 26.3). Treatment emergent adverse events were consistent with sunitinib’s mechanism of action. The non-randomized design and small number of patients are limitations of this study.
Conclusions
The differential response of chromophobe histology to sunitinib suggests a therapeutically relevant biological heterogeneity exists within nccRCC. The low ORR and short PFS with sunitinib in the other nccRCC subtypes underscore the need to enroll patients with these diverse tumors on clinical trials.
Introduction
Of the estimated 64,000 cancers of the kidney and renal pelvis which will be diagnosed in 2012 in the US,1 approximately 80% will be clear-cell renal cell carcinoma (RCC), and 20% comprised of non-clear cell subtypes, the most common of which is papillary RCC (pRCC), but which also include chromophobe RCC, renal medullary carcinoma (RMC), collecting duct carcinoma (CDC), and other subtypes recently incorporated in the World Health Organization (WHO) classification. Each of these subtypes is morphologically unique, and biologically distinct as well. Most of the recent clinical trials in RCC have either restricted enrollment to patients with clear-cell RCC (ccRCC), or have reported small numbers of patients with non-clear cell RCC (nccRCC), often without differentiating among the heterogeneous group of non-clear cell subtypes.2–9 Sunitinib was one of the first tyrosine kinase inhibitors (TKIs) to emerge in the treatment of RCC, and is currently a standard of care in the first-line setting. Retrospective series and expanded access data suggest that sunitinib may have efficacy in patients with nccRCC.10,11
By treating a histologically diverse population of nccRCC patients with a molecularly targeted agent, our aim was to provide a therapeutic framework to improve our disease classification, and to set the stage for a deeper understanding of the molecular biology of these tumor subtypes.
Patients and Methods
The trial was approved by the institutional review board at MD Anderson Cancer Center (MDACC), and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. Patients provided written informed consent at enrollment.
Eligibility
Central histological confirmation by an expert genitourinary pathologist at MDACC of any of the nccRCC subtypes recognized by the WHO classification was required. Patients who had clear-cell RCC with at least 20% sarcomatoid features were permitted to enroll on this trial. Patients were required to have adequate hematologic, hepatic, cardiac and renal functions, Eastern Cooperative Oncology Group performance status (PS) 0–2, measurable disease, maximum two prior systemic therapies, and no prior treatment with vascular endothelial growth factor (VEGF) directed TKIs. Prior therapy with angiogenesis inhibitors, other than anti-VEGF TKIs, was allowed.
Treatment Plan
Patients received sunitinib 50 mg orally, daily for 28 days, on a 4-week on, 2-week off schedule. For patients who developed toxicity, doses were reduced in single decrements of 12.5 mg to a minimum dose of 25 mg daily, 4 weeks on, 2 weeks off. Patients continued on study until progressive disease (PD), unacceptable toxicity, or withdrawal of patient consent.
Diagnostic Evaluation and Assessment of Efficacy and Safety
Baseline evaluation included a history and physical examination, computed tomography (CT) scans of the chest, abdomen and pelvis, and CT or magnetic resonance imaging of the brain. Bone scans and/or bone surveys were obtained if clinically indicated. A 2D Doppler echocardiogram and a 12-lead EKG were obtained at baseline and every 12 weeks while patients were on study.
Imaging studies were performed every 6 weeks for the first two cycles, then every 12 weeks. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0. Adverse events were graded using the National Cancer Institute Common Toxicity Criteria version 3.0.
Statistical Analysis
The primary endpoints of the study were objective response rate (ORR) and progression-free survival (PFS). Secondary endpoints were safety and overall survival (OS). A two-stage design was used. The study was designed to result in termination if, after analysis of the first 20 patients, fewer than 2 patients had a partial response (PR), and there was a greater than 70% posterior probability that 75% or more patients would have PD by 12 weeks.
