Abstract
Background
The purpose of this study was to evaluate the clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic paragangliomas by assessing reduction in tumor size, blood pressure, and improvement in overall survival.
Methods
We retrospectively reviewed the medical records of patients with metastatic pheochromocytomas-sympathetic paragangliomas who had received chemotherapy at The University of Texas MD Anderson Cancer Center
Results
Clinical benefit and overall survival (OS) were assessed. Of fifty-four patients treated with chemotherapy, fifty-two were evaluable for response. Seventeen (33%) experienced a response, defined as decreased or normalized blood pressure/decreased number and dosage of antihypertensive medications and/or reduced tumor size after the first chemotherapy regimen. The median OS time was 6.4 years (95 confidence interval (CI): 5.2–16.4) for responders and 3.7 (95% CI: 3.0–7.5) years for non-responders. Of patients who had synchronous metastatic disease, a positive response at 1 year after the start of chemotherapy was associated with a trend toward a longer overall survival (log-rank test, P-value =0.095). In a multivariate Cox proportional hazards model, the effect of response to chemotherapy on overall survival was significant (hazard ratio=0.22, 95% confidence interval: 0.05–1.0; P-value = 0.05). All responders had been treated with dacarbazine and cyclophosphamide. Vincristine was included for 14 responders and doxorubicin was included for 12 responders. We could not identify clinical factors that predicted response to chemotherapy.
Conclusion
Chemotherapy may decrease tumor size and facilitate blood pressure control in about 33% of patients with metastatic pheochromocytoma-sympathetic paraganglioma. These patients exhibit a longer survival.
Keywords: clinical benefits, chemotherapy, survival, metastatic pheochromocytoma, paraganglioma
INTRODUCTION
Pheochromocytomas and sympathetic extra-adrenal paragangliomas are rare neuroendocrine tumors that arise from chromaffin cells in, respectively, the adrenal medulla and in paraganglia in the thorax, abdomen, and pelvis. Pheochromocytomas and sympathetic paragangliomas together have an estimated incidence of 0.95 cases per 100,000 person-years in the United States.1 The metastasis rate of these tumors was long reported to be 10%.2–4However, in a recent comprehensive study of clinical predictors of metastases in pheochromocytoma and sympathetic paraganglioma patients, the estimated metastasis rate was actually 17.2%.5
Patients with metastatic tumors present a unique clinical challenge. They have high morbidity and mortality rates from excessive catecholamine secretion, hypertension, cardiovascular complications, and bulky disease, for which no curative treatment is available. The 5-year overall survival (OS) rate for patients with metastatic tumors ranges from 40% to 77%.5–7 It has been suggested that survival is affected by whether metastasis is found at the time of diagnosis of the primary tumor or later in the disease course7 but information on this is limited.
Treatments for metastatic tumors are also limited and include radiopharmaceutical agents such as iodine-131-metaiodobenzylguanidine (131I-MIBG) and systemic cytotoxic antineoplastic therapy. The first chemotherapy reported for metastatic sympathetic paragangliomas was the alkylating agent cyclophosphamide, introduced in the late 1960s.8 The use of combination antineoplastic therapy (cyclophosphamide, vincristine, and dacarbazine) for this disease was first described in 1985.9 Since then, a few small retrospective studies of chemotherapy for metastatic pheochromocytomas and paragangliomas have been reported, but no apparent survival benefits from chemotherapy have been found, perhaps because of the small number of patients available for analysis and the lack of systematic follow-up.7, 10–12 The rarity of these tumors has made it difficult, if not impossible, to perform randomized, prospective studies to compare different regimens.
The aim of this study was to assess the clinical benefits of systemic chemotherapy in a large single-institution cohort of patients with metastatic pheochromocytoma or sympathetic extra-adrenal paraganglioma.
