Abstract
OBJECTIVE
To determine the clinical activity and toxicity of combination pemetrexed and gemcitabine for locally advance and metastatic non-clear cell renal cell carcinoma.
METHODS
In this phase II study patients were treated with pemetrexed 500 mg/m2 IV infusion over 10 minutes(min) on day one followed immediately by gemcitabine 1500 mg/m2 IV over 30 min on day one, repeated every fourteen days. Planned enrollment was 40 patients. The primary endpoints were response rate and progression free survival (PFS). The secondary endpoints were toxicity and overall survival (OS) in months (mo).
RESULTS
Between December 2005 and December 2008, 16 patients with locally advanced or metastatic non clear cell renal cell carcinoma were enrolled. The trial was stopped due to lack of response and excessive toxicity. The overall response rate was 6.7% (95% exact CI: 0.2%–31.9%), no patients with renal cell carcinoma responded to therapy. The median number of cycles administered was 4 (range: 1–12 cycles), median PFS was 3.2 mo (95% CI: 1.875–>6 mo), and the 16-week PFS rate was 46.7% (95% CI: 19.8%–100%). The median OS was 23.2 mo (95% CI: 12.9–38.1 mo). The most common grade 3 or 4 toxicities were neutropenia (53%), leukopenia (53%), anemia (13%), fatigue (40%), and renal failure (13%).
CONCLUSIONS
Patients with non clear cell carcinoma metastatic renal cell carcinoma. The combination of pemetrexed and gemcitabine did not show clinical activity in this cohort of patients with non clear cell renal cell carcinoma.
Introduction
Renal cell carcinoma (RCC) represents approximately 90% of all kidney cancer cases. Approximately 58,240 cases of kidney cancer were diagnosed in 2010 in the United States, with approximately 13,040 deaths due to metastatic disease.[1] Eighty-five percent of RCC are clear cell (conventional) type, with the remaining non-clear cell tumors comprised of papillary types 1 and 2, chromophobe, collecting duct, renal medullary carcinoma, translocation, unclassified RCC, and other rare types. The current standard of care for the systemic treatment of metastatic renal cell carcinoma (mRCC) is targeted therapy with anti VEGF agents and mTOR inhibitors. [2–6] However, the majority of the patients in the pivotal trials that lead to the approval of these targeted agents had clear cell (conventional) renal cell carcinoma. Temsirolimus was approved for the treatment of patients with mRCC of any histological subtype who had poor risk features. There is no established effective therapy for good or intermediate risk non-clear cell mRCC, hence the need to explore new therapeutic approaches for this disease.
Single agent cytotoxic chemotherapy and combinations of cytotoxic agents have been extensively studied in mRCC. A phase II study of infusional fluorouracil (5-FU) in mRCC, prior to the era of targeted therapy, showed a 5% response rate and medial survival of 12 months. Gemcitabine is a pyrimidine antimetabolite which has show single agent response rates of 6% and 8% in two phase II trials.[7–9] A phase II study of infusional 5-FU with weekly gemcitabine showed an objective response rate of 17% with PFS of 28.7 weeks.[10] The combination of gemcitabine and capecitabine has been studied in patients with metastatic RCC in four single-arm phase II trials, with response rates ranging from 8% to 16%, stable disease rates ranging from 48% to 67%, and median progression-free survival ranging from 4.6 to 7.6 months.[11–14]
Pemetrexed is a more broad spectrum antifolate antineoplastic agent compared to 5FU. Pemetrexed inhibits thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase with preclinical activity against renal cell carcinoma cell lines and a partial response rate of 9% in one phase II clinical trial.[15, 16] These two cytotoxic agents have been safely combined in multiple phase II and phase III trials in advanced non-small cell lung cancer, breast cancer, bladder cancer, and pancreatic cancer.[17–20]
Herein, we report the results of a phase II trial aimed at determining the clinical activity and safety of the combination of pemetrexed and gemcitabine in patients with non-clear cell mRCC.
