Abstract
Rationale
Optimal management of complicated parapneumonic effusions (CPPE) remains controversial.
Objectives
to assess safety and efficacy of iterative therapeutic thoracentesis (ITTC), the first-line treatment of CPPE in Rennes University Hospital.
Methods
Patients with CPPE were identified through our computerized database. We retrospectively studied all cases of CPPE initially managed with ITTC in our institution between 2001 and 2010. ITTC failure was defined by the need for additional treatment (i.e. surgery or percutaneous drainage), or death.
Results
Seventy-nine consecutive patients were included. The success rate was 81% (n = 64). Only 3 patients (4%) were referred to thoracic surgery. The one-year survival rate was 88%. On multivariate analysis, microorganisms observed in pleural fluid after Gram staining and first thoracentesis volume ≥450 mL were associated with ITTC failure with adjusted odds-ratios of 7.65 [95% CI, 1.44–40.67] and 6.97 [95% CI, 1.86–26.07], respectively. The main complications of ITTC were iatrogenic pneumothorax (n = 5, 6%) and vasovagal reactions (n = 3, 4%). None of the pneumothoraces required chest tube drainage, and no hemothorax or re-expansion pulmonary edema was observed.
Conclusions
Although not indicated in international recommendations, ITTC is safe and effective as first-line treatment of CPPE, with limited invasiveness.
Introduction
Pleural infection is a common clinical problem associated with significant morbidity and mortality [1], [2]. Most guidelines recommend that complicated parapneumonic effusions (CPPE) be evacuated, in addition to appropriate antibiotics [3], but the optimal evacuation method remains controversial and poorly standardized. Current options include iterative therapeutic thoracentesis (ITTC), chest tube drainage, video-assisted thoracoscopic surgery (VATS), or thoracotomy. [4]–[6]. Few randomized studies compared evacuation methods in CPPE, but no consensus could be reached from these studies, due to their limited sample size, and heterogeneity [5], [7].
In our department, ITTC has long been the first-line treatment to remove infected pleural fluid in CPPE, in association with systemic antibiotics. The theoretical benefits associated with this procedure include shorter immobilization, and limited use of thromboprophylaxis and analgesics, as compared to chest tube drainage or surgery.
We report our experience of systematic use of ITTC as first-line treatment in CPPE, focusing on efficacy, tolerability, and risk factors for failure.
Methods
Patients
We performed an observational study of all consecutive patients managed with ITTC as first-line treatment for CPPE in the Rennes University Hospital, France, during years 2001–2010. Other aetiologies (i.e. surgery, trauma, mediastinal or sub-diaphragmatic primary infections) were excluded from this study, as well as non-complicated parapneumonic effusions. We included all patients with at least one of the following characteristic for pleural fluid: frank pus (empyema), micro-organisms observed after Gram staining, pH <7.2, glucose level <2.2 mmol/L, loculations or profuse effusion [5], [6].
The study was approved by the Rennes University Hospital Ethics Committee (project approval number 12.52) which waived the informed consent.
ITTC Protocol
The ITTC protocol was standardized in our department. Thoracentesis were performed at bedside under local anaesthesia (lidocain 1%) using 8- or 10-French disposable pleural needle (Novatech®, La Ciotat, France). Each evacuation was maximal, until no more liquid was aspirated, or until the patient could no longer tolerate the thoracentesis (irrepressible cough, chest pain, or vagal faintness). Thoracentesis were repeated every 1 to 3 days until major decrease of the pleural opacity on chest X-ray and/or until no more pleural fluid could be aspirated. A chest X-ray was performed after each thoracentesis. Intrapleural fibrinolysis protocol was standardized, based on urokinase (Eumedica®, Biarritz, France), 100 000 UI in 50 mL of saline, instilled in the pleural space via the pleural needle at the end of thoracentesis. Fibrinolysis was contra-indicated in patients at risk of severe haemorrhagic events, as recommended. The decision of using intrapleural fibrinolysis was left to the physician in charge.
Failure of Management with ITTC
Failure of ITTC was defined by the need to escalate therapy (i.e. chest tube drainage or thoracic surgery), or death due to sepsis.
Data Collection
Demographic, clinical, biological and radiological data, especially characteristics of pleural fluid and thoracentesis; and data related to complications and to patients’ vital status were collected from medical records using a standardized questionnaire. Physicians blinded to clinical data reviewed chest X-ray and CT-scan, and collected data on the location and quantity of pleural effusion, the existence of a mediastinal shift, and loculations. Pain was estimated through the use of analogic visual scale when performed, and through the prescription of analgesics.
Statistical Analysis
Statistical analysis was performed using SAS® version 9.3 software. Two-tailed p-values were reported, with p<0.05 considered as statistically significant.