The distribution of each continuous variable was summarized by its mean, standard deviation and range. For continuous variables [hemoglobin, platelet count, absolute neutrophil count (ANC), albumin, corrected serum calcium, serum lactate dehydrogenase (LDH)], values were considered abnormal, if they fell outside the upper or lower limits of normal range for our laboratory. The distribution of each categorical variable was summarized by its frequencies and percentages. Kaplan-Meier curves were used to estimate unadjusted PFS and OS time distributions. The Log rank test and Cox proportional hazards regression model were used to evaluate the various clinical and demographic factors predictive of PFS and OS. Variables with p value < 0.15 in the univariable analysis were included in the multivariable analysis for OS and PFS. Initially, a Cox proportional hazards model was fitted including variables with p value < 0.15, then the backward selection procedure was applied for model selection to identify independent significant prognostic factors for OS or PFS. All analyses were carried out in SAS version 9.1 (Cary, NC) and S-Plus version 8.04. (Palo Alto, CA). P values less than .05 were considered statistically significant.
Results
Patient Characteristics
The study opened for enrollment in April 2007. At the designated interim analysis, the study met criteria for continuation to full accrual. The study completed accrual in May 2010, with a total of 61 patients enrolled. Two patients were ineligible (one had urothelial carcinoma of the renal pelvis and one had ccRCC without sarcomatoid features); two patients (one with RMC and one with type 2 pRCC) withdrew consent and never received sunitinib. Fifty-seven patients received sunitinib and are included in this analysis. Patients’ characteristics are summarized in Table 1A. Baseline laboratory variables are summarized in Table 1B.
Table 1.
| A: Patients’ Characteristics (N=57) | ||
|---|---|---|
| Characteristic | No. | % |
| Gender | ||
| Female | 19 | 33% |
| Male | 38 | 67% |
| Age, years | ||
| Median | 57 | |
| Range | 22-85 | |
| Histology | ||
| Papillary | 27 | 47% |
| Type 1 | 2 | |
| Type II | 11 | |
| Not otherwise specified | 14 | |
| Unclassified | 8 | 14% |
| Sarcomatoid | 7 | 12% |
| Collecting Duct / Renal Medullary | 6 | 11% |
| Chromophobe | 5 | 9% |
| Other* | 4 | 7% |
| ECOG Performance Status | ||
| 0 | 20 | 35% |
| 1 | 32 | 56% |
| 2 | 5 | 9% |
| MSKCC** Risk Group | ||
| 0 (favorable) | 9 | 16% |
| 1-2 (intermediate) | 36 | 63% |
| ≥ 3 (poor) | 12 | 21% |
| Heng et al’s Risk Group | ||
| 0 (favorable) | 10 | 17.5% |
| 1-2 (intermediate) | 37 | 65% |
| ≥3 (poor) | 10 | 17.5% |
| Number of disease sites | ||
| 1 | 14 | 25% |
| 2 | 18 | 32% |
| ≥ 3 | 25 | 44% |
| Other Characteristics | ||
| Prior nephrectomy | 40 | 70% |
| Prior systemic therapy*** | 8 | 14% |
| Treated brain metastases at baseline | 2 | 4% |
| B: Summary of Baseline Laboratory Variables. | |||||
|---|---|---|---|---|---|
| Variable | 1st Quartile |
Median | 3rd Quartile | Mean | Std Dev |
| Hemoglobin | 11.20 | 12.60 | 13.90 | 12.49 | 1.79 |
| Corrected Serum calcium | 8.98 | 9.20 | 9.60 | 9.27 | 0.49 |
| Serum albumin | 4.00 | 4.20 | 4.50 | 4.15 | 0.61 |
| Serum LDH | 413.00 | 496.00 | 666.00 | 668.82 | 496.97 |
| Platelet count | 211.00 | 266.00 | 341.00 | 298.87 | 129.15 |
| Absolute neutrophil count | 3.82 | 4.85 | 6.17 | 5.31 | 2.40 |
Translocation carcinoma, mucinous tubular and spindle cell carcinoma, thyroid-like follicular carcinoma, tubulocystic carcinoma (1 patient each)
Memorial Sloan Kettering Cancer Center
pemetrexed plus gemcitabine (2 patients); erlotinib, gefitinib, ABT-510, interleukin-2 plus interferon-alfa plus 5-fluorourocil, bevacizumab, thalidomide (1 patient each)
Treatment Administration and Safety
All 57 patients were evaluable for safety and OS. The adverse events (AEs) noted in this study were similar to those reported in previous trials; however, we observed higher rates of grade 3 fatigue and hypertension than previously reported with sunitinib. The most common grade 3 AEs were fatigue, hypertension, neutropenia, and thrombocytopenia (Table 2). One patient with sickle-thalassemia and RMC died suddenly while on study; autopsy was not performed and cause of death is unknown.
