SUMMARY
Ceramides and sphingoid long-chain bases (LCBs) are precursors to more complex sphingolipids and play distinct signaling roles crucial for cell growth and survival. Conserved reactions within the sphingolipid biosynthetic pathway are responsible for the formation of these intermediates. Components of target of rapamycin complex 2 (TORC2) have been implicated in the biosynthesis of sphingolipids in S. cerevisiae; however, the precise step regulated by this complex remains unknown. Here we demonstrate that yeast cells deficient in TORC2 activity are impaired for de novo ceramide biosynthesis both in vivo and in vitro. We find that TORC2 regulates this step in part by activating the AGC kinase Ypk2 and that this step is antagonized by the Ca2+/calmodulin-dependent phosphatase calcineurin. Because Ypk2 is activated independently by LCBs, the direct precursors to ceramides, our data suggest a model wherein TORC2 signaling is coupled with LCB levels to control Ypk2 activity and, ultimately, regulate ceramide formation.
INTRODUCTION
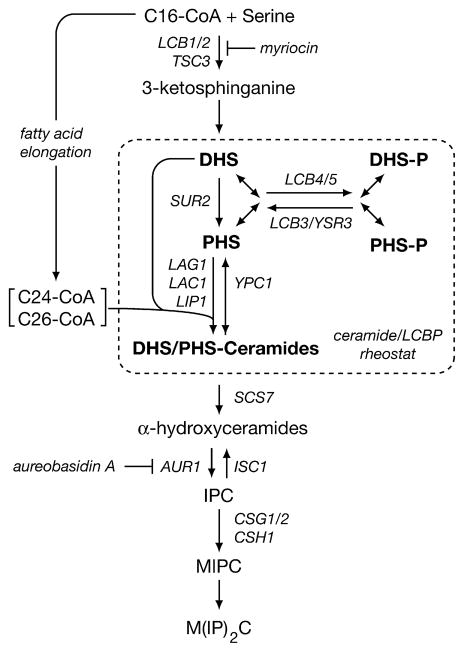
Complex sphingolipids are essential components of eukaryotic membranes, where they play a variety of roles important for cell signaling and intracellular trafficking (Cowart and Obeid, 2007; Dickson et al., 2006; Sims et al., 2004). In yeast, the early steps of sphingolipid biosynthesis involve a number of important metabolites, most notably (1) sphingoid long-chain bases (LCBs), represented by dihydrosphingosine (DHS) and phytosphingosine (PHS); (2) the phosphorylated forms of these LCBs (LCBPs), represented by DHS-P and PHS-P; and (3) dihydroceramides and phytoceramides (DHS-ceramides and PHS-ceramides, respectively) (Cowart and Obeid, 2007; Dickson et al., 2006; Sims et al., 2004) (Figure 1). In particular, LCBs and LCBPs have been identified as important mediators of heat stress (Dickson et al., 1997; Jenkins et al., 1997; Skrzypek et al., 1999; Zhang et al., 2001). PHS also participates in activation of the kinases Pkh1 and Pkh2, orthologs of mammalian PDK1, as well as their downstream signaling partners, the AGC kinases Ypk1 and Ypk2 (Friant et al., 2001; Liu et al., 2005a; Sun et al., 2000). In mammalian cells, ceramides have been implicated in diverse functions such as apoptosis, inflammation, and aging, but the roles of ceramides have not been well characterized in yeast (Hannun and Obeid, 2002; Obeid and Hannun, 2003; Venable et al., 1995). A sensitive balance exists between levels of LCBPs versus ceramides that is believed to be crucial for normal cell growth and survival (Hannun, 1996; Sims et al., 2004; Taha et al., 2006). Remarkably, the regulation of this balance, often referred to as the “ceramide/LCBP rheostat” (Kobayashi and Nagiec, 2003; Spiegel, 2000), as well as of sphingolipid metabolism in general, remains poorly characterized.
Figure 1. Simplified Schematic Diagram of the Sphingolipid Biosynthetic Pathway in S. cerevisiae.
Shown are some of the major intermediates of the pathway as well as a selected set of genes encoding proteins required for specific steps of the pathway considered here. Not shown is Ydc1, an alkaline ceramidase that catabolizes DHS-ceramides.
Previous studies have linked a number of regulatory proteins to sphingolipid biosynthesis in yeast, including components of the target of rapamycin (TOR) signaling network (Beeler et al., 1998; Tabuchi et al., 2006). TOR is an evolutionarily conserved, nutrient-regulated protein kinase that assembles into two distinct protein complexes, termed TOR complex 1 and 2 (TORC1 and TORC2) (Loewith et al., 2002; Reinke et al., 2004), of which TORC1 is uniquely inhibited by the immunosuppressive drug rapamycin (Loewith et al., 2002). Together, TORC1 and TORC2 influence a wide spectrum of cellular activities that are important for temporal as well as spatial aspects of cell growth (Wullschleger et al., 2006). An early study implicated two essential components of TORC2 in sphingolipid biosynthesis, namely Tor2 and Tsc11/Avo3 (referred to here as Avo3) (Beeler et al., 1998), the latter of which is the ortholog of the mammalian TORC2-specific component Rictor (Wullschleger et al., 2006). Specifically, genes encoding Tor2 and Avo3 were identified in a genetic screen for suppressors of a deletion of CSG2, whose gene product is involved in conversion of inositol phosphorylceramide (IPC) to mannosyl-IPC (MIPC) in a later step of the pathway (Beeler et al., 1998) (Figure 1). The precise step affected by these suppressors has not been determined, but they are likely to act upstream of IPC formation because they suppress the Ca2+ hypersensitivity of csg2Δ cells that results from the accumulation of IPC. More recently, Slm1 and Slm2, two proteins that function downstream of TORC2 (Audhya et al., 2004), have also been linked to CSG2 and to IPC formation (Tabuchi et al., 2006). Whether these results can be explained by TORC2 or its effectors acting at an earlier step within the sphingolipid biosynthetic pathway has not been addressed.
An important consideration in the studies cited above is that temperature-sensitive mutants were employed that required use of a nonpermissive temperature of 37°C or higher, wherein heat-stress conditions can dramatically affect the synthesis and/or balance of sphingolipid biosynthetic intermediates (Dickson et al., 2006; Ferguson-Yankey et al., 2002). Here we describe the characterization of an allele of AVO3 (avo3-30) that is compromised for growth and sphingolipid metabolism at 30°C. Liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS)-based profiling of sphingolipids in this mutant reveals an important and positive role for TORC2 in the regulation of ceramide biosynthesis. Our results suggest that positive control of phytoceramide and dihydroceramide synthesis by TORC2 is mediated by the AGC kinase Ypk2, an ortholog of mammalian SGK and/or Akt/PKB that is a specific substrate for TORC2 (Kamada et al., 2005). Based on our results, we suggest a model wherein the TORC2 branch of TOR signaling is coupled with PHS levels to control Ypk2 activity and, ultimately, regulate ceramide production.
