Abstract
A 1:1 mixture of (IPr)AuCl [IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidine] and AgClO4 catalyzes the intermolecular dehydrative alkoxylation of primary and secondary allylic alcohols with aliphatic primary and secondary alcohols to form allylic ethers. These transformations are regio- and stereospecific with preferential addition of the alcohol nucleophile at the γ-position of the allylic alcohol syn to the departing hydroxyl group and with predominant formation of the E stereoisomer. The minor α-regioisomer is formed predominantly through a secondary reaction manifold involving regioselective γ-alkoxylation of the initially formed allylic ether rather than via the direct α-alkoxylation of the allylic alcohol.
Keywords: Alcohols, Allylic compd., Allylation, Au, Stereoselective catalysis
Introduction
Alkyl allylic ethers are found in a number of naturally occurring and biologically active compounds and are versatile building blocks for further synthetic manipulation.[1] As a result, considerable effort has been directed toward the development of efficient and selective methods for the synthesis of allylic ethers.[2,3] The transition metal-catalyzed alkoxylation of allylic alcohol derivatives represents an attractive route to allylic ethers with the potential to form C–O bonds under mild conditions and with high levels of regio- and stereocontrol.[3,4] Indeed, Pd0,[5] RhII,[6] Ru,[7] and IrI [8] complexes catalyze the alkoxylation of allylic carboxylates, carbonates, and related substrates with metal alkoxides, silanoates, alcohols, and phenols, and a number of these transformations occur with high enantioselectivity.
With the goal of condensing synthetic sequences and minimizing byproduct formation, there has been an ongoing interest in the utilization of underivatized allylic alcohols as electrophiles for the transition metal-catalyzed allylation of heteroatom nucleophiles.[9] Regarding the formation of carbon–oxygen bonds, PdII,[10] AuI,[11-16] and RuII [17] complexes catalyze the intramolecular dehydrative alkoxylation of allylic alcohols with simple alcohol nucleophiles. Conversely, the intermolecular alkoxylation of underivatized allylic alcohols remains an unsolved challenge in homogeneous catalysis. The most notable example of such a transformation is the iridium(I)-catalyzed, regio- and enantioselective alkoxylation of secondary allylic alcohols with aliphatic alcohols to form secondary alkyl allylic ethers.[18-20] However, given the ready access to regio- and stereochemically pure allylic alcohols,[21] an effective method for the regio- and stereospecific conversion of substituted allylic alcohols to allylic ethers would also be significant, particularly if these transformations occurred with concomitant 1,3-allylic transposition.[22]
We have recently reported the intermolecular dehydrative amination of allylic alcohols with imidazolidin-2-ones catalyzed by a mixture of (1)AuCl [1 = P(t-Bu)2o-biphenyl] and AgSbF6.[23,24] The γ-regiospecificity and syn-stereospecificity displayed by this transformation distinguished it from extant methods for the amination of allylic alcohols. In this context, we considered that a similar catalyst system might also effect the regiospecific and stereospecific intermolecular dehydrative alkoxylation of allylic alcohols with alcohol nucleophiles. Indeed, here we report the γ-regioselective and syn-stereospecific intermolecular alkoxylation of underivatized allylic alcohols with primary and secondary aliphatic alcohols catalyzed by a gold N-heterocyclic carbene complex.[20]
Results and Discussion
Development of the Protocol
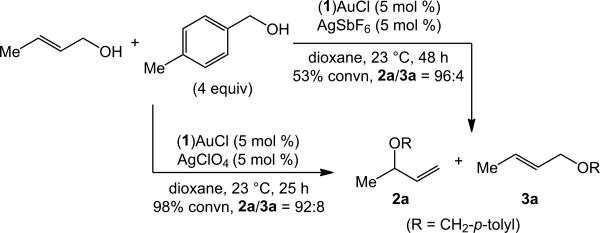
An initial experiment directed toward the gold(I)-catalyzed intermolecular dehydrative alkoxylation of allylic alcohols was encouraging. Treatment of a 1:4 mixture of trans-2-buten-1-ol and 4-methylbenzyl alcohol with a catalytic 1:1 mixture of (1)AuCl and AgSbF6 in dioxane at room temperature for 48 h led to 53% conversion to form a 96:4 mixture of the γ-addition product 2a and the α-addition product 3a (Scheme 1).[25] Substitution of AgSbF6 with AgClO4 led to 98% conversion after 25 h at room temperature to generate a 92:8 mixture of 2a and 3a (Scheme 1).
Scheme 1.
Gold-catalyzed alkoxylation of trans-2-buten-1-ol with 4-methylbenzyl alcohol.
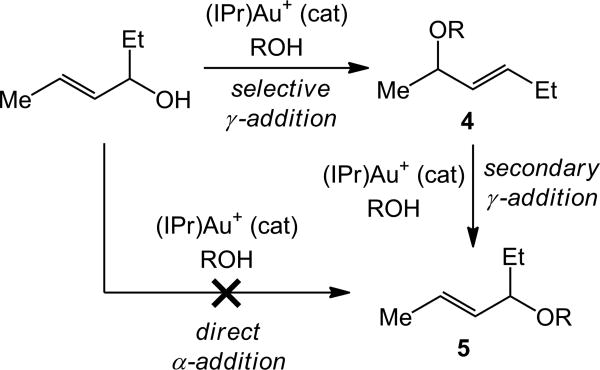
Unfortunately, continued experimentation revealed the shortcomings of the (1)AuCl/AgClO4 catalyst system. For example, reaction of a 1:4 mixture of trans-4-hexen-3-ol and 4-methylbenzyl alcohol catalyzed by a 1:1 mixture of (1)AuCl and AgClO4 in dioxane at room temperature for 24 h led to 85% conversion to form a 64:36 mixture of the γ-addition product 4 and the α-addition product 5 (Table 1, entry 1). To improve upon this result, the gold-catalyzed reaction of 4-methylbenzyl alcohol and trans-4-hexen-3-ol was screened as a function of supporting ligand, silver salt, and solvent (Table 1). Among the supporting ligands tested, the N-heterocyclic carbene ligand IPr gave the best combination of conversion (99%) and selectivity (75:25) after 24 h at room temperature (Table 1, entries 2-4). Among the different solvents screened utilizing the (IPr)AuCl/AgClO4 catalyst mixture, methylene chloride and chloroform showed reactivity and selectivity comparable to that realized in dioxane (Table 1, entries 5-9). Among the silver salts tested, AgOTf, AgOTs, and AgPF6 proved less effective than AgClO4 (Table 1, entries 10-14). However, two experiments utilizing AgOTs pointed to a potential relationship between conversion and regioselectivity. Specifically, whereas reaction of 4-methylbenzyl alcohol with trans-4-hexen-3-ol catalyzed by (IPr)AuCl/AgOTs for 24 h gave 47% conversion with 92% selectivity for 4, reaction for 4 h led to only 10% conversion, but with >99% selectivity for 4 (Table 1, entries 12 and 13).
Table 1.
Effect of ligand, counterion and reaction conditions on the gold(I)-catalyzed intermolecular allylic alkoxylation of trans-4-hexen-3-ol with 4-methylbenzyl alcohol.

