Abstract
The biochemical identification and immunocytochemical characterization of a cell surface antigen, expressed on globose basal cells (GBCs) of the rodent olfactory epithelium (OE), are described. The monoclonal antibody (MAb) GBC-3 recognizes a surface protein, confirmed by both live cell staining and fluorescence-activated cell sorting. Two-dimensional SDS-PAGE/Western blot followed by tandem mass spectrometry demonstrates that the cell surface GBC-3 antigen is the 40 kDa laminin receptor precursor protein. The MAb GBC-3 labels the vast majority of cells among the GBC population and does not stain either sustentacular cells or horizontal basal cells (HBCs) in the normal rat OE. After epithelial lesion by exposure to methyl bromide, the remaining cells, which are mostly GBCs, are heavily stained by GBC-3, and colabeled with GBC-3 and sustentacular cell or HBC markers. GBC-3 will be a potentially useful tool for identifying and characterizing GBCs.
Indexing terms: 2D IEF/SDS-PAGE, tandem mass spectrometry, cell surface marker, nonintegrin laminin receptors, tissue stem cells, progenitors
The capacity of the olfactory epithelium (OE) for sustained neurogenesis and to recover after injury implies that the adult OE retains a population of tissue stem cells. Based in part on proliferative capacity, basal cells—globose basal cells (GBCs) and horizontal basal cells (HBCs)—have been deemed likely candidates for olfactory stem cells. While still subject to debate, there are ample data to support the notion that among the GBCs are putative stem cells with the capacity to give rise to both neurons and nonneuronal cells during reconstitution of the OE after various types of injury. That GBCs are neuronal progenitors has been well established by studies in unlesioned OE or following bulbectomy including; 1) pulse-chase experiments using 3H-thymidine (Graziadei, 1973; Graziadei and Monti Graziadei, 1979); 2) increased proliferation of GBCs, but not HBCs, during the accelerated neurogenesis following bulbectomy (Schwartz Levey et al., 1991); and 3) lineage tracing using replication-incompetent, retroviral vectors (Caggiano et al., 1994; Schwob et al., 1994; Huard et al., 1998). However, a broader-than-neuronal potency (that is, multipotency) of GBCs has been demonstrated in other settings; for example, following MeBr lesion when multiple cell types are destroyed and need replacing. The most direct evidence of broad multipotency derives from experiments using transplantation of FACS-purified cell types into the MeBr-lesioned OE, which is a type of colony-forming unit (CFU) assay in vivo. In aggregate, FACS-sorted GBCs harvested from normal epithelium produce colonies containing olfactory sensory neurons (OSN), sustentacular cells, GBCs, Bowman's gland/duct (BG/D) cells, and respiratory columnar epithelial cells following transplantation into the MeBr-lesioned epithelium (Chen et al., 2004). Moreover, a subset of GBCs in the normal epithelium has the cell cycle characteristics associated with stem cells, including the retention of BrdU label for at least 4 weeks and mitotic quiescence as marked by cyclin-dependent kinase inhibit tor p27Kip1 (Chen and Schwob, 2003). Following MeBr lesion, quiescent GBCs are recruited to reenter the mitotic cycle and then label-retaining and p27(+) GBCs reappear during the recovery process. Finally, we have shown that when GBCs are destroyed by MeBr exposure the epithelium fails to reconstitute as olfactory, rather undergoing respiratory metaplasia (Jang et al., 2003).
As an easily accessible kind of neurocompetent stem cell, a better characterization of the biochemical phenotype of GBCs is warranted, as a way of further understanding the biology of cellular replacement in the OE for the purpose of subdividing and isolating the progenitor cell populations and as a means of accomplishing conditional gene mutation. Consequently, selective markers have been in demand in this field. Our laboratory is involved in an ongoing effort to identify and characterize GBC-selective markers. We describe here the pattern of immunohistochemical labeling of a monoclonal antibody, designated GBC-3, that identifies GBCs by virtue of labeling their cell surface. Using current proteomic approaches including two-dimensional electrophoresis (2D IEF/SDS-PAGE) and tandem mass spectrometry (MS/MS), we successfully identified the cell surface protein to which GBC-3 binds as the immature form of laminin receptor protein or laminin receptor precursor protein (iLRP). As a consequence of its expression in GBCs, iLRP may be useful for genetic targeting of GBCs.
Materials and Methods
Animals and tissue preparation
Sprague-Dawley rats (Taconic Farms, Germantown, NY) were used in this study. The procedures for MeBr exposure and tissue preparation were described previously (Schwob et al., 1995). For the purposes of immunohistochemistry the animals were perfused sequentially by phosphate-buffered saline (PBS) and then with a fixative solution of periodate-lysine-1% or 2% paraformaldehyde (PLP) in PB. The olfactory tissue was subsequently decalcified in saturated, neutral EDTA, cryoprotected, and sectioned on a cryostat at 8 μm in the coronal plane. All animal protocols were approved by the Institutional Animal Care and Use Committee of Tufts University School of Medicine.
Cell culture
The D3 line of mouse embryonic stem (ES) cells (ATCC, Manassas, VA) (Bain et al., 1995) was initially grown on gelatin-coated culture flasks in standard medium (knockout DMEM, 15% ES cell-screened FBS, 2 mM L-glutamine, 100 μM nonessential amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin). Leukemia inhibitory factor (LIF) (ESGRO; Chemicon International, Temecula, CA) and β-mercaptoethanol (β-ME) (Sigma, St. Louis, MO) were added (100 U/mL and 100 μM, respectively) to inhibit the ES cells from differentiation. Cells at subconfluency were then trypsinized and seeded onto bacteriological dishes in standard medium without LIF and β-ME. Cells for Western analysis were collected, lysed, and quantified for protein concentration using BCA Protein Assay Reagent (Pierce, Rockford, IL).
