Abstract
Sunitinib malate is a multi-targeted tyrosine-kinase inhibitor, currently in clinical trials for glioma. Previously developed methods for preclinical studies in species such as mice have either employed high-performance liquid chromatography (HPLC) or did not describe a detailed analytical method, which could be employed by other preclinical laboratories. In this paper, we have developed and validated a simple, sensitive high-performance liquid chromatography tandem mass-spectrometric method (LC–MS/MS) for the determination of sunitinib concentration in mouse plasma and brain tissue homogenate using dasatinib-free base as the internal standard. A single step liquid–liquid extraction method was used for both the matrices. Since sunitinib exhibits light-induced E/Z isomerism, all sample preparation was done in light-protected conditions. Separation was performed on a ZORBAX Eclipse XDB C18 column 4.6 × 50 mm, 1.8 μm. The mobile phase consisted of 20 mM ammonium formate (with 0.1 % formic acid): acetonitrile (70:30, v/v) pumped isocratically at a flow rate of 0.25 mL min−1 with a total run-time of 13 min. The retention times of sunitinib and dasatinib were 7.8 and 5.5 min, respectively. The calibration curve was linear over the range from 1.95 to 500 ng mL−1 in both plasma and brain tissue homogenate with 1.95 ng mL−1 as the lower limit of quantification (LLOQ) for both the matrices. Inter- and intra-day accuracy and precision was <15 % for low QC, med QC and high QC and <20 % for LLOQ. The method was applied to a pharmacokinetic study in FVB wild-type mice to determine the plasma and brain concentrations after a single oral sunitinib malate dose of 20 mg kg−1.
Keywords: Column liquid chromatography, LC–MS/MS, Sunitinib, Angiogenesis, Mouse plasma and brain homogenate
Introduction
Glioma is a solid neoplasm that originates in the glial cells of the brain. However, drug therapy for diseases of the brain is limited by the presence of the blood brain barrier (BBB) [1]. The first step in defining the distribution of a drug to any target organ is via pharmacokinetic studies done in preclinical species. Though the FDA recommends preclinical studies to be done in a variety of rodent and non-rodent species, the mouse remains one of the most studied species.
Sunitinib malate (N-(2-diethylaminoethyl)-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, SU011248) is an orally active multi-targeted tyrosine-kinase inhibitor (TKI) with potent anti-angiogenic and antitumor activity. In addition, it also inhibits other tumor growth pathway receptors such as PDGFR-α and -β, stem cell factor receptor (c-KIT), basic fibroblast-growth factor receptor (bFGF), FMS-related tyrosine-kinase 3 (FLT-3), a proto-oncogene RET and colony stimulating factor receptor (CSF 1-R) [2]. Its potent antitumor activity has been reported both in vitro and in vivo in several tumor cell lines and preclinical xenograft models, including brain tumor models [2, 3]. The FDA approved sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors in May 2006 [4]. Currently, there are many ongoing clinical trials to test the efficacy of sunitinib in glioma, a primary brain tumor, as a single agent and as a combination with other anti-cancer drugs. However, due to the anatomical structure of the blood–brain barrier, the brain delivery of sunitinib is limited and this could potentially impact its pharmacological action in glioma patients.
Sunitinib is an analog of SU5416 (semaxinib) and exhibits light-induced isomerization due to the presence of a double bond between 2-oxindol and the pyrrole ring. Rotation around this double bond leads to the existence of two isomers, the E-isomer and Z-isomer [9]. The powder form exists as the Z-isomer, which is also the thermodynamically stable form of the drug. However, when put into solution in light, it isomerizes and forms the E-isomer, which is an inactive form of sunitinib [5]. Furthermore, the E-isomer is not available as an analytical standard, and therefore a method for quantifying it cannot be developed. The amount of conversion of Z- to E-isomer depends upon how long the sample solution has been exposed to the light. Sunitinib is an extensively studied drug with regard to development of an assay method but most of these methods are deficient in some respect related to light-sensitivity, the type of method employed (HPLC vs. LC–MS/MS) or information with regard to its stability under laboratory conditions or the effect of matrix employed. Gotze et al. [6] reported that the formation ratio of E:Z was 1:4 and used the second peak (Z-isomer) for quantitation while Haouala et al. [7] reported ratio of 1:2 and used summation of both the peak areas for calibration purposes. Such variability in results suggests that the isomer ratio is dependent on many factors in addition to the duration of time the samples have been exposed to light. Several papers have been published on the LC–MS/MS method validation of sunitinib in human plasma [5–11], but have failed to mention the light-sensitive phenomenon [12]. Baratte et al. reported an analytical method for sunitinib and its metabolite in several tissues, including the brain. However, this method employed monkey tissues, which is not a routinely used laboratory animal [13]. Earlier attempts have been made to develop a LC–MS/MS assay for sunitinib where samples have been prepared under light-protected conditions. Zhou et al. [14] reported a LC–MS/MS method for determination of sunitinib in mouse plasma and normal and tumor brain tissue. However, their report did not discuss the stability of sunitinib under laboratory conditions such as sample preparation time stability (bench top) or long-term stability for samples frozen from pharmacokinetic studies. In addition, their report also lacked any mention of the effect of matrix on the extraction of sunitinib. Considering these deficiencies in published literature, there is a need to develop a stable and a reliable analytical method for preclinical purposes in an animal that is routinely used for brain distribution studies. Thus, the main purpose of the current study was to develop and validate the LC–MS/MS method in mouse plasma and brain homogenate, which could effectively describe preclinical systemic disposition and brain distribution of sunitinib. To minimize the E-to-Z isomerism, all the experiments in this study were performed in strict light-protected conditions.