PATIENTS AND METHODS
Patients
After obtaining approval from The University of Texas MD Anderson Cancer Center institutional review board, we retrospectively reviewed the medical records of 115 patients with metastatic pheochromocytoma or paraganglioma who were diagnosed from January 1979 through March 2010. We excluded patients with head and neck paragangliomas because of these tumors' differing clinical behavior. Parasympathetic tumors do not secrete catecholamines such as adrenaline and noradrenaline and are infrequently associated with metastases.13
We created a database using Microsoft SQL Server (version 2008; Microsoft Corporation, Redmond, WA) to include demographic, clinical, laboratory, imaging, pathologic, and outcome treatment information extracted from the patients' medical records. Primary tumor location and metastases were verified by pathology, surgical, or radiographic reports (magnetic resonance imaging, computed tomography, fluorine-18 fluorodeoxyglucose positron emission tomography/computerized tomography or 123I-MIBG scintigraphy). Metastatic disease was defined as the presence of tumor cells in anatomic sites in which chromaffin tissue is normally absent (eg, lymph nodes, liver, lung, brain, and bone). Patients were subdivided into 2 groups: those who had metastatic disease at the time of diagnosis of the primary tumor or within 6 months of the primary tumor (synchronous metastases) and those who were identified as having metastatic disease ≥6 months after diagnosis (metachronous metastases).
Study Design
Our primary objective was to evaluate the clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas and sympathetic paragangliomas by assessing the proportion of patients who experienced reduced tumor size, as demonstrated by radiographic studies (eg, computed tomography and magnetic resonance imaging) and the proportion of patients who experienced normalized blood pressure with at least a 50% reduction in the initial dosage and number of antihypertensive medications determined by reviewing clinical notes. Responses were categorized as the best response during first chemotherapy regimen.
We assessed OS duration. Time was calculated from primary tumor diagnosis date to death date or to last follow-up date. Patients were censored at the last follow-up dates if death did not occur. As the OS was expected to be shorter for patients with synchronous metastatic disease than for patients with metachronous metastatic disease, we independently analyzed OS for patients with synchronous and metachronous metastatic disease. We compared the OS of patients who experienced a response with that of patients who did not.
Radiographic Tumor Size Evaluation
We reviewed patients' computed tomography and magnetic resonance imaging reports. Response by tumor size was defined as any objective reduction in the size of the tumor on cross-sectional imaging during the first chemotherapy regimen.
Blood Pressure and Antihypertensive Medications
Blood pressure response was defined as blood pressure normalization after patients' first chemotherapy regimen, with at least a 50% reduction in the number and dosage of antihypertensive medications. We considered a complete response when all antihypertensive medications were discontinued.
Statistical Analyses
Summary statistics such as the number of non-missing observations (N), mean, median, standard deviation (std.dev), minimum, and maximum were calculated for continuous variables. Frequencies and percentages were calculated for categorical variables. The chi-square test or Fisher's exact test was used to evaluate the association between 2 categorical variables, and the Wilcoxon rank-sum test was used to compare the distributions of continuous variables between 2 patient groups. The Kaplan-Meier method was used to estimate OS duration, and the log-rank test was used to evaluate OS differences between patient groups. Landmark analysis was used when assessing the effect of response to chemotherapy on OS, defining the landmark time as 1 year from the start of chemotherapy. Multivariate Cox proportional hazards models were fitted to include important patient clinical variables. All tests were 2-sided. P-value <0.05 were considered statistically significant. Statistical software SAS version 9.1.3 (SAS, Cary, NC) and S-plus 8.0 (TIBCO Software, Inc., Palo Alto, CA) statistical software packages were used for the analyses.
RESULTS
Patient and Disease Characteristics
Fifty-four patients with progressive metastatic disease were treated with chemotherapy. Of the 54 patients, 52 had their response status recorded. Their clinicopathological characteristics are summarized in Table 1. We did not find any differences in the pretreatment characteristics of the responders and non-responders.
Table 1.