Patients and Methods
This is a single arm phase II trial, using the Simon two-stage design[21], to evaluate the efficacy of the combination of pemetrexed plus gemcitabine in patients with non-clear cell mRCC. The primary objective was to assess the efficacy of these agents in terms of response rate and progression free survival (PFS). The secondary objectives included safety and overall survival (OS). Target enrollment was 40 patients. Based on Bayesian probability modeling, the trial would be discontinued if there was a greater than 70% probability that the progression free survival at 16 weeks was less than 25%. An ad-hoc composite decision rule for assessing futility was planned after 20 patients were enrolled, with termination of the trial if fewer than 2 patients of 20 patients showed response.
Male and female patients >18 years of age with a histologically or cytologically confirmed advanced non-clear cell renal cell carcinoma were eligible for the study. Patients were required to have measurable disease, an ECOG performance status of 0–2, life expectancy of at least 8 weeks, and no prior chemotherapy. Patients may have received up to two prior targeted therapies and may have had surgical resection or prior radiation therapy for brain metastasis. Adequate organ and bone marrow function was required, defined as: hemoglobin ≥ 9 g/dL, absolute neutrophil count of ≥ 1500/μL, platelets ≥ 100,000/μL, serum total bilirubin ≤ 1.5 mg/dL, serum aspartate aminotransferase and alanine aminotransferase ≤ 2.5 times the upper limit of normal, and creatinine clearance of ≥ 45 mL/min (by Cockcroft-Gault method). Other exclusion criteria included pregnancy or lactation and prior malignancies unless treated and disease free for two years or non-melanoma skin cancer. Written informed consent was obtained before enrolment for all patients. The trial was approved by the institutional review board. Both pemetrexed and gemcitabine were supplied by Lilly and Co.
Prior to treatment, evaluation consisted of a complete medical history, physical exam, and laboratory testing as described, chest x-ray, computed tomography(CT) scans of the chest/abdomen/pelvis, and a CT or magnetic resonance imaging (MRI) scan of the brain.
Therapy consisted of pemetrexed 500 mg/m2 IV infusion over 10 minutes on day one followed immediately by gemcitabine 1500 mg/m2 IV over 30 minutes on day one, repeated every fourteen days. Folic acid, 350–1000 mcg po daily, was administered 5–7 days prior to the first dose of pemetrexed and continued throughout therapy and for 21 days after the last dose of pemetrexed. Vitamin B12 1000 mcg was given intramuscularly prior to first dose of pemetrexed and thereafter every 8–9 weeks while on therapy. Patients were followed with weekly complete blood count with differential and platelet count. Liver enzymes and complete chemistry profiles were monitored on an every 2 weeks basis. A minimum absolute neutrophil count of 1500 × 106/L was required to administer each cycle of therapy. Radiographic studies were repeated every 8 weeks (or four cycles of treatment) or as needed to confirm response or progression.
All patients were evaluated for response by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.[22] Toxicity was assessed using the NCI-CTC expanded Common Toxicity Criteria version 3.0. Dose reductions at 25% of the initial dosing for both gemcitabine and pemetrexed were employed for grade 3 or 4 non-hematologic toxicity, neutropenic fever requiring hospitalization, grade 4 thrombocytopenia, or a treatment delay of more than seven days for any toxicity, and up to three dose reductions were allowed. Dose reductions of 50% for both agents were employed for grade 3 or 4 mucositis.
The response rate was evaluated with a 95% exact confidence interval. The median PFS, 16-week PFS rate and median OS were estimated with 95% confidence interval using Kaplan-Meier product method. PFS was defined as the time from the date of registration to the date of progression of disease, patient withdrawal, or death. OS was defined as the time from the date of registration to the date of last follow-up or death.