Materials and Methods
Chemicals
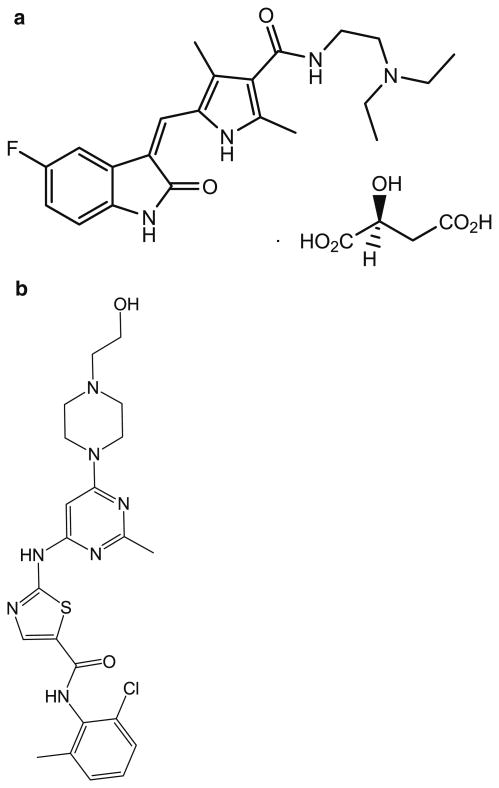
Sunitinib malate salt (Fig. 1a) and internal standard dasatinib (Fig. 1b; IS) were purchased from LC laboratories (Woburn, MA, USA). Acetonitrile (ACN), methanol (MeOH), ethyl acetate, and dimethyl sulfoxide (DMSO) were procured from Fisher Scientific (Fair Lawn, NJ, USA). Ammonium formate, formic acid, bovine serum albumin (BSA; >96 % pure from agarose gel electrophoresis) were obtained from Sigma Aldrich (St. Louis, MO, USA). Drug-free mouse plasma was obtained from Valley Biomedicals (Catalog # AP3054, Winchester, VA, USA). The mobile phase was vacuum-filtered through a 0.45-μm filter (Millipore, Milford, MA, USA).
Fig. 1. Chemical structures of a sunitinib and b internal standard, dasatinib.
Preparation of Stock Solution, Calibration Standards and Quality Controls
All preparation was done in light-protected conditions. Individual stock solutions of sunitinib malate (in black polypropylene tubes) and the IS were prepared at a concentration of 1 mg mL−1 in DMSO.
Dilutions from the stock solutions were made in methanol to yield a working stock solution of 100 μg mL−1 to then generate two 10 μg mL−1 working solutions of sunitinib malate and one 10 μg mL−1 working solution of the IS. One 10 μg mL−1 working solution of sunitinib malate was used to prepare a series of eight non-zero calibration standards ranging from 1.95 to 500 ng mL−1 in amber-colored glass vials. 10 μg mL−1 working solution of IS was diluted to get a final working concentration of 2 μg mL−1. The stock solution, sub-stocks, and the calibration standards were stored in the dark at −80 °C.
A separate 10 μg mL−1 working solution of sunitinib was used to obtain 1 μg mL−1 sub-stock solution. Quality control samples were prepared independently from this sub-stock solution in order to obtain 250 ng mL−1 (High QC; HQC), 62.5 ng mL−1 (Medium QC; MQC), 3.9 ng mL−1 (Low QC; LQC) and 1.95 ng mL−1 (lower limit of quantification; LLOQ). The QC samples were stored in the dark at −80 °C.
Sample Preparation
The blank brain homogenate was prepared by homogenizing drug-free mouse brains using a tissue homogenizer (PowerGen 125, Fisher Scientific, Pittsburgh, PA, USA) and diluting it with 3 volumes of ice-cold 5 % (w/v) BSA in phosphate-buffer saline (PBS) solution. Drug-free plasma was commercially obtained (see “Materials and Methods”).