Patient and disease characteristics by response to chemotherapya
| Characteristic | Responders (N=17) | Non-responders (N=35) | Total (N=52) | P-value |
|---|---|---|---|---|
| Age | 0.81 | |||
| Median (min to Max) | 42 (25–62) yr | 42 (6–70) yr | 42 (6 to70) yr | |
| Gender | 0.37 | |||
| Male | 11 (37.9%) | 18 (62.1%) | 29 (55.8%) | |
| Female | 6 (26.1%) | 17 (73.9%) | 23 (44.2%) | |
| Ethnicity | 0.24 | |||
| White | 16 (37.2%) | 27 (62.8%) | 43 (82.7%) | |
| Other | 1 (11.1%) | 8 (88.9%) | 9 (17.3%) | |
| Tumor size | 0.39 | |||
| Median (min to max) | 5.5 (2–18) cm | 8.0 (1–15) cm | 7.2 (1–18) cm | |
| Tumor type | 0.90 | |||
| Pheochromocytoma | 6 (31.6%) | 13 (68.4%) | 19 (36.5%) | |
| Paraganglioma | 11 (33.3%) | 22 (66.7%) | 33 (63.5%) | |
| Metastasis timing | 0.91 | |||
| Synchronous | 10 (33.3%) | 20 (66.7%) | 30 (57.7%) | |
| Metachronous | 7 (31.8%) | 15 (68.2%) | 23 (42.3%) | |
| Sites of metastasisb | ||||
| Single site of metastasis | 4 (44.4%) | 5 (55.5%) | 9 (16.9%) | 0.40 |
| Multiple sites of metastases | 13 (30.2%) | 30 (69.7%) | 43 (82.6%) |
Data are number of patients (%) unless otherwise specified,
Number of sites of metastasis at the start of chemotherapy,
24 patients in the Non-responders group had Not Available biochemical studies;
Nine patients had succinate dehydrogenase complex gene testing (Table 2). We found that none of the 4 patients with mutations on the SDHB gene (including 1 complete deletion) responded to chemotherapy. One patient with an SDHC mutation was in the group of responders.
Table 2.
Succinate dehydrogenase genetic analysis in 9 patients by response to chemotherapya
| Genetic finding | Responders (N=3) | Non-responders (N=6) | Total (N=9) |
|---|---|---|---|
| SDHB positive | 0 | 4 | 4 |
| SDHB negative | 0 | 1 | 1 |
| SDHC positive | 1 | 0 | 1 |
| SDHB, SDHD negative | 1 | 0 | 1 |
| SDHB, SDHC, SDHD negative | 1 | 1 | 2 |
Data are number of patients
The number of tumor sites was not associated with the response to chemotherapy (Table 1). All patients had normal liver and kidney function tests results before chemotherapy (data not shown).
Chemotherapy Response
Twenty-one patients received chemotherapy as initial treatment (Of these, 2 received chemotherapy with the intention of decreasing the size of the primary tumor before surgery). For 31 patients, chemotherapy was used to treat relapsing disease after surgical excision of the primary tumor. Two patients with unresectable disease were treated initially with 131I-MIBG, but chemotherapy was initiated 1 year later because of tumor progression.
Front-line chemotherapy regimens were classified as doxorubicin based, non-doxorubicin based, and other (consisting of platins; cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone or prednisolone; temozolomide; etoposide; imatinib; ifosfamide; and thalidomide). Table 3 shows the front-line regimens used, the number of responders, number of cycles and serious adverse effects.
Table 3.
Front-line chemotherapy regimens used, median number of cycles, serious adverse effects
| Protocol | Responders (N=17) | Non-Responders (N=35) | Cycles | Total (N=52) |
|---|---|---|---|---|
| CyVADic | 9 | 10 | Median=6 | 19 |
| CyADic | 3 | 9 | Median=5.5 | 12 |
| CyVDic | 5 | 5 | Median=9 | 10 |
| CyAV | 0 | 2 | Median=1.5 | 2 |
| CyA | 0 | 1 | 1 | 1 |
| CHOP | 0 | 1 | 2 | 1 |
| Imatinib | 0 | 1 | 1 | 1 |
| Cis + VP16 | 0 | 1 | 3 | 1 |
| AVDic | 0 | 1 | 6 | 1 |
| Cy + V + temozolomide | 0 | 1 | 12 | 1 |
| Carb + VP16 + ifosfamide | 0 | 1 | 4 | 1 |
| Tamoxifen | 0 | 1 | 4 | 1 |
| Temozolomide + bevacizumab + sorafenib | 0 | 1 | 4 | 1 |
Cy indicates cyclophosphamide; V, vincristine; A, doxorubicin; Dic, dacarbazine; Cis, cisplatin; VP-16, etoposide; Carb, carboplatin; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone
N= number of patients.
Seventeen patients (33%) responded to front-line chemotherapy, 9 had reduced tumor size, 4 had normalized blood pressure, and 4 had both. In 2 patients, chemotherapy shrank initially unresectable primary tumors so that they could be surgically excised. Thirty-five patients did not experience a response to chemotherapy.