Results
Between December 2005 and December 2008, 16 patients were enrolled in this study. One patient did not receive treatment. One patient received treatment with pemetrexed and gemcitabine but was subsequently found to have transitional cell carcinoma of the upper collecting system at the time of nephrectomy. Thus, a total of 14 patients were included in the efficacy analysis and a total of 15 patients were included in the toxicity analysis (excluding the patient who did not receive treatment). The characteristics of these patients are included in Table-1. Among the total of 14 patients, 5 patients were not evaluable for response (four withdrew due to unacceptable toxicity and one withdrew by personal choice). Under the intention to treat principle, all non-evaluable patients were treated as failure for the primary analysis.
The median number of cycles administered was 4 (range: 1–12 cycles). Four patients (29%) received 8 or more cycles of chemotherapy. Five patients (36%) discontinued therapy due to unacceptable toxicity. Eight patients (57%) received pemetrexed and gemcitabine in the first-line setting, with three patients (21%) and two patients (14%) receiving the combination in the second and third-line settings, respectively. Six patients (42%) received systemic therapy after they were taken off protocol, Table 2. Patients received the following other systemic therapies, either before or after treatment with pemetrexed and gemcitabine: sunitinib (n=6), sorafenib (n=4), erlotinib (n=2), temsirolimus (n=2), temsirolimus and bevacizumab (n=1), bevacizumab (n=2), gemcitabine and capecitabine (n=2), pazopanib (n=1), carboplatin and paclitaxel (n=1), doxorubicin and gemcitabine (n=1), investigational agent PX-866 (n=1).
Table 2.
Prior and subsequent therapies in study patients treated with pemetrexed and gemcitabine
| Patient | Regimen 1 | Regimen 2 | Regimen 3 | Regime 4 | Regimen 5 |
|---|---|---|---|---|---|
| 1 | Pem/Gem | ||||
| 2 | Erlotinib | Pem/Gem | Sorafinib | Everolimus | Sunitinib |
| 3 | Sorafinib | Sunitinib | Pem/Gem | ||
| 4 | Sorafinib | PemGem | Gem/Cape/Bev | Cape/Bev | Pazopanib |
| 5 | Pem/Gem | ||||
| 6 | Pem/Gem | Doxo/Gem | |||
| 7 | Pem/Gem | Cape/Bev/I-alpha | Sunitinib | Paclitaxel/Carboplatin | |
| 8 | Pem/Gem | Sunitinib | Temsirolimus | Temsirolimus, Bev | Erlotinib |
| 9 | Sorafinib | Sunitinib | Pem/Gem | Temsirolimus | PX-866 |
| 10 | Pem/Gem | ||||
| 11 | Sunitinib | Pem/Gem | |||
| 12 | Pem/Gem | ||||
| 13 | PemGem | ||||
| 14 | PemGem | Dose Dense MVAC |
PemGem= pemetrexed plus gemcitabine, Doxo/Gem= doxorubicin plus gemcitabine, Gem/Cape/Bev= gemcitabine plus capecitabine plus bevacizumab, Cape/Bev= capecitabine plus bevacizumab, Cape/Bev/I-alpha= capecitabine plus gemcitabine plus interferon alpha.
The overall response rate among all 14 patients was 6.7% (95% CI: 0.2%–31.9%). Only one patient, with histology type of upper tract transitional cell urothelial carcinoma, had a partial response. When the two patients with chromophobe histology were excluded, the overall response rate was 7.7% (95% CI: 0.2%–36%). The median PFS was 3.2 months (95% CI: 1.9–>6 months) (Figure 2). The 16-week PFS rate was 46.7% (95% CI: 19.8%–100%). The median OS was 23.2 months (95% CI: 12.9–38.1 months) (Figure 3).
Figure 2.
Kaplan-Meier estimate of progression free survival
Figure 3.
Kaplan-Meier estimate of overall survival
The safety data are summarized in Table 3. All of the 15 patients were evaluable for toxicities and 13 patients experienced grade 3 or grade 4 adverse events (87%), 7 patients (47%) experienced grade 4 toxicity. The most common grade 3 or 4 toxicities were neutropenia (53%), leukopenia (53%), and fatigue (40%). One patient developed pulmonary embolism and therapy was discontinued.
