Abstract
Dynamic monitoring of stimulus-evoked inner neural response is important for functional validation of stimulation protocols of retinal prosthetic devices. In this paper, we demonstrate label-free intrinsic optical signal (IOS) imaging of electrically stimulated inner neural response in freshly isolated mouse retinas. While single-pulse stimulation evoked rapid IOS within 20 ms, pulse-train stimulation indicated that the fast IOS response can follow frequency stimulation up to at least 8 Hz. Fast IOS imaging promises a noninvasive method for high resolution examination of electrically evoked retinal response, without artifact contamination of electrical stimulus.
Keywords: intrinsic optical signal, retinal function, retinal prosthesis, ganglion cell
Retinal photoreceptors play an essential role in the process of capturing photons and converting optical energy into biochemical signals used for visual information processing. Eye diseases, such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), may produce inevitable photoreceptor degradations which can lead to severe vision loss or even permanent blindness. It is well known that a large population of inner retinal neurons such as bipolar cells (BC) and retinal ganglion cells (RGC) may remain functional integrity for many years after the development of AMD [1,2] or RP [3,4]. Therefore, direct stimulation of inner retinal neurons provides the hope to restore vision from AMD or RP associated blindness [5–7]. Most emerging visual prosthetic devices employ electrical current [8], through implanted microelectrodes, to stimulate inner retinal neurons. Therefore, reliable evaluation of electrically evoked neural responses is essential for functional validation and optimization of the visual prosthetics. Light evoked responses of inner retinal neurons, such as RGC, can be accurately recorded by conventional electrophysiological methods [9]. However, electrophysiological measurement of electrically evoked responses can be contaminated by electrical stimulus artifact. Moreover, in vivo implementation of electrophysiological examination is complicated. An optical recording method, which is free from potential contamination of electrical stimulus artifact, may provide a useful alternative for functional monitoring of localized responses to electrical stimulation.
Stimulus-evoked intrinsic optical signals (IOSs), free from potential contamination of electrical stimulus artifact, may provide a useful alternative for functional monitoring of localized responses to electrical stimulation. In principle, transient IOSs can be a result of both stimulus-evoked retinal neural activity and corresponding hemodynamic and metabolic changes in response to a light or electrical stimulation. While hemodynamic and metabolic changes [10,11] associated slow IOSs can provide useful information for the assessment of the retinal well-being, they are relatively slow and cannot directly track fast neural activities. Stimulus-evoked fast IOSs, which have a time course comparable to retinal electrophysiological response, have been imaged in isolated retinal tissues [12,13] and intact animals [14]. In this paper, we demonstrate the feasibility of using fast IOSs to monitor electrically activated regions. In order to reduce the potential contamination of metabolic changes associated with slow IOS responses in the activated retina, dynamic differential data processing was employed [12] to reconstruct IOS images shown in this article.
Isolated mouse (Mus musculus) retinal slices were used to validate dynamic IOS imaging of localized activities evoked by the electrical stimulation. Without the complications of other ocular tissues and eye movements, isolated mouse retinas provide a simple preparation for functional test of electrical stimulation. The experimental procedures were approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham. During the sample preparation, mice were rapidly euthanized by means of cervical dislocation, and the retina was carefully isolated from the enucleated eyeball in Ames media. Then, a retinal patch (~3mm × 3 mm) was laid flat over a small piece of nitrocellulose filter (Millipore Co, 0.8 µm) with its posterior side (i.e. photoreceptors) facing upwards. A tissue slicer (Stoelting Co.) was used to slice the flat-mounted retina into thin slices of ~200 µm thicknesses. Finally, the sliced retinas were rotated 90° and transferred to a recording chamber for IOS imaging. During the recording, the sample was continuously super-fused (~2 ml/min) with oxygenated bicarbonate-buffered Ames medium, maintained at pH 7.4 and 33–37°C.