| ||||||
|---|---|---|---|---|---|---|
| Entry | L | Solvent | X– | Time [h] | Convn/yield [%][a] | 4:5[b] |
| 1 | 1 | dioxane | ClO4– | 24 | 85 (81) | 64:36 (67:33) |
| 2 | IPr | dioxane | ClO4– | 24 | 99 (90) | 75:25 (75:25) |
| 3 | PPh3 | dioxane | ClO4– | 24 | 100 (89) | 55:45 (55:45) |
| 4 | P(t-Bu) 3 | dioxane | ClO4– | 24 | 90 | 62:38 |
| 5 | IPr | toluene | ClO4– | 24 | 100 | 69:31 |
| 6 | IPr | m-xylene | ClO4– | 24 | 99 | 73:27 |
| 7 | IPr | CH3CN | ClO4– | 24 | <5 | – |
| 8 | IPr | C2H4Cl2 | ClO4– | 24 | 99 | 71:29 |
| 9 | IPr | CH2Cl2 | ClO4– | 24 | 99 | 75:25 |
| 10 | IPr | CHCl3 | ClO4– | 5 | 91 | 85:15 |
| 11 | IPr | CHCl3 | OTf– | 4 | 85 | 86:14 |
| 12 | IPr | CHCl3 | OTs– | 4 | 10 | 99:1 |
| 13 | IPr | CHCl3 | OTs– | 24 | 47 | 92:8 |
| 14 | IPr | CHCl3 | PF6– | 4 | 76 | 79:21 |
Conversion determined by GC analysis of the crude reaction mixture; value in parenthesis refers to isolated yield of a mixture of 4 and 5.
Isomer ratio determined by GC analysis of the crude reaction mixture at the indicated time; value in parenthesis refers to isomer ratio of the purified mixture as determined by GC analysis; for both 4 and 5, E:Z ≈ 9:1.
Origin of the α- and γ-regioisomers in gold-catalyzed allylic alkoxylation
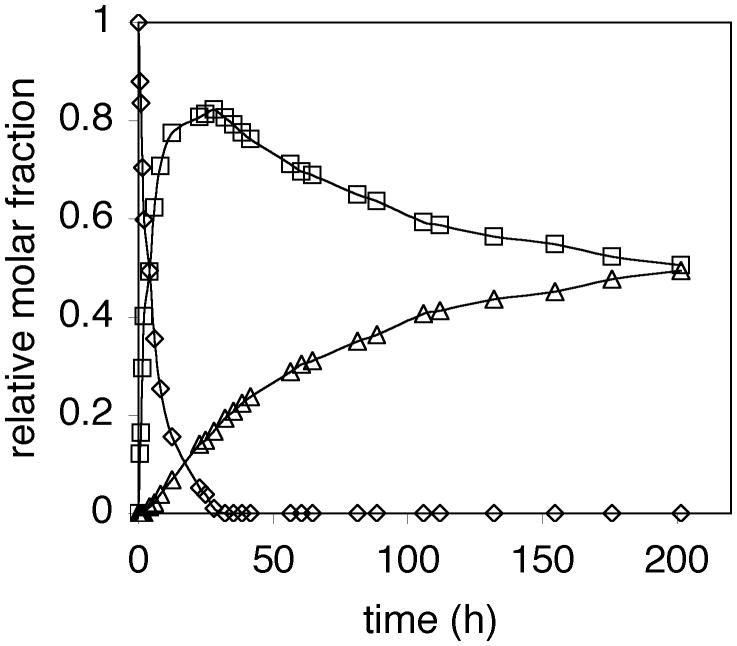
The conversion dependent regioselectivity of the reaction of 4-methylbenzyl alcohol with trans-4-hexen-3-ol catalyzed by (IPr)AuCl/AgOTs suggested that α-substitution product 5 was formed through a secondary process involving gold-catalyzed alkoxylation of γ-substitution product 4. Supporting this contention, Aponick has shown that allylic ethers are competent electrophiles for the gold(I)-catalyzed intramolecular allylation of alcohols.[12] To more thoroughly probe for the presence of primary and secondary reaction pathways in the gold-catalyzed formation of 4 and 5, a CD2Cl2 solution consisting of a 1:4 mixture of trans-4-hexen-3-ol and 4-methylbenzyl alcohol and a catalytic 1:1 mixture of (IPr)AuCl and AgClO4 was analyzed periodically by 1H NMR spectroscopy at room temperature. In accord with our expectations, the corresponding concentration versus time plot revealed rapid formation of 4 early in the reaction superimposed on slower formation of 5, which led to degradation of the 4:5 ratio with increasing conversion (Figure 1). For example, 4 constituted 97% of the product mixture at 50% conversion, 91% of the product mixture at 85% conversion, and 82% of the product mixture at 100% conversion. Following complete consumption of trans-4-hexen-3-ol, the isomerization of 4 to 5 was apparent to ultimately generate an equilibrium ∼1:1 mixture of the two regioisomers (Figure 1).
Figure 1.
Concentration versus time plot for the reaction of a 1:4 mixture of trans-4-hexen-3-ol (◊) with 4-methylbenzyl alcohol catalyzed by a 1:1 mixture of (IPr)AuCl and AgClO4 (5 mol %) in CD2Cl2 at room temperature to form 4 (□) and 5 (Δ).
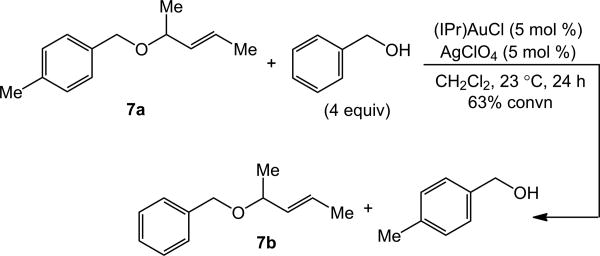
Periodic analysis of the gold-catalyzed reaction of trans-2-butene-1-ol and 4-methylbenzyl alcohol also revealed the rapid formation of γ-alkoxylation product 2a superimposed on the slower, secondary formation of α-alkoxylation product 3a (2a:3a ≥95:5 at 63% conversion; 2a:3a = 92:8 at 85% conversion; Figure S9). One additional experiment further established the reactivity of alkyl allylic ethers toward gold-catalyzed alkoxylation. Specifically, treatment of a 1:4 mixture of allylic ether 7a with catalytic 1:1 mixture of (IPr)AuCl and AgClO4 at room temperature for 24 h led to 63% conversion to the corresponding allylic ether 7b (Scheme 2). Taken together, these experiments established that the gold-catalyzed reaction of 4-methylbenzyl alcohol with either trans-2-buten-1-ol or trans-3-penten-2-ol occurs with high γ-selectivity (>95%) and that the minor α-regioisomers are formed predominantly via secondary reaction pathways involving gold-catalyzed γ-alkoxylation of the initially formed allylic ether (Scheme 3).
Scheme 2.
Gold-catalyzed alkoxylation of allylic ether 7a with benzyl alcohol.
Scheme 3.
Minor regioisomer 5 is formed primarily via secondary γ-alkoxylation of 4 and not through direct α-alkoxylation of trans-3-penten-2-ol.