All 17 patients who experienced a response had been treated with regimens that included cyclophosphamide and dacarbazine. In addition, doxorubicin had been included for 12 of 17 patients (71%) and vincristine for 14 of 17 patients (82%). The dosages of these medications were: Doxorubicin 60–75 mg/m2, cyclophosphamide 600–750 mg/m2, dacarbazine 750–1000 mg/m2 and vincristine1– 2 mg/m2. The mean number of cycles of front-line chemotherapy was 6.9. Twelve of the 17 patients who responded had skeletal metastases. Only 2 patients received external beam radiation therapy before chemotherapy.
Blood Pressure Responses
Thirty one (59.6%) of 52 patients had clinical evidence of excessive catecholamine secretion, adrenergic symptoms, and hypertension and had been treated with antihypertensive medication before chemotherapy. They required a median of 4 different antihypertensive medications (range 1–7) to maintain normal or near-normal blood pressure. The most common antihypertensives used were alpha-blockers (phenoxybenzamine, terazosin, doxazosin, and prazosin), beta blockers (eg, propranolol and metoprolol), calcium channel blockers (amlodipine, nifedipine, and nicardipine), angiotensin-converting enzyme inhibitors (captopril and enalapril), and angiotensin receptor blockers (irbesartan). Others included nitrates, labetalol, hydrochlorothiazide, hydralazine, clonidine and carvedilol.
In 6 (19.3%) of these 31 patients, the antihypertensive medication dosage and number were reduced by more than 50% after the first chemotherapy regimen. Three of the 6 patients had a complete response as they discontinued all antihypertensive medications (Table 4).
Table 4.
Blood pressure measurements, number of antihypertensive medications and biochemical markers in patients who responded to chemotherapy before and after treatment
| Patient | Blood pressure before chemo (mmHg) | Antihypertensive drugs before chemo | Blood pressure after chemo (nnHg) | Antihypertensive drugs after chemo | Catecholamines before chemo | Catecholamines after chemo |
|---|---|---|---|---|---|---|
| 1 | 201/102 | Propranolol, 160 mg; NTG prna; phenoxybenzamine, 20 mg | 130/90 | Medications suspended | U/NE= 1299 (2–100 ug/d); 7682 (11.8–591 nmol/d) U/E=1 (2–15 ug/d); 5.4 (11.8–81.8 nmol/d) U/D= 321.8 (65–400 ug/d); 2100.7 (424–2609.6 nmol/d) |
U/NE= 235 (2–100 ug/d); 1389 (11.8–591 nmol/d) U/E=1 (2–15 ug/d); 5.4 (11.8–81.8 nmol/d) U/D=413 (65–400 ug/d); 2694 (424–2609.6 nmol/d) |
| 2 | 141/100 | Propranolol 60 mg; phenoxybenzamine 30 mg | 111/60 | Medications suspended | U/NE= 766 (11–86 ug/d); 4530 (65– 508.6 nmol/d) | U/NE= 56 (11–86 ug/d); 331.1 (65–508.6 nmol/d) |
| 3 | 144/90 | Captopril 50 mg | 151/55 | Medications suspended | U/NE= 427 (11–86 ug/d); 2525.2 (65– 508.6 nmol/d) U/E= 0 (0–15 ug/d); 0 (0–81.8 nmol/d) U/D= 323 (100–440 ug/d); 2107 (652.4–2609.6 nmol/d) |
U/NE= 82 (11–86 ug/d); 484.9 (65– 508.6 nmol/d) U/E= 0 (0–15 ug/d); 0 (0–81.8 nmol/d) U/D= 124 (100–440 ug/d); 808.9 (652.4– 2609.6 nmol/d) |
| 4 | 140/90 | Amlodipine, 20 mg; furosemide, 30 mg; irbesartan, 300 mg; clonidine, 0.1 mg/prn; clonidine, transdermal /0.2 weekly ; phenoxybenzamine, 20 mg; hydralazine 50 mg | 124/76 | Phenoxybenzamine, 20 mg; all others suspended | P/NE= 16260 (80–520 pg/mL); 96161.6 (473.1 –3075.2 pmol/L) P/E= 39 (10–200 pg/mL); 212.7 (54.5–1090.8 pmol/L) P/D= 52 (0–20 pg/mL); 283.6 (0–109 pmol/L) |