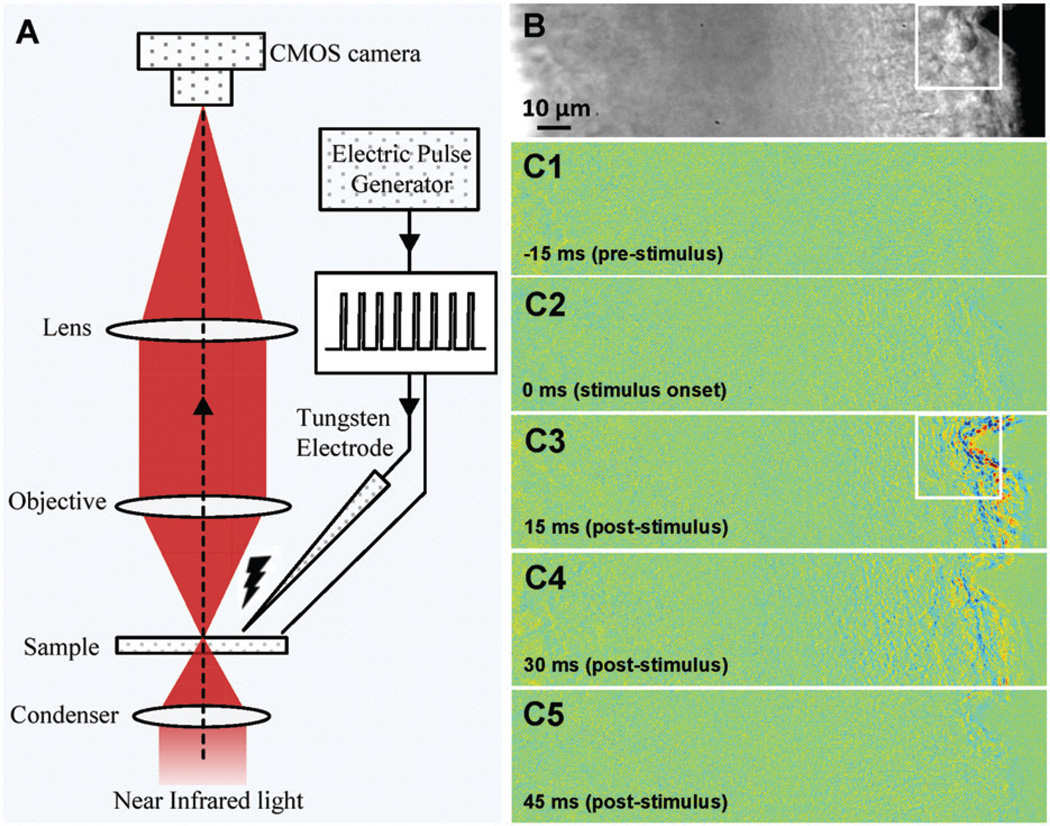
Figure 1(A) illustrates the schematic diagram of the experimental setup. Details of the optical imaging system have been reported in previous publications [12,13]. Equipped with a high-speed CMOS camera (PCO1200, PCO AG, Kelheim, Germany), the imaging system can support millisecond temporal resolution and sub-cellular spatial resolution for dynamic monitoring of fast IOSs associated with electrically stimulated localized activities. A NIR light was used IOS recording. The NIR light was produced by a 12V, 100W halogen lamp (PHILIPS7724) with a band-pass filter (wavelength band: 800–1000 nm). A tungsten electrode (FHC Inc.) was used for electrical stimulation of the retina (Figure 1B). The microelectrode tips were epoxylite insulated and had an impedance of 50–100 kΩ. An electric pulse generator (Isostim A320, WPI Inc.) was used to generate stimuli in both single and continuous pulse (train) modes.
Figure 1.
(A) Schematic diagram of the IOS imaging setup. During the measurement, the retinal slice was continuously illuminated by the NIR light for IOS recording. An electrical stimulator was used to generate single or continuous current pulses that could be delivered onto a retina though the tungsten microelectrode. (B) Representative image of retinal slices. (C1–C5) Representative IOS images recorded at the time of −15 ms (pre-stimulus), 0ms (stimulus onset), 15 ms, 30 ms and 45 ms (post-stimulus), respectively. (The color version of this figure is included in the online version of the journal.)
