Abstract
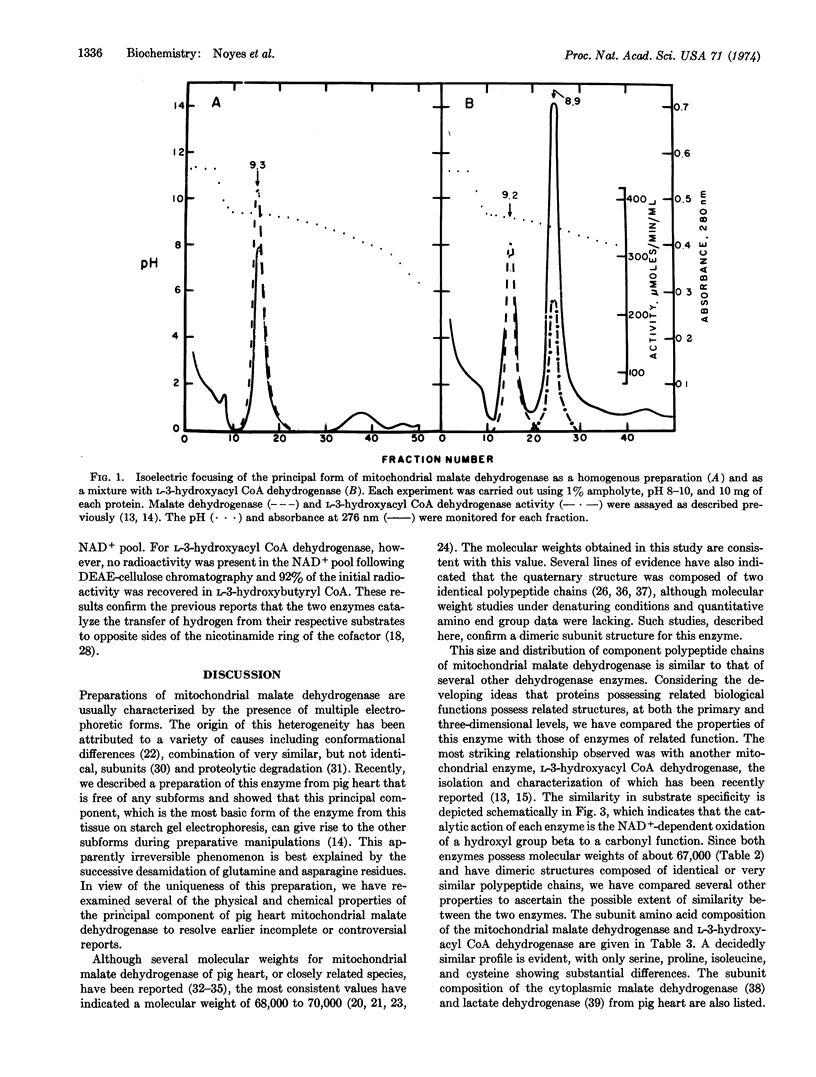
Pig heart mitochondrial malate dehydrogenase (EC 1.1.1.37), which has been obtained free of electrophoretic subforms, has been shown to have a molecular weight of 67,000 and to be composed of two polypeptide chains. Comparison of these and other properties, such as amino-acid composition, isoelectric point, and keto-substrate inhibition, with those of L-3-hydroxyaeyl CoA dehydrogenase (EC 1.1.1.35), another NAD+-dependent dehydrogenase of mitochondrial origin, suggests structural similarities of the type associated with proteins possessing common evolutionary origins. This conclusion is supported by immunological crossreactivity. In view of these observations, the dissimilarity in the stereospecificity of hydrogen transfer from cofactor to substrate catalyzed by the two enzymes is attributed to 180° rotation in the binding orientation of the nicotinamide moiety of the NAD+, rather than to gross differences in the geometry of the active site of the two enzymes.