P/NE= 500 (80–520 pg/mL); 2957 (473.1 –3075.2 pmol/L) |
| 5 | 155/110 | Metoprolol, 100 mg; doxazosin, 8 mg; nicardipine, 30 mg; phenoxybenzamine, 40 mg | 97/63 | Metoprolol 50 mg All others suspended |
U/NM=10563 (less than 900 ug/d); 57673.9 (less 4914 nmol/d) | U/NM=1010 (less than 900 ug/d); 5514.6 (less 4914 nmol/d) |
| 6 | 130/88 | Labetalol, 800 mg; HCTZ, 25 mgb; lisinopril 20 mg Amlodipine 5 mg Phenoxybenzamine 20 mg |
127/79 | Labetalol 400mg Amlodipine 5mg All others suspended |
U/NE= 11164 (58–203 ug/d); 66023.8 (343–1200.5 nmol/d) U/E= 168 (0–15 ug/d); 916.2 (0–81.8 nmol/d) |
U/NE=2266 (58–203 ug/d); 13401.1 (343–1200.5 mol/d) U/E= 325 (0–15 ug/d); 1772.5 (0–81.8 nmol/d) |
Nitroglycerin as needed;
Hydrochlorothiazide
U/NE indicates urinary norepinephrine; U/E, urinary epinephrine; U/D, urinary dopamine; P/NE, plasma norepinephrine; P/E, plasma epinephrine; P/D, plasma dopamine; U/NM, urinary normetanephrine
Conversion Factors for P/NE 5.914 pmol/L; P/E= 5.454 pmol/L; P/D 6.524 pmol/L; U/NE= 5.914 nmol/d; U/E= 5.454 nmol/d; U/D= 6.524 nmol/d; U/NM= 5.46 nmol/d
Patients underwent various measurements of urinary and plasma biochemical markers of catecholamine excess. Because tests for these markers varied among patients over time, we could not perform a standardized analysis. Individual results for patients who experienced a blood pressure response are listed in Table 4.
Survival
Among the 52 patients treated with chemotherapy and were evaluable for response, the OS rate at 5 years was 51% (95% CI: 38–67%). In the group of 17 patients who responded to chemotherapy, the median OS time was 6.4 years (95% CI: 5.2–16.4) while for non-responders was 3.7 years (95% CI: 4.0–7.5) (log-rank test from landmark analysis at 1 year of chemotherapy, P-value = 0.24). The 30 patients with synchronous metastatic disease had a median OS time of 3.7 years (95% CI: 2.9–5.1), and the 22 patients with metachronous metastatic disease had a median OS time of 9.9 years (95% CI: 7.5-NA) (log-rank test, P-value <0.001). We also analyzed the survival by treatment and found that patients, who had surgery for their primary tumor and chemotherapy, had a longer survival compared to the patients who only had chemotherapy (6.5 years vs 3 years, log-rank test, P-value <0.001).
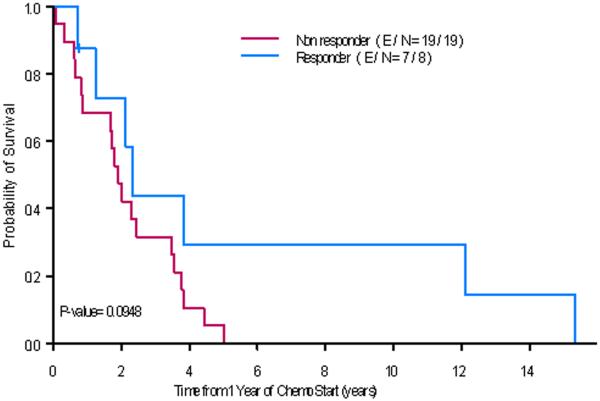
Among the 17 patients who experienced a response, 10 had synchronous metastatic disease with a median OS time of 5.6 years (95%CI: 4.4-NA). None of these patients underwent any surgical resection before chemotherapy was started. The other 7 responders had metachronous metastatic disease with a median OS time of 7.6 years (95% CI: 5.9-NA) (log-rank test, P-value = 0.433). Within the group of patients who had synchronous metastatic disease, a positive response status at one year after the start of chemotherapy was associated with a trend toward a longer survival (Fig. 1; P-value = 0.095). In addition, when we analyzed the characteristics of the patients with synchronous metastatic disease by their response to chemotherapy, we discovered that the non-responders had larger tumors than the responders (median 10 cm vs 5.2 cm P-value = 0.022) (Table 5).