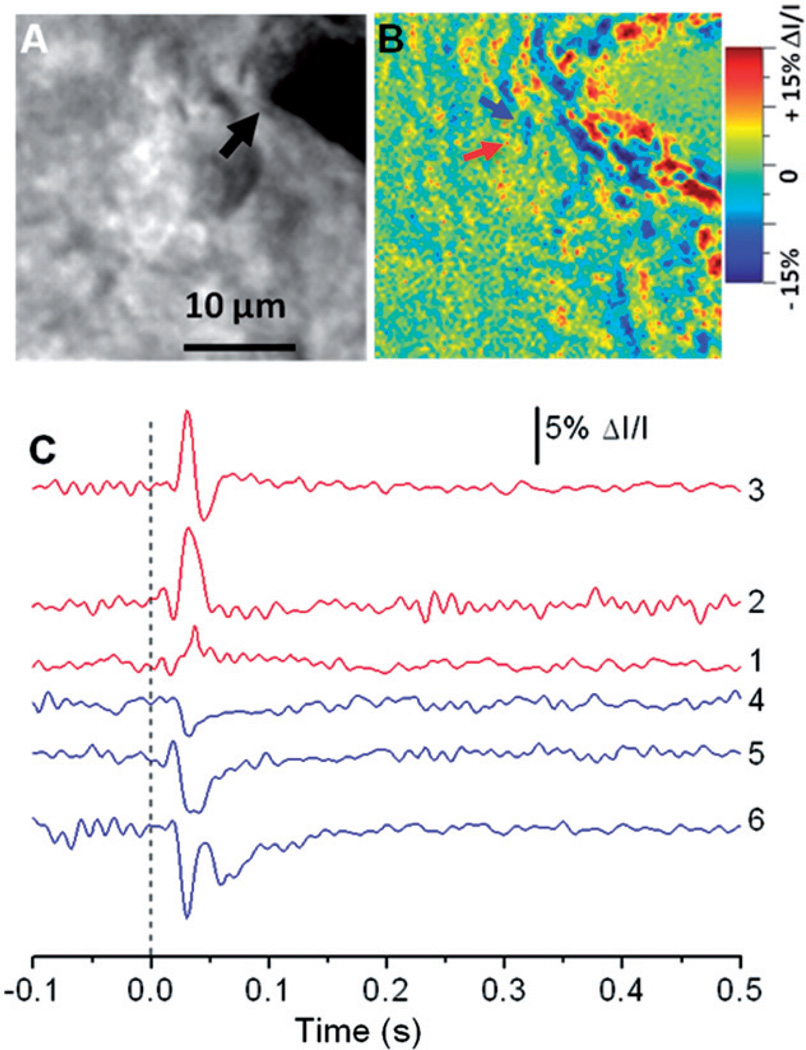
Figure 2 represents IOS responses in the retina slice activated by single pulse stimulation. The black arrowhead (in Figure 2(A)) indicates the electrode for retinal stimulation. Rapid IOS images revealed transient optical response at the stimulus activated inner retinal region, i.e. ganglion cell layer. As shown in Figure 2(B), both positive and negative IOS responses were observed. Figure 2(C) illustrates localized positive and negative IOS responses of representative retinal areas (arrowhead in Figure 2(B)) activated by three (0.05 mA, 0.1 mA, 0.2 mA) single-pulse stimulations (stimuli). As shown in Figure 2(C), the fast IOSs were tightly correlated with the electrical stimulation. Unambiguous optical response occurred almost immediately (20–30 ms) after the stimulus onset.
Figure 2.
(A) Enlarged image of the white square area in Figure 1(B). The black arrow points to the metal electrode tip. (B) Enlarged image of the white square area in Figure 1(C3). (C) IOS dynamics of two localized (5 × 5 pixels) areas indicated by the red and blue arrowheads in (B). Curves 1–3 (red) represent the dynamic positive IOS changes under 0.05 mA, 0.1mA and 0.2mA stimuli, respectively, and curves 4–6 (blue) represent the dynamic negative IOS changes under 0.05 mA, 0.1mA and 0.2mA stimuli, respectively. Vertical line indicates the delivery of the stimulus. (The color version of this figure is included in the online version of the journal.)