Microcentrifuge tubes containing 100 μL of the IS and 100 μL of each calibration standard prepared in triplicate or the QC samples prepared in six replicates were dried under a gentle stream of nitrogen. Then, 100 μL of drug-free plasma or 200 μL of blank brain homogenate was added. This was followed by a single step liquid–liquid extraction, by adding 1 mL of ice-cold ethyl acetate and the tubes were vigorously shaken on a vortex-mixer for 10 min, followed by centrifugation at 7,500 rpm for 15 min at 4 °C (SORVALL LEGEND RT, Kendro). Eight hundred microliters of the supernatant consisting of the organic solvent was transferred to a fresh set of polypropylene micro centrifuge tubes and dried under nitrogen.
The dried samples were reconstituted in 100 μL of the mobile phase (30:70:0.1, ammonium formate:acetonitrile:formic acid, %v/v) and transferred to the auto-sampler inserts in amber-colored glass vials for injection. A volume of 5 μL of each sample was injected for analysis by LC–MS/MS.
Instrumentation and Mass-Spectrometric Conditions
The LC system consisted of an Agilent 1200 series binary pump (Santa Clara, CA, USA) and analytical separation was achieved using a ZORBAX Eclipse XDB C18 column 4.6 × 50 mm, 1.8 μm (Agilent Technologies, Santa Clara, USA). The mobile phase consisted of 20 mM ammonium formate buffer (containing 0.1 % formic acid) and acetonitrile (70:30; v/v) and was delivered at a flow rate of 0.25 mL min−1 and the total run-time was 13 min.
A TSQ Quantum triple quadrupole mass spectrometer (Thermo Finnigan, San Jose, CA, USA) using selected reaction monitoring (SRM) mode and an electrospray ionization source (ESI) in positive ion mode was utilized to obtain mass spectra at a voltage of 4,500 V. The sheath gas pressure and auxillary gas pressure was maintained at 50 and 20 arbitrary units, respectively. The capillary temperature was maintained at 300 °C throughout the run. The collision energy for the analyte and IS was 28 and 16 eV, respectively. Data acquisition and analysis were performed using Xcalibur software, Version 2.0.7. The precursor to product ion transition (Q1 → Q3) for quantitation (m/z) of sunitinib and dasatinib were programmed in the spectrometer at (399.26 → 282.98) and (488.00 → 401.03), respectively, to obtain the optimum parameters.
Calibration Curve
The linear calibration curve of sunitinib was estimated using the peak area ratio of the analyte to the IS, employing a weighting factor of 1/y2 (where y = peak area ratio). Parameters obtained from the calibration curve were used to determine the concentration of the unknown samples by back-calculation.
Method Validation: Assay Characteristics
The developed method was validated for accuracy, precision, stability, linearity, matrix effect, and extraction recovery.
Inter-Assay and Intra-Assay Variability
Method validation for accuracy and precision in the mouse plasma and brain was performed on three separate days as three batches. Each batch comprised of three replicates of eight non-zero calibration standards and six replicates of each QC sample, including the LLOQ. Inter- and intra-day accuracy and precision was determined by obtaining the plasma and brain concentrations of sunitinib malate and calculating the relative standard error (%RE) and percentage coefficient of variation (%CV).
Limit of Quantification
According to the FDA guidance for bioanalytical method validation [15], the LLOQ is the lowest calibration standard and is selected on the basis that the variability in the accuracy and precision should be less than 20 % and the signal-to-noise ratio greater than 5. The signal-to-noise ratio is obtained from the peak area ratio of the LLOQ and the background noise obtained from drug-free plasma and brain homogenate run in the same time window.
Matrix Effects (Ionization Efficiency)
The effect of matrix interference caused by endogenously present substances in plasma and brain homogenate on the ionization efficiency was evaluated by determining the ratio as [(ratio of absolute peak area of post-extracted spiked sample/absolute peak area of non-extracted samples reconstituted in the mobile phase)−1] × 100. Matrix effect on the ionization efficiency of the IS was also determined in a similar way [16].
The post-extracted spiked samples were prepared as follows: three replicates (N = 3 each matrix) of 100 μL of the drug-free plasma and 200 μL of drug-free brain homogenate were extracted by liquid–liquid extraction using 1 mL of ice-cold ethyl acetate. 800 μL of the supernatant was transferred to a microcentrifuge tube and dried under nitrogen. To the dried residue, 100 μL of the analyte at three concentration levels (HQC, MQC and LQC) and 100 μL of the IS were added and dried again under nitrogen. The dried samples were reconstituted in 100 μL of the mobile phase and were injected into the LC–MS/MS for analysis.