Fig. 1.
Overall survival for patients with synchronous metastatic disease and chemotherapy: responders and not responders at one year after the start of chemotherapy. E/ N= number of events/total number of patients
Table 5.
Characteristics in patients with synchronous metastatic disease who received chemotherapy: responders and non-responders.
| Patient demographics | Responders (N=10) | Non-responders (N=20) | Total (N=30) | P-value |
|---|---|---|---|---|
| Age | ||||
| Median (min to max) | 44 (33–50) yr | 42 (13–70) yr | 42 (13 to70)yr | 0.81 |
| Gender | ||||
| Male | 5 (29.4%) | 12 (70.6%) | 17 (56.7%) | 0.71 |
| Female | 5 (38.5%) | 8 (61.5%) | 13 (43.3%) | |
| Primary tumor size | 0.022 | |||
| Median (min to max) | 5.2 (3–12) cm | 10 (4.1–15) cm | 10 (3–16) cm | |
| Tumor type | ||||
| Pheochromocytoma | 3 (30%) | 7 (70%) | 10 (33.3%) | 1.0 |
| Paraganglioma | 7 (35%) | 13 (65%) | 20 (66.7%) | |
| Metastasis site | ||||
| Single site | 4 (36.3%) | 7 (63.6%) | 11 (60%) | 0.70 |
| Multiple sites | 6 (31.5%) | 13 (68.4%) | 19 (40%) |
We performed a multivariate Cox proportional hazard model analyses with response status at one year of chemotherapy initiation (yes versus no) and tumor size (fitted as continuous variable) as covariates. The effect of response to chemotherapy at one year on OS was significant (P-value = 0.05; HR=0.22, 95% CI: 0.05–1.0) with the adjustment of tumor size (P-value=0.77) in the model for the patients with synchronous metastatic disease.
DISCUSSION
To our knowledge, this is the largest single-institution review of the clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas and sympathetic paragangliomas. In our study, 33% of patients with metastatic pheochromocytoma or sympathetic paraganglioma, experienced improvements in hypertension and/or tumor size after front-line chemotherapy. The improvement in hypertension was evidenced by a substantial dosage reduction or discontinuation of antihypertensive medications. We also found that the clinical benefits observed were associated with a trend towards a longer survival in the responders to chemotherapy that had synchronous metastatic disease than the non-responders who had synchronous metastases. Cyclophosphamide and dacarbazine combined with vincristine, and/or doxorubicin based chemotherapy was the regimen provided to these patients. Forty percent of patients treated with this particular regimen exhibited clinical benefits.
We chose tumor size reduction and/or blood pressure control as response endpoints in this study. The diagnosis of pheochromocytoma and sympathetic paragangliomas relies partly on the biochemical demonstration of excessive catecholamines or their metabolites in the blood and urine.14, 15 These biochemical markers are strongly correlated with tumor size and are the cause of hypertension observed in these patients.16 Therefore, the tumor destruction caused by chemotherapy could decrease catecholamine synthesis and normalize blood pressure, decreasing the need for antihypertensive medications. Hypertension is an objective, easy-to-measure variable and it is well recognized that a small reduction in the blood pressure of hypertensive patients, dramatically reduces cardiovascular complications, thus improving OS.17, 18 Hypertension can be difficult to control in patients with metastatic pheochromocytomas and sympathetic paragangliomas and these patients often take multiple medications. Thus, an improvement in blood pressure control may be associated with OS improvement in these patients. In fact, a current phase II clinical trial of Ultratace iobenguane I-131 (MIBG without carrier) in malignant pheochromocytomas and sympathetic paragangliomas is using blood pressure control as the primary endpoint of therapeutic success (www.clinicaltrials.gov)
Analyzing differences in survival by tumor response to therapy using standard statistical approaches and measuring the event of interest from the start of treatment can lead to biased and incorrect conclusions.19 For instance, responders could have shown a longer survival than non-responders because of more favorable pretreatment characteristics rather than the therapy itself. To avoid such bias, we compared OS by response to therapy using the landmark method,20 in which patient's response is fixed at 1 predetermined time point. Furthermore, when we compared pretreatment characteristics of responders and non-responders, we could not find any differences.