RESULTS
Isolation of a Temperature-Sensitive Allele of AVO3
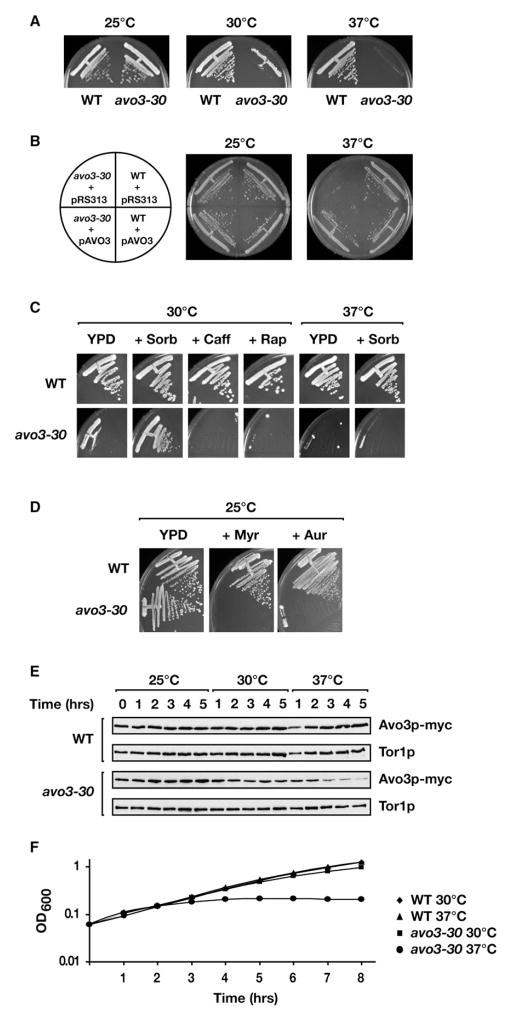
To investigate the role of TORC2 in sphingolipid biosynthesis, we isolated a temperature-sensitive (ts) allele of AVO3, termed avo3-30, as described in Experimental Procedures. We chose to focus on Avo3 rather than Tor2 because only the former is found exclusively in TORC2 (Loewith et al., 2002; Reinke et al., 2004). From among several avo3 mutants isolated, avo3-30 was unique in that it grew like wild-type at 25°C but displayed significant growth defects at both 30°C and 37°C (Figure 2A). These growth defects were due specifically to mutations in AVO3 because they were completely suppressed by introduction of a plasmid-expressed wild-type copy of the gene (Figure 2B). In addition to temperature-sensitive growth, avo3-30 cells displayed a number of phenotypes at 30°C, similar to other reported avo3 (Ho et al., 2005) and tor2 ts alleles (Helliwell et al., 1998), suggestive of defects in cell integrity signaling, including partial restoration of growth by inclusion of the osmotic stabilizer sorbitol, hypersensitivity to rapamycin and caffeine, and depolarization of the actin cytoskeleton (Figure 2C; see also below). The mutant was also hypersensitive to low concentrations of myriocin, an inhibitor of serine palmitoyltransferase, which catalyzes the first step in the sphingolipid biosynthetic pathway, as well as aureobasidin A, an inhibitor of IPC synthase, consistent with it playing a role within this biosynthetic pathway (Figure 2D). Western blot analysis of extracts prepared from avo3-30 cells demonstrated that there were diminished but stable levels of mutant Avo3 protein at 30°C but a drastic reduction following prolonged incubation at 37°C (Figure 2E), consistent with the more severe growth phenotype observed at 37°C (Figures 2A and 2F). Also in agreement with these findings was the observation that sorbitol fails to rescue the growth of avo3-30 cells at 37°C (Figure 2C). Taken together, we conclude from these results that avo3-30 confers partial loss of function at 30°C and complete loss of function at 37°C, most likely with respect to TORC2 activity.
Figure 2. Characterization of avo3-30, a Temperature-Sensitive Allele of AVO3.
(A) Phenotype of avo3-30 cells on solid medium. Wild-type (WT; LHY291) and avo3-30 cells were streaked onto YPD agar plates and incubated at the indicated temperatures for 2–3 days.
(B) The temperature-sensitive (ts) phenotype of avo3-30 is rescued by plasmid-expressed WT AVO3. WT and avo3-30 cells were transformed with either the control vector pRS313 (Sikorski and Heiter, 1989) or pAVO3 and grown on SCD agar plates lacking histidine at the indicated temperatures for 2–3 days.
(C) Cell integrity-related phenotypes of avo3-30 cells. Cells were streaked onto YPD agar plates or plates containing 0.8 M sorbitol (+Sorb), 1 mM caffeine (+Caff), or 0.2 μg/ml rapamycin (+Rap) and incubated at 30°C or 37°C, as indicated.
(D) Hypersensitivity of avo3-30 cells to inhibitors of the sphingolipid biosynthetic pathway. Cells were streaked onto YPD agar plates or plates containing 0.5 μM myriocin (+Myr) or 9 nM aureobasidin A (+Aur) and incubated at 25°C.
(E) Protein levels of Avo3 in WT and avo3-30 cells. Cells were grown at 25°C and were either left at 25°C or shifted to 30°C and 37°C for a further 5 hr. Aliquots were collected each hour and processed for western blot analysis. Tor1 protein levels were analyzed as a loading control.
(F) Growth curves of WT and avo3-30 cells in liquid culture. Cells were grown in YPD medium at 25°C, diluted to OD600 = 0.08, and shifted to 30°C or 37°C for 8 hr. Growth was monitored by A600, and growth curves were plotted on a semilogarithmic scale.
avo3-30 Cells Display Reduced Levels of Major Ceramides
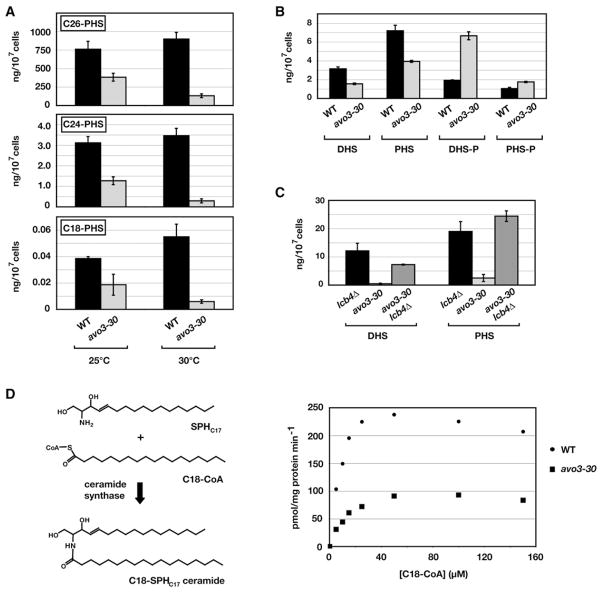
The growth defect observed in avo3-30 cells at 30°C provided an important and unique advantage for metabolic profiling of sphingolipids in conditions free of heat stress encountered at 37°C. Moreover, because no significant growth defect was observed until approximately 5–6 hr following a shift to 30°C, by performing analyses at early time points we could be confident that any differences observed in comparison to wild-type cells were unlikely to be due to secondary effects associated with altered growth rate (Figure 2F). We first applied this strategy to examine the role of AVO3 in ceramide synthesis. Ceramides in yeast are formed by N-acylation of sphingoid bases, primarily phytosphingosine (PHS) but also dihydrosphingosine (DHS), using C26-CoA and C24-CoA as preferred substrates (Cowart et al., 2006; Guillas et al., 2003) (Figure 1). Accordingly, we first used LC-MS/MS to measure absolute levels of C26-PHS and C24-PHS ceramides in wild-type versus avo3-30 cells (see Experimental Procedures). We observed that steady-state levels of both phytoceramides were decreased 5- to 10-fold in avo3-30 cells relative to wild-type following a 3 hr shift to 30°C (p < 0.01; Figure 3A). Minor differences were also observed at 25°C, suggesting that avo3-30 cells possess a constitutive defect in ceramide production at the permissive temperature (Figure 3A). In addition to these results, we observed a reduction in C26-DHS-ceramide in avo3-30 cells following a shift to 30°C (p < 0.01; see Figure S1A available online). With respect to C24-DHS ceramide, this species could be detected at low levels in wild-type cells but was not detectable in avo3-30 cells at 30°C (data not shown).
