Summary
Although pancreatic cancer is the fourth leading cause of cancer death, it has received much less attention compared to other malignancies. There are several transgenic animal models available for studies of pancreatic carcinogenesis, but most of them do not recapitulate, histologically, human pancratic cancer. Here we review some detailed molecular complexity of human pancreatic cancer and their reflection in histomorphological complexities of pancreatic lesions developed in various transgenic mouse models with a special concern for studying the effects of chemotherapeutic and chemopreventive agents. These studies usually require a large number of animals that are at the same age and gender and should be either homozygote or heterozygote but not a mixture of both. Only single-transgene models can meet these special requirements, but many currently available models require a mouse to simultaneously bear several transgene alleles. Thus it is imperative to identify new gene promoters or enhancers that are specific for the ductal cells of the pancreas and are highly active in vivo so as to establish new single-transgene models that yield pancreatic ductal adenocarcinomas for chemotherapeutic and chemopreventive studies.
Keywords: Carcinogenesis, Animal model, Molecular signaling
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer death in the United States and many other western countries. According to 2006 statistics of American Cancer Society, more Americans die of pancreas cancer than prostate cancer (American Cancer Society, 2006; Jemal et al., 2006). Pancreatic cancer has dismal prognosis; most patients are dead within six months after diagnosis, and the five-year survival rate is less than 5% in most countries, mainly because the tumors are usually diagnosed only at an advanced stage and are resistant to various treatments (American Cancer Society, 2006; Jemal et al., 2006). The vast majority of human PCs are ductal adenocarcinomas, whereas acinar cell carcinomas and other histological types are much less common.
The cell origin of ductal adenocarcinomas is still under debate (Pour et al., 2003). Some studies have suggested that it arises from metaplasia (transdifferentiation) of acinar cells or even endocrine(islet) cells to ductal cells leading to ductal adenocarcinoma (Pour et al., 2002; Schmid, 2002; Miyatsuka et al., 2006). While this has been the impression based on cell-lines and animal studies, observations on human carcinomas, however, imply a different scenario. Hyperplastic and dysplastic epithelial lesions of the pancreatic ducts have been identified frequently in association with ductal adenocarcinomas (Cubilla and Fitzgerald, 1979; Andea et al., 2003), and these are now referred to as pancreatic intraepithelial neoplasia (PanINs) (Hruban et al., 2001, 2004). These evidences in human pancreas suggest that ductal adenocarcinomas may simply be originated from ductal cells, although it cannot be ruled out that ductal metaplasia of other cell types, especially acinar cells (transdifferentiation or formation of ductular structures) could also be involved in the development of ductal adenocarcinoma under different situations. It is certain that the etiology of pancreatic ductal adenocarcinomas is fairly complex and poorly understood. Here we provide an overview of the histological and molecular complexities of pancreatic neoplasia as they apply to and correlate with different animal models, with a focus on transgenic mice, and their potential usefulness for developing preventive and/or therapeutic strategies.
Histological and genetic complexities of PC
There are several chemical- or transgene-induced animal models of pancreatic carcinogenesis available. The morphologic traits of pancreatic lesions from most transgenic models have been reviewed by a bevy of pathologists in a workshop in 2004 (Hruban et al., 2006b) and further summarized in a recent review article (Hruban et al., 2006a). However, few of these transgenic models have really been studied in a systematic manner, and thus the morphologic details related to age, gender, genetic background, and hetero-/homo-zygous status are inadequate. Moreover, ultra-structural data are basically lacking as well. Human PC exhibits changes in a variety of genetic markers (Maitra et al., 2006). In order to understand the complexities of histological findings in humans and their complex reflection in different animal models of PC, one must also appreciate the molecular complexities of PC. Therefore, we shall first summarize what is known thus far regarding the role of specific genetic markers relevant to human and animal PC.