Table 2.
Treatment Emergent Adverse Events Grade >=3 Occuring At Least Once Among all 57 Patients
| Adverse Event | Grade 3 | Grade 4 | Total |
|---|---|---|---|
| Fatigue (asthenia, lethargy, malaise) | 16 | 0 | 16 |
| Hypertension | 16 | 0 | 16 |
| Neutropenia | 10 | 1 | 11 |
| Pain | 7 | 0 | 7 |
| Mucositis/stomatitis | 5 | 1 | 6 |
| Thrombocytopenia | 3 | 2 | 5 |
| Anemia | 5 | 0 | 5 |
| Leukopenia | 3 | 0 | 3 |
| Hyperuricemia | 0 | 3 | 3 |
| Hyponatremia | 3 | 0 | 3 |
| Diarrhea | 3 | 0 | 3 |
| Sensory Neuropathy | 3 | 0 | 3 |
| Nausea | 3 | 0 | 3 |
| Anorexia | 2 | 0 | 2 |
| Hypophosphatemia | 2 | 0 | 2 |
| Vomiting | 2 | 0 | 2 |
| Dysphagia | 0 | 1 | 1 |
| Motor Neuropathy | 1 | 0 | 1 |
| Hemorrhage/Bleeding | 0 | 1 | 1 |
| Nonneutropenic Fever | 1 | 0 | 1 |
| Left ventricular diastolic dysfunction | 1 | 0 | 1 |
| Hyperbilirubinemia | 1 | 0 | 1 |
| Hemolysis | 1 | 0 | 1 |
| Transaminitis | 1 | 0 | 1 |
| Elevated Lipase | 1 | 0 | 1 |
| Myocardial Infarct | 0 | 1 | 1 |
| Hypoalbuminemia | 1 | 0 | 1 |
| Hypomagnesemia | 1 | 0 | 1 |
| Thrombosis/embolism | 0 | 1 | 1 |
| Dyspnea | 1 | 0 | 1 |
| Rash/Desquamation | 1 | 0 | 1 |
| Hand-Foot Skin Reaction | 1 | 0 | 1 |
| Febrile Neutropenia | 1 | 0 | 1 |
| Hypokalemia | 1 | 0 | 1 |
| Syncope/near syncope | 1 | 0 | 1 |
| Dehydration | 1 | 0 | 1 |
| Death within 30 Days of Discontinuing Study Drug | 0 | 0 | 1 |
| Hypocalcemia | 1 | 0 | 1 |
Efficacy
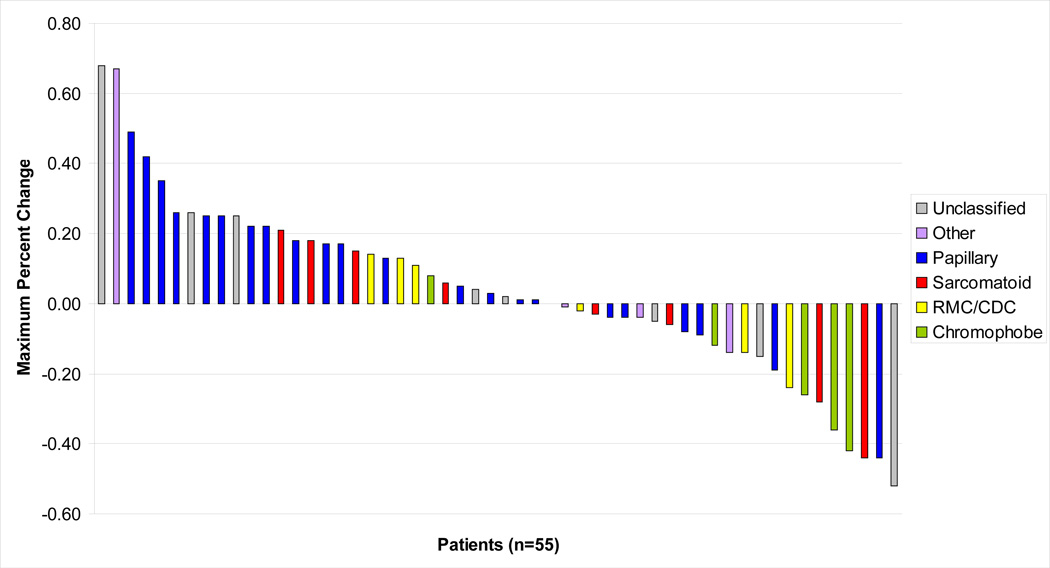
Two patients with pRCC came off study prior to their first tumor evaluation for reasons other than PD: one patient sustained a non-fatal myocardial infarction 12 days after starting sunitinib, and one patient withdrew consent due to grade 3 rash after two doses of sunitinib. Of the remaining 55 patients, one patient showed clinical PD before the first restaging scans were obtained. The 54 patients with follow up scans had an independent masked radiology review for assessment of tumor response. Overall, 43% of patients had some degree of shrinkage of target lesions, with a median decrease of 14% (Figure 1). Three previously untreated patients had a confirmed PR; two had chromophobe and one had unclassified RCC. The rate of tumor shrinkage among the three responders was slow. Time to PR was 9.7 months and 14.9 months for each of the responders with chromophobe RCC, and 12.6 months for the responder with unclassified RCC. These three patients have maintained their PR and were still