Scope of Dehydrative Alkoxylation
The procedure used for the preparatory-scale, gold-catalyzed dehydrative alkoxylation of allylic alcohols draws from our observations regarding the intermolecular alkoxylation of trans-2-buten-1-ol and trans-3-penten-2-ol. Preparative-scale reactions were typically run with a four-fold excess of the nucleophilic alcohol to enhance the reaction rate and minimize self condensation and utilized the (IPr)AuCl/AgClO4 catalyst system in methylene chloride at room temperature.[25] Reactions were run to 80-95% conversion to maximize the formation of the primary γ-addition product while minimizing the formation of the α-addition byproduct generated in the secondary reaction manifold. In all cases, the minor α-regioisomer was removed by a single column chromatographic separation to provide the regiochemically pure γ-addition product in good to excellent yield.
The scope of the gold-catalyzed intermolecular alkoxylation of allylic alcohols was evaluated as a function of both the allylic and nucleophile alcohol component (Table 2). Intermolecular alkoxylation of primary (E)-allylic alcohols that contained a single methyl, ethyl or n-propyl substituent at the γ-position with primary or secondary alcohol nucleophiles led to isolation of the corresponding secondary allylic ethers 2, 6, and 8 in 79-95% yield as single regioisomers (Table 2, entries 1-7). Similarly, both (E)-9 and (Z)-9 that possessed a more electron deficient γ-benzyloxymethyl substitutent underwent gold-catalyzed alkoxylation with n-butanol with complete regioselectivity, albeit in modest yield (Table 2, entries 8 and 9). This pair of transformations revealed no significant impact of the C=C bond geometry on the rate or regioselectivity of allylic alkoxylation. Further establishing the γ-regiospecificity of gold(I)-catalyzed allylic alkoxylation, gold-catalyzed alkoxylation of 3-buten-2-ol with 4-methylbenzyl alcohol formed the primary allylic ether 3a in 98% yield with 89% regioselectivity, from which regiochemically pure 3a was isolated in 85% yield as an 84:16 mixture of E/Z isomers (Table 2, entry 10).
Table 2.
Intermolecular alkoxylation of allylic alcohols with alcohol nucleophiles (ROH, 4 equiv) catalyzed by a mixture of (IPr)AuCl (5 mol %) and AgClO4 (5 mol %) in methylene chloride (0.6 M) at 23 °C.
| Entry | R of ROH | Allylic alcohol | Allylic ether | Time [h] | Convn/Yield [%][a] | γ/α[b] | E/Z[c] |
|---|---|---|---|---|---|---|---|
|
|

|
||||||
| 1 | CH2-4-C6H4Me | R′ = Me | 2a | 4 | 96 (76) | 94:6 | – |
| 2 | CH2-Ph | R′ = Me | 2b | 5 | 98 (95) | >99:1 | – |
| 3 | CH2-4-C6H4Me | R′ = Et | 6a | 9 | 95 (83) | 92:8 | – |
| 4 | CH2-Ph | R′ = Et | 6b | 14 | 83 (81) | >99:1 | – |
| 5 | CH2CH2Ph | R′ = Et | 6c | 11 | 88 (87) | 93:7 | – |
| 6[d] | Cy | R′ = Et | 6d | 21 | 87 (76) | 90:10 | – |
| 7 | CH2-4-C6H4Me | R′ = n-Pr | 8 | 18 | 82 (79) | 92:8 | – |
| 8[e] | n-Bu |
(E)-9 |
 10 |
24 | ND (52) | >99:1 | – |
| 9[e] | n-Bu |
(Z)-9 |
10 | 24 | ND (57) | >99:1 | – |
| 10 | CH2-4-C6H4Me |

|
3a |
12 | 98 (85) | 89:11 | 84:16 |
| 11 | CH2-4-C6H4Me |

|
 4 
|
4 | 82 (76) | 92:8 | 90:10 |
| 12[d] | n-Bu | R′ = Bn (11) | 12a | 24 | ND (80) | 84:16 | 89:11 |
| 13[d] | Me | R′ = Bn (11) | 12b | 7 | ND (77) | 84:16 | 91:9 |
| 14[f] | n-Bu | R′ = Bz (13)
|
14
|
24 | ND (53) | 94:6 | 93:7 |
| 15 | CH2-4-C6H4Me | 7a | 7 | 100 (96) | – | 90:10 | |
| 16 | CH2-Ph | 7b | 14 | 100 (99) | – | 91:9 | |
| 17 | CH2-4-C6H4Me |

|
 15 |
1 | 100 (88) | >99:1 | – |
Conversion determined by GC analysis of the crude reaction mixture at the indicated time; ND = not determined. Value in parentheses refers to isolated yield of the γ-regioisomer in >95% regioisomeric and chemical purity.
γ/α Ratio determined by GC analysis of the crude reaction mixture at the indicated time point.
E/Z ratio determined by 1H NMR analysis of the purified reaction mixture.
Reaction temperature = 40 °C.
Catalyst system = (1)AuCl (5 mol %) and AgClO4 (5 mol %) in dioxane at 60 °C.
Reaction performed in neat n-BuOH (0.5 M) at 50 °C.
We likewise explored the gold-catalyzed dehydrative alkoxylation of secondary allylic alcohols substituted at the γ-position. In addition to the gold-catalyzed formation of 4 from reaction of trans-4-hexen-3-ol and 4-methylbenzyl alcohol (Table 2, entry 11), gold-catalyzed alkoxylation of the mono-benzyl protected trans-2-pentene-1,4-diol 11 with either n-butanol or methanol led to isolation of 1,2-diethers 12 as single regioisomers in >75% yield (Table 2, entries 12 and 13). The analogous reaction of n-butanol with the mono-benzyloxy protected trans-2-pentene-1,4-diol 13 formed allylic ether 14 with 94% regioselectivity, but with diminished yield (Table 2, entry 14). 1,2-Dioxygenated compounds such as 12 and 14 are potentially important synthetic building blocks.[24] Both the symmetrically disposed allylic alcohol (E)-pent-3-en-2-ol and the tertiary allylic alcohol 2-methyl-3-penten-2-ol also underwent efficient gold-catalyzed dehydrative alkoxylation to form allylic ethers 7 and 15, respectively (Table 2, entries 15-17).