Table 3.
Summary of Grade 3 or 4 adverse events
| N (%) | |
|---|---|
| Hematologic Toxicities | |
| Leukocytopenia | 8 (53%) |
| Neutropenia | 8 (53%) |
| Anemia | 2 (13%) |
| Prolonged INR* | 1 (7%) |
| Non Hematologic Toxicities | |
| Fatigue | 6 (40%) |
| Renal Failure | 2 (13%) |
| Hyperglycemia | 1 (7%) |
| Nausea | 1 (7%) |
| Vomiting | 1 (7%) |
| Constipation | 1 (7%) |
| ALT, SGPT (serum glutamic pyruvic transaminase) | 1 (7%) |
| AST, SGOT (serum glutamic oxaloacetic transaminase) | 1 (7%) |
| Pulmonary Embolism | 1 (7%) |
| Non-neutropenic fever | 1 (7%) |
International Normalized Ratio of prothrombin time
Discussion
In this phase II study, gemcitabine and pemetrexed were administered to patients with non-clear cell mRCC to assess efficacy and safety in this patient population. The combination of gemcitabine and pemetrexed had not previously been studied in mRCC. The current standard of care for the treatment of mRCC is VEGF targeted therapy or mTOR inhibitors. The targeted agents sunitinib, pazopanib, and bevacizumab with interferon alfa are approved for frontline treatment of metastatic disease.[2, 3, 6] Sorafenib was approved for the treatment of mRCC following cytokine failure and everolimus, an oral mTOR inhibitor, was approved for the setting of TKI failure.[4, 5] These agents were studied in clear cell renal cell carcinoma. Temsirolimus is the only approved agent which was studied in patients with both clear cell and non-clear cell histology.[23]
The treatment of non clear cell mRCC has been studied in several retrospective and phase II trials to date. A retrospective study of 64 patients with non clear cell mRCC treated with cytokine therapy had a median OS of 9.4 months.[24] Another retrospective study of 53 patients treated with targeted therapy (sunitinib or sorafenib) showed median PFS of 7.6 months for papillary mRCC and 10.6 months for chromophobe mRCC.[25] A phase II trial of erlotinib in patients with papillary mRCC showed an 11% response rate and median OS of 27 months.[26] Two phase II trials of sunitinib, one trial of 23 patients with non clear cell mRCC and one trial of 61 patients with papillary mRCC, showed median PFS of 5.6 months and 2.7 months, respectively.[27, 28] Thus the treatment of non-clear cell renal cell carcinoma remains an unmet need in the management of advanced kidney cancer, unlike the advances of targeted therapy in clear cell renal cell carcinoma.
Renal cell carcinoma has traditionally been viewed as resistant to cytotoxic chemotherapy.[29] However, multiple phase I and phase II studies showed modest activity against this disease with single agent 5-FU, capecitabine, or gemcitabine- with objective response rates ranging from 5% to 8%. The combination of gemcitabine and capecitabine or infusional 5FU has shown improved response rates, with occasional durable responses.[12, 13, 30] A retrospective study of 28 patients with mRCC (35% non-clear cell histology) treated with the combination of gemcitabine, capecitabine, and bevacizumab showed a median PFS of 6.2 months and median OS of 11.7 months.[31] We chose to combine pemetrexed, a multi-enzyme inhibiting antimetabolite, with gemcitabine in an attempt to improve upon the activity of fluorouracil/gemcitabine combinations.