A descriptive analysis with medians and 25th and 75th percentiles (IQR) for quantitative variables, and absolute and relative frequencies for qualitative variables were performed. The global survival rate of the cohort was estimated using the Kaplan-Meier estimator. The association between the ITTC outcomes and the characteristics of both patients and ITTC procedures was then studied using the Fischer exact test or the Pearson chi-squared test, and the Mann-Whitney U test. Finally, a binomial logistic model was built with the ITTC outcomes as dependent variable, and the overall significant variables associated with the ITTC outcomes in bivariate analysis. Factors with more than 10% of missing data were excluded from the multivariate analysis, and quantitative variables were first categorized in quartile.
Results
Patients Characteristics
Seventy-nine consecutive patients were included. Patients and pleural fluids’ characteristics are described in Table 1 and 2. Median age was 54 years (IQR 46–71), and male-to-female sex ratio was 2.59. Most infections were community-acquired (n = 72, 91%). The most frequent comorbidities were alcohol abuse (n = 25, 32%), and neurological disorders (n = 25, 32%). Only 32% of patients did not have any comorbidity.
Table 1. Characteristics of patients.
| Total (n = 79) | Success (n = 64) | Failure (n = 15) | p | ||
| Demographical and Clinical characteristics | |||||
| Median age, years (IQR) | 54 (46–71) | 56 (45.5–71) | 53 (47–72) | 0.803 | |
| Male gender, n (%) | 57 (72%) | 45 (70%) | 12 (80%) | 0.539 | |
| Community-acquired infection, n (%) | 72 (91%) | 59 (92%) | 13 (87%) | 0.612 | |
| Delay symptoms-admission, days (IQR) | 10 (5–22) | 10 (4.5–21) | 8 (5–25) | 0.837 | |
| Smoker, n (%) | 49 (62%) | 39 (61%) | 10 (67%) | 0.774 | |
| COPD, n (%) | 7 (9%) | 6 (9%) | 1 (7%) | 1.000 | |
| Heart disease, n (%) | 11 (14%) | 9 (14%) | 2 (13%) | 1.000 | |
| Diabetes mellitus, n (%) | 8 (10%) | 5 (8%) | 3 (20%) | 0.171 | |
| Immunodepression, n (%) | 6 (8%) | 4 (6%) | 2 (13%) | 0.319 | |
| Alcohol abuse, n (%) | 25 (32%) | 19 (30%) | 6 (40%) | 0.540 | |
| Cancer, n (%) | 13 (16.5%) | 10 (16%) | 3 (20%) | 0.704 | |
| Chronic liver disease, n (%) | 9 (11%) | 5 (8%) | 4 (27%) | 0.061 | |
| Neurological impairment, n (%) | 25 (32%) | 19 (30%) | 6 (40%) | 0.540 | |
| Non-steroid anti-inflammatory drugs, n (%) | 15 (19%) | 14 (22%) | 1 (7%) | 0.279 | |
| Corticosteroids >3 weeks, n (%) | 9 (11%) | 8 (12.5%) | 1 (7%) | 1.000 | |
| Antibiotics initiated before thoracentesis, n (%) | 37 (47%) | 32 (50%) | 5 (33%) | 0.268 | |
| Radiological data | |||||
| Right side location, n (%) | 46 (58%) | 36 (56%) | 10 (67%) | 0.567 | |
| Large effusion (> ½ thorax), n (%) | 43 (55%) | 33 (52%) | 10 (67%) | 0.509 | |
| Bilateral effusion, n (%) | 5 (6%) | 5 (8%) | 0 (0%) | 0.576 | |
| Mediastinal shift, n (%) | 17 (22%) | 10 (16%) | 7 (47%) | 0.016 | |
| Loculations, n (%) | 47 (66%) | 38 (68%) | 9 (60%) | 0.557 | |
| Index of diseases severity | |||||
| Respiratory failure, n (%) | 12 (15%) | 9 (14%) | 3 (20%) | 0.689 | |
| Severe sepsis, n (%) | 4 (5%) | 3 (5%) | 1 (7%) | 0.577 | |
| Impaired consciousness-Confusion | 8 (10%) | 6 (9%) | 2 (13%) | 0.643 | |
| Urea, mmol/L (IQR) | 5.4 (3.4–8.1) | 5.3 (3.4–8.0) | 5.9 (3.6–8.2) | 0.762 | |
| Albumin, g/L (IQR)(†) | 22.1 (20.0–24.0) | 22.2 (21.7–24.5) | 20.9 (19.0–23.0) | 0.177 | |
IQR: Interquartile range; COPD: chronic obstructive pulmonary disease.
Quantitative variables are indicated as median (IQR), qualitative variables are indicated as numbers (%). (†) Variables with >10% of missing data.