1,3-Chirality transfer in gold-catalyzed dehydrative alkoxylation
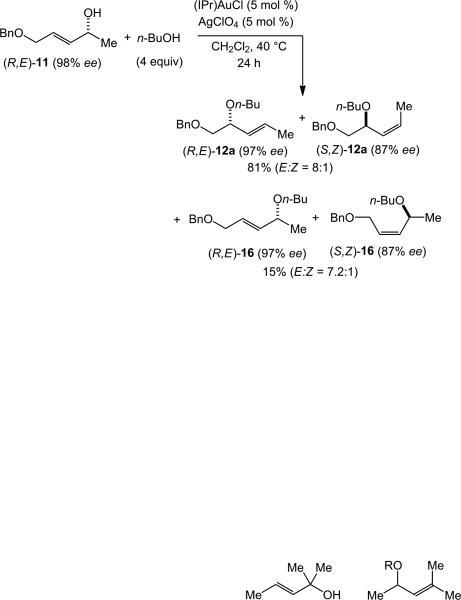
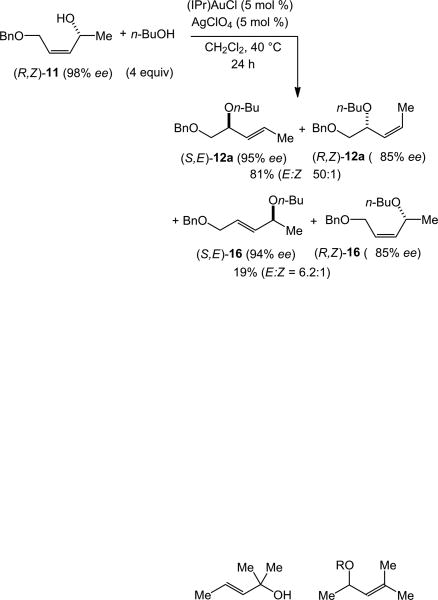
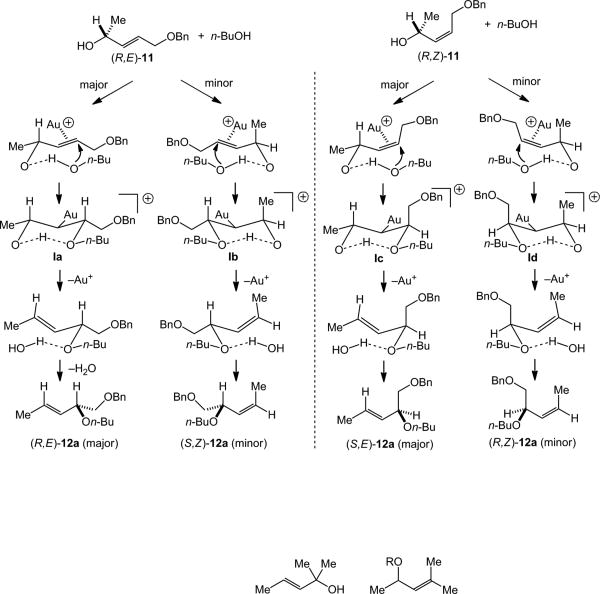
Efficient 1,3-chirality transfer from chiral, non-racemic secondary allylic alcohols has been previously observed in the gold(I)-catalyzed inter-[23] and intramolecular[27] dehydrative amination and intramolecular dehydrative alkoxylation of allylic alcohols[13,14] We therefore sought to evaluate the extent of 1,3-chirality transfer in the gold-catalyzed intermolecular dehydrative alkoxylation of allylic alcohols. These experiments were conducted so that sufficient quantities of both the γ- and α-regioisomeric allylic ethers were isolated so that the stereoselectivity of both the primary and secondary reaction pathways could be evaluated. In one experiment, gold(I)-catalyzed reaction of (R,E)-11 (98% ee) with n-butanol (4 equiv) at 40 °C for 24 h led to isolation of two chromatographic fractions. The first fraction consisted of an 8:1 mixture of (R,E)-12a (97% ee) and (S,Z)-12a (87% ee) in 81% combined yield and the second fraction consisted of a 7.2:1 mixture of (R,E)-16 (97% ee) and (S,Z)-16 (87% ee) in 15% combined yield (Scheme 4).[28] A second experiment involving the gold-catalyzed reaction of (R,Z)-11 (98% ee) with n-butanol likewise led to isolation of two chromatographic fractions. The first fraction consisted of a >50:1 mixture of (S,E)-12a (95% ee) and (R,Z)-12a (≥85% ee) in 81% combined yield and the second fraction consisted of a 6.2:1 mixture of (S,E)-16 (94% ee) and (R,Z)-16 (≥85% ee) in 19% combined yield (Scheme 5).[28]
Scheme 4.
Stereochemical analysis of the gold-catalyzed reaction of (R,E)-11 with n-butanol.
Scheme 5.
Stereochemical analysis of the gold-catalyzed reaction of (R,Z)-11 with n-butanol.
Several conclusions can be drawn from the gold-catalyzed alkoxylation of enantiomerically enriched (R,E)-11 and (R,Z)-11. Firstly, high levels of chirality transfer were observed in the primary reaction pathway (11→ 12a) for both (R,E)-11 and (R,Z)-11, particularly in the pathways leading to major diastereomer (E)-12a, which occurred with ≥97% transfer of chirality; the pathways leading to the minor diastereomer (Z)-12a occurred with ∼88% chirality transfer. Also worth noting is the high E-selectivity of the alkoxylation of (R,Z)-11, which was not revealed by the alkoxylation of primary allylic alcohol (Z)-9 (Table 2, entry 9). Secondly, the stereochemical outcome of these primary alkoxylation pathways, specifically the conversion of (R,E)-11 to (R,E)-12a and (S,Z)-12a and the conversion of (R,Z)-11 to (S,E)-12a and (R,Z)-12a, establish the net syn-displacement of the hydroxyl group by n-butanol. Thirdly, high levels of chirality transfer were likewise observed in the secondary reaction pathways that convert the initially formed γ-allylic ethers (R,E)-12a/(S,Z)-12a and (S,E)-12a/(R,Z)-12a to the regioisomeric α-allylic ethers (R,E)-16/(S,Z)-16 and (S,E)-16/(R,Z)-16, respectively (≥97% chirality transfer leading to (E)-16, ∼88% chirality transfer leading to (Z)-16). Fourthly, this stereochemical outcome likewise establishes the net syn-displacement of the butoxy group of (E)-12a or (Z)-12a by n-butanol.[29] Together these observations indicate that the mechanism and origin of stereocontrol in the secondary alkoxylation pathway is similar to that of the primary alkoxylation pathway.