Extraction Recovery
A liquid–liquid extraction method was employed to efficiently extract the drug from the biological matrices, plasma and brain. Three replicates (N = 3) at three concentration levels (HQC, MQC and LQC) for the analyte and the working concentration of the IS were studied. Extraction recovery was evaluated by comparing the absolute peak areas of the extracted and post-extracted spiked samples reconstituted in mobile phase. The processed samples consist of samples extracted from plasma and brain as mentioned earlier.
Extraction recovery was determined by (ratio of processed samples/post-extracted spiked samples in mobile phase) × 100.
Stability
Stability of sunitinib malate was evaluated in five replicates at three concentration levels (HQC, MQC and LQC) in both plasma and brain homogenate. Analysis was performed for short-term, long-term, freeze–thaw, auto-sampler, and stock solution stability.
For short-term or bench-top stability, samples were kept for 5 h at ambient temperature (room temperature) in light-protected conditions. The time period was chosen on the basis of the maximum time the samples will be exposed to room temperature during sample preparation.
Three freeze–thaw cycles were performed to assess stability. Samples were prepared and thawed unassisted at the bench on day one, then frozen again at −80 °C. This cycle was repeated three times and then the samples were extracted on day three.
Long-term stability was assessed by storing the samples at −80 °C for 2 months (60 days) followed by extraction on day 61.
Auto-sampler stability of the samples was determined for the extracted and reconstituted QC samples by re-injecting the third day validation run samples, which were additionally stored at 4 °C for 48 h and compared to freshly prepared QC samples.
Since we had prepared the stock solution of all calibration standards and QC's, and stored them at −80 °C, we also determined the stock solution stability by preparing fresh QC's on the day of the experiment and analyzing them with the QC's stored in −80 °C for 6 months. Stock solution stability was determined only in non-extracted neat samples.
All stability studies were determined by comparing the peak area of freshly prepared samples and stability samples expressed as percent recovery.
Method Application
The LC–MS/MS assay was applied to an oral pharmacokinetic study of sunitinib malate in FVB wild-type mice. Plasma and brain concentration profiles were determined following an oral dose of sunitinib at 20 mg kg−1 administered as a 1 % CMC (carboxymethyl cellulose) suspension via oral gavage in 24 mice. All mice were between 8 and 10 weeks old at the time of the experiment and the average weight was ∼30 g. The experiment was conducted in accordance with the University of Minnesota (UMN) Institutional Animal Care and Use Committee (IACUC). Animals were euthanized at various time points 0.5, 1, 2, 4, 6, 11, and 16 h post-dose (N = 4 at each time point). Blood was collected via cardiac puncture (∼500 μL) and immediately transferred into microcentrifuge tubes containing 20 μL (100 U mL−1) of heparinized saline. Plasma was separated by centrifuging the blood at 7,500 rpm at 4 °C for 15 min. Whole brain was immediately removed and rinsed with ice-cold saline to remove extraneous blood and blot-dried. Brain was snap-frozen in liquid nitrogen and stored in pre-weighted and labeled vials at −80 °C until analysis. On the day of analysis, all brain samples were thawed and weighed to obtain the brain weight expressed as the difference between the pre- and post-vial weights. The brains were homogenized with 3 volumes of 5 % BSA using a tissue homogenizer. Pharmacokinetic parameters were calculated using non-compartmental analysis by Phoenix WinNonlin Version 6.3. (Pharsight, CA).
Results and Discussion
Chromatography and Mass-Spectrometric Conditions
Dasatinib belongs to the same class of tyrosine-kinase inhibitors as sunitinib and was chosen as the IS due to its similar chromatographic behavior. We chose not to use deuterated sunitinib malate as the IS in part because we did not want to have the complication of interfering peaks due to isomerism for the IS. Moreover, at the time we began our experiments; deuterated sunitinib was not readily available and was very expensive. Therefore, we wanted to use an IS, which is readily available for laboratory purposes. Even though all experiments were done in light-protected conditions, still we could not totally avoid the Z-to-E isomerism of sunitinib. The Z-isomer is the stable form of sunitinib and the E-isomer formed is too small in peak area to account for any significant effect. However, we have determined that after a light exposure time of 5–6 h, equal peak areas of E- and Z-isomer were obtained (1:1 ratio; data not shown). Optimal resolution for analyte and IS was achieved with 70 volumes of aqueous buffer and 30 volumes of acetonitrile, when run isocratically at 0.25 mL min−1.