Immunohistochemistry
Production of hybridoma clone GBC-3, an IgM, was described previously (Goldstein et al., 1997). Briefly, Balb/c mice were immunized with the rat olfactory epithelium-derived cell line called neuroblasts in culture (NIC), a line that shows multiple similarities with GBCs in vitro (Goldstein et al., 1997). After the final immunization, mouse splenocytes were fused with NS-1 myeloma cells to produce the hybridoma line, which was subsequently cloned by limiting dilution. Sections were pre-treated with 0.05% trypsin and blocked with 2.25% fish gelatin (Sigma) in PBS plus 0.1% Triton-X (blocking solution) for 20 minutes. Sections were then incubated with GBC-3 diluted 1:100 in blocking solution overnight at 4°C. After washing three times with 0.05% Tween-20, sections were incubated with FITC-conjugated mu-chain specific goat antimouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:50 in blocking solution plus 10% normal goat serum (NGS). After washing three times and reblocking with a solution of 4% BSA and 10% NGS with 0.1% Triton-X, sections were double-labeled with the following markers for the other cell types in the OE: rabbit polyclonal anti-NCAM (neural cell adhesion molecule; 1:200; a generous gift by Dr. J. Covault; Covault and Sanes, 1986) that was raised against 180, 140, and 120 kD forms of NCAM from adult rat brain that labels both immature and mature olfactory neurons (Goldstein and Schwob, 1996); mouse monoclonal IgG SUS-4 (1:50; generated in our laboratory; Goldstein and Schwob, 1996) that was raised against rat OE cells harvested 7 days after MeBr lesion and labels both sustentacular cells and duct/gland cells; or biotinylated Bandeiraea (Griffonia) simplicifolia I lectin (bBS-I; 1:50; Vector Laboratories, Burlingame, CA) that has been shown to bind carbohydrate moieties expressed selectively by HBCs (Holbrook et al., 1995). Bound reagent was visualized with Texas Red-conjugated goat antirabbit IgG for anti-NCAM, antimouse IgG (gamma chain-specific) for SUS-4, Texas Red streptavidin for bBS-I (all from Jackson ImmunoResearch Laboratories). Goat anti-ICAM (intercellular adhesion molecule also known as CD54) antibody (R&D Systems, Minneapolis, MN) that was raised against recombinant rat ICAM extracellular domain was also used to label HBCs (Carter et al., 2004); in this case normal donkey serum was used for blocking and AMCA-conjugated donkey antigoat IgG (Jackson ImmunoResearch Laboratories) was used for secondary antibody.
Mouse MAb 43515 (the generous gift of Drs. J. Coggin and A. Barsoum, University of South Alabama) that was raised against mouse recombinant iLRP (A. Barsoum, University of South Alabama, pers. commun.; Zelle-Rieser et al., 2001) was used to identify an immature form of laminin receptor protein, iLRP, for immunohistochemistry and Western blot analysis. Rabbit polyclonal antibody Ab711 (Cambridge, MA) that was raised against synthetic peptide corresponding to amino acids 263–282 of human laminin receptor (LR) was also used to probe 67-kD mature LR on the immunoblot (Baloui et al., 2004).
Cell dissociation
Protocols for cell dissociation have been described previously (Chen et al., 2004). Briefly, after the animals were perfused with cold low Ca2+/Mg2+ Ringer's solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM EDTA, 10 mM glucose, 1 mM sodium pyruvate, pH 7.2), septum and turbinates were exposed. The olfactory mucosa from normal rats was peeled off from the septum and the turbinate bones and minced into small 1–2 mm pieces in cold low Ca2+/Mg2+ Ringer's solution. The mucosa from MeBr-lesioned rats was left on the bone, since at that time the OE lacks intercellular junctions and is readily removed from the basal lamina by enzymatic digestion, leaving intact the lamina propria and its cellular constituents. The lesioned mucosa or the minced tissues were incubated with 0.05% trypsin/EDTA (Gibco BRL, Gaithersburg, MD) in low Ca2+/Mg2+ Ringer's solution for 5–10 minutes at 37°C. The tissue was then transferred to a dissociation enzyme cocktail consisting of collagenase/hyaluronidase/trypsin inhibitor at 1, 1.5, 0.1 mg/mL, respectively (Worthington Biochemical, Freehold, NJ) plus papain (5 U/mL, Sigma) at 37°C for 15 minutes if MeBr-lesioned epithelia or 30 minutes if minced tissues and occasionally triturated. After the incubation period cells were heavily triturated, rinsed in regular Ringer's solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 1 mM sodium pyruvate, pH 7.2) supplemented with 0.025 mg/mL DNase I (Worthington) to prevent aggregation of the cells, and filtered with 35 μm mesh before use. The dissociated cells were used for Western blot analysis and flow cytometry (see below).
Two-dimensional electrophoresis (2-D IEF/SDS-PAGE) and Western blot analysis
Protein was prepared from ES-D3 cells and dissociated cells from OE of 4 days post-MeBr lesioned rats as follows. Cell pellets were homogenized with a motorized pestle in M-PER Mammalian Protein Extraction Reagent (Pierce) plus protease inhibitors, (100 mM PMSF, 1.5 mM pepstatin A, 10 mg/mL leupeptin, 1.8 mg/mL aprotinin; all from Sigma). Cell lysate was centrifuged at 27,500g and the supernatant was collected. These protein preparations were precipitated with cold acetone, washed with cold ethanol, and resuspended with a rehydration buffer containing 7M urea, 2M thiourea, 2% CHAPS, and 18mM DTT with 0.5% IPG buffer of choice (either pH gradient 3–10, nonlinear or 4–7, Amersham Pharmacia Biotech, Piscataway, NJ). IPG strips (13 cm) with a pH gradient from 3–10 (nonlinear) or 4–7 were soaked with protein samples in rehydration buffer for 12 hours at 20°C. Isoelectric focusing (IEF) was conducted at 70,000–75,000 Vhr, 20°C, on the Ettan IPGphor Isoelectric Focusing System (Amersham Pharmacia Biotech). After IEF, IPG strips were equilibrated in SDS equilibration buffer (50 mM Tris, pH 8.8, 6M urea, 30% v/v glycerol, 2% SDS, 10 mg/mL DTT) for 30 minutes at room temperature before running onto the second dimension SDS-PAGE (10%) using Hoefer SE 600 gel electrophoresis apparatus (Amersham Pharmacia Biotech). Gels were either silver-stained using SilverQuest Silver Staining Kit–Mass Spectrometry Compatible (Invitrogen, Carlsbad, CA) or transferred onto PVDF membrane and then immunoblotted with GBC-3 (1:20,000) or MAb 43515 (1:30,000). Blots were visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce) using HRP-conjugated mu-specific antimouse IgM or HRP-conjugated antimouse IgG (Jackson ImmunoResearch Laboratories) at a concentration of 1:30,000. For reprobing, the blot was incubated in stripping buffer (62.5 mM Tris, pH 6.7, 2% SDS and 100 mM β-ME) for 30 minutes at 50°C, then rinsed and reblocked.
Mass spectrometry
An adaptation of previous methods was used for protein identification (Peng and Gygi, 2001). Protein spots were excised from silver-stained SDS-PAGE gels, destained, and digested with sequencing-grade trypsin (Promega, Madison, WI) as described (Shevchenko et al., 1996). Digested samples were loaded onto a fused silicamicrocapillary C18 column (Magic; Michrom BioResources, Auburn, CA) prepared inhouse (75 μm inner diameter, 10 cm long). An Agilent 1100 high-pressure liquid chromatography (HPLC) system (Agilent Technologies, Palo Alto, CA) was used to deliver a gradient across a flow splitter to the column over 40 minutes. The column eluant was directed into an LTQ electrosprayion-trap mass spectrometer (ThermoFinnigan, San Jose, CA) and eluting peptides were dynamically selected for fragmentation by the operating software. The acquired MS/MS data was analyzed with the nonredundant mouse database from NCBI using the SEQUEST database search tool for peptide identification (Eng et al., 1994). Modifications were permitted to allow for the detection of the following (mass shift shown in daltons): oxidized methionine (+16) and carboxymethylated Cysteine (+57).