Figure 3. Monitoring Intermediates in the Sphingolipid Biosynthetic Pathway in Wild-Type and avo3-30 Cells.
(A) Levels of phytoceramides were analyzed in extracts derived from WT and avo3-30 cells grown in YPD medium at 25°C following a shift to 30°C for 3 hr.
(B) Levels of LCBs (DHS and PHS) and LCBPs (DHS-P and PHS-P) were analyzed in extracts derived from WT and avo3-30 cells in YPD medium following a shift to 30°C for 3 hr.
(C) Levels of DHS and PHS were analyzed in extracts derived from WT, avo3-30, and avo3-30 lcb4Δ cells in YPD medium following a shift to 30°C for 3 hr. In (A–C), error bars indicate the SD of separately extracted samples (n = 3).
(D) Monitoring ceramide synthesis in vitro. Microsomal membranes were isolated and ceramide synthase activity was measured as described in Experimental Procedures. Left panel: schematic diagram of the in vitro reaction, showing the structure of the C18-SPHC17 ceramide product and the substrates SPHC17 and C18-CoA used for this reaction. Right panel: C18-CoA concentration dependence of ceramide synthase activity in microsomal membranes isolated from WT versus avo3-30 cells.
C26-CoA and C24-CoA used in ceramide synthesis are derived from shorter C18-CoA or C16-CoA molecules by a series of fatty acid elongation steps (Kohlwein et al., 2001) (Figure 1). Thus, we considered the possibility that downregulation of this elongation branch could account for the decreased levels of ceramides in avo3-30 cells described above. If this were the case, we expected to observe preferential accumulation of ceramides containing shorter fatty acids in the avo3-30 mutant (Kohlwein et al., 2001). By contrast, however, we observed that following a 3 hr shift to 30°C, the steady-state levels of both C18-PHS and C18-DHS ceramides were significantly decreased in avo3-30 cells relative to wild-type (p < 0.01; Figure 3A; Figure S1A). A reduction, although less prominent, was also observed in the levels of C16-PHS and C16-DHS ceramides in avo3-30 cells at 30°C (Figures S1A and S1B).
Most ceramides undergo α-hydroxylation in their fatty acid residues, in a reaction controlled by Scs7, prior to incorporation into IPC (Dunn et al., 1998; Haak et al., 1997) (Figure 1). We therefore next considered the possibility that the decrease in ceramide levels in avo3-30 cells was a consequence of upregulation of the α-hydroxylation step of the pathway. If this were the case, we expected to observe preferential accumulation of α-hydroxylated ceramide species in avo3-30 cells at 30°C. By contrast, however, we observed decreased levels of C26(OH)-PHS and C24(OH)-PHS relative to wild-type cells following a shift to 30°C (Figure S1C). Together with previous results placing TORC2 upstream of IPC synthesis (Beeler et al., 1998), these findings suggested that the reduction in ceramide levels in avo3-30 cells is due to a defect prior to or at the step of ceramide formation. This conclusion is consistent with previous findings showing that cells lacking ceramide synthase fail to synthesize α-hydroxylated C26-PHS (Guillas et al., 2001).
Reduction in Ceramide Levels in avo3-30 Cells Is Due to a Defect Downstream of LCB Formation
We next considered the possibility that the reduction in ceramide levels in avo3-30 cells is a consequence of limiting amounts of ceramide precursors, i.e., DHS and PHS (Figure 1). Accordingly, we used LC-MS/MS to examine steady-state levels of both DHS and PHS in avo3-30 cells. Because a dynamic balance exists with respect to the levels of LCBs and their phosphorylated forms (Hannun, 1996; Kobayashi and Nagiec, 2003; Spiegel and Milstien, 2000) (Figure 1), we also monitored steady-state levels of DHS-P and PHS-P. Intriguingly, following a shift to 30°C for 3 hr, we observed a reproducible decrease in the levels of both DHS and PHS (p < 0.01) and a corresponding increase in the levels of DHS-P and PHS-P (p < 0.01and p < 0.05, respectively) in avo3-30 cells relative to wild-type (Figure 3B). One possible explanation for these results was that LCBs are formed normally in avo3-30 cells at 30°C but are preferentially shunted toward LCBP formation. A prediction of this hypothesis was that preventing LCBP formation would result in accumulation of DHS and/or PHS in avo3-30 cells. To test this prediction, we combined the avo3-30 allele with a deletion of LCB4, which encodes the major LCB kinase required for the synthesis of the majority of LCBPs (Nagiec et al., 1998) (Figure 1). Indeed, we now observed accumulation of LCBs, in particular PHS, in avo3-30 lcb4Δ cells at 30°C (p < 0.01; Figure 3C). No significant corresponding increase in levels of phytoceramides was detected in the double mutant, however, consistent with avo3-30 cells being impaired at the step of ceramide synthesis (data not shown; see below).
avo3-30 Cells Have Reduced Ceramide Synthase Activity In Vitro
To test directly whether ceramide synthesis is affected in avo3-30 cells, we monitored ceramide formation in isolated microsomes in vitro. Previous studies have demonstrated the feasibility of this approach (Guillas et al., 2001, 2003; Kobayashi and Nagiec, 2003; Schorling et al., 2001), which we modified in order to monitor ceramide formation using LC-MS/MS (see Experimental Procedures). As substrates, we used C18-CoA and an unnatural C17 derivative of mammalian sphingosine (SPHC17) (Figure 3D, left panel). We chose these substrates because the expected product (C18-SPHC17 ceramide) is not produced in yeast, which facilitates its identification within an expected background of endogenous ceramide species. A previous study has demonstrated that sphingosine can be used as a substrate by yeast ceramide synthase in vivo (Tani et al., 2006), which we have confirmed is also the case for SPHC17 (unpublished data). In membranes prepared from wild-type cells, we observed that ceramide formation in this assay was linear over time (data not shown) and reached a plateau at saturating concentrations of C18-CoA (Figure 3D, right panel). The overall kinetic parameters observed in this reaction were consistent with a previous study using radiolabeled DHS as a substrate (Kobayashi and Nagiec, 2003). Remarkably, we consistently observed a reduction in ceramide synthase activity of membranes prepared from temperature-shifted avo3-30 cells (Figure 3D, right panel).