EGFR signaling with TGFα as the preferred ligand plays crucial roles in PC
It has been reported that 43%, 46% and 54% of human PC show over-expression of epidermal growth factor receptor (EGFR), EGF and TGFα, respectively (Yamanaka et al., 1993). A marked increase in EGFR expression (Friess et al., 1999; Tobita et al., 2003) or a concomitant expression of EGFR with its ligand EGF, TGFα or amphiregulin (Kleeff et al., 2000) has been shown to be associated with a poor prognosis of PC. Other reports also confirm high expression of both TGFα and EGFR in human PC (Korc et al., 1992; Srivastava et al., 2001). In vitro studies showed that EGF, TGFα and amphiregulin significantly enhanced the proliferation of human PC cell lines (Liu et al., 1998). TGFα expressed in human PC cells is secreted to the circulation and excreted into the urine, which leads to a suggestion that measurement of serum or urinary TGFα levels may have a prognostic value as tumor marker (Chuang et al., 1994; Moskal et al., 1995). Conversely, spontaneous or butyrate-induced differentiation of cultured human PC cells is associated with decrease in the expression of TGFα (Estival et al., 1992). TGFα has been shown to be expressed in ducts, acini and islets of the human fetal pancreas (Miettinen and Heikinheimo, 1992; Hormi and Lehy, 1994) and is considered to be important for development of the organs that undergo branching, such as the mammary gland, lung and pancreas. However, our own immunohistochemical staining (Liao et al., 2006) revealed that in addition to the cells of ductal adenocarcinomas (Fig. 1a), TGFα was expressed only in ductal cells, not acinar cells, in the normal tissue adjacent to the cancer. However, “ductalized” acini in the adjacent tissue also showed positive staining (Fig. 1b), suggesting that TGFα is also expressed in acinar-ductal metaplasia. These results indicate that in adult pancreas, probably only ductal cells retain the ability to express TGFα. Moreover, TGFα positivity in the lineage from normal ductal cells to acini-ductal metaplasia and ductal adenocarcinomas seems to support the notions that the cancer can originate from ductal cells and ductal-acinal metaplasia.
Fig. 1.
Immunohistochemical staining shows TGFα expression in human ductal adenocarcinoma and its adjacent normal ductal cells as well as ductalized acini. c-myc shows positive nuclear staining in cancer cells of two human ductal adenocarcinomas. (see Liao, et al., 2006 for detail).
In one interesting study, a stronger immunohistochemical staining, relative to the adjacent normal tissue and chronic pancreatitis, was observed in 95% of the tumors for TGFα but only in 12% of the tumors for EGF (Barton et al., 1991). An in vitro study also showed that TGFα was more potent than EGF in enhancing the anchorage-independent growth of several human PC cell lines (Korc, 1991). It has been considered that PC cells may be more sensitive than normal pancreatic cells in response to TGFα (Kullenberg et al., 2000). Carcinogen-induced pancreatic cancer in the hamster and rat expressed only TGFα and EGFR, but not EGF (Visser et al., 1995, 1996). Administration of TGFα to the hamsters bearing xenograft tumors of hamster-derived ductal PC cells induced DNA synthesis, whereas treatment with EGF failed to do so (Morita et al., 1998). Collectively, these data suggest that TGFα may be the preferred growth factor over EGF or other EGFR ligands for normal ductal cells and cancer cells of the pancreas (Vaughan et al., 1992, 1993; Barnard et al., 1995; Giraud, 2000). TGFα/EGFR singling is well known to initiate Ras signaling (Harris et al., 2003) and induce transcription of cyclin D1 (Yan et al., 1997) and many other genes to effect cancer promotion. Thus the cross-talk between TGFα/EGFR singling with other cellular signaling pathways may play important roles in human PC and is thereby considered an important target for the development of animal models of PCs.
