Abstract
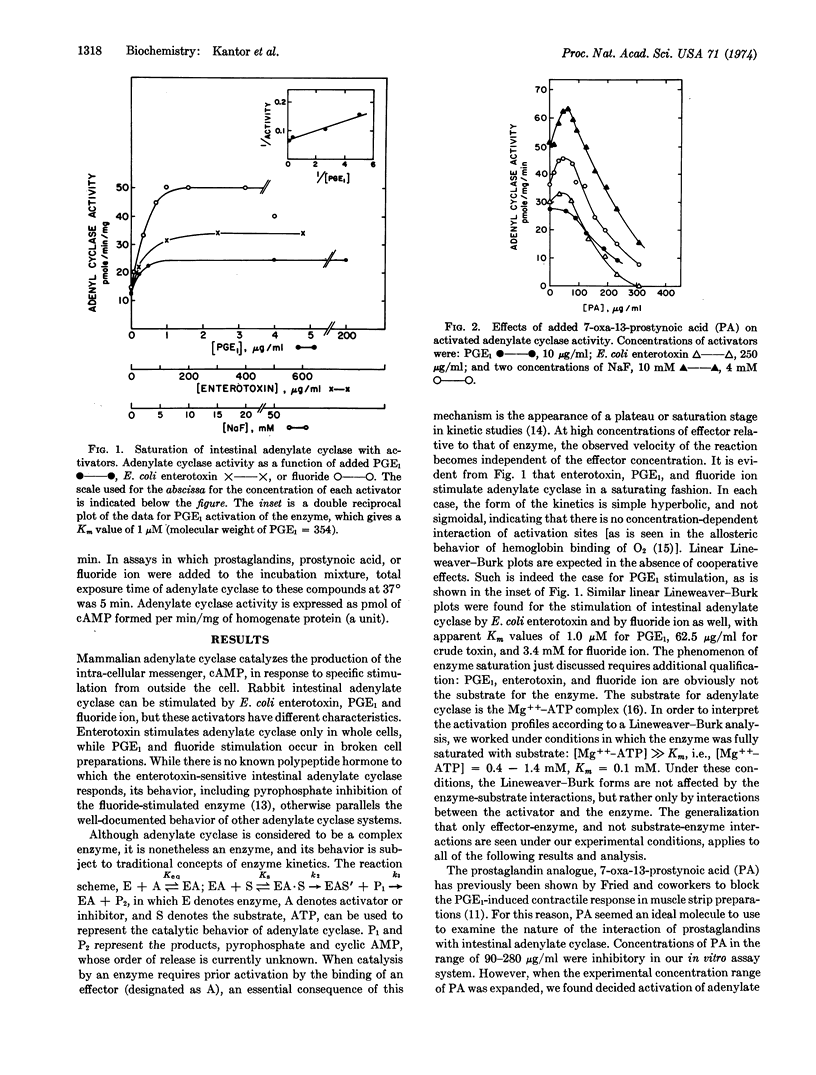
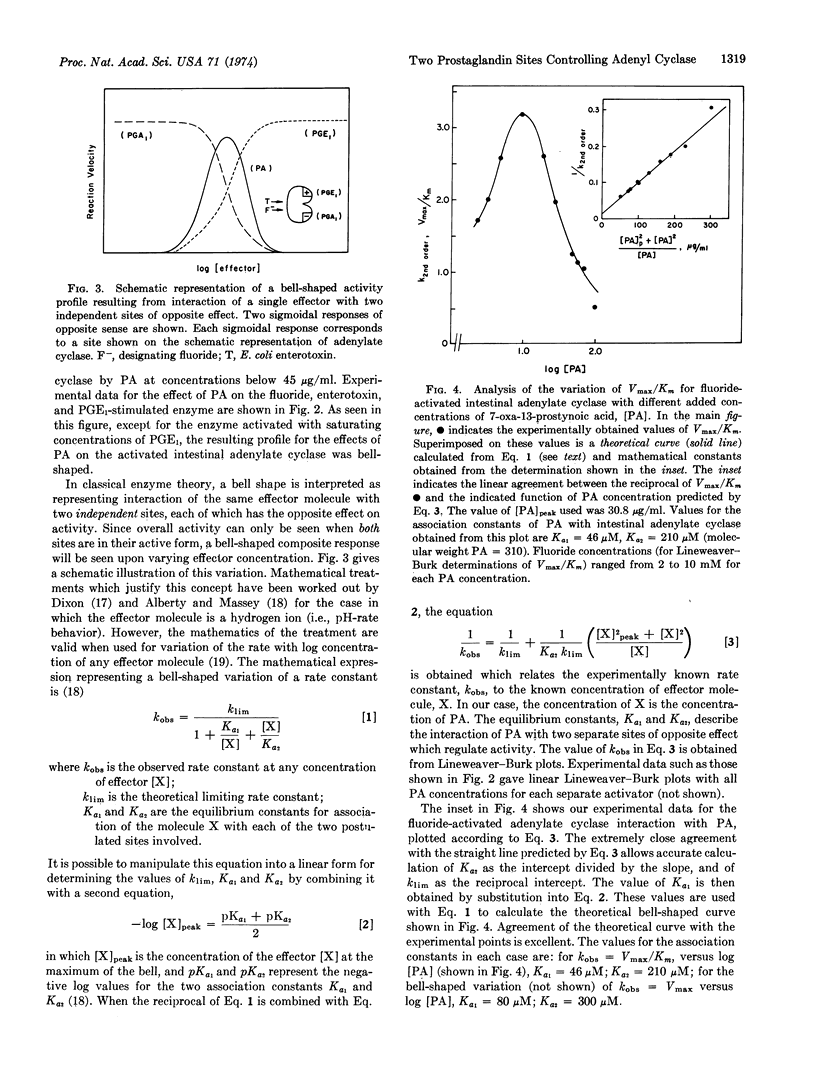
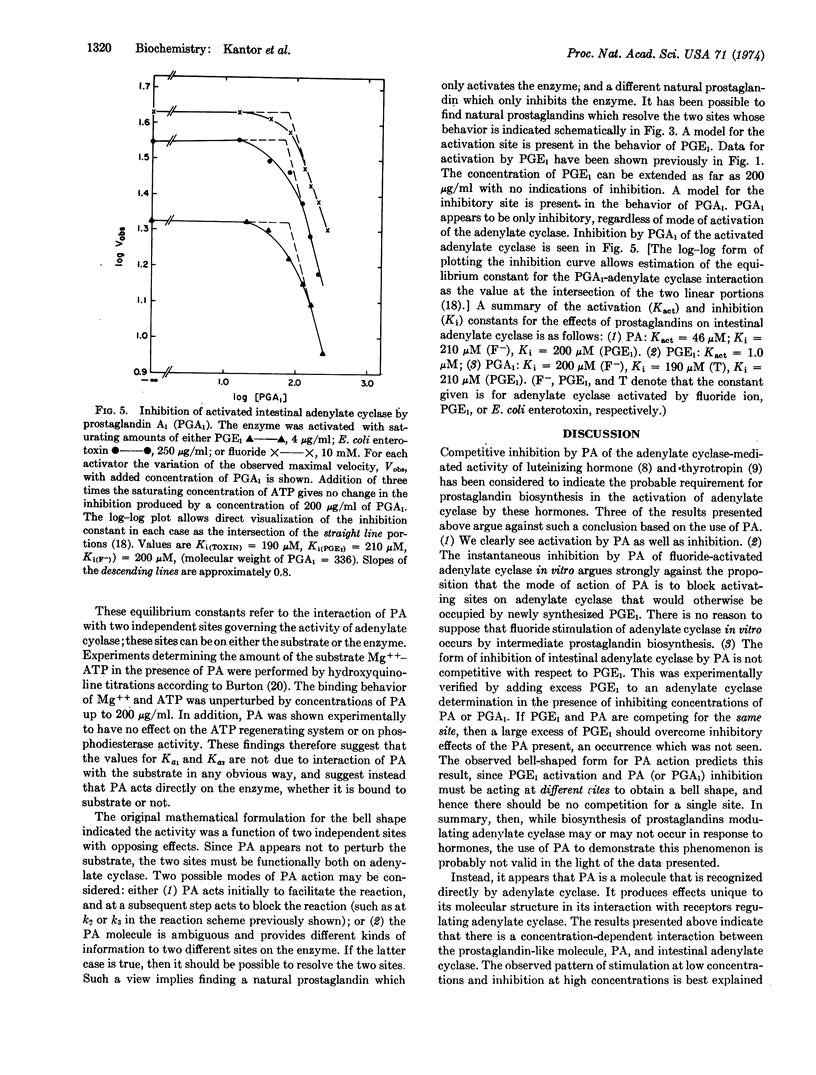
Kinetic behavior most consistent with the presence of two independent, but simultaneously acting, regulatory effector sites for prostaglandins has been presented for adenylate cyclase (EC 4.6.1.1) of rabbit intestinal epithelial cells. One site regulates activation of the catalytic site, while the other site regulates inhibition. A synthetic prostaglandin analogue, 7-oxa-13-prostynoic acid, is recognized at both sites in a concentration-dependent manner. At concentrations of 7-oxa-13-prostynoic acid less than 45 μg/ml, activation is seen, while at higher concentrations, inhibition is seen. Different naturally occurring prostaglandins appear to be site-specific. Prostaglandin E1 gives only activation of the cyclase, while prostaglandin A1 gives only inhibition of the activated cyclase. When saturating concentrations of prostaglandin E1 are used to activate adenylate cyclase, no further activation by 7-oxa-13-prostynoic acid can be elicited, indicating that both molecules activate at the same site. The similarity of inhibition constants for both 7-oxa-13-prostynoic acid and prostaglandin A1 suggests that the mode of binding is the same for both compounds and that they probably inhibit by acting at the same