Pemetrexed was approved for first and second line treatment of locally advanced or metastatic non-squamous non-small cell lung cancer and malignant pleural mesothelioma, in combination with a platinum agent.[32–35] The combination of gemcitabine and pemetrexed was studied in a phase II trial of patients with locally advanced bladder cancer, showing a response rate of 28%, but with grade 3 or 4 toxicities including neutropenia (38%), febrile neutropenia (17%), sepsis (3%), anemia (19%) and thrombocytopenia (9%). In this study, gemcitabine 1250 mg/m2 was administered i.v. over 30 minutes on days 1 and 8 with pemetrexed 500 mg/m2 administered on day 1 of a 21 day cycle.[19] A phase II trial in patients with advanced non-small cell lung cancer used a 14 day schedule of pemetrexed 500 mg/m2 and gemcitabine 1500 mg/m2 administered on day 1. The response rate was 26%, and the most common grade 3 or 4 toxicities were neutropenia (47%), leukopenia (25%), fatigue (25%), and anemia (8%).[17] Another phase II trial of this combination in patients with metastatic breast cancer, showed a similar response rate but required a protocol amendment due to excessive toxicities (grade 3 or 4 neutropenia 71%) in the planned 21-day administration cycle. The most common toxicities in the amended 14 day cycle group were neutropenia (32.7%), leukopenia (13.5%) and anemia/thrombocytopenia (9.5%). A phase III trial of gemcitabine and pemetrexed versus gemcitabine in patients with locally advanced pancreatic cancer on a 21 day schedule showed a similar toxicity profile.[20] A phase II trial conducted in thirty-nine patients with mRCC treated with single-agent pemetrexed showed an overall partial response rate of 9% and median time to progression of 10.5 months. [16]
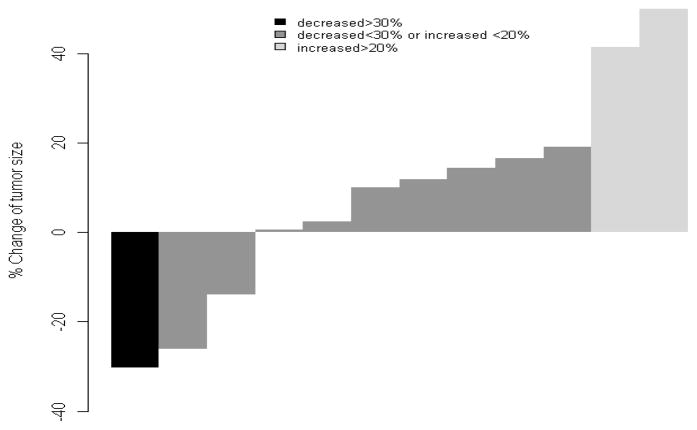
In this study cohort, we observed an overall response rate of 6.7% (95% exact CI: 0.2%–31.9%) by RECIST criteria. The only patient in the cohort with a partial response to therapy did not have renal cell carcinoma, but was found to have upper tract transitional cell urothelial carcinoma upon nephrectomy following 8 cycles of pemetrexed and gemcitabine. Two additional patients had tumor shrinkage less than 30% as the best response; one patient with collecting duct carcinoma (26% reduction) and one patient with papillary renal cell carcinoma (14% reduction). However, seven patients (50%) had progression of disease as their best response and the median PFS was 3.2 months (95% CI: 1.9–>6 months) for the cohort. The median OS was 23.2 months, however most patient to received other therapies prior to receiving or beyond progression on pemetrexed and gemcitabine. The study was terminated early based on lack of response and clinical benefit. The toxicity profile in this cohort was more intense that described in the previously mentioned studies combining these two agents. We observed that thirteen patients (87%) developed at least one grade 3 or 4 toxicity. Four patients (29%) were taken off study due to excessive toxicity. The most common grade 3 or 4 toxicities were neutropenia (53%), leukopenia (53%), anemia (13%), fatigue (40%), and renal failure (13%). There were no deaths on therapy due to toxicity and there were no cases of neutropenic fever.