Table 2. Pleural fluid and microbiology characteristics (first thoracentesis).
| Total (n = 79) | Success (n = 64) | Failure (n = 15) | p | ||
| Pleural fluid analysis | |||||
| Frank pus (empyema), n (%) | 52 (66%) | 39 (61%) | 13 (87%) | 0.073 | |
| Protein, g/L (IQR)(†) | 46 (41–50) | 45 (41–50) | 47 (34–48) | 0.534 | |
| LDH, IU/L (IQR)(†) | 4336 (1721–17810) | 3932 (1134–14872) | 17284 (9452–26068) | 0.079 | |
| pH (IQR)(†) | 7.19 (7.0–7.5) | 7.10 (7.0–7.5) | 7.50 (7.15–7.75) | 0.422 | |
| Glucose level, mmol/L (IQR)(†) | 1.0 (0.1–3.8) | 1.0 (0.1–4.1) | 0.6 (0.1–1.1) | 0.716 | |
| Micro-organisms observed on Gram staining, n (%) | 41 (52%) | 28 (44%) | 13 (87%) | 0.003 | |
| Positive culture, n (%) | 36 (46%) | 26 (41%) | 10 (67%) | 0.088 | |
| Positive culture on aerobic atmosphere, n (%) | 25 (32%) | 20 (31%) | 5 (36%) | 0.759 | |
| Positive culture on anaerobic atmosphere, n (%) | 16 (20%) | 11 (17%) | 5 (36%) | 0.036 | |
| Polymicrobial culture, n (%) | 12 (15%) | 8 (12.5%) | 4 (27%) | 0.227 | |
| Microbiological characteristics | |||||
| Positive blood culture, n (%) | 7 (9%) | 5 (8%) | 2 (14%) | 0.612 | |
| Positive pneumococcal urine antigen, n (%) | 7 (37%) | 5 (38.5%) | 2 (33%) | 1.000 | |
| Identified bacteria | |||||
| Anaerobic bacteria, n (%) | 16 (20%) | 11 (17%) | 5 (33%) | 0.171 | |
| Streptococcus milleri, n (%) | 15 (19%) | 12 (19%) | 3 (20%) | 1.000 | |
| Streptococcus pneumoniae, n (%) | 12 (15%) | 9 (14%) | 3 (20%) | 0.689 | |
| Other Streptococcus sp., n (%) | 8 (10%) | 5 (8%) | 3 (20%) | 0.171 | |
| Staphylococcus aureus, n (%) | 3 (4%) | 2 (3%) | 1 (7%) | 0.473 | |
| Gram-negative bacteria, n (%) | 3 (4%) | 2 (3%) | 1 (7%) | 0.473 | |
Quantitative variables are indicated as median (IQR), qualitative variables are indicated as numbers (%). (†) Variables with >10% of missing data.
Loculations were observed on chest X-ray and/or CT scan in 47 (66%) of patients. At least one microbiological documentation was obtained in 45 (57%) patients, from pleural fluid, blood culture, or pneumococcal urinary antigen.
Fifteen patients (19%) were classified as ‘failure’ of the ITTC strategy, including 12 (15%) who were cured by chest tube drainage, and 3 (4%) who finally required surgical drainage.
Iterative Therapeutic Thoracenteses (ITTC)
ITTC modalities are detailed in Table 3. The median number of thoracenteses was 3 [IQR 2–5]. The median duration of ITTC management was 8 [IQR 4–15] days and the median delay between admission and first therapeutic thoracentesis was 1 [IQR 0–4] day. Blank thoracenteses were observed in 23 (29%) patients.
Table 3. Iterative therapeutic thoracentesis (ITTC) modalities and secondary treatments.