Brønsted- and Lewis acid-catalyzed pathways for dehydrative alkoxylation
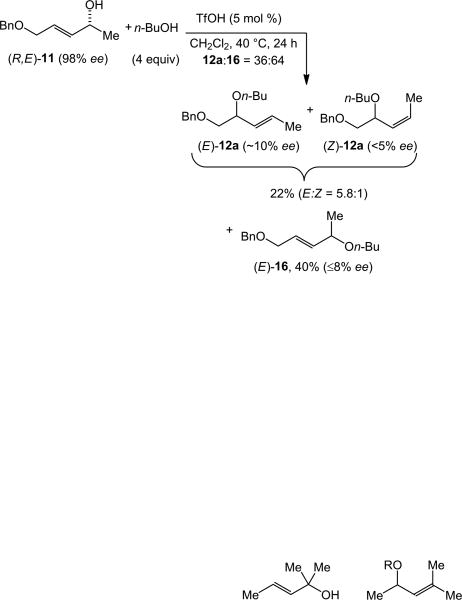
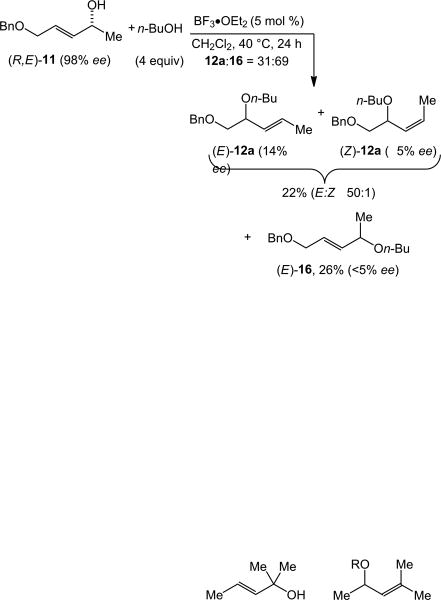
The syn-stereoselectivity observed for the gold(I)-catalyzed alkoxylation of both (R,E)-11 and (R,Z)-11 with n-butanol is characteristic of a concerted SN2’ substitution reaction[30] involving the σ-activation of the hydroxyl group by gold(I) or Brønsted acid generated under reaction conditions. Importantly, Toste has demonstrated that bis(gold) phosphine complexes are sufficiently Lewis acidic to acidify the hydroxyl group of an alcohol.[31] For this reason, we investigated the efficiency and regio- and stereoselectivity of Brønsted- and Lewis acid-catalyzed pathways for intermolecular alkoxylation of allylic alcohols. In one experiment, a 1:4 mixture of (R,E)-11 (98% ee) and n-butanol was treated with a catalytic amount of triflic acid (5 mol %) in methylene chloride at 40 °C and analyzed periodically by GC. After 5 h, 30% of (R,E)-11 was consumed to form a 40:60 mixture of regioisomeric allylic ethers 12a and 16, indicating that both regioisomers were formed directly from 11 (Scheme 6).[32] After 24 h, the regioisomeric ratio had not changed significantly (12a:16 = 36:64) and purification of the reaction mixture at this time led to isolation of two chromatographic fractions. One fraction consisted of a 5.8:1 mixture of (E)-12a (≤10% ee) and (Z)-12a (<5% ee) in 22% combined yield while the second fraction consisted of (E)-16 (8% ee) in 40% yield (Scheme 6). In a second experiment, treatment of a 1:4 mixture of (R,E)-11 and n-butanol with a catalytic amount of BF3·OEt2 (5 mol %) in methylene chloride at 40 °C for 24 h generated a ∼30:70 mixture of 12a and 16 (Scheme 7). Purification of the reaction mixture led to isolation of two chromatographic fractions. One fraction consisted of a >50:1 mixture of (E)-12a (14% ee) and (Z)-12a (≤5% ee) in 22% combined yield while the second fraction consisted of (E)-16 (≤5% ee) in 26% yield (Scheme 7).
Scheme 6.
Regio- and stereochemical outcomes of the triflic acid catalyzed intermolecular alkoxylation of (R,E)-11.
Scheme 7.
Regio- and stereochemical outcomes of the BF3·OEt2 catalyzed intermolecular alkoxylation of (R,E)-11.
Although HOTf catalyzed the intermolecular dehydrative alkoxylation of (R,E)-11 with n-butanol, this transformation occurred with neither the regioselectivity nor stereoselectivity displayed by the gold(I)-catalyzed alkoxylation of (R,E)-11. Also worth noting is that triflic acid failed to catalyze the intermolecular alkoxylation of trans-2-buten-1-ol with 4-methylbenzyl alcohol.[32] Therefore, HOTf is neither a selective nor general catalyst for the intermolecular dehydrative alkoxylation of allylic alcohols. Furthermore, there is no indication that the acidity of a gold-complexed hydroxyl group approaches that of triflic acid.[31] Taken together, we can confidently rule out a mechanism for gold-catalyzed intermolecular alkoxylation involving σ-activation of the hydroxyl group by Brønsted acid. Similarly, because gold(I) is a much weaker and less oxophilic Lewis acid than is BF3·OEt2, the sluggish dehydrative alkoxylation of (R,E)-11 catalyzed by BF3·OEt2 argues strongly against a mechanism for gold-catalyzed dehydrative alkoxylation involving σ-activation of the hydroxyl group by gold(I).
Mechanism of dehydrative alkoxylation
In 2010 we proposed a mechanism to account for the γ-regiospecificity and syn-stereospecificity of the gold(I)-catalyzed intermolecular dehydrative amination of allylic alcohols,[23] which was inspired by the computational analyses of Maseras.[33] This mechanism invoked initial π-complexation of the C=C bond of the allylic alcohol to gold followed by outer-sphere, anti-addition of the nucleophile facilitated by an intramolecular N–H•••O hydrogen bond, anti-elimination of water, and decomplexation of gold.[23] Aponick later invoked an analogous mechanism to account for the gold(I)-catalyzed intramolecular dehydrative alkoxylation of allylic alcohols[13] and provided further support for this mechanism through a combined experimental/computational study.[14] Therefore, it appears likely that a similar π-activation mechanism is responsible for the gold(I)-catalyzed intermolecular dehydrative alkoxylation of allylic alcohols. Complexation of gold to either of the diastereotopic C=C π-faces of (R,E)-11 or (R,Z)-11 followed by outer-sphere, anti-addition of n-butanol facilitated by an O–H•••O hydrogen bond would form the gold σ-alkyl intermediates I (Scheme 8). Subsequent anti-elimination of water and displacement of gold would form allylic ethers 12a with γ-regioselectivity and net syn-stereoselectivity (Scheme 8).
Scheme 8.
Proposed mechanism for the gold-catalyzed γ-regioselective and syn-stereoselective alkoxylation of (R,E)-11 and (R,Z)-11.
In addition to accounting for the regio- and stereospecificity of gold-catalyzed intermolecular alkoxylation of (R,E)-11 and (R,Z)-11, the proposed mechanism provides a rationale for the predominant formation of the (E)-12a stereoisomers in these transformations (Scheme 8). For example, in the gold(I)-catalyzed alkoxylation of (R,E)-11, intermediate Ia, which leads to major product (R,E)-12a, possess a trans,trans arrangement of the benzyloxymethyl, gold phosphine, and methyl groups, whereas in intermediate Ib, which leads to minor product (S,Z)-12a, the methyl group and gold phosphine moiety are arranged in a less favorable cis orientation (Scheme 8). Likewise, in the gold(I)-catalyzed alkoxylation of (R,Z)-11, intermediate Ic, which leads to major product (S,E)-12a, possesses a cis,trans arrangement of the benzyloxymethyl, gold phosphine, and methyl groups, whereas intermediate Id, which leads to minor product (R,Z)-12a, possesses a cis,cis arrangement of these groups and what appears to be a particularly destabilizing 1,3-diaxial interaction that may help explain the high E/Z selectivity (>50:1) observed in this transformation (Scheme 8).
Conclusion
In summary, we have developed a gold(I)-catalyzed method for the intermolecular dehydrative alkoxylation of primary and secondary allylic alcohols with primary and secondary aliphatic alcohols to form allylic ethers in good yield under mild conditions. These transformations occur via regio- and stereospecific addition of the alcohol nucleophile to the γ-position of the allylic alcohol on the C=C π-face syn to the departing hydroxyl group. Analysis of concentration versus time plots for the gold(I)-catalyzed alkoxylation of trans-4-hexen-3-ol and stereochemical analysis of the gold-catalyzed alkoxylation of (R,E)-11 and (R,Z)-11 revealed that the minor α-substitution products are formed predominantly through secondary reaction pathways involving γ-regiospecific and syn-stereospecific alkoxylation of the allylic ethers formed in the primary reaction pathway.