What factors predict response to chemotherapy in this disease remains unknown. Tumors larger than 5 cm and extraadrenal location have been recognized as clinical predictors of decreased overall survival in patients with pheochromocytomas and sympathetic paragangliomas.5 Our univariate analysis suggested that patients with large primary tumors are less likely to have a response to chemotherapy. However, in the multivariate Cox proportional hazards model, the effect of response at 1 year on overall survival was independent from tumor size. Additionally, response to chemotherapy did not correlate with any other factors, particular primary tumor location.
Another reported clinical predictor of survival is the presence of germline mutations of the SDHB gene.21 However, since genetic testing for succinate dehydrogenase germline mutations was not introduced at our institution until 2005 we found 9 patients who underwent genetic testing. Therefore, we could not determine whether a particular genotype was associated with higher response to chemotherapy. Of note, none of the patients that tested positive for SDHB mutations were in the responders group. Nonetheless, future studies will need to explore this further.
Performance status is a strong predictor of survival for cancer patients, but we could not evaluate it in this retrospective study. This information should be used to select patients for systemic therapy, as the tolerability of a regimen may depend upon the individual's performance status.
We found that patients with synchronous metastases at diagnosis exhibited a shorter median OS than patients whose metastatic disease was indentified later (metachronous metastases). This contrasts with the results of the most recent study of chemotherapy and pheohromocytomas by Nomura et al7, that described that patients with metastases found at diagnosis had better survival than those whose metastases were found later. However, in other malignancies a poorer survival has been reported when metastatic disease is present at diagnosis than when it occurs later.22, 23
Unfortunately, most patients with metastatic pheochromocytomas and sympathetic paragangliomas do not respond to cytotoxic chemotherapy (67% in our series) and the effect of chemotherapy on OS is small. Currently,131I-MIBG, remains as the only other nonsurgical treatment that has shown some benefits in treating symptomatic disease with tumor responses above 30%.24, 25 However, this radiopharmaceutical agent has not been shown to be effective in tumors that lack MIBG uptake (approximately 30%).26 Furthermore, there is evidence that some patients with metastases that are avid for I131-MIBG often harbor concurrent metastases that are not avid and may remain undetected even on post-therapy whole-body scanning after high-dose131I-MIBG therapy.26 Additionally, there are only few specialized centers in the United States and worldwide that have131I-MIBG as a treatment. Therefore, for patients with non-MIBG avid tumors and patients with MIBG avid tumors that do not response to131I-MIBG therapy, systemic chemotherapy may be the only therapeutic option.
Recent advances in the study of genetics and molecular pathways have helped elucidate the signal transduction abnormalities that occur in these tumors, and some of these may serve as targets for new therapies. Moderate to strong vascular endothelial growth factor receptor expression and platelet-derived growth factor receptor-β expression have been found in malignant pheochromocytomas and sympathetic paragangliomas.21 Thus, a considerable proportion of patients with these tumors may benefit from therapies that target hypoxia and angiogenic factors. A few case reports have described clinical benefits in patients treated with sunitinib.27, 28
The main limitation of our study is its retrospective design and the lack of a control group. In addition, most medical records lacked universal descriptions of performance status, such as Karnofsky performance status or Eastern Cooperative Oncology Group scores, so we could not asses that factor's effect on response or OS. Nonetheless, this study has multiple strengths. All pathology specimens and radiologic images were reviewed by specialists who confirmed the diagnosis. Hypertension and medication changes were well documented by nurses, pharmacists, and physicians. Furthermore, our study represents the largest single institutional experience.
CONCLUSION
Pheochromocytomas and sympathetic paragangliomas are rare tumors; therefore, retrospective studies may be the only feasible way to inform current multidisciplinary treatment strategies. We found that cyclophosphamide and dacarbazine with vincristine and/or doxorubicin was associated with blood pressure reduction, and longer OS in some patients. The information presented here is useful to develop therapeutic guidelines.