Conclusions
In summary, patients with non clear cell mRCC should still be enrolled on clinical trials, as there is no established effective therapy. The combination of pemetrexed and gemcitabine did not show clinically meaningful activity in patients with metastatic non clear cell renal cell carcinoma. Further investigation of this chemotherapy combination in non-clear cell renal cell carcinoma is not warranted. (words 2387)
Figure 1.
Waterfall plot of best response
Table 1.
Patient Characteristics
| (n=14) | |
|---|---|
| Age | |
| Median (Range) | 56.5 (18–70) |
| Gender | |
| Male | 11 (78.57%) |
| Female | 3 (21.43%) |
| Performance Status (ECOG) | |
| 0 | 4(29%) |
| 1 | 8(57%) |
| 2 | 2(14%) |
| Histology | |
| Papillary | 5 (36%) |
| Collecting Duct Carcinoma | 3 (22%) |
| Chromophobe | 2 (14%) |
| Unclassified | 2 (14%) |
| Translocation carcinoma | 2 (14%) |
| Sarcomatoid dedifferentiation | |
| No | 13 (93%) |
| Yes | 1 (7%) |
| Histologic Grade (Furhman) | |
| 1 | 0 |
| 2 | 1 (7%) |
| 3 | 5 (36%) |
| 4 | 3 (22%) |
| High Grade | 1 (7%) |
| Unknown | 4 (28%) |
| Clinical T stage | |
| T1a | 2(14%) |
| T1b | 1(7%) |
| T2 | 3(22%) |
| T3a | 6(43%) |
| T3b | 1(7%) |
| T4 | 1(74%) |
| Clinical Nodal Status | |
| N1 | 2(14%) |
| N2 | 4(28%) |
| Metastatic Sites | |
| Retroperitoneal Lymph Nodes | 9 (64%) |
| Supra-diaphragmatic Lymph Nodes | 9 (64%) |
| Renal Fossa | 5 (36%) |
| Lung | 5 (36%) |
| Bone | 5 (36%) |
| Liver | 4 (28%) |
| Adrenals | 4 (28%) |
| Spleen | 1 (7%) |
| Contralateral Kidney | 1 (7%) |
| Number of involved sites | |
| 1 | 0 |
| 2 | 3 (22%) |
| 3 | 5 (36%) |
| 4 | 4 (28%) |
| 5 | 2 (14%) |
Acknowledgments
This study was funded by Eli Lily and Company, Indianapolis, Indiana, USA.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Society AC. American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; American Cancer Society; 2009. http://www.cancer.org/acs/groups/content/@nho/documents/document/500809webpdf.pdf. [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007 Jan 11;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009 Aug 1;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine. 2007 Jan 11;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007 Dec 22;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Rohde D, De Mulder PH, Weissbach L, et al. Experimental and clinical efficacy of 2′, 2′-difluorodeoxycytidine (gemcitabine) against renal cell carcinoma. Oncology. 1996 Nov-Dec;53(6):476–81. doi: 10.1159/000227623. [DOI] [PubMed] [Google Scholar]
- 8.Mertens WC, Eisenhauer EA, Moore M, et al. Gemcitabine in advanced renal cell carcinoma. A phase II study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 1993 Apr;4(4):331–2. doi: 10.1093/oxfordjournals.annonc.a058494. [DOI] [PubMed] [Google Scholar]
- 9.De Mulder PH, Weissbach L, Jakse G, et al. Gemcitabine: a phase II study in patients with advanced renal cancer. Cancer chemotherapy and pharmacology. 1996;37(5):491–5. doi: 10.1007/s002800050417. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000 Jun;18(12):2419–26. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 11.Waters JS, Moss C, Pyle L, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. British journal of cancer. 2004 Nov 15;91(10):1763–8. doi: 10.1038/sj.bjc.6602209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadler WM, Halabi S, Rini B, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: a report of Cancer and Leukemia Group B protocol 90008. Cancer. 2006 Sep 15;107(6):1273–9. doi: 10.1002/cncr.22117. [DOI] [PubMed] [Google Scholar]