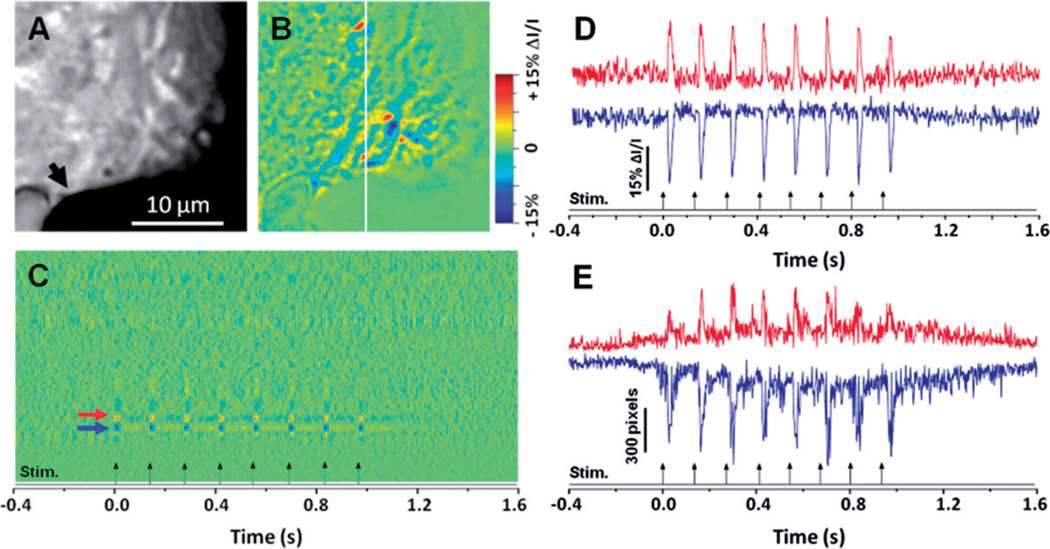
In order to test repeatability of the fast IOSs, IOS imaging of the retinal slice with pulse train stimuli were conducted. 8Hz pulse trains were applied to evoke the fast IOS response. Representative raw and IOS images are shown in Figures 3(A) and 3(B), respectively; the black arrow head in Figure 3(A) represents the location of the stimulating electrode. A line area (white line in Figure 3(B)) was selected to produce a dynamic spatiotemporal image of the IOSs in Figure 3(C), which displays the dynamic IOS changes of the same line area throughout the entire recording period. Figure 3(D) shows representative IOS responses of local retinal areas (arrowheads in Figure 3(C)), and the examination at sub-cellular level further confirmed that transient IOSs are tightly correlated with each pulse onset. Each dynamic IOS peak consistently tracked with the stimulus pulse within 20 ms. Figure 3(E) shows statistics of activated pixel numbers, with positive and negative IOS responses, in the image area. During data processing, standard deviation (σ) of each pixel in the pre-stimulus baseline images was calculated first. For each post-stimulus IOS image, if a pixel value is larger than the pre-stimulus average plus 3σ, the pixel will be considered as positive response. If a pixel value is smaller than the pre-stimulus average minus 3σ, the pixel will be considered negative response. As shown in Figure 3(E), transient IOS peaks were observed corresponding to individual current pulses of the stimulation.
Figure 3.
Raw (A) and IOS (B) images of a retinal slice with pulse train stimulation. The black arrow points to the metal electrode tip. (C) Spatiotemporal image produced from line area (white line in (B)). (D) Dynamic differential IOS of local retinal area (5 × 5 pixels) indicated by the red and blue arrows in (C). Red curve represents the positive signals and blue curve represents the negative signals. (E) Dynamic differential IOS curves produced from the entire frame area shown in (A). Red curve represents the positive signals and blue curve represents the negative signals. (The color version of this figure is included in the online version of the journal.)
In summary, we have demonstrated the fast IOS imaging of localized retinal responses in electrically stimulated retinal slices. High spatial and temporal resolution images of the mouse retinal slices revealed consistent patterns of IOSs at the stimulated regions. Both single pulse and pulse train stimuli were utilized for localized electrical stimulation of the retina.
Ultra-fast high resolution imaging of the retinal slice under single pulse stimulus revealed transient IOSs that were tightly correlated with the electrical stimulus. With an onset time of less than 30 ms and a recovery time of less than 50 ms, the temporal courses of the fast IOS responses displayed a spike-like pattern in response to the stimulus (Figure 2). In addition, the results showed that fast IOSs are capable of tracking pulse train stimulation of at least 8 Hz. The robustness and repeatability of the fast IOSs in response to high frequency pulse train stimulation may provide a practical strategy for functional evaluation and optimization of the electrical stimulation protocols of retinal prosthetics.