| Total (n = 79) | Success (n = 64) | Failure (n = 15) | p | |||
| ITTC modalities | ||||||
| Number of thoracentesis (IQR) | 3 (2–5) | 4 (2–5.5) | 3 (2–3) | 0.062 | ||
| Duration of management with ITTC, days (IQR) | 8 (4–15) | 9 (5–16) | 5 (2–7) | 0.030 | ||
| Delay admission –1st thoracentesis, day (IQR) | 1 (0–4) | 1 (0–4) | 1 (0–1) | 0.165 | ||
| Delay symptoms –1st thoracentesis, days (IQR) | 12.5 (7–25) | 13 (8–27) | 9 (6–25) | 0.360 | ||
| Ultrasonography-guided procedure, n (%) | 42 (53%) | 37 (58%) | 5 (33%) | 0.149 | ||
| Urokinase use, n (%) | 52 (66%) | 42 (66%) | 10 (67%) | 1.000 | ||
| Number of urokinase injection (IQR) | 2 (1–3) | 2 (1–3) | 1 (1–2) | 0.071 | ||
| Volume 1st thoracentesis, mL (IQR) | 300 (100–450) | 200 (100–400) | 450 (240–700) | 0.009 | ||
| Volume 2nd thoracentesis, mL (IQR) | 200 (140–350) | 200 (150–310) | 275 (45–400) | 0.806 | ||
| Volume 3rd thoracentesis, mL (IQR) | 250 (150–425) | 250 (150–375) | 425 (100–600) | 0.511 | ||
| Total volume of pleural fluid retrieved, mL (IQR) | 875 (500–1600) | 847 (500–1545) | 1000 (450–1700) | 1.000 | ||
| Blank thoracentesis | 23 (29%) | 20 (31%) | 3 (20%) | 0.5332 | ||
| Secondary treatments | ||||||
| Chest tube drainage, n (% total patients) | 12 (15.2%) | |||||
| Surgery, n (% total patients) | 3 (3.8%) | |||||
| Other treatments | ||||||
| Duration of IV antibiotics, days (IQR) | 18.5 (11–32) | 16.5 (11–29) | 31.5 (20–49) | 0.012 | ||
| Total duration of antibiotics, days (IQR) | 47 (38–56) | 46 (38–53) | 49 (44–60) | 0.290 | ||
| Co-amoxiclav use, n (%) | 36 (60%) | 30 (60%) | 6 (67%) | 0.720 | ||
Ultrasonography-guidance was performed in 42 (53%) patients, and intrapleural fibrinolysis in 52 (66%) patients. Median total volume of pleural fluid removed was 875 mL [IQR 500–1600].
Median duration of antibiotics was 18.5 days [IQR 11–32] for intravenous use, and 47 days [IQR 38–56] when oral treatment was taken into account.
Complications
Most frequent complications of ITTC included 5 (6%) iatrogenic pneumothoraces and 3 (4%) vagal faintness (Table 4). No pneumothorax required chest tube drainage, and no re-expansion oedema was observed. We did not observe any allergic reaction related to fibrinolytic agent and no hemothorax. However, two major haemorrhagic events occurred (hemoptysis, and gastrointestinal bleeding, one patient each). The median hospital length of stay was 21 days [IQR 14–34]. The median duration of fever was 10 days [IQR 7–15] in total and 7.5 days [IQR 4–13] after the first thoracentesis. Thirteen patients (16%) were admitted in intensive care unit.
Table 4. Complications during hospitalisation.
| Total (n = 79) | Success (n = 64) | Failure (n = 15) | p | |
| Thoracentesis | ||||
| Step 3 analgesic use | 22 (29%) | 13 (21%) | 9 (60%) | 0.008 |
| Duration of step 3 analgesic use, days (IQR) | 0 (0–1) | 0 (0–0) | 1 (0–13) | 0.019 |
| Vasovagal reaction, n (%) | 3 (4%) | 3 (5%) | 0 (0%) | 1.000 |
| Iatrogenic pneumothorax, n (%) | 5 (6%) | 4 (6%) | 1 (7%) | 1.000 |
| Confinement to bed, days (IQR) | 4 (1–11) | 3 (1–6.5) | 11.5 (8–21) | 0.007 |
| Thromboembolism prophylaxis, days (IQR) | 7 (0–17) | 5 (0–12) | 22 (11–30) | 0.005 |
| General | ||||
| In-hospital death, n (%) | 3 (4%) | 2 (3%) | 1 (7%) | 0.473 |
| Hospital stay, days (IQR) | 21 (14–34) | 21 (13–29) | 33 (18–56) | 0.036 |
| Fever duration, days (IQR) | 10 (7–15) | 10 (7–15) | 9 (5–32) | 0.749 |
| Fever duration after 1st thoracentesis, days (IQR) | 7.5 (4–13) | 7 (4–13) | 9 (4–31) | 0.500 |
| ICU admission, n (%) | 13 (16%) | 5 (8%) | 8 (53%) | <10−3 |
| Re-hospitalisation rate, n (%) | 10 (13.5%) | 7 (12%) | 3 (21%) | 0.388 |
Survival
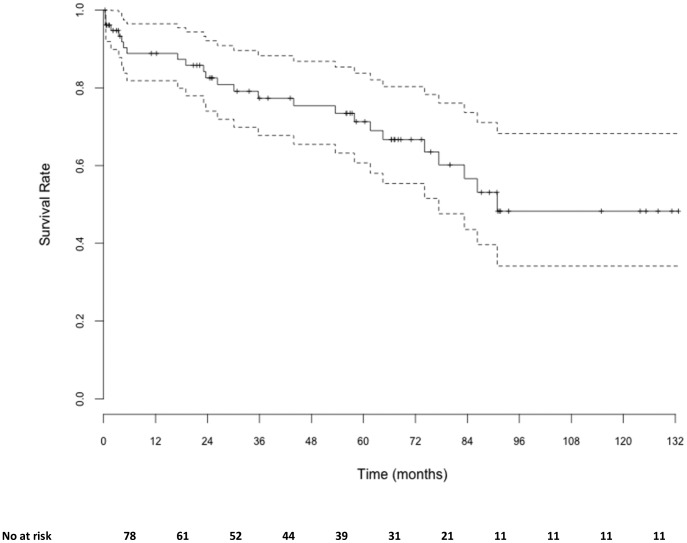
Three patients (4%) died during hospitalisation and the one year survival rate was estimated at 88.8% [95% CI, 81.8–96.5] (Fig. 1). All early death but one (septic shock) occurred in patients under palliative care due to end-stage comorbidities.