Flow cytometry
Fluorescence-activated cell sorting (FACS) was performed in a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and events were acquired using CELLQuest software program (Becton Dickinson). Cells from normal or post-MeBr rat OE were dissociated as described above and resuspended in PBS (pH 7.4). The dissociated cells were then incubated with either GBC-3 (1:50) or MAb 43515 (1:50) for 20 minutes on ice followed by washing with PBS. Fluorescein or R-phycoerythrin (PE)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) was applied for 20 minutes on ice followed by washing with PBS. Forward and side scatters were monitored to eliminate dead/aggregated cells and debris. FACS profiles were then analyzed using Summit software program (Cytomation, Fort Collins, CO).
Deglycosylation
A protein preparation generated from the ES-D3 as described above was digested by N-glycosidase F (PNGase F; Sigma) as follows. Protein samples from ES-D3 cells (200 μg) were mixed with the final concentration of 50 mM sodium phosphate buffer (pH 7.5). Samples were then added with the final concentrations of 0.1% SDS and 50 mM β-ME and heated for 5 minutes. After cooling to room temperature, Triton-X 100 was added to the final concentration of 0.75% before enzyme incubation. Samples without heat denaturation were also incubated with the enzyme. Nondenatured and denatured protein samples were incubated with PNGase F (5 units/100μg protein) overnight at 37°C and were analyzed by Western blotting probed with the GBC-3 antibody.
Preparation of images
Images of immunohistochemical staining were photographed using a digital SPOT camera (Diagnostic Instruments, Sterling Heights, MI) and processed using Adobe Photoshop (San Jose, CA) where only balance, contrast, and evenness of illumination were altered. Images of protein gels and Western blots were obtained using the Eagle Eye II Still Video System (Stratagene, La Jolla, CA).
Results
We generated a series of monoclonal antibodies—GBC-1, GBC-2, and GBC-3—that label GBCs in both normal and lesioned epithelium, and we reported the uses of GBC-1 and GBC-2 antibodies for tissue staining, cell sorting, and staining of cell lines in culture (Goldstein and Schwob, 1996; Goldstein et al., 1997; Jang et al., 2003; Chen et al., 2004). In this study the protein backbone for the antigen recognized by GBC-3 was identified biochemically and subsequently characterized histochemically to examine how the antigen is regulated in its expression during the course of reconstitution of OE after MeBr lesion.
First, we found that in the normal OE GBC-3 labels cells close to the basal lamina (Fig. 1A,B). Because of their morphology and location superficial to flat, unlabeled cells resembling HBCs, we considered it likely that the heavily labeled cells were GBCs (confirmed below). There is a decreasing gradient of staining toward the apex of the OE. After MeBr lesion, most of the residual cells are heavily labeled with GBC-3 at intensity comparable to that of the highly labeled cells in the normal OE (Fig. 1C,D). In addition to the cells in the OE, neurons and progenitor cells of the vomeronasal organ (VNO) are also stained with GBC-3 (Fig. 1E). In particular, cells at the margin of the sensory epithelium of the VNO are more intensely stained with GBC-3 than the neurons in the middle region of the epithelium. That GBC-3 labels GBCs led us to elucidate biochemical and molecular identity of its antigen.
Fig. 1.

In rat olfactory epithelium GBC-3 heavily labels cells deep in the epithelium that resemble GBCs with respect to their shape and position superficial to HBCs. Sections of OE were stained with GBC-3 (visualized with diaminobenzidine in A and C) and counterstained with hematoxylin (B,D). In the normal epithelium (A,B), GBC-3 strongly stains cells in the basal zone of the epithelium that are occupied by GBCs and lightly labels less mature and more mature neurons. Located just above the basal lamina, but below the GBC-3 (+) cells, are flat, unlabeled cells that are likely to be HBCs (arrows in B). In the lesioned epithelium at 2 days post-MeBr (C,D), most, if not all, of cells above the basal lamina (arrowheads) are heavily labeled with GBC-3. In the vomeronasal organ (VNO), cells in the sensory epithelium are labeled, while cells in the nonsensory epithelium (asterisks) are not. Cells at the boundary between sensory and nonsensory epithelium (thick arrows) are strongly labeled, and the intensity of the labeling is attenuated on the neurons in the middle region of the epithelium. Scale bars = 25 μm.
Cell surface expression of the GBC-3 antigen
Close examination of GBC-3 labeling in the OE suggests that the corresponding antigen is membrane-bound. Live cells were stained with GBC-3 for direct observation and for FACS analysis to demonstrate that the antigen is indeed exposed on the cell surface. By both measures a subpopulation of living, acutely dissociated cells is labeled on its surface by GBC-3 (Fig. 2). In contrast, incubation with a negative control antibody against a known internal antigen, vimentin, did not label cells until their membranes were permeabilized by incubation with detergent (data not shown). When the cells dissociated from normal rat OE were stained with GBC-3 and subjected to FACS analysis (“Normal” in Fig. 2E), two populations of labeled cells could be distinguished by the intensity of labeling: moderately labeled (“M” in Fig. 2E) and heavily labeled (“H” in Fig. 2E) populations. FACS analysis of cells from lesioned OE was undertaken as well. A shift to a higher percentage of labeled cells, both moderately and heavily labeled, is evident. The percentage of cells (per total dissociated OE cells) in the M and H populations is 7.5 and 0.2 in normal OE, respectively, but increases several-fold to 12.5 and 1.0 at 1 day, 30.2 and 1.6 at 2 days, and 28.2 and 1.0 at 3 days post-MeBr, respectively. A further point emerges from these FACS data: peak fluorescence intensity of OE cells due to GBC-3 labeling (i.e., the position along the FL1 axis) of OE cells does not change after MeBr lesion from that observed in normal and two populations of moderately and highly labeled cells remain evident, suggesting that the expression of the GBC-3 antigen by individual cells is not shifted markedly following lesion. The FACS profiles in the acute postlesion period confirm the enhanced immunohistochemical staining of GBCs that we observed early in the reconstitution of the lesioned epithelium, and, by virtue of the elimination of neurons from the early postlesion OE and the concomitant reduction in the numbers of GBC-3 (−) cells, reinforce the interpretation that expression tends to decline as the cells progress from progenitor through immature to mature olfactory neuron (see below). Finally, declining surface expression with maturation was addressed directly by subjecting sorted cells to post-FACS immunostaining. In this case, sorted cells that are heavily labeled with GBC-3 from the normal OE (population labeled H in Fig. 2) are devoid of OMP expression as shown by post-FACS immunostaining with anti-OMP; some of the sorted cells that are moderately labeled with GBC-3 (population M, Fig. 2) are OMP-positive (data not shown).
Fig. 2.