Exogenous PHS Compensates for Diminished Levels of Phytoceramides in avo3-30 Cells
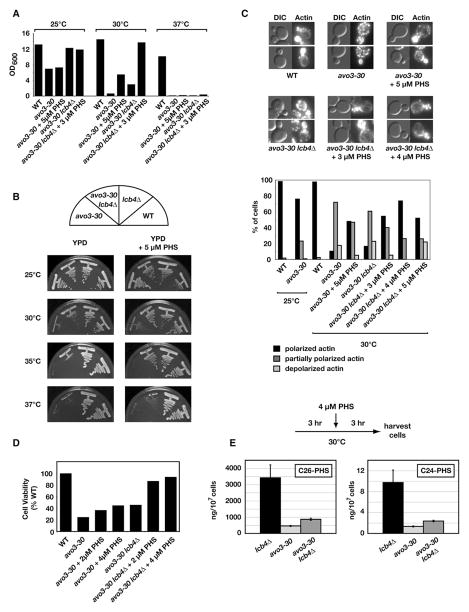
Accumulation of LCBPs has previously been shown to correlate with cell growth inhibition in yeast (Kim et al., 2000; Zhang et al., 2001). In this regard, it is possible that accumulation of LCBPs in avo3-30 cells contributes to the growth phenotype of this mutant, based on our observation that deletion of LCB4 partially improves its growth at 30°C (Figures 4A and 4B). On the other hand, deletion of LCB4 did not significantly improve actin polarization, because similar defects were observed in both avo3-30 as well as avo3-30 lcb4Δ cells at 30°C (Figure 4C). Remarkably, we found that addition of exogenous PHS significantly rescued defects in actin polarization in both avo3-30 and, to a greater extent, avo3-30 lcb4Δ cells (Figure 4C). Moreover, exogenous PHS partially improved the growth of avo3-30 cells and significantly improved the growth of avo3-30 lcb4Δ cells at 30°C (Figures 4A and 4B). Simultaneous deletion of LCB4 and addition of PHS also largely restored the viability of avo3-30 cells when shifted to 30°C (Figure 4D). That PHS addition was more efficacious when added to avo3-30 lcb4Δ versus avo3-30 cells is presumably related to the fact that it cannot be converted efficiently to PHS-P in the former strain. Deletion of LCB4 and/or PHS addition could partially restore growth of avo3-30 cells at 35°C but not 37°C, indicating that these conditions can compensate for a partial but not complete loss of function of AVO3 (Figure 4B). Interestingly, we observed that addition of PHS following a temperature shift to 30°C resulted in a minor but statistically significant restoration of ceramide levels in avo3-30 lcb4Δ cells relative to lcb4Δ control cells (p < 0.01; Figure 4E). Thus, it is possible that the positive effects of adding exogenous PHS are due to a partial rescue of phytoceramide levels in avo3-30 lcb4Δ cells. Alternatively, exogenous PHS could act as an independent signaling molecule, as has been suggested previously (Dickson and Lester, 2002; Liu et al., 2005b).
Figure 4. Beneficial Effects of Deleting LCB4 and/or Adding Exogenous PHS with Respect to avo3-30 Phenotypes.
(A and B) Growth in liquid YPD medium (A) and on YPD agar plates (B) of indicated strains in the absence or presence of different concentrations of PHS and at different temperatures, as indicated.
(C) Effect of PHS addition on actin polarization in the indicated strains grown at 30°C (upper panels). Bar graph represents the quantification of this experiment and includes data for WT and avo3-30 cells grown at 25°C for comparison.
(D) Influence of LCB4 deletion and/or PHS addition on viability of avo3-30 cells. The indicated strains were grown to early log phase in liquid media that either lacked or contained the indicated concentration of PHS at 25°C and were then transferred to 30°C for 4 hr. Cultures were diluted and plated onto solid YPD medium at 25°C to determine colony-forming units (expressed as % of WT).
(E) Addition of PHS partially rescues phytoceramide levels in avo3-30 and avo3-30 lcb4Δ cells. Cells were grown at 25°C and shifted to 30°C for 3 hr. PHS was then added to a final concentration of 4 μM, and cells were incubated for an additional 3 hr at 30°C. Sphingolipids were extracted, and ceramide levels were analyzed by LC-MS/MS. Error bars indicate the SD of separately extracted samples (n = 3).
Ypk2 Functions Downstream of TORC2 to Regulate Ceramide Levels
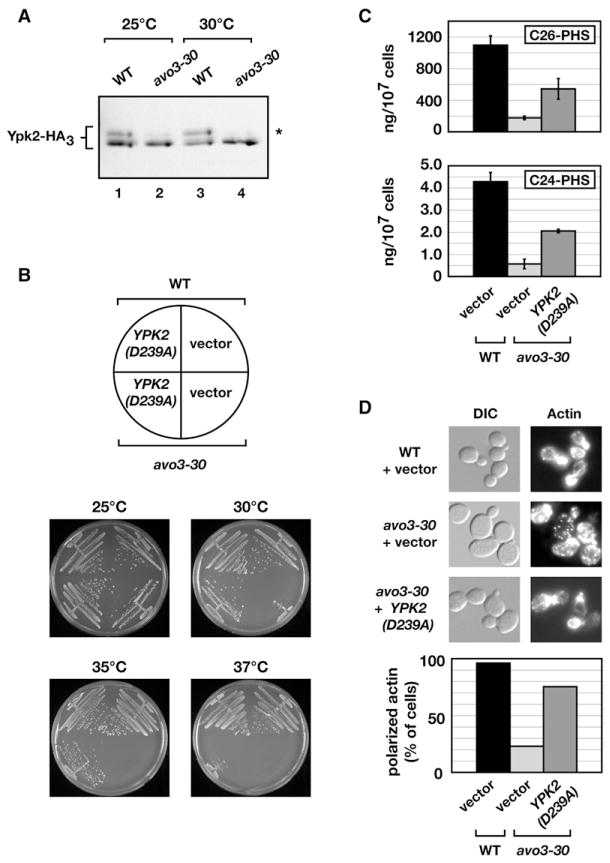
It has recently been proposed that PHS directly activates a number of protein kinases, in particular members of the AGC family of kinases (Liu et al., 2005a). Among these, Ypk2 is known to play a role in cell integrity and actin polarization and moreover has been shown to be a direct target of TORC2 (Kamada et al., 2005). Given our present results, we therefore investigated whether Ypk2 might be involved in any of the phenotypes associated with avo3-30 cells. First, we asked whether the phosphorylation state of Ypk2 was altered in avo3-30 cells by monitoring its mobility using SDS-PAGE and western blotting, as has been used previously to examine the phosphorylation state of this protein (Kamada et al., 2005). Indeed, we observed that Ypk2 was constitutively hypophosphorylated in avo3-30 cells in comparison to wild-type (Figure 5A). Interestingly, hypophosphorylation of Ypk2 was not affected by either deletion of LCB4 or addition of exogenous PHS, consistent with it functioning directly downstream of TORC2 (data not shown). These observations prompted us to examine avo3-30-specific phenotypes when the mutant was transformed with a plasmid expressing a constitutively active allele of YPK2 (YPK2D239A) (Kamada et al., 2005). We found that expression of this allele rescued the ts phenotype of avo3-30 cells completely at 30°C, and to a lesser extent at higher temperatures (Figure 5B). Moreover, expression of YPK2D239A restored C24-PHS and C26-PHS ceramide levels (p < 0.01 and p < 0.05, respectively) as well as actin polarization in avo3-30 cells (Figures 5C and 5D). From these results, we conclude that Ypk2 acts downstream of TORC2 to regulate ceramide levels as well as actin polarization.
Figure 5. Expression of a Constitutively Active Allele of YPK2 Rescues Multiple Phenotypes of the avo3-30 Mutant.
(A) WT and avo3-30 cells expressing a HA3-tagged version of Ypk2 were grown in YPD medium at 25°C and either shifted to 30°C for 3 hr or left at 25°C and were then processed for western blot analysis, probing for Ypk2 using anti-HA antibody. The asterisk indicates the phosphorylated form of Ypk2 (Kamada et al., 2005).
(B) WT and avo3-30 cells harboring either pYE352[YPK2D239A] or vector plasmid were streaked onto selective dropout plates and incubated at the indicated temperatures for 3–4 days.
(C) WT and avo3-30 cells harboring either pYE352[YPK2D239A] or vector plasmid were grown in selective dropout medium at 25°C overnight, followed by a shift to 30°C for 3 hr. Sphingolipids were extracted and ceramide levels were analyzed by LC-MS/MS as described in Experimental Procedures. Error bars indicate the SD of separately extracted samples (n = 3).
(D) WT and avo3-30 cells harboring either pYE352[YPK2D239A] or vector plasmid were grown as described in (C), fixed, and stained for actin with rhodamine-coupled phalloidin. The graph below shows the percentage of cells with completely polarized actin patches.