Figure 1. Global survival. Doted lines represent 95% confidence interval.
Predictive Factors of ITTC Failure
Four variables were significantly associated with ITTC failures (Table 5): mediastinal shift (p = 0.016), positive anaerobic culture (p = 0.036), micro-organisms observed after Gram staining in pleural fluid (p = 0.003), and volume of the first thoracentesis ≥450 mL (p = 0.009).
Table 5. Factors associated with outcome of iterative therapeutic thoracentesis.
| Univariate | Multivariate | Adjusted odds-ratio | 95% CI | |
| Age | NS | NS | – | – |
| Mediastinal shift | p = 0.016 | NS | – | – |
| Positive culture on anaerobic atmosphere | p = 0.036 | NS | – | – |
| Micro-organisms observed in pleural fluid on Gram staining | p = 0.003 | p = 0.017 | 7.65 | 1.44–40.67 |
| 1st thoracentesis volume ≥450 mL | p = 0.009 | p = 0.004 | 6.97 | 1.86–26.07 |
CI: confidence interval; NS: non-significant.
After fitting the data within the logistic model, only two covariates remained associated with the failures of ITTC (Table 5): micro-organisms observed after Gram staining in pleural fluid (OR = 7.65 [95% CI = 1.44–40.67]) and volume of first thoracentesis ≥450 mL [95% CI = 1.86–26.07]).
Discussion
We analysed 79 consecutive patients with complicated parapneumonic effusion (CPPE) managed with iterative therapeutic thoracentesis (ITTC). Our success rate was 81%, with in-hospital mortality at 4%, and a need for secondary surgery in less than 4% of cases. The median number of thoracentesis needed to achieve cure was 3. Factors associated with failure of ITTC on multivariate analysis were the observation of microorganisms after Gram staining on pleural fluid, and volume of the first thoracentesis ≥450 mL.
Demographic data from our study (median age 54 years, 72% male) and prevalence of comorbidities (68%) are comparable to other studies dealing with CPPE [8]–[13]. Likewise, the high prevalence of empyema (66%), and loculations (66%) are in agreement with previous studies [13], [14]. The relatively low yield of conventional microbiology in pleural fluid is well known [13], [14] and our study highlights the same results, in these settings where patients are frequently on antibiotics before the first thoracentesis (47% of cases in our study). The advent of molecular identification techniques is likely to expand the rate of CPPE with microbiological documentation [15].
In our series, 81% of patients with CPPE could be cured with the combination of antibiotics and ITTC, and did not require chest tube drainage or surgery. The success rates of ITTC are highly variable in the literature, ranging from 2.4% to 100% (Table 6). This variability may be explained by i) differences in patient characteristics; ii) use of heterogeneous protocols (e.g. systematic daily thoracentesis until resolution, versus additional thoracentesis based on clinical and radiological criteria; use of saline irrigation or local antibiotics); iii) the heterogeneity of the criteria used to define success and failure; iv) variability in follow-up duration and methods. The success rate of ITTC in our institution is similar to that of other methods of pleural evacuation in the literature, where success rates ranged from 70% to 94% for chest tube drainage guided by the imaging [16]–[23], and from 71% to 93% for VATS [24]–[26].
Table 6. Success rates of iterative therapeutic thoracentesis in clinical studies: a literature review.
| Author | Characteristics | Number ofpatients | Type of pleuraleffusion | Success n (%) | Mortality n (%) |
| Viana et al. [39] | Monocentric 1964–1968 | 41 | NR | 1 (2.4%) | 8 (19.5%) |
| Benfield et al. [40] | Monocentric 1968–1978 | 24 | NR | 8 (33.3%) | 3 (12.5%) |
| Lemmer et al. [41] | Monocentric 1978–1982 | 4 | NR | 3 (75%) | 1 (25%) |
| Mandal et al. [42] | Monocentric 1972–1984 | 28 | 50% CPPE | 28 (100%) | 0 (0%) |
| Wehr et al. [28] | Monocentric 1974–1984 | 27 | NR | 6 (22.2%) | 2 (7.4%) |
| Storm et al. [43] | Monocentric 1984–1989 | 51 | 100% CPPE and empyema | 48 (94.1%) | 4 (7.8%) |
| Ferguson et al. [8] | Multicentric 1986–1990 | 46 | 100% empyema | 19 (41%) | 3 (6.5%) |
| Simmers et al. [44] | Monocentric 1999 | 29 | 100% CPPE and empyema | 25 (86%) | 4 (14%) |
| Letheulle et al. | Monocentric 2013 | 79 | 100% CPPE and empyema | 64 (81%) | 4 (12%) |
NR: not reported; CPPE: complicated parapneumonic effusions.