Both positive (increasing) and negative (decreasing) IOSs were observed with complex spatiotemporal patterns. In principle, both cellular and molecular changes may occur during retinal activation correlated with the electrical stimulation. With visible light flash stimulation, previous studies have detected IOSs from inner retinal [13,15] and individual ganglion cells [16]. Early studies with other neural tissues have suggested that several stimulation associated processes [17], such as reorientation of membrane proteins and phospholipids, and refractive index change of neural tissues, may contribute to the IOSs. Water influx in response to ionic currents through gated channels during depolarization causes cellular swelling that may produce changes in tissue light scattering [18–20] as well as polarization IOS changes [21]. We anticipate that further studies with the fast IOS imaging method would shed more light into characterizing the neural behavior of inner retinal cells. Fast IOS imaging may lead to a new, non-invasive methodology for in-depth evaluation of the inner retinal neuron responses to electrical stimulation, and detailed understanding of the retinal neural responses may promise better visual prosthesis development. In turn, this could provide more efficient vision restoration in blind patients that developed AMD, retinitis pigmentosa (RP), and other eye diseases that can produce functional damages of retinal photoreceptors.
Acknowledgments
This research is supported partially by Dana Foundation (Brain and Immuno-Imaging Grant program), Eyesight Foundation of Alabama, NSF CBET-1055889, NIH R21 RR025788, NIH R21 EB012264.
References
- 1.Kim SY, Sadda S, Humayun MS, de Juan E, Jr, Melia BM, Green WR. Retina. 2002;22:464–470. doi: 10.1097/00006982-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros NE, Curcio CA. Invest. Ophthalmol. Visual Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- 3.Santos A, Humayun MS, de Juan E, Jr, Greenburg RJ, Marsh MJ, Klock IB, Milam AH. Arch. Ophthalmol. 1997;115:511–515. doi: 10.1001/archopht.1997.01100150513011. [DOI] [PubMed] [Google Scholar]
- 4.Stone JL, Barlow WE, Humayun MS, de Juan E, Jr, Milam AH. Arch. Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 5.Weiland JD, Liu W, Humayun MS. Annu. Rev. Biomed. Eng. 2005;7:361–401. doi: 10.1146/annurev.bioeng.7.060804.100435. [DOI] [PubMed] [Google Scholar]
- 6.Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G, de Juan E. Vision Res. 2003;43:2573–2581. doi: 10.1016/s0042-6989(03)00457-7. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Invest. Ophthalmol. Visual Sci. 2003;44:5362–5369. doi: 10.1167/iovs.02-0817. [DOI] [PubMed] [Google Scholar]
- 8.Dobelle WH, Mladejovsky MG. J. Physiol. 1974;243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutter EE, Tran D. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DA, Krupsky S, Pollack A, Aloni E, Belkin M, Vanzetta I, Rosner M, Grinvald A. Ophthalmic Surg. Lasers Imaging. 2005;36:57–66. [PubMed] [Google Scholar]
- 11.Abramoff MD, Kwon YH, Ts’o D, Soliz P, Zimmerman B, Pokorny J, Kardon R. Invest. Ophthalmol. Visual Sci. 2006;47:715–721. doi: 10.1167/iovs.05-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YG, Zhang QX, Liu L, Amthor F, Yao XC. Opt. Express. 2010;18:7210–7218. doi: 10.1364/OE.18.007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao XC, Zhao YB. Opt. Express. 2008;16:12446–12459. doi: 10.1364/oe.16.012446. [DOI] [PubMed] [Google Scholar]
- 14.Zhang QX, Lu RW, Li YG, Yao XC. Opt. Lett. 2011;36:4692–4694. doi: 10.1364/OL.36.004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawis SM, Rossetto M. Visual Neurosci. 1993;10:687–692. doi: 10.1017/s0952523800005381. [DOI] [PubMed] [Google Scholar]
- 16.Li YC, Strang C, Amthor F, Liu L, Li YG, Zhang QX, Keyser K, Yao XC. Opt. Lett. 2010;35:1810–1812. doi: 10.1364/OL.35.001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foust AJ, Rector DM. Neuroscience. 2007;145:887–899. doi: 10.1016/j.neuroscience.2006.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao XC, Rector DM, George JS. Appl. Opt. 2003;42:2972–2978. doi: 10.1364/ao.42.002972. [DOI] [PubMed] [Google Scholar]
- 19.Tasaki I, Byrne PM. Biochem. Biophys. Res. Commun. 1992;188:559–564. doi: 10.1016/0006-291x(92)91092-5. [DOI] [PubMed] [Google Scholar]
- 20.Li YC, Cui WX, Wang XJ, Amthor F, Lu RW, Thompson A, Yao XC. Opt. Express. 2011;19:99–106. doi: 10.1364/OE.19.000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao XC, Foust A, Rector DM, Barrowes B, George JS. Biophys. J. 2005;88:4170–4177. doi: 10.1529/biophysj.104.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]