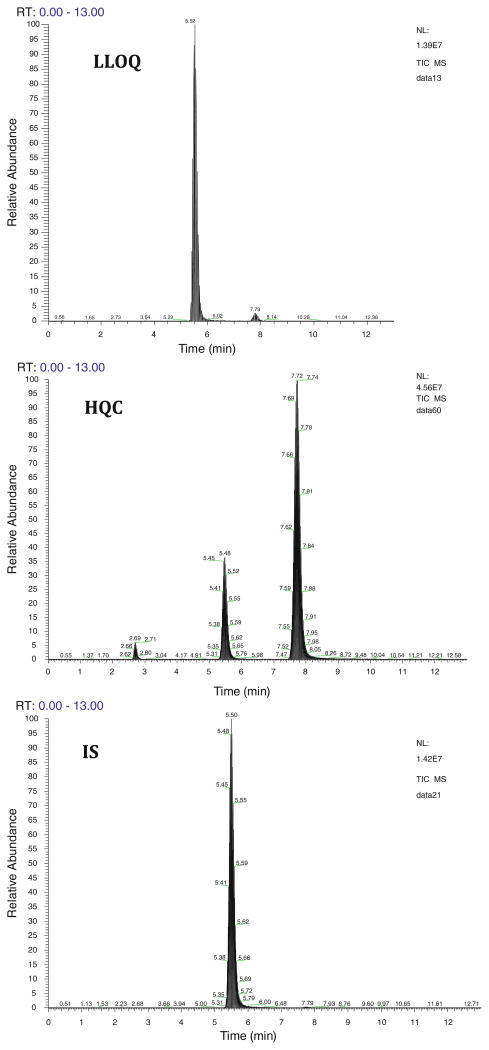
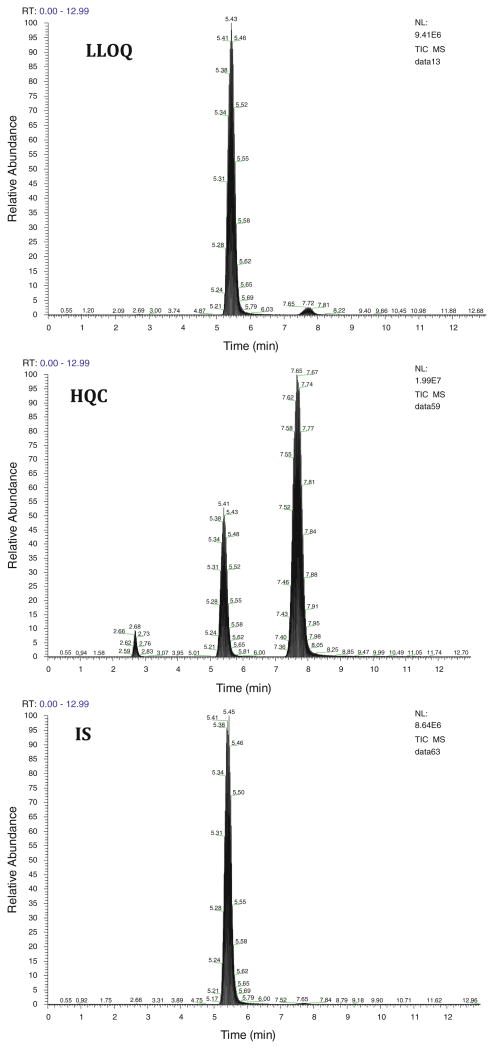
Mass-spectrometric conditions were optimized to obtain the maximum stable response of the parent analyte and the production. Three productions were obtained for sunitinib with m/z of 326, 283, and 238. We have chosen the product ion with m/z of 283 as it gave the highest percentage fragmented. The use of selected reaction monitoring (SRM) over selected ion monitoring (SIM), afforded a better advantage in reducing interference and increasing sensitivity. The retention time achieved for E-isomer and Z-isomer of sunitinib and dasatinib was 2.7, 7.8, and 5.5 min, respectively. Low background noise from the biological matrix showed good selectivity of the method. Typical chromatograms of HQC, LLOQ, and IS in both plasma (Fig. 2) and brain (Fig. 3) are shown.
Fig. 2. Representative HPLC–MS/MS chromatograms of high QC (250 ng mL−1), LLOQ (1.95 ng mL−1) and IS (dasatinib, 2 μg mL−1) in plasma extracts. Two peaks observed for sunitinib in the high QC sample indicate E-isomer (2.7 min) and Z-isomer (7.8 min).
Fig. 3. Representative HPLC–MS/MS chromatograms of high QC (250 ng mL−1), LLOQ (1.95 ng mL−1) and IS (dasatinib, 2μg mL−1) in brain tissue homogenate extracts. Two peaks observed for sunitinib in the high QC sample indicate E-isomer (2.7 min) and Z-isomer (7.8 min).