Our criteria for failure were death, and/or therapeutic escalation, from ITTC to more invasive procedures (i.e. chest tube drainage or thoracic surgery). The most recent studies [13], [14] defined success as the resolution of CPPE without surgery. With this criterion, our rate of success would have been 96% in our series. In comparison with previous studies relating ITTC, the need for surgery in our study was lower than in Storm et al. [27] and Wehr et al. [28] series (respectively, 6% and 18%). In comparison with the MIST studies, the need for surgery in our study is lower than in MIST1 [13] (16% in the intervention group) and similar to MIST2 [14] (4%). Overall these data suggest that surgery can be avoided in most cases of CPPE [29], which may be preferable given the high prevalence of comorbidities in these populations [8], [30], [31].
In our series, the in-hospital mortality was 4%, with a mortality of 12% at one year. Those results are in accordance with epidemiologic data [30], [32].
In our study, two parameters were independently associated with failure: microorganisms observed on Gram stain of pleural fluid, and first thoracentesis volume ≥450 mL. These two items are available at the beginning of the management and could therefore be used as a guide for closer monitoring, or earlier switch to more invasive strategy. Of note, the observation of micro-organisms after Gram stain on pleural fluid was not affected by antibiotic use prior to first thoracentesis (p = 0.320), and first thoracentesis volume was not correlated to imaging findings such as the estimated size of effusion (p = 0.066), the presence of mediastinal shift (p = 0.203), loculations (p = 1.000), and was not affected by ultrasound guidance (p = 0.790). Finally, as observed in the MIST studies [13], [14], fibrinolytic use was not associated with improved outcome. To our knowledge, only one study analysed factors associated with ITTC failure [8], and found that an estimated pleural volume ≥40% of the thorax was associated with worse outcome. The factors identified as significantly predictive of chest tube drainage failure to date are the presence of empyema, and loculations [12], [30].
In our series as in others, ITTC was well tolerated. Pain during thoracentesis has been reported in 15% to 28% of cases [33]–[37]. WHO step 3 analgesics were used in 29% of patients in our series, for less than 24 hours (IQR = 1). Vasovagal reactions have been reported in 2% to 4% of thoracentesis [36], [38], as in our series (4%). Pneumothorax complicated 4 to 30% of thoracentesis in the literature, and required chest tube drainage in 20% to 50% of cases [33]–[36]. The rate of post-ITTC pneumothorax was 6% in our series, and never required chest tube drainage. In addition, no hemothorax was reported despite the use of intrapleural fibrinolytics in two thirds of cases. These low rates of local complications may be related to the systematic use of atraumatic needles, and the large experience accumulated over years, through the protocolized management of ITTC.
The main limitations of our study are inherent to its design (retrospective, observational, and monocentric). Failure was defined as escalation, which was not protocolized, and left to the physician in charge, based on the combination of clinical, microbiological, and imaging data. Hence, classification biases may have occurred (e.g. patients classified as ‘failure’ could have been successfully controlled with ITTC). This means that our estimated 81% success rate is conservative. The rate of missing data did not exceed 10% except for a few variables which were not included in multivariate analysis.
The main caveats of our strategy based on ITTC are the following: i) its reliance on experimented staff, which means that these encouraging results may not be replicated in every institutions; ii) the significant duration of hospital stay (median, 21 days in our study), although we are currently reducing hospital stay by earlier discharge, and the development of ambulatory care units; iii) the need to repeat the procedure (median, three times in our series), which means that ITTC may be more time-consuming for physicians, globally, than chest tube drainage.
Our study has several strengths. To our knowledge, this study included the largest number of patients treated with ITTC (Table 6).
Our selection criteria enabled the constitution of an homogeneous group of patients with CPPE requiring pleural drainage according to current recommendations [5], [6]. Finally, throughout the study period, standardized protocols for thoracentesis and pleural fibrinolysis were applied.
In conclusion, our study demonstrates that ITTC is safe and effective as first-line treatment of CPPE. However, the design of our study (observational, monocentric), precludes comparison of ITTC with other common therapeutic strategies, such as chest tube drainage or surgery. The identification of variables available with the first thoracentesis and independently associated with failure of the ITTC strategy led us to implement the following protocol: The first thoracentesis is performed for both diagnostic and therapeutic purposes. If micro-organisms are observed after Gram stain on pleural fluid and/or if the volume evacuated during this first procedure is ≥450 mL, patients will be closely monitored, and chest tube drainage or VATS will be considered in case of uncontrolled sepsis at day 4. In the absence of both failure criteria (microorganisms observed on Gram stain of pleural fluid, and first thoracentesis volume ≥450 mL), ITTC was associated with a success rate of 97% in our series. A randomized study comparing ITTC vs. chest tube drainage would be required to establish the optimal first-line treatment of CPPE.