receiving treatment as of last follow up. Two additional patients, one with type 2 pRCC and one with ccRCC and 50% sarcomatoid features, achieved greater than 30% reduction in their target lesions, but had PD by their next tumor evaluation 12 weeks later, and are categorized as having stable disease (SD) by RECIST.11 Median PFS for all 55 evaluable patients was 2.7 months (95% CI: 1.4, 5.4). The efficacy results are summarized in Table 3A. There were no significant differences in ORR, PFS, and OS between previously untreated and previously treated patients (Tables 3B/C).
Figure 1.
Maximum Percent Change in Target Lesions by RECIST 1.0
Table 3.
| A: Efficacy Results (N=55) | ||||||
|---|---|---|---|---|---|---|
| Histology | Median PFS in Months (95% CI) |
Best Response by RECIST | ||||
| Objective Response Rate |
Disease Control Rate (PR+SD) |
PR | SD | PD | ||
| Entire Group (n=55) | 2.7 (1.4, 5.4) | 5% | 58% | 3 | 29 | 23 |
| Papillary (n=25) | 1.6 (1.4, 5.4) | 0% | 48% | 0 | 12 | 13 |
| Unclassified (n=8) | 3.2 (1.4, NA) | 13% | 63% | 1 | 4 | 3 |
| Sarcomatoid (n=7) | 1.4 (1.3, NA) | 0% | 43% | 0 | 3 | 4 |
| Collecting Duct / Renal Medullary (n=6) | 3.1 (1.4, NA) | 0% | 67% | 0 | 4 | 2 |
| Chromophobe (n=5) | 12.7 (8.5, NA) | 40% | 100% | 2 | 3 | 0 |
| Thyroid-like Follicular (n=1)* | 33+ | 0% | 100% | 0 | 1 | 0 |
| Translocation (n=1)# | 1.0 | 0% | 0% | 0 | 0 | 1 |
| Tubulocystic (n=1)# | 9.8 | 0% | 100% | 0 | 1 | 0 |
| Mucinous Tubular and Spindle (n=1)# | 6.5 | 0% | 100% | 0 | 1 | 0 |
| B. Objective Response Rate (ORR) by pretreatment | |||
|---|---|---|---|
| Response | Pre-treatment | p value | |
| Naïve | Pre-treated | ||
| PR | 3 | 0 | 1 |
| SD | 24 | 5 | |
| PD | 20 | 3 | |
| C: Overall Survival (OS) and Progression Free Survival (PFS) According to Prior Systemic Therapy Status | ||||||
|---|---|---|---|---|---|---|
| Prior systemic therapy |
N | Event | Median OS time (95% CI) (Months) |
OS Rate at 3 Years (95% CI) |
P-value | |
| OS | No | 49 | 32 | 14.0 ( 8.6 , 28.6 ) | 0.2 ( 0.1 , 0.4 ) | 0.8 |
| Yes | 8 | 7 | 21.0 ( 12.9 , NA ) | 0.1 ( 0.0 , 0.7 ) | ||
| N | Event |
Median PFS time (95% CI) (Months) |
PFS Rate at 3 Years (95%CI) |
P-value | ||
| PFS | No | 47 | 40 | 2.7 ( 1.3 , 5.4 ) | 0.1 ( 0.0 , 0.2 ) | 0.9 |
| Yes | 8 | 8 | 5.3 ( 1.4 , NA ) | |||
Time for thyroid-like follicular carcinoma patient was the SD duration, no PD observed.
Time to PD