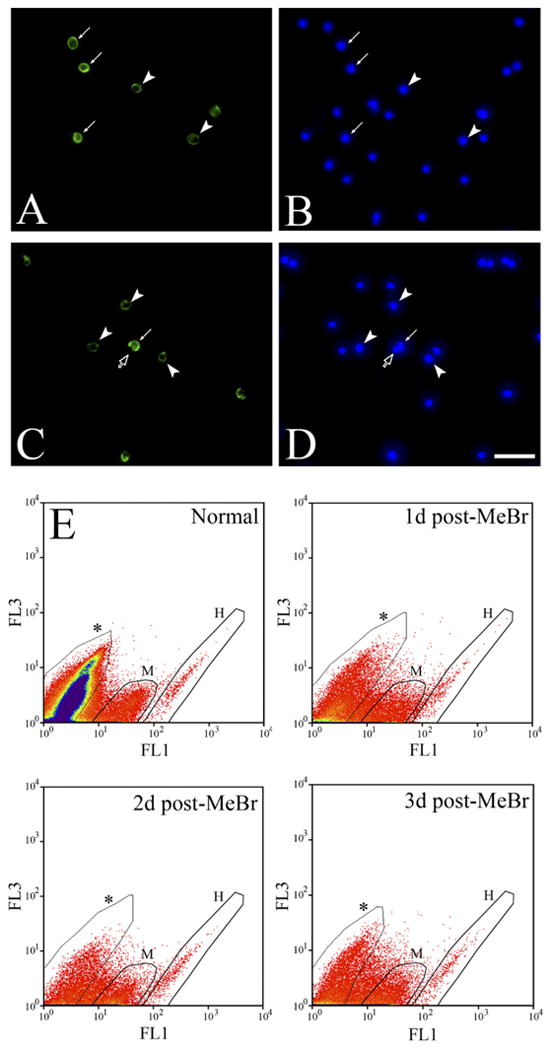
Cell surface labeling with GBC-3 on acutely dissociated OE cells demonstrates that the GBC-3 antigen is membrane bound. A,B: Live staining on the dissociated cells from normal OE with GBC-3 (A) and Hoechst dye (B) shows that GBC-3 labels a surface antigen. It is noted that there are strongly GBC-3 (+) (arrows) and lightly GBC-3 (+) cells (arrowheads), as well as unlabeled cells. C,D: Another example of live staining with GBC-3 shows an unlabeled cell (open arrow) right next to a GBC-3 (+) cell (arrow). Arrowheads indicate lightly labeled cells. E: FACS profiles of GBC-3 on the dissociated cells from normal and MeBr-lesioned OE (1–3 days) encompass two distinct GBC-3 (+) populations—highly labeled (H) and moderately labeled (M)—consistent with the live staining of the cells shown in A and C. Asterisks indicate unstained population.
Biochemical identification of the GBC-3 antigen
In an attempt to isolate and identify the antigen recognized by GBC-3, we performed 2D SDS-PAGE/Western blot analysis with protein samples from various sources: a rat olfactory epithelium-derived NIC cell line, which was the immunogen used to generate the antibody (Coon et al., 1989; Goldstein et al., 1997); dissociated olfactory epithelial cells from rats at 4 days after MeBr lesion, when most OE cells are intensely stained with GBC-3; and mouse ES-D3 cell line (Bain et al., 1995), cells of which also express the GBC-3 antigen (see below). On immunoblots from dissociated OE cells and ES-D3 cells, three different proteins of sizes of 37, 40, and 50 kDa are recognized by GBC-3 (Fig. 3). A similar pattern is observed in protein preparations from the NIC cell line, although the spots at 40 and 50 kDa are relatively weaker (data not shown).
Fig. 3.

GBC-3 immunolabeled protein spots are identified by 2D IEF/SDS-PAGE. Protein preparations from dissociated OE cells at 4 days postlesion (A) and the embryonic stem cell line ES-D3 (B) were isolectrofocused from pH 3–10 (nonlinear) range with acidic end to the left (see Materials and Methods). Three spots (at 52, 40, and 37 kDa) were labeled with GBC-3. The protein spots were identified by tandem mass spectroscopy (MS/MS) and confirmed by immunohistochemistry and Western blots using appropriate antibodies (see Figs. 4–6; see also Results).
Tandem mass spectrometry (MS/MS) demonstrated that the 40 kDa spot found in blots of both rat OE cells and mouse ES-D3 cells is the precursor for the 67 kDa nonintegrin laminin receptor, also known as immature laminin receptor precursor protein (iLRP) and variably described as 34–40 kDa in size (primary accession number: P38983 [rat] and P14206 [mouse]; Swiss-Prot entry: RSP4, which later changed to RSSA), a known cell surface antigen (Gauczynski et al., 2001; Baloui et al., 2004) (Fig. 4). Eight tryptic peptide fragments that match iLRP were identified with Xcorr > 2.5 and ΔCn > 0.1, and they were accepted as positive identification of the target spot. Coverage of amino acids in the fragments positively identified by MS/MS is ≈24% and estimated mass of the protein based is 32.7 kDa. The correspondence of 2D SDS-PAGE (Fig. 4A), MS identification of the 40-kDa spot, and subsequent MS/MS analysis of the peptide fragment from mouse ES-D3 cell lines confirm the identification of the GBC-3-labeled spot as iLRP (Fig. 4). The amino acid sequence of OE- and ES cell-derived iLRP is also 99% identical to a protein known as oncofetal antigen (OFA), which has a reported size of 32–44 kDa (Zelle-Rieser et al., 2001).
Fig. 4.

GBC-3 antigen is identified as laminin receptor precursor protein (iLRP) by 2D IEF/SDS-PAGE and MS/MS. A: The protein preparation from mouse ES-D3 was electrofocused from pH 4–7 range (acidic end is to the left). The 40 kDa spot was recognized by GBC-3 on Western blot and the gel spot was silver-stained preparatory to MS/MS identification. B: A representative MS/MS spectrum of a tryptic peptide (amino acid 64–80) with a series of b and y ions resulting from peptide fragmentation reveals actual amino acid sequence, AIVAIENPADVSVISSR. C: SEQUEST database search result based on eight identified peptide fragments. The scores of cross correlation (Xcorr) and delta correlation (ΔCn) for each identified peptide are presented. The search indicates that the identified protein is 34/67 kDa laminin receptor, i.e., iLRP (average mass is 32719 and pI 4.74) with 24.1% sequence coverage by identified peptides. The identified amino acid sequences are indicated in red (Note: The entry number in SWISS-PROT has been changed from RSP4 to RSSA). This was also repeated with the same spot from protein preparation of the dissociated cells of the OE from rat at 4 days post-MeBr lesion, and yielded the same identification.