Acknowledgments
The authors thank Boris Campilo-Gimenez for his help with the statistics, Jean-Sébastien Poineuf for the pilot study leading to the present work, Arnaud Gacouin and James Norwood for the review of this article.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hasley PB, Albaum MN, Li YH, Fuhrman CR, Britton CA, et al. (1996) Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 156: 2206–2212. [PubMed] [Google Scholar]
- 2. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, et al. (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 3. Light RW, Girard WM, Jenkinson SG, George RB (1980) Parapneumonic effusions. The American journal of medicine 69: 507–512. [DOI] [PubMed] [Google Scholar]
- 4. Koegelenberg CFN, Diaconi AH, Bolligeri CT (2008) Parapneumonic Pleural Effusion and Empyema. Respiration 75: 241–250. [DOI] [PubMed] [Google Scholar]
- 5. Colice GL (2000) Medical and Surgical Treatment of Parapneumonic Effusions: An Evidence-Based Guideline. Chest 118: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 6. Davies HE, Davies RJO, Davies CWH (2010) on behalf of the BTS Pleural Disease Guideline Group (2010) Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 65: ii41–ii53. [DOI] [PubMed] [Google Scholar]
- 7. Wait MA, Sharma S, Hohn J, Nogare AD (1997) A Randomized Trial of Empyema Therapy. Chest 111: 1548–1551. [DOI] [PubMed] [Google Scholar]
- 8. Ferguson AD, Prescott RJ, Selkon JB, Watson D, Swinburn CR (1996) The clinical course and management of thoracic empyema. QJM 89: 285–289. [DOI] [PubMed] [Google Scholar]
- 9. Alfageme I, Muñoz F, Peña N, Umbría S (1993) Empyema of the thorax in adults. Etiology, microbiologic findings, and management. Chest 103: 839–843. [DOI] [PubMed] [Google Scholar]
- 10. Chapman SJ, Davies RJO (2004) Recent advances in parapneumonic effusion and empyema. Curr Opin Pulm Med 10: 299–304. [DOI] [PubMed] [Google Scholar]
- 11. Chen KY (2000) A 10-Year Experience With Bacteriology of Acute Thoracic Empyema: Emphasis on Klebsiella pneumoniae in Patients With Diabetes Mellitus. Chest 117: 1685–1689. [DOI] [PubMed] [Google Scholar]
- 12. Huang HC (1999) Predicting Factors for Outcome of Tube Thoracostomy in Complicated Parapneumonic Effusion or Empyema. Chest 115: 751–756. [DOI] [PubMed] [Google Scholar]
- 13. Maskell NA, Davies CWH, Nunn AJ, Hedley EL, Gleeson FV, et al. (2005) UK controlled trial of intrapleural streptokinase for pleural infection. New England Journal of Medicine 352: 865–874. [DOI] [PubMed] [Google Scholar]
- 14. Rahman NM, Maskell NA, West A, Teoh R, Arnold A, et al. (2011) Intrapleural use of tissue plasminogen activator and DNase in pleural infection. New England Journal of Medicine 365: 518–526. [DOI] [PubMed] [Google Scholar]
- 15. Maskell NA (2006) The Bacteriology of Pleural Infection by Genetic and Standard Methods and Its Mortality Significance. American Journal of Respiratory and Critical Care Medicine 174: 817–823. [DOI] [PubMed] [Google Scholar]
- 16. Moulton JS, Benkert RE, Weisiger KH, Chambers JA (1995) Treatment of complicated pleural fluid collections with image-guided drainage and intracavitary urokinase. Chest 108: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 17. Crouch JD, Keagy BA, Delany DJ (1987) “Pigtail” catheter drainage in thoracic surgery. Am Rev Respir Dis 136: 174–175. [DOI] [PubMed] [Google Scholar]
- 18. Silverman SG, Mueller PR, Saini S, Hahn PF, Simeone JF, et al. (1988) Thoracic empyema: management with image-guided catheter drainage. Radiology 169: 5–9. [DOI] [PubMed] [Google Scholar]
- 19. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA (1988) Radiographically guided percutaneous catheter drainage of pleural fluid collections. AJR Am J Roentgenol 151: 1113–1116. [DOI] [PubMed] [Google Scholar]
- 20. Hunnam GR, Flower CD (1988) Radiologically-guided percutaneous catheter drainage of empyemas. Clin Radiol 39: 121–126. [DOI] [PubMed] [Google Scholar]
- 21. Ulmer JL, Choplin RH, Reed JC (1991) Image-guided catheter drainage of the infected pleural space. J Thorac Imaging 6: 65–73. [DOI] [PubMed] [Google Scholar]