Keywords: subunit structure, immunological cross-reactivity, amino-acid composition, specificity of hydrogen transfer, evolution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Ford G. C., Koekoek R., Lentz P. J., McPherson A., Jr, Rossmann M. G., Smiley I. E., Schevitz R. W., Wonacott A. J. Structure of lactate dehydrogenase at 2-8 A resolution. Nature. 1970 Sep 12;227(5263):1098–1103. doi: 10.1038/2271098a0. [DOI] [PubMed] [Google Scholar]
- Anderton B. H. Identification of an essential, reactive histidine in pig heart mitochondrial malate dehydrogenase. Eur J Biochem. 1970 Sep;15(3):562–567. doi: 10.1111/j.1432-1033.1970.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Browne W. J., North A. C., Phillips D. C., Brew K., Vanaman T. C., Hill R. L. A possible three-dimensional structure of bovine alpha-lactalbumin based on that of hen's egg-white lysozyme. J Mol Biol. 1969 May 28;42(1):65–86. doi: 10.1016/0022-2836(69)90487-2. [DOI] [PubMed] [Google Scholar]
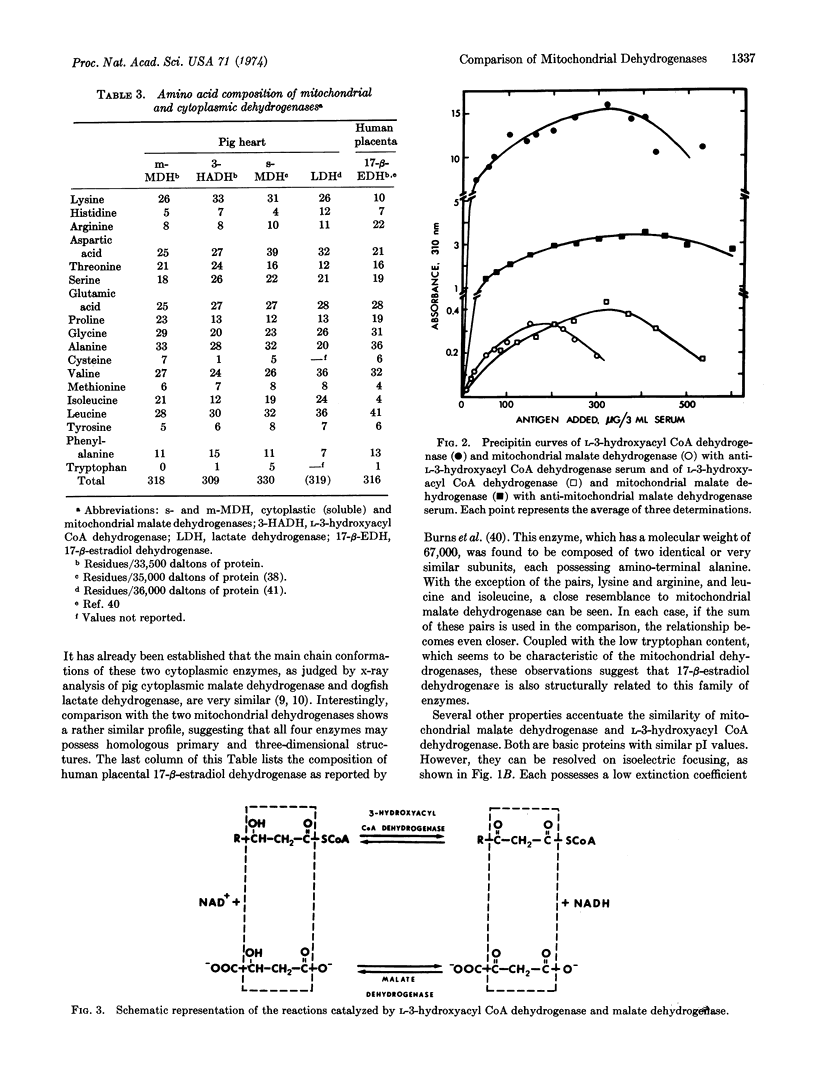
- Burns D. J., Engel L. L., Bethune J. L. Amino acid composition and subunit structure. Human placental 17 -estradiol dehydrogenase. Biochemistry. 1972 Jul 4;11(14):2699–2703. doi: 10.1021/bi00764a023. [DOI] [PubMed] [Google Scholar]
- Cassman M., King R. Molecular weight studies on the supernatant malate dehydrogenase isolated from beef heart. Evidence for a proteolytic contaminant in the purified preparation. Biochim Biophys Acta. 1972 Jan 26;257(1):143–149. doi: 10.1016/0005-2795(72)90263-2. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kitto G. B., Kaplan N. O. Factors affecting the reversible dissociation of dehydrogenases. Proc Natl Acad Sci U S A. 1965 May;53(5):1006–1014. doi: 10.1073/pnas.53.5.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Teixeira A., Kenworthy P. The stereospecificity of nicotinamide-adenine dinucleotide-dependent oxidoreductases from plants. Biochem J. 1972 Apr;127(2):335–343. doi: 10.1042/bj1270335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dévényi T., Rogers S. J., Wolfe R. G. Structural studies of pig heart malate dehydrogenase. Nature. 1966 Apr 30;210(5035):489–491. doi: 10.1038/210489a0. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- GRIMM F. C., DOHERTY D. G. Properties of the two forms of malic dehydrogenase from beef heart. J Biol Chem. 1961 Jul;236:1980–1985. [PubMed] [Google Scholar]
- Glatthaar B. E., Barbarash G. R., Noyes B. E., Banaszak L. J., Bradshaw R. A. The preparation of the cytoplasmic and mitochondrial forms of malate dehydrogenase and aspartate aminotransferase from pig heart by a single procedure. Anal Biochem. 1974 Feb;57(2):432–451. doi: 10.1016/0003-2697(74)90099-2. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Rohrbach M. S., Harrison J. H. Selective chemical modification of malate dehydrogenase. N-ethylmaleimide modification of active center sulfhydryl residues. J Biol Chem. 1971 Sep 10;246(17):5491–5497. [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Heyde E., Ainsworth S. A reappraisal of some structural features of bovine heart malate dehydrogenase. Biochem J. 1968 Oct;109(4):663–668. doi: 10.1042/bj1090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Brew K., Vanaman T. C., Trayer I. P., Mattock P. The structure, function, and evolution of alpha-lactalbumin. Brookhaven Symp Biol. 1968 Jun;21(1):139–154. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kitto G. B., Lewis R. G. Purification and properties of tuna supernatant and mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1967 May 16;139(1):1–15. doi: 10.1016/0005-2744(67)90107-6. [DOI] [PubMed] [Google Scholar]