Experimental Section
1-{(Hex-3-en-2-yloxy)methyl}-4-methylbenzene (4)
A mixture of (IPr)AuCl (9.3 mg, 1.5 × 10–2 mmol] and AgClO4 (3.1 mg, 1.5 × 10–2 mmol) in CH2Cl2 (0.3 mL) was stirred at room temperature for 5 min. To this was added a solution of 4-methylbenzyl alcohol (147 mg, 1.20 mmol) and trans-4-hexen-3-ol (36 μL, 0.30 mmol) in CH2Cl2 (0.2 mL), and the resulting mixture was stirred at 23 °C for 4 h. Flash column chromatography of the crude reaction mixture (pentane–CH2Cl2 = 4:1 → 1:1) gave 4 (47 mg, 76%, E/Z = 9.1:1) as a colorless oil. The E-configuration of the major stereoisomer of 4 was established by the vicinal olefinic sp2-sp2 coupling constant of 3JHH = 15.6 Hz in the 1H NMR spectrum. TLC (hexanes–CH2Cl2 = 1:1): Rf = 0.47. 1 H NMR: δ = 7.23 (d, J = 8 Hz, 2 H), 7.14 (d, J = 7.6 Hz, 2 H), [5.66 (td, J = 6, 15.6 Hz), 5.55 (td, J = 7.6, 10.8 Hz), 9.1:1, 1 H], 5.38 (tdd, J = 1.6, 8, 15.6 Hz, 1 H), 4.51 (d, J = 12 Hz, 1 H), [4.33 (d, J = 12 Hz), 4.31 (d, J = 11.6 Hz), 9.1:1, 1 H], 3.87 (qd, J = 6.4, 7 Hz, 1 H), 2.34 (s, 3 H), 2.08 (qd, J = 7.6, 6.2 Hz, 2 H), [1.26 (d, J = 6.8 Hz), 1.24 (d, J = 6.8 Hz), 9.1:1, 3 H], [1.02 (t, J = 7.6 Hz), 0.98 (t, J = 7.6 Hz), 9.1:1, 3 H]. 13C{1H} NMR: δ = 136.9, 135.9, 134.7, 130.9, 129.0, 127.8, 75.5, 69.4, 25.2, 21.7, 21.1, 13.6. IR (neat, cm–1): 2967, 2928, 2863, 1454, 1369, 1074, 969, 910, 799, 732, 475. HRMS calcd (found) for C14H19 [(MH–H2O)+]: 187.1481 (187.1178).
Supplementary Material
Acknowledgments
We thank the NIH (GM-080422) for support of this research. P.M. thanks Duke University for support through the Burroughs Welcome and C.R. Hauser Fellowships.
References
- 1.a) Buckingham J. Dictionary of Natural Products. Vol. 1. University Press; Cambridge, MA: 1994. [Google Scholar]; b) Elliott MC, Williams E. J Chem Soc Perkin Trans. 2001;1:2303–2340. [Google Scholar]
- 2.a) Mitsunobu O. In: Comprehensive Organic Chemistry. Trost BM, Fleming I, editors. Vol. 6. Pergamon Press; New York: 1991. pp. 1–31. [Google Scholar]; b) Feuer H, Hooz J. In: The Chemistry of the Ether Linkage. Patai S, editor. Interscience Publishers; London, UK: 1967. pp. 445–468. [Google Scholar]; c) Baggett N. In: Comprehensive Organic Chemistry. Stoddart JF, editor. Vol. 1. Pergamon Press; New York: 1979. pp. 799–850. [Google Scholar]
- 3.a) Tsuji J. Palladium Reagents and Catalysts. Wiley; New York: 1996. pp. 290–404. [Google Scholar]; b) Trost BM, Lee C. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley-VCH; New York: 2000. pp. 593–649. [Google Scholar]
- 4.a) Trost BM, Zhang T, Sieber JD. Chem Sci. 2010;1:427–440. [Google Scholar]; b) Lu Z, Ma S. Angew Chem. 2008;120:264–303. [Google Scholar]; Angew Chem Int Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]; c) Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]; d) Hartwig JF. In: Organotransition Metal Chemistry. Hartwig JF, editor. University Science Books; Sausalito, California: 2010. pp. 967–1014. [Google Scholar]; e) Trost BM, McEachern EJ, Toste FD. J Am Chem Soc. 1998;120:12702–12703. [Google Scholar]; f) Trost BM. Chem Pharm Bull. 2002;50:1–14. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]; g) Trost BM, Van Vranken DL. Chem Rev. 1996;96:395. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 5.a) Cannon JS, Kirsch SF, Overman LE, Sneddon HF. J Am Chem Soc. 2010;132:15192–15203. doi: 10.1021/ja106688j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Z, Du H. Org Lett. 2010;12:3054–3057. doi: 10.1021/ol101069y. [DOI] [PubMed] [Google Scholar]; c) Lam FL, Au-Yeung TTL, Kwong FY, Zhou Z, Wong KY, Chan ASC. Angew Chem. 2008;120:1300–1303. doi: 10.1002/anie.200703955. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:1280–1283. doi: 10.1002/anie.200703955. [DOI] [PubMed] [Google Scholar]; d) Kirsch SF, Overman LE, White NS. Org Lett. 2007;9:911–913. doi: 10.1021/ol070110b. [DOI] [PubMed] [Google Scholar]; e) Uozumi Y, Kimura M. Tetrahedron: Asymmetry. 2006;17:161–166. [Google Scholar]; f) Roberts JP, Lee C. Org Lett. 2005;7:2679–2682. doi: 10.1021/ol0508328. [DOI] [PubMed] [Google Scholar]; g) Kim H, Men H, Lee C. J Am Chem Soc. 2004;126:1336–1337. doi: 10.1021/ja039746y. [DOI] [PubMed] [Google Scholar]; h) Kim H, Lee C. Org Lett. 2002;4:4369–4371. doi: 10.1021/ol027104u. [DOI] [PubMed] [Google Scholar]; i) Trost BM, Toste FD. J Am Chem Soc. 1999;121:4545–4554. [Google Scholar]
- 6.a) Evans PA, Leahy DK. J Am Chem Soc. 2002;124:7882–7883. doi: 10.1021/ja026337d. [DOI] [PubMed] [Google Scholar]; b) Evans PA, Leahy DK, Slieker LM. Tetrahedron: Asymmetry. 2003;14:3613–3618. [Google Scholar]; c) Evans PA, Leahy DK, Andrews WJ, Uraguchi D. Angew Chem. 2004;116:4892–4895. doi: 10.1002/anie.200460612. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:4788–4791. doi: 10.1002/anie.200460612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Mbaye MD, Renaud JL, Demerseman B, Bruneau C. Chem Commun. 2004:1870–1871. doi: 10.1039/b406039c. [DOI] [PubMed] [Google Scholar]; b) Onitsuka K, Okuda H, Sasai H. Angew Chem Int Ed. 2008;120:1476–1479. doi: 10.1002/anie.200704457. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:1454–1457. doi: 10.1002/anie.200704457. [DOI] [PubMed] [Google Scholar]; c) Saporita M, Bottari G, Bruno G, Drommi D, Faraone F. J Mol Catal A. 2009;309:159–165. [Google Scholar]; d) Ammar HB, Hassine BB, Fischmeister C, Dixneuf PH, Bruneau C. Eur J Inorg Chem. 2010:4752–4756. [Google Scholar]; e) Austeri M, Linder D, Lacour J. Chem Eur J. 2008:14, 5737–5741. doi: 10.1002/chem.200800619. [DOI] [PubMed] [Google Scholar]