Further confirmation that the 40 kDa spot recognized by GBC-3 is indeed iLRP was provided by labeling the GBC-3-reactive spot with an antibody against iLRP (MAb 43515) on the same 2D Western blot (Fig. 5A–D). In this case, a blot prepared from dissociated OE cells from 4 days post-MeBr rats was probed with GBC-3 and imaged before stripping and reprobing with MAb 43515. The same spot at 40 kDa MW is labeled by both antibodies. It is worth noting that GBC-3 does not label the entirety of the protein spot, indicating that there are multiple isoforms of the iLRP protein (Fig. 5D). Indeed, our data suggest that the epitope recognized by GBC-3 is likely to be a carbohydrate moiety on this glycoprotein. When protein preparations from the ES-D3 cell line were subjected to enzymatic digestion by N-glycosidase (PNGase F), the GBC-3 band on the Western blot disappeared (Fig. 5E), indicating that the carbohydrate moiety recognized by the exoglycosidase (i.e., asparagine-linked high mannose and other oligosaccharides) was released from the backbone of the protein. This confirmed the fact that the immunostaining is blocked by either simple sugars or nonfat dry milk (with its multiple glycoproteins and sugars). As the GBC-3 antigen is resistant to extraction by alcohols, it cannot be a glycolipid. Consistent with the presence of iLRP in the epithelium, the mature nonintegrin laminin receptor (LR) is also observed in preparations of cells of the dissociated OE and of the mouse ES cell line (Fig. 5F).
Fig. 5.

MS identification of GBC-3 antigen as iLRP is confirmed by 2D IEF/SDS-PAGE and the GBC-3 epitope is characterized. A–D: Proteins from the dissociated cells of 4 days post-MeBr OE were isoelectrofocused in the pH 4–7 range, gel electrophoresed, and immunoblotted with GBC-3. The blot was then stripped and reprobed with MAb 43515 against iLRP. A: Silver-stained gel; B: GBC-3 staining of blot prepared in parallel with the gel in A; C: After stripping bound GBC-3 with stripping buffer, the blot was reprobed with MAb 43515; D: Both blots were shown with reduced opacity and overlapped for the purpose of matching (as in “merged”). The 40-kDa spot recognized by GBC-3 (indicated by number 1) was positively identified by MAb 43515 (indicated by number 1′). Note that the GBC-3 (+) spot represents only a part of the iLRP spot. The numbers 2 and 3 in B and D indicate the spots of acidic-calponin and β-tubulin, respectively, which label only with GBC-3 (see also Fig. 3). E: After proteins from the ES-D3 cell line were SDS-denatured and then digested with N-glycosidase, the GBC-3 band on the Western blot disappeared. The digestion was less efficient for nondenatured proteins but was still evident as a marked decline in the intensity of the band on Western blot. Thus, the epitope recognized by GBC-3 is a carbohydrate moiety. F: Western blots show that both mouse ES-D3 line (lane “m”) and rat OE at 4 days after MeBr-lesion (lane “r”) express both iLRP (probed with MAb 43515 and indicated by arrowhead) and laminin receptor, LR (probed with Ab711; rabbit polyclonal antibody against 67 kDa LR, and indicated by open arrowhead).
Two other protein spots (37 and 50 kDa in Fig. 3) were identified by MS/MS and confirmed by subsequent immunoblotting as acidic calponin and β-tubulin, respectively (MS data and 2D Western data not shown). As these two protein species are known to be cytoplasmic, it is unlikely that they are the source of the staining on the cell surface of OE cells (see Fig. 2). To confirm that acidic calponin is not the antigen responsible for surface labeling with GBC-3, we performed FACS after incubation of live cells with polyclonal anti-acidic calponin antibody (Covance, Berkeley, CA); no surface labeling was observed (data not shown). The crossreactivity of GBC-3 with other proteins does not appear to be specific to the amino acid sequence. When the sequences of laminin receptor, acidic calponin, and β-tubulin were aligned using Blast 2 (National Center for Biotechnology Information, NCBI, NIH, Bethesda, MD), no similarity was found among them, a further indication that the epitope recognized by GBC-3 is posttranslational in nature.
The identification of iLRP as the cell surface antigen recognized by GBC-3 was supported by immunohistochemistry (Fig. 6) and FACS profiling (Fig. 7) using MAb 43515, the anti-iLRP antibody. In normal OE the immunostaining of MAb 43515 is virtually identical to that of GBC-3 (Fig. 6). The MAb clearly labels cells below the NCAM (+) neuronal layer and above the CD54 (+) HBCs (Fig. 6E–H), as does GBC-3 (Fig. 6A–D). When analyzed on flow cytometry, the vast majority of GBC-3 (+) cells (i.e., surface labeled) from normal OE were also stained by MAb 43515, as shown by the shift of the fluorescent signal toward the diagonal in the FACS plot, which corresponds to double labeling (Fig. 7). Cells that are positive with other GBC markers—GBC-1 and GBC-2—are also double-labeled with MAb 43515, confirming that most, if not all, GBCs express iLRP (data not shown). These data, along with our Western blot results, strongly support our conclusion that GBC-3 does indeed recognize the same antigen protein, i.e., iLRP, as does MAb 43515. In an attempt to block the labeling of GBCs by GBC-3, an excess amount of MAb 43515 (10 times the molar amount of GBC-3) was used to challenge the binding of GBC-3 (data not shown). However, excess MAb 43515 did not interfere with GBC-3 labeling, indicating that the GBC-3 epitope is a distinct moiety from the one recognized by MAb 43515. That conclusion is also supported by the greater extent of labeling of the 40-kDa spot by MAb 43515 as compared to GBC-3.
Fig. 6.

Staining patterns with GBC-3 and MAb 43515 (anti-iLRP) are indistinguishable, when compared on adjacent sections. Labeling of normal OE with GBC-3 (green) or MAb 43515 (green) and with anti-NCAM (red) and anti-CD54 antibodies (blue) (A–D) and (E–H), respectively. Some of both GBC-3 (+) cells and MAb 43515 (+) cellsare NCAM (−) and CD54 [ICAM] (−), confirming their expression on GBC (indicated by arrows). Arrowheads point to basal lamina. Higher magnification of the boxed area is shown below the corresponding panel. Scale bar = 25 μm in H (10 μm in higher magnification areas).
Fig. 7.
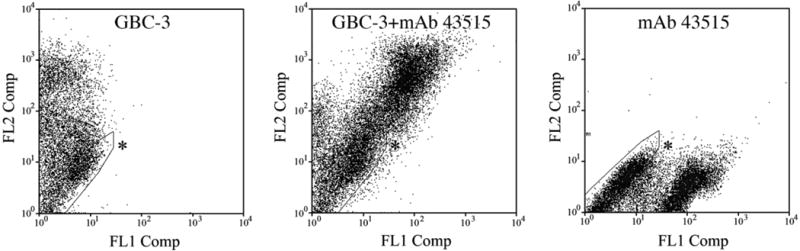
FACS profiles obtained with MAb 43515 and GBC-3 demonstrate that both antibodies label the same cells. The acutely dissociated cells from normal OE were surface-labeled with GBC-3 (followed by PE-conjugated secondary antibody, registering on FL2) and MAb 43515 (followed by FITC-conjugated secondary antibody, registering on FL1). Asterisks indicate unstained population.
Expression of GBC-3 antigen: normal OE
The characterization and verification that the antigen iLRP is recognized by GBC-3 is potentially useful for identifying a population of GBCs in the OE when its labeling is combined with markers of other cell types in the OE. Moreover, the immunohistochemical profiles in the lesioned OE (i.e., by MeBr) may also give us insight on how the expression of iLRP is regulated during reconstitution of the OE by lesion-activated multipotent progenitor cells (i.e., GBCs).