Linearity, Accuracy, Precision and LLOQ
The assay was found to be linear over the calibration range from 1.95 to 500 ng mL−1 for both plasma and brain homogenate using a weighting scheme of 1/y2 (y = peak area ratio). Selection of the weighting scale was based on the best estimation of coefficient of determination (r2) and deviation of back-calculated concentrations from calibrators expressed as % difference. The calibration curves for sunitinib in plasma and brain had coefficients of determination (r2) of about 0.99 and 0.97 for plasma and brain, respectively (N = 3 in each run) (Table 1).
Table 1. Precision and accuracy of calibration standards of sunitinib in mouse plasma and brain tissue homogenate.
| Conc. (ng mL−1) | Brain | Plasma | ||
|---|---|---|---|---|
|
|
|
|||
| RE (%) | CV (%) | RE (%) | CV (%) | |
| 1.95 | 12.9 | 6.15 | −0.076 | 3.08 |
| 3.9 | −12 | 3.5 | 1.4 | 3.6 |
| 15.6 | −1.72 | 1.92 | 0.572 | 1.21 |
| 31.25 | 1.870 | 3.980 | 8.365 | 3.083 |
| 62.5 | 6.54 | 8.67 | −0.858 | 2.53 |
| 125 | −4.52 | 1.50 | −1.78 | 0.640 |
| 250 | 1.09 | 3.49 | −13.2 | 12.2 |
| 500 | −1.07 | 2.38 | −0.867 | 1.94 |
Inter- and intra-assay variability at four different concentrations, HQC (250 ng mL−1), MQC (62.5 ng mL−1), LQC (3.9 ng mL−1), and LLOQ (1.95 ng mL−1) was determined in plasma and brain with six replicates on each day for three separate days. Inter- and intra-assay bias (%CV) and precision (%RE) were within ±15 % for all QC's, except at LLOQ (±20 %), which is in agreement with FDA guidelines. This indicates that this assay is suitable in terms of accuracy and precision. The detailed results for inter-assay accuracy and precision in plasma and brain and intra-assay accuracy and precision in plasma and in brain are summarized (Table 2).
Table 2. Inter-assay and intra-assay accuracy and precision of sunitinib in mouse plasma and brain tissue homogenate.
| Matrix | Conc. (ng mL−1) | Intra-day (N = 6) | Inter-day (N = 18) | ||
|---|---|---|---|---|---|
|
|
|
||||
| RE (%) | CV (%) | RE (%) | CV (%) | ||
| Plasma | LLOQ_1.953 | −4.38 | 4.15 | −4.83 | 4.94 |
| Low QC_3.906 | 1.41 | 2.88 | −0.12 | 8.63 | |
| Med QC_62.5 | −5.92 | 5.17 | −3.87 | 5.84 | |
| High QC_250 | −8.72 | 7.86 | −8.97 | 1.33 | |
| Brain | LLOQ_1.953 | 11.81 | 10.73 | 9.05 | 5.91 |
| Low QC_3.906 | −12.85 | 5.39 | −7.66 | 7.45 | |
| Med QC_62.5 | −1.43 | 3.27 | 3.53 | 5.44 | |
| High QC_250 | −6.46 | 4.35 | 0.05 | 5.82 | |
Recovery
Recovery was calculated by comparing the absolute peak area of the processed samples at 3.9, 62.5, and 250 ng mL−1, using the extraction method described earlier, with those of post-extracted samples at the same concentrations in mobile phase, expressed as a percentage. Extraction recovery of the IS was also determined at 2 μg mL−1 using the same procedure. Average recovery for sunitinib in plasma and brain was 112.3 and 94.3 %, respectively. The recovery results are summarized in Table 3.
Table 3. Matrix effect of plasma and brain homogenate on the ionization efficiency of sunitinib and IS and extraction recovery of sunitinib and IS in mouse plasma and brain homogenate.
| Matrix | Conc. (ng mL−1) | Matrix effect, mean (%) ± SD (%) N = 3 | Extraction efficiency, mean (%) ± SD (%) N = 3 |
|---|---|---|---|
| Plasma | Low QC_3.9 | −38.1 ± 5.4 | 126 ± 0.1 |
| Med QC_62.5 | −43.8 ± 4.3 | 119 ± 0.1 | |
| High QC_250 | −33.5 ± 3.5 | 92 ± 0.1 | |
| Dasatinib (IS)_2000 | 0.04 ± 0.03 | 79 ± 0.02 | |
| Brain | Low QC_3.9 | −43 ± 2.4 | 113 ± 0.1 |
| Med QC_62.5 | −45 ± 2.6 | 102 ± 0.1 | |
| High QC_250 | −34.1 ± 3.1 | 68 ± 0.04 | |
| Dasatinib (IS)_2000 | −1.7 ± 0.9 | 72 ± 0.02 |
Matrix Effects
Effect of endogenous substances in the biological matrix was evaluated at three different concentrations, 3.9, 62.5, and 250 ng mL−1 for sunitinib and at a single concentration of 2 μg mL−1 for the IS. The suppression of ionization in both plasma and brain homogenate was highest in medium QC (approx. −44 %) and lowest for the high QC (approx. −34 %). Plasma extracts enhanced the ionization of the IS by ∼0.04 %, but was decreased by −1.7 % in the brain homogenate extracts. These results suggest that plasma and brain homogenate interfered with the ionization of the analyte (Table 3).