- 22. Keeling AN, Leong S, Logan PM, Lee MJ (2008) Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 31: 135–141. [DOI] [PubMed] [Google Scholar]
- 23. Shankar S, Gulati M, Kang M, Gupta S, Suri S (2000) Image-guided percutaneous drainage of thoracic empyema: can sonography predict the outcome? Eur Radiol 10: 495–499. [DOI] [PubMed] [Google Scholar]
- 24. Sahn SA (2007) Diagnosis and Management of Parapneumonic Effusions and Empyema. Clinical Infectious Diseases 45: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 25. Lawrence DR, Ohri SK, Moxon RE, Townsend ER, Fountain SW (1997) Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg 64: 1448–1450. [DOI] [PubMed] [Google Scholar]
- 26. Luh SP, Chou MC, Wang LS, Chen JY, Tsai TP (2005) Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 127: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 27. Storm HK, Krasnik M, Bang K, Frimodt-Møller N (1992) Treatment of pleural empyema secondary to pneumonia: thoracocentesis regimen versus tube drainage. Thorax 47: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wehr CJ, Adkins RB Jr (1986) Empyema thoracis: a ten-year experience. South Med J 79: 171–176. [DOI] [PubMed] [Google Scholar]
- 29.Riquet M, Badia A (2003) Pleurésies purulentes aiguës à germes banals. Encycl Méd Chir (Editions Scientifiques et Médicales Elsevier SAS, Paris, tous droits réservés), Pneumologie, 6–041-A-40, 2003, 13 p. [Google Scholar]
- 30. Davies CWH, Kearney SE, Gleeson FV, Davies RJO (1999) Predictors of outcome and long-term survival in patients with pleural infection. American journal of respiratory and critical care medicine 160: 1682–1687. [DOI] [PubMed] [Google Scholar]
- 31. Cham CW, Haq SM, Rahamim J (1993) Empyema thoracis: a problem with late referral? Thorax 48: 925–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmed RA, Marrie TJ, Huang JQ (2006) Thoracic Empyema in Patients with Community-Acquired Pneumonia. The American Journal of Medicine 119: 877–883. [DOI] [PubMed] [Google Scholar]
- 33. Grogan DR, Irwin RS, Channick R, Raptopoulos V, Curley FJ, et al. (1990) Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 150: 873–877. [DOI] [PubMed] [Google Scholar]
- 34. Seneff MG, Corwin RW, Gold LH, Irwin RS (1986) Complications associated with thoracocentesis. Chest 90: 97–100. [DOI] [PubMed] [Google Scholar]
- 35. Collins TR, Sahn SA (1987) Thoracocentesis. Clinical value, complications, technical problems, and patient experience. Chest 91: 817–822. [DOI] [PubMed] [Google Scholar]
- 36. Bartter T, Mayo PD, Pratter MR, Santarelli RJ, Leeds WM, et al. (1993) Lower risk and higher yield for thoracentesis when performed by experienced operators. Chest 103: 1873–1876. [DOI] [PubMed] [Google Scholar]
- 37. Jones PW (2003) Ultrasound-Guided Thoracentesis: Is It a Safer Method? Chest 123: 418–423. [DOI] [PubMed] [Google Scholar]
- 38. Colt HG, Brewer N, Barbur E (1999) Evaluation of patient-related and procedure-related factors contributing to pneumothorax following thoracentesis. Chest 116: 134–138. [DOI] [PubMed] [Google Scholar]
- 39. Vianna NJ (1971) Nontuberculous bacterial empyema in patients with and without underlying diseases. JAMA 215: 69–75. [PubMed] [Google Scholar]
- 40. Benfield GF (1981) Recent trends in empyema thoracis. Br J Dis Chest 75: 358–366. [DOI] [PubMed] [Google Scholar]
- 41. Lemmer JH, Botham MJ, Orringer MB (1985) Modern management of adult thoracic empyema. J Thorac Cardiovasc Surg 90: 849–855. [PubMed] [Google Scholar]
- 42. Mandal AK, Thadepalli H (1987) Treatment of spontaneous bacterial empyema thoracis. J Thorac Cardiovasc Surg 94: 414–418. [PubMed] [Google Scholar]
- 43. Storm HK, Krasnik M, Bang K, Frimodt-Møller N (1992) Treatment of pleural empyema secondary to pneumonia: thoracocentesis regimen versus tube drainage. Thorax 47: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simmers TA, Jie C, Sie B (1999) Minimally invasive treatment of thoracic empyema. Thorac Cardiovasc Surg 47: 77–81. [DOI] [PubMed] [Google Scholar]