- 8.a) López F, Ohmura T, Hartwig JF. J Am Chem Soc. 2003;125:3426–3427. doi: 10.1021/ja029790y. [DOI] [PubMed] [Google Scholar]; b) Shu C, Hartwig JF. Angew Chem. 2004;116:4898–4901. [Google Scholar]; Angew Chem Int Ed. 2004;43:4794–4797. doi: 10.1002/anie.200460214. [DOI] [PubMed] [Google Scholar]; c) Stanley LM, Bai C, Ueda M, Hartwig JF. J Am Chem Soc. 2010;132:8918–8920. doi: 10.1021/ja103779e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ueno S, Hartwig JF. Angew Chem. 2008;120:1954–1957. [Google Scholar]; Angew Chem Int Ed. 2008;47:1928–1931. doi: 10.1002/anie.200705267. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kimura M, Uozumi Y. J Org Chem. 2007;72:707–714. doi: 10.1021/jo0615403. [DOI] [PubMed] [Google Scholar]; f) Lyothier I, Defieber C, Carreira EM. Angew Chem. 2006;118:6350–6353. doi: 10.1002/anie.200602408. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:6204–6207. doi: 10.1002/anie.200602408. [DOI] [PubMed] [Google Scholar]
- 9.For a review and recent references regarding the amination of allylic alcohols see: Muzart J. Eur J Org Chem. 2007:3077–3089.Das K, Shibuya R, Nakahara Y, Germain N, Ohshima T, Mashima K. Angew Chem. 2012;124:154–158. doi: 10.1002/anie.201106737.Angew Chem Int Ed. 2012;51:150–154.Thoumazet C, Grützmacher H, Deschamps B, Ricard L, le Floch P. Eur J Inorg Chem. 2006:3911–3922.Piechaczyk O, Thoumazet C, Jean Y, le Floch P. J Am Chem Soc. 2006;128:14306–14317. doi: 10.1021/ja0621997.Mora G, Deschamps B, van Zutphen S, Le Goff XF, Ricard L, le Floch P. Organometallics. 2007;26:1846–1855.Usui I, Schmidt S, Keller M, Breit B. Org Lett. 2008;10:1207–1210. doi: 10.1021/ol800073v.Ohshima T, Miyamoto Y, Ipposhi J, Nakahara Y, Utsunomiya M, Mashima K. J Am Chem Soc. 2009;131:14317–14328. doi: 10.1021/ja9046075.Utsunomiya M, Miyamoto Y, Ipposhi J, Ohshima T, Mashima K. Org Lett. 2007;9:3371–3374. doi: 10.1021/ol071365s.Mora G, Piechaczyk O, Houdard R, Mezailles N, Le Goff XF, le Floch P. Chem Eur J. 2008;14:10047–10057. doi: 10.1002/chem.200801197.Yang H, Fang L, Zhang M, Zhu C. Eur J Org Chem. 2009:666–672.Qin HB, Yamagiwa N, Matsunaga S, Shibasaki M. Angew Chem. 2007;119:413–417. doi: 10.1002/anie.200602909.Angew Chem Int Ed. 2007;46:409–413.Guo S, Song F, Liu Y. Synlett. 2007:964–968.
- 10.a) Kawai N, Lagrange JM, Ohmi M, Uenishi J. J Org Chem. 2006;71:4530–4537. doi: 10.1021/jo060415o. [DOI] [PubMed] [Google Scholar]; b) Kawai N, Lagrange JM, Uenishi J. Eur J Org Chem. 2007:2808–2814. [Google Scholar]; c) Uenishi J, Vikhe YS, Kawai N. Chem Asian J. 2008;3:473–484. doi: 10.1002/asia.200700390. [DOI] [PubMed] [Google Scholar]; d) Uenishi J, Ohmi M, Ueda A. Tetrahedron: Asymmetry. 2005;16:1299–1303. [Google Scholar]; e) Uenishi J, Ohmi M. Angew Chem. 2005;117:2816–2820. [Google Scholar]; Angew Chem Int Ed. 2005;44:2756–2760. doi: 10.1002/anie.200500029. [DOI] [PubMed] [Google Scholar]
- 11.a) Aponick A, Biannic B. Synthesis. 2008:3356–3359. [Google Scholar]; b) Aponick A, Li CY, Palmes JA. Org Lett. 2009;11:121–124. doi: 10.1021/ol802491m. [DOI] [PubMed] [Google Scholar]; c) Aponick A, Li CY, Biannic B. Org Lett. 2008;10:669–671. doi: 10.1021/ol703002p. [DOI] [PubMed] [Google Scholar]; d) Aponick A, Biannic B, Jong MR. Chem Commun. 2010;46:6849–6851. doi: 10.1039/c0cc01961e. [DOI] [PubMed] [Google Scholar]; e) Bandini M, Monari M, Romaniello A, Tragni M. Chem Eur J. 2010;16:14272–14277. doi: 10.1002/chem.201002606. [DOI] [PubMed] [Google Scholar]
- 12.Biannic B, Aponick A. Bielstein J Org Chem. 2011;7:802–807. doi: 10.3762/bjoc.7.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aponick A, Biannic B. Org Lett. 2011;13:1330–1333. doi: 10.1021/ol200203k. [DOI] [PubMed] [Google Scholar]
- 14.Ghebreghiorgis T, Biannic B, Kirk BH, Ess DH, Aponick A. J Am Chem Soc. 2012;134:16307–16318. doi: 10.1021/ja306333a. [DOI] [PubMed] [Google Scholar]
- 15.For recent reviews of the gold-catalyzed dehydrative functionalization of allylic alcohols see: Cera G, Chiarucci M, Bandini M. Pure Appl Chem. 2012;84:1673–1684.Biannic B, Aponick A. Eur J Org Chem. 2011:6605–6617.
- 16.See also: Chiarucci M, Locritani M, Cera G, Bandini M. Beilstein J Org Chem. 2011;7:1198–1204. doi: 10.3762/bjoc.7.139.Marion N, Gealageas R, Nolan SP. Org Lett. 2007;9:2653–2656. doi: 10.1021/ol070843w.
- 17.Tanaka S, Seki T, Kitamura M. Angew Chem. 2009;121:9110–9113. doi: 10.1002/anie.200904671. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:8948–8951. doi: 10.1002/anie.200904671. [DOI] [PubMed] [Google Scholar]
- 18.Roggen M, Carreira EM. Angew Chem. 2011;123:5683–5686. [Google Scholar]; Angew Chem Int Ed. 2011;50:5568–5571. doi: 10.1002/anie.201007716. [DOI] [PubMed] [Google Scholar]
- 19.For a review of catalytic enantioselective alkylation utilizing alllylic alcohols see: Bandini M, Cera G, Chiarucci M. Synthesis. 2012:504–512.
- 20.a) A related method for the dehydrative alkoxylation of allylic alcohols with carbinols catalyzed by Ph3PAuNTf2 has recently appeared.[19b] However, the regiospecificity of the method was modest and stereospecificity was not demonstrated; Young PC, Schopf NA, Lee AL. Chem Commun. 2012 Oct 17; doi: 10.1039/c2cc36760b. 5 10.1039/b000000x.
