Abstract
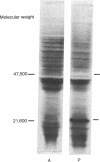
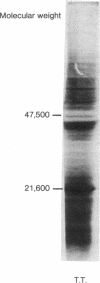
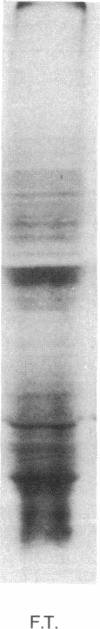
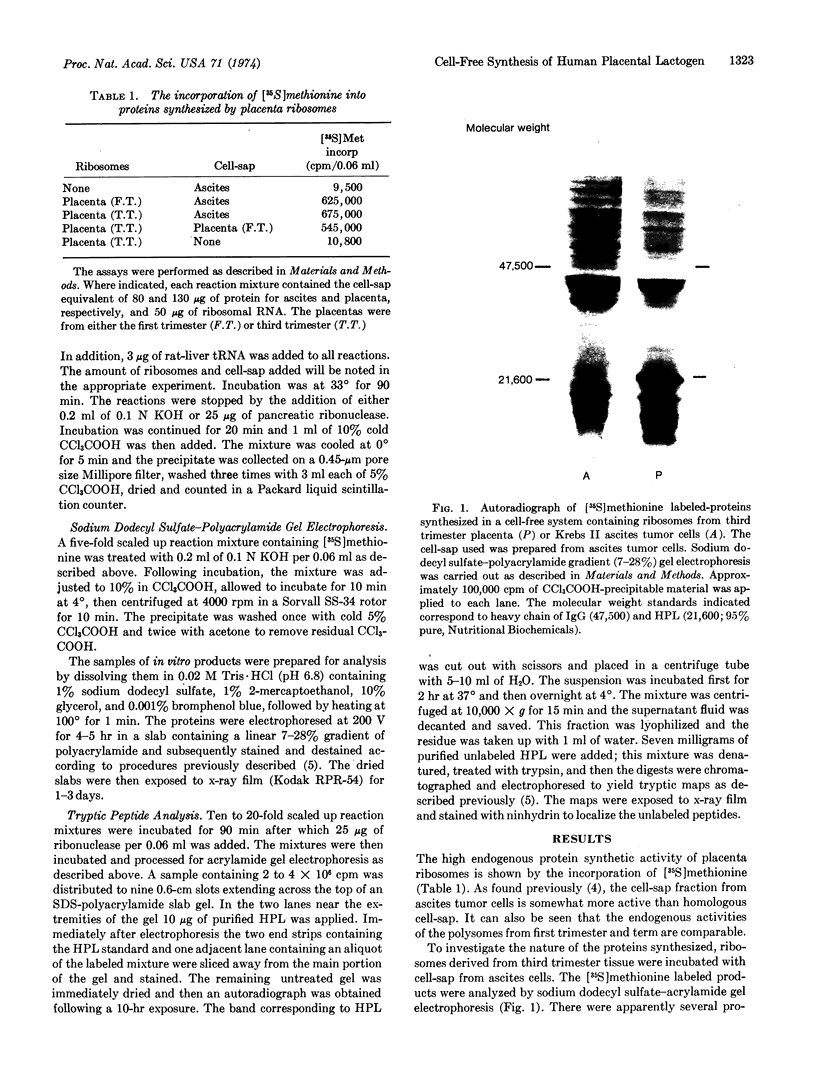
In a very active cell-free system containing polysomes derived from human placenta and a cell-sap fraction prepared from ascites tumor cells, the synthesis of the hormone human placental lactogen (HPL) was detected. The identification was based on the following: (a) The in vitro synthesized protein labeled with [35S]methionine migrated at the same rate as authentic HPL on sodium dodecyl sulfate-polyacrylamide gels and (b) tryptic fingerprint analysis of the labeled protein yielded peptides having the same mobilities as seen with the same analysis of purified HPL.
The amount of HPL synthesized in a cell-free system containing polysomes derived from term placenta was about 10% of the total proteins synthesized and in a comparable system containing first trimester ribosomes the level of synthesis was about 5%. These data suggest the potential for quantitating the HPL mRNA activity as a function of the period of gestation and for isolating the mRNA itself.
Keywords: reproduction, protein synthesis, hormone, pregnancy
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Friesen H. G., Suwa S., Pare P. Synthesis and secretion of placental lactogen and other proteins by the placenta. Recent Prog Horm Res. 1969;25:161–205. doi: 10.1016/b978-0-12-571125-8.50007-7. [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Kaplan S. L., Sciarra J. J., Burr I. M. Chorionic growth hormone-prolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the "growth hormone" of the 2d half of pregnancy. Ann N Y Acad Sci. 1968 Feb 5;148(2):501–531. doi: 10.1111/j.1749-6632.1968.tb20372.x. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Gurpide E., Sciarra J. J., Grumbach M. M. Metabolic clearance rate and production rate of chorionic growth hormone-prolactin in late pregnancy. J Clin Endocrinol Metab. 1968 Oct;28(10):1450–1460. doi: 10.1210/jcem-28-10-1450. [DOI] [PubMed] [Google Scholar]
- Li C. H., Dixon J. S., Chung D. Amino acid sequence of human chorionic somatomammotropin. Arch Biochem Biophys. 1973 Mar;155(1):95–110. doi: 10.1016/s0003-9861(73)80012-8. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]