In the normal OE, GBC-3 stains the vast majority of, if not all, GBCs (arrows in Fig. 8). The staining pattern of GBC-3 is highly similar to that of GBC-1 or GBC-2 (see Goldstein and Schwob, 1996; Jang et al., 2003). In normal OE, GBC-3 strongly labels GBCs, although a population of neurons, less mature ones based on their relative proximity to the basal lamina and on the post-FACS-staining of the separated populations with anti-OMP referred to above, is also weakly labeled such that the GBC-3 produces a gradient of labeling from basal to apical side of the OE (Fig. 1). This carryover of GBC-3 immunoreactivity to neurons in the normal epithelium, especially less mature ones, is also characteristic of the other GBC-reactive monoclonal antibodies generated in our laboratory, GBC-1 and GBC-2 (Goldstein and Schwob, 1996; Goldstein et al., 1997). When merged with the nuclear staining with Hoechst dye, it appears that a very small number of what appear to be GBCs are not labeled by GBC-3, based on their position and the lack of staining by any of the markers that are characteristic of cell types in the OE (thick arrows in Fig. 8). Immunostaining of sections with GBC-3 does not extend to HBCs, which are labeled with bBS-I in the normal OE (arrows in Figs. 8E–H). Instead, the round, strongly labeled GBC-3 (+) GBCs are clearly above the layer of flattened HBCs, as confirmed in nuclear staining (Fig. 8H). In the normal OE, more apically located SUS-4 (+) sustentacular cells are also not labeled with GBC-3 (Fig. 8I–L). GBC-3 (+) GBCs are located near foot processes of sustentacular cells (stained with SUS-4) but are distinguishable with careful microscopy.
Fig. 8.

In normal OE immunostaining of GBC-3 and other cell markers (NCAM for neurons, bBS-I for HBCs and SUS-4 for sustentacular cells and duct/gland cells) shows that GBC-3 labels GBCs and some less mature neurons. A–D: GBC-3 clearly labels cells located below the NCAM (+) neuronal layer, i.e., basal cells. Rounded in shape (indicated by thin arrows) as opposed to the flattened HBCs below them, the stained cells are GBCs. Some putative NCAM (−) GBCs are also GBC-3 (−) as indicated by thick arrows. GBC-3 also weakly labels neurons, both less mature and more mature, in the middle of the epithelium. E–H: Flat HBCs, stained by bBS-I, lack labeling with GBC-3, which is found in the GBCs located right above bBS-I (+) HBCs (thin arrows). As in A–D, one can find examples of GBC-3 (−) GBCs (thick arrows), identified as the same by their location and morphology. I–L: Double labeling of GBC-3 and SUS-4 shows that GBC-3 does not label sustentacular cells in normal epithelium. Thin arrows indicate GBC-3 (+)/SUS-4 (−) GBCs based on location and morphology. Arrowheads mark the basal lamina. Higher magnification of the boxed area in A–H is shown below the corresponding panel. Scale bar = 25 μm in L (10 μm in higher magnification areas).
Expression of GBC-3 antigen after MeBr-lesion: dorsal vs. ventral
MeBr lesion destroys neurons and sustentacular cells throughout the OE and depletes, but does not eliminate, the populations of GBCs and HBCs (Schwob et al., 1995). Consequent to the destruction of the differentiated elements, the majority of the remaining cells are heavily labeled with GBC-3 in sections of the OE (Figs. 1, 9, 10). At early times after lesion (2 days), virtually all the spared cells in the OE are GBC-3 (+), and neurons are absent (Figs. 1, 9A–D). As the newly generated NCAM (+) neurons come to occupy the apical portion of the OE (cf. first new neurons begin to appear and are very sparse at 3 days postlesion) (Schwob et al., 1995), GBC-3 (+) cells remain concentrated near the basal lamina (7 days postlesion; arrows in Fig. 10). There are no apparent differences in GBC-3 immunoreactivity when comparing the dorsal and ventral OE.
Fig. 9.

In the OE at early time points (2 and 4 days) after MeBr-lesion immunostaining with GBC-3 and other cell markers (NCAM for neurons, bBS-I for HBCs, and SUS-4 for sustentacular cells and duct/gland cells) shows that GBC-3 labels the spared cells after lesion. A-D: At 2 days after MeBr exposure, a majority of the remaining cells in the OE (now neuron-less) are GBCs and they are heavily labeled with GBC-3. The labeling of the residual basal cells with GBC-3 in the lesioned OE is comparable to that of the heavily labeled cells in the normal OE, although the heavily labeled cells are more numerous (compare to Fig. 8). E–H: At 2 days after MeBr lesion two cell types populate the reconstituting epithelium: GBC-3 (+) / bBS-I (−) cells indicated by asterisks and GBC-3 (+) / bBS-I (+) cells. In the ventral OE bBS-I(+) cells are rare (see Results), and all the remaining cells are GBC-3 (+) / bBS-I (−). I–L: At 4 days after MeBr lesion when the SUS-4 immunoreactivity begins to show, some regenerating SUS-4 (+) cells are colabeled with GBC-3 (double arrows). Arrowheads mark basal lamina. Higher magnification of the boxed area in E–L is shown below the corresponding panel. Scale bar = 25 μm in L (10 μm in higher magnification areas).
Fig. 10.

In MeBr-lesioned OE at later timepoints (7 and 10 days postlesion) immunostaining of GBC-3 and other cell markers (NCAM for neurons, bBS-I for HBCs, and SUS-4 for sustentacular cells and duct/gland cells) shows that GBC-3 labels GBCs, HBCs, and regenerating neurons. A–D: At 7 days after MeBr lesion, most cells in the regenerating OE remain labeled with GBC-3. At this time reappearing neurons are colabeled with GBC-3 (B), while the layer of GBC-3 (+) cells which is NCAM (−) rests (indicated by thin arrows) deep to neurons. E–H: In dorsal OE at 7 days after lesion most of the regenerating cells are GBC-3 (+), and some bBS-I (+) HBCs are still labeled with GBC-3 (double arrows). Note that the regenerating gland/duct cells are devoid of GBC-3 labeling (open arrows). I–L: In ventral OE at 7 days after lesion where bBS-I (+) HBCs begin to reappear, one can find some GBC-3 (+) / bBS-I (+) cells (double arrows), suggesting differentiation of HBCs from GBCs. M–P: At 10 days after lesion, regenerating SUS-4 (+) cells form a distinct at the apical surface of the OE, like the normal OE. Arrowheads mark basal lamina. Higher magnification of the boxed area in A-H is shown below the corresponding panel. Scale bar = 25 μm in P (applies to A–H and M–P) (10 μm in I–L and higher magnification areas).