- 13.Tannir NM, Thall PF, Ng CS, et al. A phase II trial of gemcitabine plus capecitabine for metastatic renal cell cancer previously treated with immunotherapy and targeted agents. The Journal of urology. 2008 Sep;180(3):867–72. doi: 10.1016/j.juro.2008.05.017. discussion 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Veldhuizen PJ, Hussey M, Lara PN, Jr, et al. A Phase II Study of Gemcitabine and Capecitabine in Patients With Advanced Renal Cell Cancer: Southwest Oncology Group Study S0312. American journal of clinical oncology. 2009 May 29; doi: 10.1097/COC.0b013e3181925176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adjei AA. Pemetrexed: a multitargeted antifolate agent with promising activity in solid tumors. Ann Oncol. 2000 Oct;11(10):1335–41. doi: 10.1023/a:1008379101017. [DOI] [PubMed] [Google Scholar]
- 16.Thodtmann R, Sauter T, Weinknecht S, et al. A phase II trial of pemetrexed in patients with metastatic renal cancer. Investigational new drugs. 2003 Aug;21(3):353–8. doi: 10.1023/a:1025480914273. [DOI] [PubMed] [Google Scholar]
- 17.Spigel DR, Hainsworth JD, Barton JH, et al. Phase II study of biweekly pemetrexed and gemcitabine in patients with previously untreated advanced non-small cell lung cancer. J Thorac Oncol. 2010 Jun;5(6):841–5. doi: 10.1097/JTO.0b013e3181d737e3. [DOI] [PubMed] [Google Scholar]
- 18.Pippen J, Elias AD, Neubauer M, et al. A phase II trial of pemetrexed and gemcitabine in patients with metastatic breast cancer who have received prior taxane therapy. Clinical breast cancer. 2010 Apr;10(2):148–53. doi: 10.3816/CBC.2010.n.020. [DOI] [PubMed] [Google Scholar]
- 19.von der Maase H, Lehmann J, Gravis G, et al. A phase II trial of pemetrexed plus gemcitabine in locally advanced and/or metastatic transitional cell carcinoma of the urothelium. Ann Oncol. 2006 Oct;17(10):1533–8. doi: 10.1093/annonc/mdl154. [DOI] [PubMed] [Google Scholar]
- 20.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005 Oct;16(10):1639–45. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 21.Cheung YK, Thall PF. Monitoring the rates of composite events with censored data in phase II clinical trials. Biometrics. 2002 Mar;58(1):89–97. doi: 10.1111/j.0006-341x.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000 Feb 2;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007 May 31;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002 May 1;20(9):2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 25.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008 Jan 1;26(1):127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol. 2009 Dec 1;27(34):5788–93. doi: 10.1200/JCO.2008.18.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Investigational new drugs. 2010 Aug 14; doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plimack ER, Jonasch E, Bekele BN, et al. Sunitinib in papillary renal cell carcinoma (pRCC): Results from a single-arm phase II study. Journal of Clinical Oncology 2010 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2010;28(15_suppl May 20 Supplement):4604. [Google Scholar]
- 29.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. The Journal of urology. 2000 Feb;163(2):408–17. [PubMed] [Google Scholar]
- 30.Richey SL, Ng C, Lim ZD, et al. Durable Remission of Metastatic Renal Cell Carcinoma With Gemcitabine and Capecitabine After Failure of Targeted Therapy. J Clin Oncol. 2010 Dec 20; doi: 10.1200/JCO.2010.31.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonasch E, Lal LS, Atkinson BJ, et al. Treatment of metastatic renal carcinoma patients with the combination of gemcitabine, capecitabine and bevacizumab at a tertiary cancer centre. BJU international. 2011 Mar;107(5):741–7. doi: 10.1111/j.1464-410X.2010.09626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008 Jul 20;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 33.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004 May 1;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 34.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003 Jul 15;21(14):2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 35.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009 Oct 24;374(9699):1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]