Deletion of the Calcineurin Regulatory Subunit B Restores Ceramide Levels in avo3-30 Cells
Calcineurin is an evolutionarily conserved Ca2+/calmodulin-regulated protein phosphatase that has recently been identified as a negative regulator of TORC2 activity and has also been reported to be a negative regulator of the synthesis of complex sphingolipids (Mulet et al., 2006; Tabuchi et al., 2006). We therefore sought to determine whether calcineurin might also influence TORC2 activity with respect to maintenance of ceramide levels. Indeed, we observed that deletion of CNB1, which encodes the calcineurin regulatory subunit B, both rescued the growth phenotype of avo3-30 at 30°C and restored phytoceramide levels in avo3-30 cells (p < 0.05), although not to levels observed in wild-type cells (Figure 6). These results thus extend the antagonistic relationship between calcineurin and TORC2 activity to include regulation of ceramides. Interestingly, however, no improvement in actin polarization was observed in avo3-30 cnb1Δ cells compared to avo3-30 cells (data not shown), suggesting that regulation of ceramides levels and control of actin polarization represent distinct and separable readouts of TORC2.
Figure 6. Deletion of the Calcineurin Regulatory Subunit CNB1 Restores a Subset of the Phenotypes of the avo3-30 Mutant.
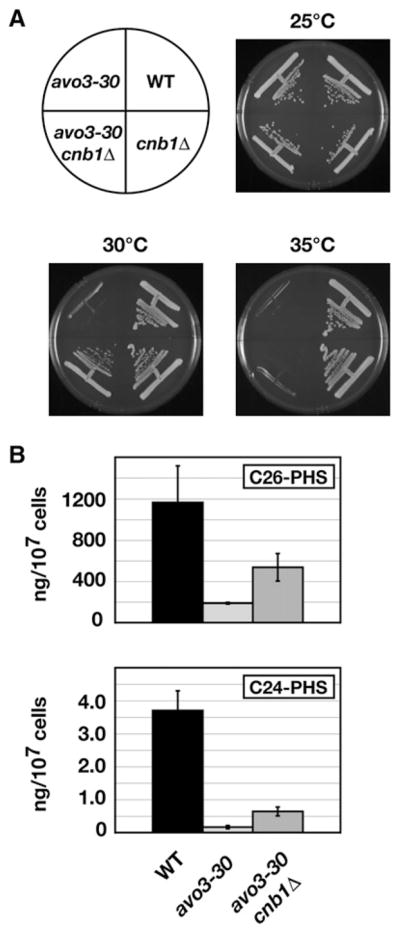
(A) WT, avo3-30, avo3-30 cnb1Δ, and cnb1Δ cells were streaked onto YPD plates and incubated at the indicated temperatures.
(B) avo3-30 and avo3-30 cnb1Δ cells were grown in liquid YPD medium to mid-log phase, sphingolipids were extracted, and ceramide levels were analyzed by LC-MS/MS. Error bars indicate the SD of separately extracted samples (n = 3).
DISCUSSION
TORC2 has previously been implicated in the regulation of sphingolipid biosynthesis at a step (or steps) that occurs prior to formation of IPC (Beeler et al., 1998; Tabuchi et al., 2006) (Figure 1). The findings presented here significantly extend our understanding of this regulation by demonstrating that a functional Avo3 protein, an essential and specific component of TORC2, is required for maintenance of normal levels of ceramides in vivo, which in turn allows for the synthesis of more complex sphingolipids that are required for cell growth. Our observation that ceramide synthesis is impaired in vitro in microsomal membranes isolated from avo3-30 cells suggests further that the activity of ceramide synthase is directly affected in this mutant. Acyl-CoA-dependent ceramide synthase activity is carried out by the highly similar Lag1 and Lac1 enzymes (Schorling et al., 2001; Guillas et al., 2001), as well as the recently characterized regulatory protein Lip1 (Vallee and Riezman, 2005). Our results now raise the important question of whether TORC2 signaling affects one or more of these subunits.
Concomitant with a reduction in ceramides in avo3-30 cells are a decrease in the steady-state levels of sphingoid LCBs and an increase in LCBPs, both of which are likely to contribute to the impaired growth phenotype of this mutant. These findings therefore suggest that TORC2 is likely to influence more than simply ceramide synthase and may play a more general role in the “ceramide/LCBP rheostat,” wherein the relative levels of these components are controlled to regulate cell growth and survival in both yeast and mammals (Hannun, 1996; Kobayashi and Nagiec, 2003; Spiegel and Milstien, 2000) (Figure 1). This conclusion is consistent with the extreme phylogenetic conservation of TORC2 as well as the fact that the initial steps of sphingolipid biosynthesis, including ceramide formation, involve enzymes that are conserved among eukaryotes (Obeid et al., 2002).
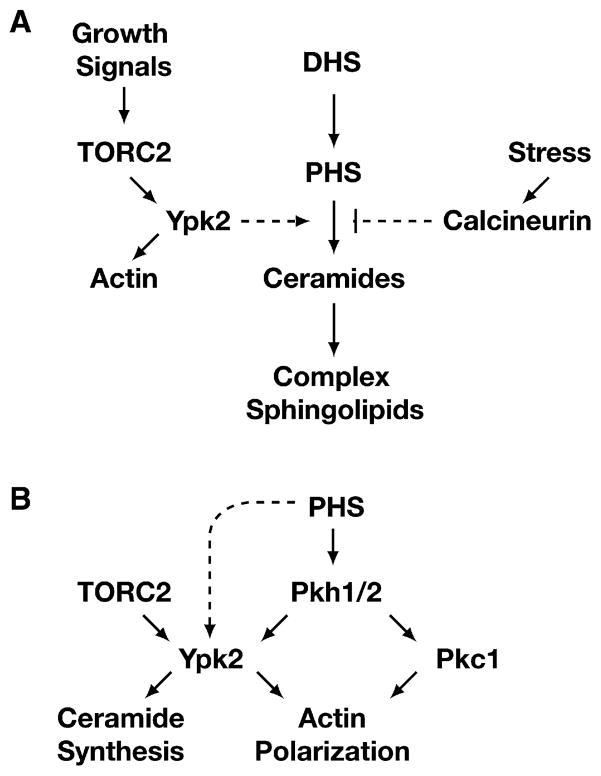
We suggest that TORC2 regulates ceramide biosynthesis at least in part by signaling through the AGC kinase Ypk2 (Figure 7A). This conclusion is based on our findings that Ypk2 is hypophosphorylated in avo3-30 cells and that expression of a constitutively active version of this kinase rescues both the growth defect and ceramide levels in this mutant. We have also found that deletion of CNB1 partially restores ceramide levels in avo3-30 cells, demonstrating that calcineurin plays an antagonistic role within the pathway (Figure 7A). Together, these observations extend the results of previous studies that have established both a positive link between TORC2 and Ypk2 (Kamada et al., 2005) and a negative link between TORC2 and calcineurin (Mulet et al., 2006) to include regulation of ceramide synthesis. In addition, our finding that expression of the constitutively active YPK2D239A allele rescues the actin polarization defect in avo3-30 cells whereas deletion of CNB1 does not argues that actin polarization and ceramide synthesis are separable activities regulated independently by TORC2/Ypk2 (Figure 7A).
Figure 7. Models Illustrating Connections between TORC2 Signaling and Regulation of the Sphingolipid Biosynthetic Pathway.
(A) TORC2 signals to Ypk2 to positively regulate de novo synthesis of ceramides at a step that is antagonized by calcineurin.
(B) Interplay between TORC2 and PHS for activation of Ypk2 for ceramide synthesis. See text for details.