site. The inhibition by 7-oxa-13-prostynoic acid and by prostaglandin A1 overrides enzyme activation produced by either Escherichia coli enterotoxin, prostaglandin E1, or sodium fluoride, suggesting that in intestinal adenylate cyclase this site is the primary regulatory site (i.e., primary allosteric effector site) for enzyme activity. These data suggest that sites exist on adenylate cyclase which would allow prostaglandins to serve as the intracellular messengers by which the cell controls its adenylate-cyclase-mediated response to extracellular stimulation, as with hormones.
Keywords: 7-oxa-13-prostynoic acid, prostaglandin E1, prostaglandin A1, cholera toxin, rabbit
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTY R. A., MASSEY V. On the interpretation of the pH variation of the maximum initial velocity of an enzyme-catalyzed reaction. Biochim Biophys Acta. 1954 Mar;13(3):347–353. doi: 10.1016/0006-3002(54)90340-6. [DOI] [PubMed] [Google Scholar]
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell J. G., Gorbach S. L., Pierce N. F., Mitra R., Mondal A. Acute undifferentiated human diarrhea in the tropics. II. Alterations in intestinal fluid and electrolyte movements. J Clin Invest. 1971 Apr;50(4):890–900. doi: 10.1172/JCI106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried J., Santhanakrishnan T. S., Himizu J., Lin C. H., Ford S. H., Rubin B., Grigas E. O. Prostaglandin antagonists: synthesis and smooth muscle activity. Nature. 1969 Jul 12;223(5202):208–210. doi: 10.1038/223208a0. [DOI] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Gorbach S. L. Stimulation of intestinal adenyl cyclase by Escherichia coli enterotoxin: comparison of strains from an infant and an adult with diarrhea. J Infect Dis. 1974 Jan;129(1):1–9. doi: 10.1093/infdis/129.1.1. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L., Tarnoff J., Cirillo V. J., Ham E. A. Prostaglandin receptor site: evidence for an essential role in the action of luteinizing hormone. Science. 1970 Aug 28;169(3948):883–886. doi: 10.1126/science.169.3948.883. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Adenyl cyclase. II. The enzymatically catalyzed formation of adenosine 3',5'-phosphate and inorganic pyrophosphate from adenosine triphosphate. J Biol Chem. 1962 Apr;237:1228–1232. [PubMed] [Google Scholar]
- Sato S., Szabo M., Kowalski K., Burke G. Role of prostaglandin in thyrotropin action on thyroid. Endocrinology. 1972 Feb;90(2):343–356. doi: 10.1210/endo-90-2-343. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Jessup S. J., Ramwell P. W. Prostaglandin--adenyl cyclase relationships. Adv Cyclic Nucleotide Res. 1972;1:479–491. [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]