The univariable analyses suggested that PFS was significantly associated with serum albumin, platelet count, ANC and number of disease sites. The multivariable analysis showed number of disease sites as the only independent significant prognostic factor for PFS (HR=1.51, p=0.008; 95% CI: 1.1, 1.9). Median follow up of surviving patients is 21.7 months (range: 13.8, 50.4). For all 57 patients, median OS was 16.8 months (95% CI: 10.7, 26.3 months). The univariable analyses suggested that OS was significantly associated with PS, serum albumin, serum LDH, platelet count, ANC, number of disease sites, and Heng et al’s prognostic group12. A multivariable analysis showed that PS, ANC, and number of disease sites were independent prognostic factors for OS. PS of 2 (HR = 8.06, p = 0.0015), higher number of disease sites (HR = 1.66, p = 0.0007), and neutrophilia (HR = 1.36, p = 0.002), were significantly associated with a higher risk of death (Table 4).
Table 4.
Multivariable Cox Model for OS
| Variable | HR (95% CI) | P value |
|---|---|---|
| PS | ||
| 1 vs. 0 | 2.48 (1.08, 5.72) | 0.033 |
| 2 vs. 0 | 8.06 (2.22, 29.22) | 0.0015 |
| Absolute neutrophil count | 1.36 (1.12, 1.66) | 0.002 |
| Number of disease sites | 1.66 (1.24, 2.22) | 0.0007 |
For the 25 patients with pRCC who were evaluable for PFS, median PFS was 1.6 months (95% CI: 1.4, 5.4) and median OS was 12.6 months (95% CI: 7.3, 36.9). A minority of patients with pRCC (28%) had tumor shrinkage of target lesions, with no confirmed PRs. Three patients with sarcomatoid features had SD. The median percent sarcomatoid component was 50% (range: 20%, 100%). Median PFS for the sarcomatoid group was 1.4 months (95% CI: 1.3, NA).
Discussion
The broad histological diversity of nccRCC presents unique challenges. Emerging data suggest that several of the hereditary nccRCC entities are linked by defects in nutrient sensing and cellular energy metabolism; the role of angiogenesis as a driver and potential therapeutic target is less well defined. The activity of the targeted agents, standard in ccRCC, has not been well described in nccRCC, as patients with nccRCC have often been excluded from large studies. A concerted effort to define the clinical and molecular characteristics of nccRCC in the context of a therapeutic trial will permit the development of a clinically relevant taxonomy, prioritize disease subgroups for further study using existing agents, and facilitate the matching of other disease subtypes with novel agents.