In addition to TORC2, activation of Ypk2 requires phosphorylation by Pkh1 or Pkh2, the yeast orthologs of mammalian PDK1 (Kamada et al., 2005). Indeed, dual activation of AGC kinase family members by TOR and PDK1 is emerging as a conserved regulatory theme in eukaryotes (Powers, 2007). Interestingly, there is also significant evidence to indicate that PHS activates Pkh1/2 and, to a lesser extent, other AGC kinases, including Ypk2 (Liu et al., 2005a). Given our present findings, we suggest the existence of a “feed-forward” regulatory loop wherein PHS both acts as a substrate and stimulates the signaling pathway directly involved in ceramide synthesis (Figure 7B). Our observation that addition of exogenous PHS can largely restore actin polarization within avo3-30 cells but does not restore Ypk2 phosphorylation or ceramide levels underscores the importance of TORC2 in Ypk2 activation and additionally suggests that PHS must influence other routes for actin polarization downstream of Pkh1/2, for example via activation of Pkc1 (Liu et al., 2005b) (Figure 7B).
We have identified what appears to be a distinct point of regulation compared to that described in another recent study, in which TORC2 and its downstream effectors Slm1 and Slm2 were also linked to sphingolipid biosynthesis (Tabuchi et al., 2006). The authors concluded that Slm1 and Slm2 negatively regulate a later step in the pathway, carried out by Isc1, which catalyzes a reverse reaction that catabolizes IPC back to free ceramides (Tabuchi et al., 2006) (Figure 1). A number of other studies have also now linked Slm1/2 to sphingolipids and/or calcineurin (Bultynck et al., 2006; Daquinag et al., 2007). Importantly, a prediction of the Tabuchi et al. (2006) model is that loss of TORC2 activity should result in elevated levels of both PHS- and α-hydroxylated ceramides; however, as described above, we instead observe a significant reduction in these species within avo3-30 cells. Moreover, whereas deletion of ISC1 was observed to rescue several phenotypes associated with slm1ts/slm2Δ cells (Tabuchi et al., 2006), we have observed no such rescue of avo3-30-specific phenotypes in an isc1Δ background (unpublished data). On the other hand, deletion of CNB1 results in the partial rescue of phenotypes associated with slm1ts/slm2Δ cells, in agreement with our present findings that ceramide levels are partially restored in avo3-30 cnb1Δ cells (Figure 6). Additional studies will be required to clarify the relationship between the TORC2/Ypk2 and the TORC2/Slm1/2 branches with respect to sphingolipid biosynthesis. Interestingly, de novo biosynthesis of ceramides has also been identified as a target for regulation by casein kinase II, where deletion of CKA2, encoding the α′ catalytic subunit, results in decreased levels of ceramides formed in microsomes in vitro (Kobayashi and Nagiec, 2003). In yeast, casein kinase II is required for cell-cycle progression as well as maintenance of cell polarity (Canton and Litchfield, 2006; Hanna et al., 1995; Rethinaswamy et al., 1998), activities also positively influenced by TOR. Thus, it will be of interest to examine whether TORC2 and casein kinase II collaborate to regulate ceramide biosynthesis.
Might TORC2 regulate de novo ceramide formation in higher eukaryotes? An apparent paradox with this suggestion is that TORC2 acts within a pro-growth signaling network, whereas ceramides are associated with promoting stress-induced growth inhibition and apoptosis in mammalian cells (Hannun and Obeid, 2002; Obeid and Hannun, 2003; Venable et al., 1995). However, it is important to consider that the major pathway responsible for production of ceramides involved in apoptosis and stress-induced signaling in mammalian cells is catabolism of sphingomyelin by neutral sphingomyelinase rather than de novo biosynthesis (Clarke and Hannun, 2006; Posse de Chaves, 2006). Moreover, in mammalian cells, both the major sphingoid base sphingosine and its phosphorylated form, sphingosine-1-P, the latter of which is positively involved in proliferation and survival signaling, are synthesized exclusively via a ceramide recycling pathway (Huwiler and Zangemeister-Wittke, 2007). Thus, ceramide synthesis is required for the production of the entire set of bioactive sphingolipids. Finally, an adequate level of ceramides must be maintained in order to facilitate synthesis of more complex sphingolipids, which are required for normal cell growth (Dickson and Lester, 1999; Yamaoka et al., 2004). We speculate that a positive role for ceramides in growth may be more apparent in a rapidly dividing organism such as yeast growing under rich nutrient conditions.
EXPERIMENTAL PROCEDURES
Yeast Strains, Media, and Antibodies
Yeast strains used in this study are listed in Table S1. Unless indicated otherwise, cells were grown in rich YPD medium (2% yeast extract, 1% peptone, and 2% dextrose supplemented with adenine and tryptophan at 5 mg/l). All yeast transformations were conducted using a lithium acetate procedure (Geitz and Woods, 1998). Construction of deletion strains by replacement of complete open reading frames (ORFs) with a selectable marker as well as epitope tagging of YPK2 was performed as described previously (Dilova et al., 2004). Monoclonal antibodies specific for HA (12CA5) and c-Myc (9E10) were purchased from Roche Diagnostics and Covance, respectively. Polyclonal antibodies specific for Tor1 were from Santa Cruz Biotechnology.
Isolation of Avo3-30
Error-prone PCR was used to generate mutations within the 3′ end of chromosomally encoded AVO3, following previously established protocols (Muhlrad et al., 1992; Umen and Guthrie, 1996). In a first step, error-prone PCR conditions were used to amplify ~1 kb of the 3′ end of the AVO3 ORF using pAVO3 (a generous gift of L. Hicke) as a template plasmid. In a second step, standard PCR conditions were used to amplify a fragment that contained the last 70 bp of the AVO3 ORF, followed by sequences encoding multiple copies of the Myc epitope and the TRP1 gene, using pFA6a-13Myc-TRP1 (Longtine et al., 1998) as a template plasmid. In a third step, these two fragments were fused together using SOEing (splice overlap extension) (Horton et al., 1993) to generate a single fragment that was used to transform strain LHY291. Transformed cells were selected for by growth on SCD agar plates lacking tryptophan for 3–4 days at 25°C. Colonies were screened for temperature-sensitive growth at both 30°C and 37°C. Of 1856 transformants screened, 4 displayed a strong temperature-sensitive (ts) phenotype at 37°C. One of these also displayed a strong ts phenotype at 30°C. This allele was named avo3-30 and was used for the studies reported here. Genetic backcross analyses demonstrated that this allele was recessive and segregated in a 2:2 manner. In addition, the temperature-sensitive behavior of avo3-30 was completely suppressed by transformation with pAVO3, and western blot analysis demonstrated that avo3-30 cells produced full-length Avo3 protein. Finally, DNA sequence analysis identified a number of predicted amino acid substitutions throughout the last 1 kb of the 3′ end of the AVO3 ORF. A full description of these mutations will be published elsewhere.
Chemicals for LC-MS/MS
DHS, PHS, DHS-P, PHS-P (all C18 species), SPHC17 base, C18:0-PHS, C16:0-DHS, C18:0-DHS, C24:0-DHS, and C17:0-SPH ceramide standards were purchased from Avanti Polar Lipids. C24:0-PHS and C26:0-PHS were synthesized as described previously (Hwang et al., 2001; Ramjit et al., 2005) and purified by preparative high-performance liquid chromatography. Sphingolipid standards were dissolved in ethanol and stored at −80°C.
Extraction of Lipids
All experiments were performed multiple times, and all samples were prepared in triplicate for each experiment. Forty A600 units of cell culture were killed by 5% trichloroacetic acid (TCA) for 10 min on ice. LCBs, LCBPs, and ceramides were extracted as described previously (Lester and Dickson, 2001). SPHC17 base, used as internal standard, was dissolved in ethanol to a concentration of 5 ng/ml and added to each cell pellet during extraction. Samples were transferred to autosampler vials and analyzed by LC-MS/MS.