Previous results show that HBCs (i.e., marked by anti-cytokeratin 5 or 14 antibodies or bBS-I) accumulate in the dorsal OE on the first few days after lesion (Schwob et al., 1995). Since GBC-3 stains the vast majority of the spared cells in the OE after lesion, the GBC-3 (+) cells in the dorsal OE at early times after lesion are likely to be a heterogeneous population of GBCs and HBCs. Indeed, some GBC-3 (+) cells are also labeled with bBS-I in the dorsal OE, although a significant number of the remaining cells are labeled only with GBC-3 (2 days post-MeBr; Fig. 9E–H). At the end of the first postlesion week, HBCs in the dorsal OE withdraw toward the basal lamina, but some cells remain labeled with both GBC-3 and the HBC marker (7 days post-MeBr; Fig. 10E–H). In contrast to the foregoing, HBCs in the ventral OE completely disappear by 3 days after exposure. HBCs reappear in the ventral OE at 7 days postlesion, at which time bBS-I (+) HBCs are also colabeled with GBC-3 (Fig. 10I–L). By 14 days after lesion, immunoreactivity of GBC-3 is separated from HBCs as in normal OE (data not shown).
Even though SUS-4 appears to label more mature sustentacular cells, we were able to find some SUS-4 (+) / GBC-3 (+) cells at 4 days postlesion OE (double arrows in Fig. 9I–L). We were limited in our capacity to show immature GBC-3 (+) sustentacular cells with another sustentacular specific marker, CK-18 (Schwob et al., 1995), due to the incompatibility of the fixation conditions required for each antibody. After the first postlesion week, SUS-4 (+) sustentacular cells are beginning to align at the apical line of the OE, and are not labeled with GBC-3 (10 days post-MeBr; Fig. 10M–P). The duct cells that invade the OE at this recovery time are not GBC-3 (+) (open arrows in Fig. 10E–H).
Discussion
The study of olfactory stem and progenitor cells, the study of stem and progenitor cells in general, and their isolation and/or genetic manipulation have been limited by a lack of markers that are selectively expressed in them. Recent data, which indicate that a kind of tissue stem cell of the olfactory epithelium resides within the functionally heterogeneous population of GBCs, have led to ongoing efforts to develop markers for GBCs in general and the stem cells among them in particular (Goldstein and Schwob, 1996; Goldstein et al., 1997; Jang et al., 2003; Chen et al., 2004). Maximal usefulness (e.g., as a molecular target for genetic manipulation or as a means of producing conditional regulation of gene expression) dictates the identification of the antigens that are recognized by the markers.
Of the three markers that we have generated so far, we report in this study the biochemical and immunohistochemical characterization of GBC-3. We took advantage of advances in 2D IEF/SDS-PAGE and mass spectrometry to identify the GBC-3 antigen that is expressed on the cell surface and is selective to GBCs and their immediate descendents. Although three distinct protein spots were identified in protein preparations from the rat NIC cell line, dissociated rat OE cells, and mouse ES-D3 cells, our results indicate that the relevant GBC-3 antigen corresponds to the 40 kDa spot labeled by the antibody and is iLRP: 1) MS/MS shows a significant sequence match; 2) the 40-kDa spot colabels with both GBC-3 and anti-iLRP antibody on the 2D Western blot; 3) both the GBC-3 antigen and iLRP are expressed on the cell surface of GBCs of normal epithelium; 4) the same population of living, dissociated OE cells stains with both GBC-3 and anti-iLRP antibody as shown by FACS; 5) the pattern of immunohistochemical staining with GBC-3 and iLRP in the OE is similar; and 6) antibodies selective for the other two GBC-3 (+) spots on the blots (calponin and tubulin) produce staining and FACS results that are dissimilar to the labeling of tissues and living cells with GBC-3. The epitope of iLRP that is recognized by GBC-3 is a different moiety from that recognized by an anti-iLRP MAb 43515 because 1) GBC-3 labels only a part of the entire MAb 43515 (+) spot on 2D Western blot, and 2) MAb 43515 does not interfere with GBC-3 labeling of cells in FACS analysis. These data suggest that there might be multiple isotypes of the protein, quite possibly differing in their carbohydrate composition. That suggestion is given additional credence by the demonstration that the epitope recognized by GBC-3 is an oligosaccharide removed by incubation with N-glycosidase, as shown above. The presence of subtypes of iLRP could in turn explain a subtle difference of its expression pattern within GBCs, less mature and more mature neurons.
To the best of our knowledge, the data reported here are the first demonstrations of the presence of the nonintegrin iLRP and of the mature receptor in the OE. The nonintegrin iLRP is a highly conserved, multifunctional protein, found in both humans and rodents (Ardini et al., 1998). The protein has also been identified as OFA (Coggin et al., 1999; Zelle-Rieser et al., 2001). The mature form of the receptor, nonintegrin LR, is generated by the dimerization of the precursor via a noncovalent association fostered by acylation, specifically via palmitoylation (Buto et al., 1998). Although the mature LR is present on many healthy cells, OFA-iLRP is highly expressed by many human tumors, including breast, lung, ovary, prostate, renal cancer, and lymphoma (Siegel et al., 2003). Data suggest that OFA-iLRP can specifically activate both T and B lymphocytes (Zelle-Rieser et al., 2001; Siegel et al., 2003) and acts as the cell-surface receptor for prion protein (PrP) subserving its uptake into eukaryotic cells (Rieger et al., 1997; Gauczynski et al., 2001). Interestingly, local differences in the expression of iLRP and LR in the central nervous system (CNS) have been reported, and the distribution of iLRP in the CNS is restricted to a subset of neurons known to be associated with the onset of prion diseases (Baloui et al., 2004). In addition, prions have been identified in the OE of patients with Creutzfeldt-Jakob disease (Zanusso et al., 2003).
Since the reported functions of iLRP are highly diverse in different organisms and tissues (Ardini et al., 1998), we can only speculate on the possible function of the protein in the OE. For a highly organized epithelium such as the OE, structural integrity may require interactions among the various cell types and between cells and extracellular matrix (ECM). Although HBCs are tightly anchored onto the basal lamina by hemidesmosomes (Holbrook et al., 1995), GBCs in normal OE may use a different means to maintain contact with others cell types within the OE, perhaps through interaction between LR and its ligand, i.e., laminin. In that respect, it is worth noting that the γ3 subunit of laminin is distributed throughout the normal OE (pers. commun., Dr. William Brunken, SUNY Downstate Medical Center). Although direct evidence of the binding of LR to laminin in the OE and its physiological significance is lacking, it has been reported that LR mediates high-affinity interactions between cells and components of ECM such as laminin, fibronactin, collagen, and elastin by other cell types (Menard et al., 1998). Therefore, it would not be surprising to find other components of ECM in the normal OE. The expression of the GBC-3 antigen after lesion suggests that the nonintegrin iLRP and LR may play an important role in the reassembly of the epithelium. In addition, it has been proposed that binding of LR and integrin to ECM mediates growth factor signaling onto hematopoietic cells (i.e., granulocytes and macrophages) leading to proliferation, survival, and differentiation effects (Gaynor, 2003) and could play a similar role here. Therefore, LR might be directly involved in the proliferation of GBCs during reconstitution of the OE.