Stability
Five replicates of HQC, MQC, and LQC were used to assess the stability of sunitinib under various conditions. The results are summarized in Table 4. Results from bench-top stability suggest that the sample preparation time of 5 h did not lead to significant degradation of sunitinib in both the plasma and the brain samples (<±15 %). Results from auto-sampler, freeze–thaw and long-term stability indicate that the degradation of sunitinib might occur under these conditions and therefore determining reliable concentrations from such samples must take this into consideration. Storing the samples after an experiment for long-term (≥60 days) under frozen conditions (−80 °C) can lead to deterioration of the drug. It is thus advisable to analyze the samples as soon as possible. Furthermore, results from auto-sampler stability suggest that it is not ideal to use reconstituted samples for determination of sunitinib concentrations if they are kept for more than 48 h in the auto-sampler. With the given run-time in this assay, we were able to analyze up to 120 samples overnight without observing degradation of the analyte. The standard stock solution was found to be stable over the time period of storage (6 months). This is important as it avoids the need to make fresh standards everyday (Table 5).
Table 4. Stability of analyte and IS under various conditions examined.
| Matrix | Concentration (ng mL−1) | Mean ± SD | |||
|---|---|---|---|---|---|
|
| |||||
| Bench-topb (% recovery) N = 5 | Freeze–thawc (% recovery) N = 5 | Auto-samplerd (% recovery) N = 5 | Long-terme (% recovery) N = 6 | ||
| Plasma | 3.9 | 94.79 ± 4.04a | 84.37 ± 7.38 | 98.4 ± 6.05a | 112 ± 21.64 |
| 62.5 | 91.8 ± 9.74 | 85.6 ± 9.69 | 68.66 ± 8.92 | 67.42 ± 13.80 | |
| 250 | 82.74 ± 20.08 | 100.73 ± 5.16 | 100.5 ± 6.13 | 70.19 ± 5.91 | |
| Brain | 3.9 | 95.26 ± 9.84 | 71.68 ± 5.73 | 121.08 ± 8.88 | 122.26 ± 12.14 |
| 62.5 | 96.35 ± 3.16 | 82.42 ± 9.88 | 119.08 ± 8.44 | 111.62 ± 11.47 | |
| 250 | 98.79 ± 5.05 | 92.62 ± 4.15 | 102.18 ± 9.02 | 107.59 ± 16.11 | |
No. of replicates = 4
At least 5 h at room temperature
At least three freeze–thaw cycles
At least 48 h at 4 °C
At least 2 months (60 days) at −80 ± 5 °C
Table 5. Stock solution stability of the analyte.
| Nominal Conc. (ng mL−1) | % Recovery Mean ± SD |
|---|---|
| Low QC_3.9 | 102.3 ± 0.61 |
| Medium QC_62.5 | 103.51 ± 0.22 |
| High QC_250 | 99.54 ± 3.57 |
Method Application
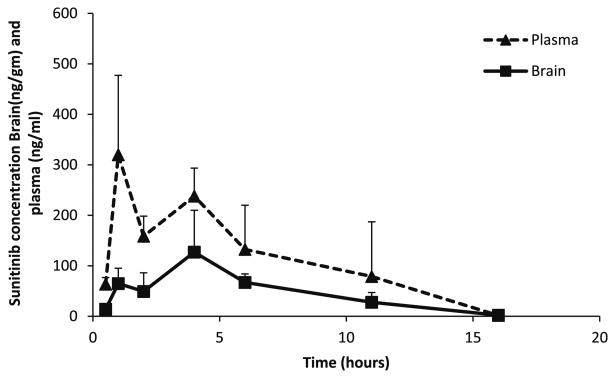
The established method was successfully used to determine pharmacokinetic parameters in plasma and brain following a single oral dose of 20 mg kg−1 sunitinib administered as a 1 % CMC suspension in FVB wild-type mice. Plasma and brain samples were collected at predetermined time points and analyzed using this assay. The assay was found to be sensitive in determining sunitinib concentrations in both plasma and brain. A non-compartmental analysis approach yielded a terminal half-life of 1.8 h in plasma and 2 h in brain. All measured concentrations were above the LLOQ. The plasma and brain area under the curve from time zero to infinity (AUC0–∞) was 1.85 and 0.77 h-μg mL−1, respectively, and AUC brain-to-plasma ratio was 0.42, i.e., 42 % of the drug in plasma reaches the brain, suggesting limited delivery of sunitinib into the brain (measured as the total sunitinib concentrations, Fig. 4). The peak concentration and Cmax were 320 and 127 ng mL−1 in both plasma and brain, respectively.