LC-MS/MS Analysis of LCBs, LCBPs, and Ceramides
LC-MS/MS analysis of sphingolipids was performed using a Quattro Premier tandem quadrupole mass spectrometer (Waters) coupled to an Acquity Ultra Performance LC system (Waters). LCBs, LCBPs, and ceramides were resolved in a single 20 min run by reverse-phase chromatography using an Acquity BEH C18 column (Waters) at a flow rate of 0.5 ml/min with a column size of 2.1 × 50 mm and a 1.7 μm particle size. The solvent system consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid. The initial solvent composition was 55% solvent B. The gradient program further involved 1 min linear gradient to 65% solvent B, followed by 4.5 min linear gradient to 75% solvent B, followed by 0.5 min linear gradient to 95% solvent B held for 3 min, followed by 1 min linear gradient to 99% solvent B held for 10 min. The column was re-equilibrated to initial conditions for 1 min.
Electrospray ionization (ESI) of sphingolipids was performed in positive mode with capillary voltage 3 kV, source temperature 125°C, and desolvation temperature 350°C using nitrogen as desolvation and cone gas. Sphingolipids were quantitatively analyzed in multiple reaction monitoring (MRM) mode (specific MRM parameters are shown in Table S2 and selected ion chromatograms in Figure S2) using 2.2 × 10−3 millibars argon as collision gas. External calibration curves of synthetic standards were built using 5–6 concentration points in the range from 1 ng/ml to 100 μg/ml. Integration and data analysis were performed using the QuantLynx module of MassLynx 4.1 software (Waters). The level of significance of all LC-MS/MS data was determined by unpaired t test.
In Vitro Ceramide Synthesis Assay
Wild-type and avo3-30 cells were grown in YPD medium at 25°C and shifted to 30 °C for 6 hr. Microsomal membranes were prepared from 250 OD600 units of each culture as described previously (Vallee and Riezman, 2005) and resuspended in B88 buffer (20 mM HEPES [pH 6.8], 150 mM KAc, 5 mM MgAc2, and 250 mM sorbitol) to a protein concentration of 2 mg/ml. For in vitro ceramide synthesis, 10 μl of protein lysate was combined in a 100 μl reaction volume with 20 μM SPHc17 base (dissolved in a 40 μM solution of defatted BSA) and 2–150 μM C18-CoA (dissolved in DMSO). The reactions were incubated at 22°C for 25 min and stopped by addition of 500 μl cold ethanol containing C17-ceramide (C17-SPH) as an internal standard at a concentration of 50 ng/ml. Lipids were extracted into ethanol solution and analyzed by LC-MS/MS. A calibration curve was constructed using C17-SPH as a standard to quantify the product of this reaction.
Fluorescence Microscopy
Rhodamine phalloidin (Invitrogen) staining of polymerized actin was performed essentially as described previously (Pringle et al., 1989). For experiments demonstrating actin polarization at the permissive temperature, cells were grown in YPD at 25°C overnight to approximately OD600 = 0.3, followed by fixation and staining with rhodamine phalloidin and visualization by fluorescence microscopy. For experiments at the nonpermissive temperature, cells were grown in YPD at 25°C overnight, diluted to approximately OD600 = 0.1 in YPD pre-warmed to 30°C with or without various concentrations of PHS, grown at 30 °C for various lengths of time to approximately OD600 = 0.3, and then fixed and stained as described above. For quantification of the status of actin polarization as represented by actin patch localization, approximately 100 small or midsize budded cells were counted for each condition. Cells were considered as having polarized actin patches if patches were concentrated in the bud and five or fewer patches were found in the mother cell. Cells were considered as having partially polarized patches if patches were concentrated in the bud and there were more than five patches in the mother cell. Cells were considered depolarized if patches were evenly distributed throughout both the bud and mother cell. Fluorescence microscopy was performed using a Nikon E600 fluorescence microscope and an Orca ER charge-coupled device camera (Hamamatsu) controlled by Simple PCI software. All images of actin staining are Z series projections of optical sections.
Supplementary Material
Acknowledgments
We thank L. Hicke for the generous gift of plasmid pAVO3 and R. Loewith and Y. Kamada for plasmid pYE352[YPK2D239A]. We are grateful to K.C. Ahn for valuable advice during the synthesis of ceramide standards, S. Lockwood for assistance with experiments involving Ypk2, K. Kaplan for the use of his fluorescence microscope, Y.-T. Chang for assistance with genetic analysis of avo3-30, and A. Hill and J. Nunnari for valuable discussions. This work was supported by National Sciences Foundation grant MCB-1031221 and American Cancer Society Research Scholar Grant RSG-1031221 (to T.P.) and by National Institute of Environmental Health Sciences (NIEHS) grant R37 ES02710, NIEHS Superfund Basic Research Program grant P42 ES004699, and NIEHS Center for Environmental Health Sciences grant P30 ES05707 (to B.D.H.). P.A.A. was supported by NIEHS Advanced Training in Environmental Toxicology grant T32 ES007059.
Footnotes
Supplemental Data include two figures and two tables and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/7/2/148/DC1/.
References
- Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 2004;23:3747–3757. doi: 10.1038/sj.emboj.7600384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, Dunn T. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Delta mutant. J Biol Chem. 1998;273:30688–30694. doi: 10.1074/jbc.273.46.30688. [DOI] [PubMed] [Google Scholar]
- Bultynck G, Heath VL, Majeed AP, Galan JM, Haguenauer-Tsapis R, Cyert MS. Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol. 2006;26:4729–4745. doi: 10.1128/MCB.01973-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–1901. doi: 10.1016/j.bbamem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Cowart LA, Obeid LM. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim Biophys Acta. 2007;1771:421–431. doi: 10.1016/j.bbalip.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart LA, Okamoto Y, Lu X, Hannun YA. Distinct roles for de novo versus hydrolytic pathways of sphingolipid biosynthesis in Saccharomyces cerevisiae. Biochem J. 2006;393:733–740. doi: 10.1042/BJ20050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag A, Fadri M, Jung SY, Qin J, Kunz J. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol. 2007;27:633–650. doi: 10.1128/MCB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RC, Lester RL. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1438:305–321. doi: 10.1016/s1388-1981(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Lester RL. Sphingolipid functions in Saccharomyces cerevisiae. Biochim Biophys Acta. 2002;1583:13–25. doi: 10.1016/s1388-1981(02)00210-x. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Nagiec EE, Skrzypek M, Tillman P, Wells GB, Lester RL. Sphingolipids are potential heat stress signals in Saccharomyces. J Biol Chem. 1997;272:30196–30200. doi: 10.1074/jbc.272.48.30196. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Sumanasekera C, Lester RL. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog Lipid Res. 2006;45:447–465. doi: 10.1016/j.plipres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Dilova I, Aronova S, Chen CY, Powers T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1p/Rtg3p-dependent genes. J Biol Chem. 2004;279:46527–46535. doi: 10.1074/jbc.M409012200. [DOI] [PubMed] [Google Scholar]
- Dunn TM, Haak D, Monaghan E, Beeler TJ. Synthesis of monohydroxylated inositolphosphorylceramide (IPC-C) in Saccharomyces cerevisiae requires Scs7p, a protein with both a cytochrome b5-like domain and a hydroxylase/desaturase domain. Yeast. 1998;14:311–321. doi: 10.1002/(SICI)1097-0061(19980315)14:4<311::AID-YEA220>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ferguson-Yankey SR, Skrzypek MS, Lester RL, Dickson RC. Mutant analysis reveals complex regulation of sphingolipid long chain base phosphates and long chain bases during heat stress in yeast. Yeast. 2002;19:573–586. doi: 10.1002/yea.861. [DOI] [PubMed] [Google Scholar]
- Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 2001;20:6783–6792. doi: 10.1093/emboj/20.23.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitz RD, Woods RA. Transformation of yeast by the lithium acetate/single-stranded carrier DNA/PEG method. In: Brown AJP, Tuite MF, editors. Methods in Microbiology. New York: Academic Press; 1998. [Google Scholar]
- Guillas I, Kirchman PA, Chuard R, Pfefferli M, Jiang JC, Jazwinski SM, Conzelmann A. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 2001;20:2655–2665. doi: 10.1093/emboj/20.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillas I, Jiang JC, Vionnet C, Roubaty C, Uldry D, Chuard R, Wang J, Jazwinski SM, Conzelmann A. Human homologues of LAG1 reconstitute Acyl-CoA-dependent ceramide synthesis in yeast. J Biol Chem. 2003;278:37083–37091. doi: 10.1074/jbc.M307554200. [DOI] [PubMed] [Google Scholar]
- Haak D, Gable K, Beeler T, Dunn T. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J Biol Chem. 1997;272:29704–29710. doi: 10.1074/jbc.272.47.29704. [DOI] [PubMed] [Google Scholar]
- Hanna DE, Rethinaswamy A, Glover CV. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J Biol Chem. 1995;270:25905–25914. doi: 10.1074/jbc.270.43.25905. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HL, Shiau YS, Chen MY. Saccharomyces cerevisiaeTSC11/AVO3 participates in regulating cell integrity and functionally interacts with components of the Tor2 complex. Curr Genet. 2005;47:273–288. doi: 10.1007/s00294-005-0570-8. [DOI] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63:150–159. doi: 10.1016/j.critrevonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Hwang O, Kim G, Jang YJ, Kim SW, Choi G, Choi HJ, Jeon SY, Lee DG, Lee JD. Synthetic phytoceramides induce apoptosis with higher potency than ceramides. Mol Pharmacol. 2001;59:1249–1255. doi: 10.1124/mol.59.5.1249. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J Biol Chem. 1997;272:32566–32572. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol. 2005;25:7239–7248. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fyrst H, Saba J. Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae. Genetics. 2000;156:1519–1529. doi: 10.1093/genetics/156.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Nagiec MM. Ceramide/long-chain base phosphate rheostat in Saccharomyces cerevisiae: regulation of ceramide synthesis by Elo3p and Cka2p. Eukaryot Cell. 2003;2:284–294. doi: 10.1128/EC.2.2.284-294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlwein SD, Eder S, Oh CS, Martin CE, Gable K, Bacikova D, Dunn T. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:109–125. doi: 10.1128/MCB.21.1.109-125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RL, Dickson RC. High-performance liquid chromatography analysis of molecular species of sphingolipid-related long chain bases and long chain base phosphates in Saccharomyces cerevisiae after derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Anal Biochem. 2001;298:283–292. doi: 10.1006/abio.2001.5368. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang X, Lester RL, Dickson RC. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J Biol Chem. 2005a;280:22679–22687. doi: 10.1074/jbc.M502972200. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang X, Sumanasekera C, Lester RL, Dickson RC. Signalling functions for sphingolipid long-chain bases in Saccharomyces cerevisiae. Biochem Soc Trans. 2005b;33:1170–1173. doi: 10.1042/BST20051170. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Mulet JM, Martin DE, Loewith R, Hall MN. Mutual antagonism of target of rapamycin and calcineurin signaling. J Biol Chem. 2006;281:33000–33007. doi: 10.1074/jbc.M604244200. [DOI] [PubMed] [Google Scholar]
- Nagiec MM, Skrzypek M, Nagiec EE, Lester RL, Dickson RC. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases. J Biol Chem. 1998;273:19437–19442. doi: 10.1074/jbc.273.31.19437. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Hannun YA. Ceramide, stress, and a “LAG” in aging. Sci Aging Knowledge Environ. 2003:PE27. doi: 10.1126/sageke.2003.39.pe27. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Okamoto Y, Mao C. Yeast sphingolipids: metabolism and biology. Biochim Biophys Acta. 2002;1585:163–171. doi: 10.1016/s1388-1981(02)00337-2. [DOI] [PubMed] [Google Scholar]
- Posse de Chaves EI. Sphingolipids in apoptosis, survival and regeneration in the nervous system. Biochim Biophys Acta. 2006;1758:1995–2015. doi: 10.1016/j.bbamem.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Powers T. TOR signaling and S6 kinase 1: Yeast catches up. Cell Metab. 2007;6:1–2. doi: 10.1016/j.cmet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AE, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Ramjit HG, Newton R, Guare JP. A novel coaxial electrospray ionization method for characterizing hexacosanoylceramides by Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1257–1262. doi: 10.1002/rcm.1926. [DOI] [PubMed] [Google Scholar]
- Reinke A, Anderson S, McCaffery JM, Yates J, 3rd, Aronova S, Chu S, Fairclough S, Iverson C, Wedaman KP, Powers T. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J Biol Chem. 2004;279:14752–14762. doi: 10.1074/jbc.M313062200. [DOI] [PubMed] [Google Scholar]
- Rethinaswamy A, Birnbaum MJ, Glover CV. Temperature-sensitive mutations of the CKA1 gene reveal a role for casein kinase II in maintenance of cell polarity in Saccharomyces cerevisiae. J Biol Chem. 1998;273:5869–5877. doi: 10.1074/jbc.273.10.5869. [DOI] [PubMed] [Google Scholar]
- Schorling S, Vallee B, Barz WP, Riezman H, Oesterhelt D. Lag1p and Lac1p are essential for the Acyl-CoA-dependent ceramide synthase reaction in Saccharomyces cerevisae. Mol Biol Cell. 2001;12:3417–3427. doi: 10.1091/mbc.12.11.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Heiter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims KJ, Spassieva SD, Voit EO, Obeid LM. Yeast sphingolipid metabolism: clues and connections. Biochem Cell Biol. 2004;82:45–61. doi: 10.1139/o03-086. [DOI] [PubMed] [Google Scholar]
- Skrzypek MS, Nagiec MM, Lester RL, Dickson RC. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J Bacteriol. 1999;181:1134–1140. doi: 10.1128/jb.181.4.1134-1140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S. Sphingosine 1-phosphate: a ligand for the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci. 2000;905:54–60. doi: 10.1111/j.1749-6632.2000.tb06537.x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476:55–57. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, Fujita T, Kawasaki T, Kozutsumi Y. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol. 2000;20:4411–4419. doi: 10.1128/mcb.20.12.4411-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol. 2006;26:5861–5875. doi: 10.1128/MCB.02403-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemicaland cellularregulation and roleindisease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Tani M, Kihara A, Igarashi Y. Rescue of cell growth by sphingosine with disruption of lipid microdomain formation in Saccharomyces cerevisiae deficient in sphingolipid biosynthesis. Biochem J. 2006;394:237–242. doi: 10.1042/BJ20051354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen JG, Guthrie C. Mutagenesis of the yeast gene PRP8 reveals domains governing the specificity and fidelity of 3′ splice site selection. Genetics. 1996;143:723–739. doi: 10.1093/genetics/143.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B, Riezman H. Lip1p: a novel subunit of acyl-CoA ceramide synthase. EMBO J. 2005;24:730–741. doi: 10.1038/sj.emboj.7600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Miyaji M, Kitano T, Umehara H, Okazaki T. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J Biol Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Skrzypek MS, Lester RL, Dickson RC. Elevation of endogenous sphingolipid long-chain base phosphates kills Saccharomyces cerevisiae cells. Curr Genet. 2001;40:221–233. doi: 10.1007/s00294-001-0259-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.