The pattern of immunohistochemical labeling of the GBC-3 antigen, i.e., iLRP, is similar to that with two other GBC markers—GBC-1 and 2 (Goldstein and Schwob, 1996; Jang et al., 2003). In all these cases the immunoreactivity carries over in normal OE to the population of differentiating neurons at a progressively attenuated level (Figs. 1, 8). The carryover of GBC-3 antigen during differentiation may be explained by 1) continuous expression of the antigen after differentiation, or 2) the persistence (perdurance) of the antigen without de novo synthesis, hence resulting in a “dilution” of its level after cell division. In the MeBr-lesioned epithelium, some GBC-3 (+) cells also express either an HBC or a sustentacular cell marker (Figs. 9, 10). In the setting of the lesioned epithelium, the coexpression of a GBC marker with that of nonneuronal cell type has been interpreted previously as indicative of a lineage relationship during reconstitution of the tissue. That the population of GBCs encompasses a set of multipotent progenitors has been shown more directly by transplantation and lineage tracing experiments as well as studies of transcription factor expression (Huard et al., 1998; Murray et al., 2003; Chen et al., 2004; Manglapus et al., 2004). The present data are consistent with, but do not compel, that interpretation, with the possible exception of the observation in the ventral OE. In this part of the epithelium the elimination of HBCs from ventral epithelium and the colabeling of cells with GBC-3 during the reemergence of HBCs 1 week after lesion is best explained by the differentiation of HBCs from GBCs in this setting, in keeping with transplantation (Goldstein et al., 1998) and lineage tracing analysis (Huard et al., 1998). Moreover, the carryover to nonneuronal cells and the pattern of expression acutely after lesion both suggest that GBC-3, like GBC-2 and GBC-1, labels multipotent GBCs (and others more downstream) within the GBC population. In conclusion, we have demonstrated the presence of iLRP on GBCs in the adult OE, which is recognized by monoclonal antibody GBC-3. Although the function of the protein remains to be determined, GBC-3 provides a practical boost to attempts to isolate a relatively pure population of GBCs. We believe that the quest to characterize GBC populations is aided by developing markers like GBC-3, and combining with other identifying molecular markers, such as transcription factors (now in progress in our laboratory). Using these biochemical and genetic markers, the heterogeneous population of GBCs can be dissected into its functionally distinct progenitor types.
Supplementary Material
Acknowledgments
The authors thank Drs. J. Coggin and A. Barsoum (anti-iLRP MAb 43515) and Dr. J. Covault (anti-NCAM) for generous gifts of antibodies, and Dr. W. Brunken for sharing unpublished data. We also thank Dr. S. Gygi, Department of Cell Biology, Harvard University, in whose laboratory the mass spectrometry analysis was carried out, for generosity and assistance.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: R01 DC002167 and R21 DC006517.
Footnotes
This article includes Supplementary Material available via the Internet at http://www.interscience.wiley.com/jpages/0021-9967/suppmat.
Liturature Cited
- Ardini E, Pesole G, Tagliabue E, Magnifico A, Castronovo V, Sobel ME, Colnaghi MI, Menard S. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol Biol Evol. 1998;15:1017–1025. doi: 10.1093/oxfordjournals.molbev.a026000. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Baloui H, von Boxberg Y, Vinh J, Weiss S, Rossier J, Nothias F, Stettler O. Cellular prion protein/laminin receptor: distribution in adult central nervous system and characterization of an isoform associated with a subtype of cortical neurons. Eur J Neurosci. 2004;20:2605–2616. doi: 10.1111/j.1460-9568.2004.03728.x. [DOI] [PubMed] [Google Scholar]
- Buto S, Tagliabue E, Ardini E, Magnifico A, Ghirelli C, van den Brule F, Castronovo V, Colnaghi MI, Sobel ME, Menard S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J Cell Biochem. 1998;69:244–451. doi: 10.1002/(sici)1097-4644(19980601)69:3<244::aid-jcb2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Schwob JE. Quiescent globose basal cells are present in the olfactory epithelium. Chem Senses. 2003;28:A5. doi: 10.1002/cne.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Coggin JH, Jr, Barsoum AL, Rohrer JW. 37 kiloDalton oncofetal antigen protein and immature laminin receptor protein are identical, universal T-cell inducing immunogens on primary rodent and human cancers. Anticancer Res. 1999;19:5535–5542. [PubMed] [Google Scholar]
- Coon HG, Curcio F, Sakaguchi K, Brandi ML, Swerdlow RD. Cell cultures of neuroblasts from rat olfactory epithelium that show odorant responses. Proc Natl Acad Sci U S A. 1989;86:1703–1707. doi: 10.1073/pnas.86.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Sanes JR. Distribution of N-CAM in synaptic and extra-synaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986;102:716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, Rieger R, Krase-mann S, Deslys JP, Dormont D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001;20:5863–5875. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor RB. A role for extracellular matrix binding receptors in regulating hematopoietic growth factor signaling. Proc Natl Acad Sci U S A. 2003;100:13737–13738. doi: 10.1073/pnas.2536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16:4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Wolozin BL, Schwob JE. FGF2 suppresses neurono-genesis of a cell line derived from rat olfactory epithelium. J Neurobiol. 1997;33:411–428. [PubMed] [Google Scholar]
- Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9:1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- Graziadei PP. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5:113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–140. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Menard S, Tagliabue E, Colnaghi MI. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat. 1998;52:137–145. doi: 10.1023/a:1006171403765. [DOI] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, Lander AD, Calof AL. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Gygi SP. Proteomics: the move to mixtures. J Mass Spectrom. 2001;36:1083–1091. doi: 10.1002/jms.229. [DOI] [PubMed] [Google Scholar]
- Rieger R, Edenhofer F, Lasmezas CI, Weiss S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med. 1997;3:1383–1388. doi: 10.1038/nm1297-1383. [DOI] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Huard JM, Luskin MB, Youngentob SL. Retroviral lineage studies of the rat olfactory epithelium. Chem Senses. 1994;19:671–682. doi: 10.1093/chemse/19.6.671. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Siegel S, Wagner A, Kabelitz D, Marget M, Coggin J, Jr, Barsoum A, Rohrer J, Schmitz N, Zeis M. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood. 2003;102:4416–4423. doi: 10.1182/blood-2003-01-0198. [DOI] [PubMed] [Google Scholar]
- Zanusso G, Ferrari S, Cardone F, Zampieri P, Gelati M, Fiorini M, Fari-nazzo A, Gardiman M, Cavallaro T, Bentivoglio M, Righetti PG, Pocchiari M, Rizzuto N, Monaco S. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;348:711–719. doi: 10.1056/NEJMoa022043. [DOI] [PubMed] [Google Scholar]
- Zelle-Rieser C, Barsoum AL, Sallusto F, Ramoner R, Rohrer JW, Holtl L, Bartsch G, Coggin JJ, Thurnher M. Expression and immunoge-nicity of oncofetal antigen-immature laminin receptor in human renal cell carcinoma. J Urol. 2001;165:1705–1709. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