In the expanded access experience with sunitinib, an 11% ORR was reported for 588 patients with nccRCC.11 However, pathology was not centrally reviewed, and results were not coded separately for each of the nccRCC subtypes. We report a 5% ORR, a median PFS of 2.7 months, and a median OS of 16.8 months for 57 patients with nccRCC. These disappointing results reflect the biology of advanced nccRCC, particularly high-grade papillary type 2 and unclassified RCC, and ccRCC with sarcomatoid features. Our prospective data suggest that sunitinib does not produce the same level of activity in nccRCC, compared with ccRCC, where ORR with sunitinib is 47% and median OS is 26.4 months.13 We should point out that our patient cohort was comprised predominantly of subjects with intermediate- and poor-risk features, a 70% nephrectomy rate, a relatively high number of metastatic sites, and 14% prior systemic therapy exposure. These characteristics should be considered in the interpretation of this analysis.
Interest in sunitinib as therapy for pRCC arose based on its efficacy in ccRCC, and retrospective data suggesting efficacy in pRCC.10 In our prospective study, which included 27 patients with pRCC, we were not able to confirm the retrospective data by Choueiri et al.10 No confirmed PRs were noted, and PFS was short. In a pooled analysis of our study and two other prospective studies of sunitinib in pRCC, 14, 15 only one PR was observed among 61 patients. In contrast, a Korean phase II study of sunitinib in nccRCC reported a 36% ORR, including eight PRs among 22 patients with pRCC, and a median PFS of 6.4 months.16 These conflicting data suggest that ethnic differences may explain the variable responses to sunitinib in nccRCC. Alternative pathways have been investigated in pRCC, with MET emerging as a target in type 1 pRCC.17, 18 The MET gene is mutated in the germline of patients with hereditary type 1 pRCC, and in 13% of sporadic type 1 pRCC.18, 19 Mutations identified to date have been activating of the MET protein.18, 19 However, over-expression of MET in pRCC tumors is not limited to cases of mutation. In one series of 50 sporadic cases of pRCC, 80% were found to over-express MET, and correlated with worse prognosis.20 Studies are investigating several c-met inhibitors in pRCC (NCT00726323).
Participation in clinical trials with a sound biological rationale is the preferred treatment approach for pRCC. Alternatively, temsirolimus or erlotinib may be considered for pRCC. In the phase III trial of temsirolimus in poor-risk patients, patients with pRCC were identified and analyzed as a subset.21 In this analysis, PFS and OS were approximately 6 and 11 months, respectively. These results are similar to those for the ccRCC cohort in the same study, suggesting that temsirolimus is an appropriate treatment for patients with poor-risk pRCC.21 Two ongoing clinical trials are comparing everolimus, an oral mTOR inhibitor, to sunitinib (NCT01185366 and NCT01108445). In a phase II trial in pRCC, erlotinib produced an 11% ORR and a disease control rate of 64%, making it worthy of further evaluation.22
Although the small patient numbers in our study make subgroup analyses difficult, anti-tumor response and durable disease control were seen predominantly in the chromophobe cohort, compared with other histological subtypes. Chromophobe RCC is typically indolent, rarely metastasizes, and is usually cured with resection.23 All five patients with chromophobe RCC in our study derived clinical benefit from treatment, with a 40% ORR and a median PFS of 12.7 months. Although a prolonged PFS could be explained by the indolent course of this disease, the observed tumor shrinkage, which included two durable PRs, indicates drug effect. Biologically, this is possibly a reflection of the KIT inhibitory activity of sunitinib, as KIT over-expression is common in chromophobe RCC.24 The lengthy time to PR noted among the two chromophobe responders is a phenomenon also described in gastrointestinal stromal tumor patients treated with imatinib, another KIT targeting agent.25
Sarcomatoid RCC is not a separate histological entity. Sarcomatoid features can be present with virtually any of the RCC histological subtypes, at a rate of approximately 10%. A debatable matter is whether therapy should be tailored to the underlying tumor histology, or to its sarcomatoid component. Sarcomatoid features imply high-grade disease and aggressive clinical behavior, therefore, we chose to analyze patients with sarcomatoid features as a discrete entity. The sarcomatoid component is typically reported as an overall percent of the total histology, and can range from focal to pure sarcomatoid within the specimen. A case series and a small clinical trial described treatment of this disease with a combination of gemcitabine and doxorubicin.26, 27 Although most responses with this regimen are of short duration, there is a small subset of patients who experience durable complete remissions.28 Golshayan et al and Molina et al reported separate retrospective series suggesting benefit with sunitinib in sarcomatoid RCC, with response rates of 29% and 14%, PFS of 5.3 and 4.4 months, and a median percent sarcomatoid component of 14% and 20% in each study, respectively.29, 30 In our sarcomatoid cohort, no responses were seen, and median PFS was 1.4 months. The 50% median percent sarcomatoid component seen in our study and the fact that five of the seven patients with sarcomatoid features had nccRCC may explain the poor results.