Aknowledgments
We thank Melissa G. Burkett and John H. McCool of the MD Anderson Department of Scientific Publications for editorial assistance
Funding source: This study received financial support from The University of Texas MD Anderson Cancer Center Support Grant (CA016672), from the National Institutes of Health
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- 1.Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58(12):802–4. [PubMed] [Google Scholar]
- 2.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc. 1981;56(6):354–60. [PubMed] [Google Scholar]
- 3.Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999;141(6):619–24. doi: 10.1530/eje.0.1410619. [DOI] [PubMed] [Google Scholar]
- 4.Melicow MM. One hundred cases of pheochromocytoma (107 tumors) at the Columbia-Presbyterian Medical Center, 1926–1976: a clinicopathological analysis. Cancer. 1977;40(5):1987–2004. doi: 10.1002/1097-0142(197711)40:5<1987::aid-cncr2820400502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical Risk Factors for Malignancy and Overall Survival in Patients with Pheochromocytomas and Sympathetic Paragangliomas: Primary Tumor Size and Primary Tumor Location as Prognostic Indicators. J Clin Endocrinol Metab. 2011;96(3):717–25. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 6.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14(3):569–85. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 7.Nomura K, Kimura H, Shimizu S, Kodama H, Okamoto T, Obara T, et al. Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy. J Clin Endocrinol Metab. 2009;94(8):2850–56. doi: 10.1210/jc.2008-2697. [DOI] [PubMed] [Google Scholar]
- 8.Joseph L. Malignant phaeochromocytoma of the Organ of Zuckerkandl with functioning metastases. British Journal of Urology. 1967;39(2):221–25. doi: 10.1111/j.1464-410x.1967.tb09801.x. [DOI] [PubMed] [Google Scholar]
- 9.Keiser HR, Goldstein DS, Wade JL, Douglas FL, Averbuch SD. Treatment of malignant pheochromocytoma with combination chemotherapy. Hypertension. 1985;7(3 Pt 2):I18–24. doi: 10.1161/01.hyp.7.3_pt_2.i18. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113(8):2020–8. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SR, Winchester DJ, Benjamin RS. A 15-year experience with chemotherapy of patients with paraganglioma. Cancer. 1995;76(8):1476–80. doi: 10.1002/1097-0142(19951015)76:8<1476::aid-cncr2820760827>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Vassilopoulou-Sellin R. Clinical outcome of 50 patients with malignant abdominal paragangliomas and malignant pheochromocytomas. Endocrine-Related Cancer. 1998;5(1):59–68. [Google Scholar]
- 13.Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, et al. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94(3):730–37. doi: 10.1002/cncr.10252. [DOI] [PubMed] [Google Scholar]
- 14.Lenders JW, Pacak K, Eisenhofer G. New advances in the biochemical diagnosis of pheochromocytoma: moving beyond catecholamines. Ann N Y Acad Sci. 2002;970:29–40. doi: 10.1111/j.1749-6632.2002.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 15.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Lenders JW, Goldstein DS, Mannelli M, Csako G, Walther MM, et al. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin Chem. 2005;51(4):735–44. doi: 10.1373/clinchem.2004.045484. [DOI] [PubMed] [Google Scholar]
- 17.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24):3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 21.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, et al. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87(10):4771–4. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 22.Rasalkar DD, Chu WC, Lee V, Paunipagar BK, Cheng FW, Li CK. Pulmonary metastases in children with osteosarcoma: characteristics and impact on patient survival. Pediatr Radiol. 2011;41(2):227–36. doi: 10.1007/s00247-010-1809-1. [DOI] [PubMed] [Google Scholar]
- 23.Tan EK, Ooi LL. Colorectal cancer liver metastases - understanding the differences in the management of synchronous and metachronous disease. Ann Acad Med Singapore. 2010;39(9):719–15. [PubMed] [Google Scholar]
- 24.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20(11):648–58. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 25.Gedik GK, Hoefnagel CA, Bais E, Olmos RA. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2008;35(4):725–33. doi: 10.1007/s00259-007-0652-6. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald PA, Goldsby RE, Huberty JP, Price DC, Hawkins RA, Veatch JJ, et al. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–90. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez C, Cabanillas ME, Santarpia L, Jonasch E, Kyle KL, Lano EA, et al. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94(2):386–91. doi: 10.1210/jc.2008-1972. [DOI] [PubMed] [Google Scholar]
- 28.Hahn NM, Reckova M, Cheng L, Baldridge LA, Cummings OW, Sweeney CJ. Patient with malignant paraganglioma responding to the multikinase inhibitor sunitinib malate. J Clin Oncol. 2009;27(3):460–3. doi: 10.1200/JCO.2008.19.9380. [DOI] [PubMed] [Google Scholar]