- 21.For a review and recent references see: Hodgson DM, Humphreys PG. In: Science of Synthesis. Clayden JP, editor. Vol. 36. Thieme; Stuttgart, Germany: 2007. pp. 583–665.Gärtner M, Mader S, Seehafer K, Helmchen G. J Am Chem Soc. 2011;133:2072–2075. doi: 10.1021/ja109953v.Ely RJ, Morken JP. J Am Chem Soc. 2010;132:2534–2535. doi: 10.1021/ja910750b.Jiang H, Holub N, Jørgensen KA. Proc Natl Acad Sci. 2010;107:20630–20635. doi: 10.1073/pnas.0914523107.Ely RJ, Morken JP. Org Lett. 2010;12:4348–4351. doi: 10.1021/ol101797f.Biradar DB, Gau HM. Org Lett. 2009;11:499–502. doi: 10.1021/ol801999u.Burks HE, Kliman LT, Morken JP. J Am Chem Soc. 2009;131:9134–9135. doi: 10.1021/ja809610h.Aikawa K, Hioki Y, Mikami K. J Am Chem Soc. 2009;131:13922–13923. doi: 10.1021/ja906164p.Yang Y, Zhu SF, Zhou CY, Zhou QL. J Am Chem Soc. 2008;130:14052–14053. doi: 10.1021/ja805296k.Arai N, Azuma K, Nii N, Ohkuma T. Angew Chem. 2008;120:7567–7570. doi: 10.1002/anie.200802533.Angew Chem Int Ed. 2008;47:7457–7460. doi: 10.1002/anie.200802533.Wu HL, Wu PY, Uang BJ. J Org Chem. 2007;72:5935–5937. doi: 10.1021/jo0709403.Hong YT, Cho CW, Skucas E, Krische MJ. Org Lett. 2007;9:3745–3748. doi: 10.1021/ol7015548.Bogar K, Vidal PH, Leon ARA, Backvall JE. Org Lett. 2007;9:3401–3404. doi: 10.1021/ol071395v.Salvi L, Jeon SJ, Fisher EL, Carroll PJ, Walsh PJ. J Am Chem Soc. 2007;129:16119–16125. doi: 10.1021/ja0762285.Ngai MY, Barchuk A, Krische MJ. J Am Chem Soc. 2007;129:280–281. doi: 10.1021/ja0670815.Sato I, Asakura N, Iwashita T. Tetrahedron: Asymmetry. 2007;18:2638–2642.
- 22.For a review and lead references regarding the 1,3-isomerization of allylic alcohols catalyzed by metal oxo complexes see: Cadierno V, Crochet P, Gimeno J. Synlett. 2008:1105–1124.Herrmann AT, Saito T, Stivala CE, Tom J, Zakarian A. J Am Chem Soc. 2010;132:5962–5963. doi: 10.1021/ja101673v.Akai S, Hanada R, Fujiwara N, Kita Y, Egi M. Org Lett. 2010;12:4900–4903. doi: 10.1021/ol102053a.Morrill C, Beutner GL, Grubbs RH. J Org Chem. 2006;71:7813–7825. doi: 10.1021/jo061436l.Uyanik M, Fukatsu R, Ishihara K. Org Lett. 2009;11:3470–3473. doi: 10.1021/ol9013188.Akai S, Tanimoto K, Kanao Y, Egi M, Yamamoto T, Kita Y. Angew Chem. 2006;118:2654–2657. doi: 10.1002/anie.200503765.Angew Chem Int Ed. 2006;45:2592–2595. doi: 10.1002/anie.200503765.Bellemin-Laponnaz S, Gisie H, Le Ny JP, Osborn JA. Angew Chem. 1997;109:1011–1013.Angew Chem Int Ed. 1997;36:976–978.Morrill C, Grubbs RH. J Am Chem Soc. 2005;127:2842–2843. doi: 10.1021/ja044054a.
- 23.Mukherjee P, Widenhoefer RA. Org Lett. 2010;12:1184–1187. doi: 10.1021/ol902923e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.For examples of gold(I)-catalyzed allene hydroalkoxylation as a route to allylic ethers see: Hadfield MS, Lee AL. Org Lett. 2010;12:484–487. doi: 10.1021/ol902675k.Cui DM, Yu KR, Zhang C. Synlett. 2009:1103–1106.Cui DM, Zheng ZL, Zhang C. J Org Chem. 2009;74:1426–1427. doi: 10.1021/jo802513a.Zhang Z, Widenhoefer RA. Org Lett. 2008;10:2079–2081. doi: 10.1021/ol800646h.Nishina N, Yamamoto Y. Tetrahedron. 2009;65:1799–1808.Nishina N, Yamamoto Y. Tetrahedron Lett. 2008;49:4908–4911.
- 25.Employment of less than four equivalents of nucleophilic alcohol led to diminished reaction and rate and lower yields, presumably due to competitive self condensation of the allylic alcohol. Bis(benzyl) ethers did not constitute a significant impurity (<5%) in any of these gold-catalyzed allylic alkoxylation reactions.
- 26.a) Solladie G. Heteroat Chem. 2002;13:443–452. [Google Scholar]; b) Boaz NW, Zimmerman RL. Tetrahedron: Asymmetry. 1994;5:153–156. [Google Scholar]; c) Liang Y, Hnatiuk N, Rowley JM, Whiting BT, Coates GW, Rablen PR, Morton M, Howell AR. J Org Chem. 2011;76:9962–9974. doi: 10.1021/jo201565h. [DOI] [PubMed] [Google Scholar]; d) Masamune S, Choy W. Aldrichimica Acta. 1982;5:47–63. [Google Scholar]; e) Martin A, Reyes B, Cintas P, Jimenez JL, Palacios JC. Recent Res Dev Org Chem. 1997;1:159. [Google Scholar]
- 27.a) Mukherjee P, Widenhoefer RA. Angew Chem. 2012;124:1434–1436. doi: 10.1002/anie.201107877. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:1405–1407. doi: 10.1002/anie.201107877. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mukherjee P, Widenhoefer RA. Org Lett. 2011;13:1334–1337. doi: 10.1021/ol103175w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The absolute configurations of (E)-12a, (Z)-12a, (E)-16, and (Z)-16 were determined by HPLC correlation with authentic samples obtained through independent synthesis. Experiment procedures for the independent synthesis of (R,E)-12a, (R,Z)-12a, (R,E)-16, and (R,Z)-16 are described in the Supporting Information.
- 29.Note the stereoconvergence of the secondary reaction manifold. Syn-selective alkoxylation of both (R,E)-12a and (S,Z)-12a form a mixture of (R,E)-16 and (R,Z)-16. Likewise, syn-selective alkoxylation of both (S,E)-12a and (R,Z)-12a form a mixture of (S,E)-16 and (R,Z)-16.
- 30.a) Magid RM. Tetrahedron. 1980;36:1901–1930. [Google Scholar]; b) Paquette LA, Stirling CJM. Tetrahedron. 1992;48:7383–7423. [Google Scholar]
- 31.Cheon CH, Kanno O, Toste FD. J Am Chem Soc. 2011;133:13248–13251. doi: 10.1021/ja204331w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.See Supporting Information for additional details regarding control experiments with HOTf and silver salts.
- 33.Paton RS, Maseras F. Org Lett. 2009;11:2237–2240. doi: 10.1021/ol9004646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.