Conclusions
The differential response of chromophobe RCC to sunitinib suggests that a therapeutically relevant biologic heterogeneity exists within nccRCC. The disappointing results in other nccRCC subtypes underscore the need to enroll these patients on clinical trials. In future trials of novel agents for nccRCC, in-depth characterization of the molecular features underpinning these subtypes will help identify rational therapeutic strategies targeting relevant pathways.
Acknowledgments
This study was funded by Pfizer Pharmaceuticals Award Number CS2006-00018400JW
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg CN, Szczylik C, Lee E, et al. A randomized, double-blind phase III study of pazopanib in treatment-naïve and cytokine-pretreated patients with advanced renal cell carcinoma (RCC) J Clin Oncol. 2009;27:5021. [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 11.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renalcell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 12.Heng DY, Xie W, Regan MM. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-tareted agents: results from a large, multicenter study. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravaud A, Oudard S, Gravis-Mescam G, et al. First-line sunitinib in type I and II papillary renal cell carcinoma (PRCC): SUPAP, a phase II study of the French Genito-Urinary Group (GETUG) and the Group of Early Phase trials (GEP) J Clin Oncol. 2009;27:5146. [Google Scholar]
- 15.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30(1):335–340. doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JL, Ahm J-H, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012 Jan 6; doi: 10.1093/annonc/mdr586. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Jeffers M, Schmidt L, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney P, El-Naggar AK, Lin SH, Pisters LL. Biological significance of c-met over expression in papillary renal cell carcinoma. J Urol. 2002;168:51–55. [PubMed] [Google Scholar]
- 21.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol. 2009;27:5788–5793. doi: 10.1200/JCO.2008.18.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin MB, Paner GP, Alvarado-Cabrero I, et al. Chromophobe renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 145 cases. Am J Surg Pathol. 2008;32(12):1822–1834. doi: 10.1097/PAS.0b013e3181831e68. [DOI] [PubMed] [Google Scholar]
- 24.Huo L, Sugimura J, Tretiakova MS, et al. C-kit expression in renal oncocytomas and chromophobe renal cell carcinomas. Hum Pathol. 2005;36:262–268. doi: 10.1016/j.humpath.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 26.Nanus DM, Garino A, Milowsky MI, Larkin M, Dutcher JP. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer. 2004;101:1545–1551. doi: 10.1002/cncr.20541. [DOI] [PubMed] [Google Scholar]
- 27.Haas N, Lin X, Manola J, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2011 Feb 6; doi: 10.1007/s12032-011-9829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutcher JP, Nanus D. Long-term survival of patients with sarcomatoid renal cell cancer treated with chemotherapy. Med Oncol. 2011;28(4):1530–1533. doi: 10.1007/s12032-010-9649-2. [DOI] [PubMed] [Google Scholar]
- 29.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235–241. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 30.Molina AM, Tickoo SK, Ishill N, et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am J Clin Oncol. 2011;34(5):454–459. doi: 10.1097/COC.0b013e3181f47aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]