Fig. 4. Plasma and brain sunitinib concentration–time profiles in FVB wild-type mice after a single oral dose of 20 mg kg−1.
Conclusion
In conclusion, we have developed and validated a robust, sensitive and reproducible LC–MS/MS method for analysis of sunitinib in mouse plasma and brain tissue homogenate. This study reports a validated method for sunitinib analysis in mouse plasma and brain considering its light-sensitive nature and has been successfully employed for preclinical pharmacokinetic investigations in mice. High extraction efficiency using a liquid–liquid extraction method in this assay is simple to apply and does not involve the need for an additional protein precipitation step. The described method was found to be linear over a wide range, from 1.95 to 500 ng mL−1. This method requires only small amount of sample (100 μL for plasma and 200 μL for brain homogenate), therefore it is also feasible for analysis of sunitinib from in vitro cell culture studies and using this assay we have determined sunitinib from cellular accumulation studies in our lab.
Acknowledgments
We would like to thank Dr. Tianli Wang and Dr. Sagar Agarwal for their valuable discussions. This work was supported by grants, CA 138437, NS 077921 and CA 108961.
Contributor Information
Rajneet K. Oberoi, Department of Pharmaceutics, University of Minnesota, 9-177 Weaver Densford Hall, 308 Harvard Street SE, Minneapolis, MN 55455, USA; Brain Barriers Research Center, University of Minnesota, Minneapolis, MN, USA
Rajendar K. Mittapalli, Department of Pharmaceutics, University of Minnesota, 9-177 Weaver Densford Hall, 308 Harvard Street SE, Minneapolis, MN 55455, USA; Brain Barriers Research Center, University of Minnesota, Minneapolis, MN, USA
James Fisher, Clinical Pharmacology and Analytical Services Laboratory, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
William F. Elmquist, Email: elmqu011@umn.edu, Department of Pharmaceutics, University of Minnesota, 9-177 Weaver Densford Hall, 308 Harvard Street SE, Minneapolis, MN 55455, USA; Brain Barriers Research Center, University of Minnesota, Minneapolis, MN, USA.
References
- 1.Mellinghoff IK, Lassman AB, Wen PY. Glia. 2011;59:1205–1212. doi: 10.1002/glia.21137. [DOI] [PubMed] [Google Scholar]
- 2.Faivre S, Demetri G, Sargent W, Raymond E. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Gallo JM. Neuro-oncology. 2009;11:301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 5.de Bruijn P, Sleijfer S, Lam MH, Mathijssen RH, Wiemer EA, Loos WJ. J Pharm Biomed Anal. 2010;51:934–941. doi: 10.1016/j.jpba.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Gotze L, Hegele A, Metzelder SK, Renz H, Nockher WA. Clin Chim Acta. 2012;413:143–149. doi: 10.1016/j.cca.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, Ris HB, Leyvraz S, Widmer N, Decosterd LA. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1982–1996. doi: 10.1016/j.jchromb.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 8.Bouchet S, Chauzit E, Ducint D, Castaing N, Canal-Raffin M, Moore N, Titier K, Molimard M. Clin Chim Acta. 2011;412:1060–1067. doi: 10.1016/j.cca.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Honeywell R, Yarzadah K, Giovannetti E, Losekoot N, Smit EF, Walraven M, Lind JS, Tibaldi C, Verheul HM, Peters GJ. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1059–1068. doi: 10.1016/j.jchromb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Rodamer M, Elsinghorst PW, Kinzig M, Gutschow M, Sorgel FJ. Chromatogr B Anal Technol Biomed Life Sci. 2011;879:695–706. doi: 10.1016/j.jchromb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Lankheet NA, Hillebrand MJ, Rosing H, Schellens JH, Beijnen JH, Huitema AD. Biomed Chromatogr. 2013;27(4):466–476. doi: 10.1002/bmc.2814. [DOI] [PubMed] [Google Scholar]
- 12.Minkin P, Zhao M, Chen Z, Ouwerkerk J, Gelderblom H, Baker SD. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;874:84–88. doi: 10.1016/j.jchromb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baratte S, Sarati S, Frigerio E, James CA, Ye C, Zhang Q. J Chromatogr A. 2004;1024:87–94. doi: 10.1016/j.chroma.2003.10.085. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Gallo JM. J Pharm Biomed Anal. 2010;51:958–964. doi: 10.1016/j.jpba.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA. 2001 http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
- 16.Wang T, Oberoi RK, Elmquist WF. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3812–3817. doi: 10.1016/j.jchromb.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]