- Kitto G. B., Wassarman P. M., Kaplan N. O. Enzymatically active conformers of mitochondrial malate dehydrogenase. Proc Natl Acad Sci U S A. 1966 Aug;56(2):578–585. doi: 10.1073/pnas.56.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick R. J., Barnes F. W. Heterogeneity of supernatant malate dehydrogenase. Biochim Biophys Acta. 1968 Aug 27;167(1):1–8. doi: 10.1016/0005-2744(68)90272-6. [DOI] [PubMed] [Google Scholar]
- MARCUS A., VENNESLAND B., STERN J. R. The enzymatic transfer of hydrogen. VII. The reaction catalyzed by beta-hydroxybutryl dehydrogenase. J Biol Chem. 1958 Sep;233(3):722–726. [PubMed] [Google Scholar]
- Murphey W. H., Kitto G. B., Everse J., Kaplan N. Malate dehydrogenases. I. A survey of molecular size measured by gel filtration. Biochemistry. 1967 Feb;6(2):603–610. doi: 10.1021/bi00854a031. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Bradshaw R. A. L-3-hydroxyacyl coenzyme A dehydrogenase from pig heart muscle. I. Purification and properties. J Biol Chem. 1973 May 10;248(9):3052–3059. [PubMed] [Google Scholar]
- Noyes B. E., Bradshaw R. A. L-3-hydroxyacyl coenzyme A dehydrogenase from pig heart muscle. II. Subunit structure. J Biol Chem. 1973 May 10;248(9):3061–3066. [PubMed] [Google Scholar]
- SAN PIETRO A., KAPLAN N. O., COLOWICK S. P. Pyridine nucleotide transhydrogenase. VI. Mechanism and stereospecificity of the reaction in Pseudomonas fluorescens. J Biol Chem. 1955 Feb;212(2):941–952. [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. I. Properties of the enzyme purified from extracts of acetone-dried powders. Biochim Biophys Acta. 1961 Nov 25;54:67–76. doi: 10.1016/0006-3002(61)90938-6. [DOI] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Shotton D. M., Watson H. C. Three-dimensional structure of tosyl-elastase. Nature. 1970 Feb 28;225(5235):811–816. doi: 10.1038/225811a0. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The crystal and molecular structure of DIP-inhibited bovine trypsin at2.7Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:125–140. doi: 10.1101/sqb.1972.036.01.018. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., GROSSMAN L. I., KAPLAN N. O. Starch-gel electrophoresis of malate dehydrogenase. Biochim Biophys Acta. 1963 Jun 11;73:193–203. doi: 10.1016/0006-3002(63)90303-2. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- THORNE C. J. Properties of mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1962 Jun 4;59:624–633. doi: 10.1016/0006-3002(62)90642-x. [DOI] [PubMed] [Google Scholar]
- WACHSMUTH E. D., PFLEIDERER G., WIELAND T. AMINOSAEUREZUSAMMENSETZUNG VON ISOZYMEN DER LACTATDEHYDROGENASE AUS MENSCHLICHEN UND TIERISCHEN ORGANEN. Biochem Z. 1964 Jul 8;340:80–94. [PubMed] [Google Scholar]
- WOLFE R. G., NEILANDS J. B. Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem. 1956 Jul;221(1):61–69. [PubMed] [Google Scholar]