Human PC shows high frequencies of overexpression and amplification of c-myc
Voluminous studies have shown that c-myc is over-expressed at high frequencies in various human cancers because it is frequently amplified or it is a downstream effector of many growth signaling pathways such as Ras, Notch, TGFα/EGFR, NF-κB or PI3-Akt signaling that are known to be activated in various types of malignancies, including PC (Bachireddy et al., 2005; Mimeault et al., 2005). Overexpression of c-myc mRNA (Han et al., 2002) and protein (Schleger et al., 2002) has been found in 50% and 43.5% of human pancreatic adenocarcinomas, respectively. In our immunohistochemical study, 13 of 15 ductal adenocarcinomas showed strong nuclear staining of c-myc in about 20-70% of cancer cells (Fig 1c,d). Gene amplification occurs in about one-third of the human PC biopsies (Schleger et al., 2002), accounts for the aberrant expression in certain cases (Adsay et al., 1999), and is positively associated with the tumor grade (Nagy et al., 2001). In one report, 54% and 28% of 31 PC cell lines show c-myc and cyclin D1 gene amplification, respectively (Mahlamaki et al., 2002), whereas another study showed concomitant amplification of activated K-ras and c-myc in both primary tumor and lymph node metastasis (Yamada et al., 1986). These data suggest that c-myc may frequently collaborate with K-ras or its downstream effector cyclin D1 during formation or progression of human PC (Brackett et al., 2003; Asano et al., 2004; Holzmann et al., 2004). Animal studies have also revealed that PC induced by chemical carcinogen in the rat manifests increased c-myc overexpression (Silverman et al., 1990; Calvo et al., 1991). Schleger et al reported that c-myc overexpression was found in 43.5% of the primary pancreatic adenocarcinomas but only in 31.6% of the metastatic tumors (Schleger et al, 2002). A possible explanation for the slightly lower rate of c-myc overexpression in the metastases could be due in part to the fact that a lower c-myc level provides survival advantage, since c-myc expression can signal apoptotic cell death (Ponzielli et al., 2005).
Alterations in c-myc have dual prognostic values for various types of cancer
Numerous studies have been published addressing the prognostic values of overexpression and/or amplification of c-myc in various malignancies, but the data are still largely controversial and confusing. Positive, null and negative correlation of c-myc overexpression or amplification with prognosis or patient survival of various types of cancer has been reported (Sklar and Prochownik, 1991; Mizutani et al., 1994; Chana et al., 1999, 2001; Donzelli et al., 1999; Arango et al., 2001, 2003; Soh et al., 2002; Vijayalakshmi et al., 2002; Akervall et al., 2003; Chang et al., 2003; Grover et al., 2003; Nagy et al., 2003; Vora et al., 2003; Yu et al., 2003). Good prognostic value of c-myc is exemplified by the reports that colon cancer patients with amplified c-myc gene show improved disease-free and overall survival in response to 5-fluorouracil (5-FU) treatment (Donzelli et al., 1999) whereas down-regulation of c-myc using c-myc anti-sense decreases the sensitivity of colon cancer cells to 5-FU (Arango et al., 2001). The dual functions of c-myc, i.e. promotion of both cell proliferation and apoptosis (Liu and Levens, 2006), may be one of the main reasons for the controversial reports.
We have previously observed that in the mammary tumors from MMTV-c-myc transgenic mice, the c-myc transgene is silenced when some tumor cells progress to a more aggressive phenotype, coined as “tumor focus”, that is less apoptotic and more proliferative (Liao et al., 2000). It has also been reported that expression of c-myc transgene may not be needed for sustaining the mammary, pancreatic islet and skin tumors (Boxer et al., 2004; Pelengaris et al., 2004), although it may not be the case for c-myc transgenic tumors in other tissues (Jain et al., 2002; Shachaf et al., 2004). These data raise a question whether in certain types of cancer, c-myc plays its role in a “hit-and-run” fashion for some unique reason that tumor cells need to silence the c-myc to decrease its apoptotic potential when they advance to a certain stage (Liao and Dickson, 2000). If this is proved to be the case by more thorough studies, it may explain why roughly half of the cases of the human PCs and other types of cancer do not show elevated c-myc expression at diagnosis: in some of the c-myc negative cases, c-myc might have been elevated initially and then silenced to gain survival advantage. If some tumor cells somehow cannot silence the c-myc during advanced stages of carcinogenesis then c-myc-induced apoptosis may lead to a good prognosis.
A second reason for the controversial reports could be related to the interactions of c-myc with different protein factors under different situations. For instance, many studies have shown that concomitant expression of c-myc and TGFα in double transgenic mouse models makes mammary (Liao et al., 2000; Liao and Dickson, 2000), liver (Cavin et al., 2005) and pancreatic (Sandgren et al., 1993; Liao et al., 2006) tumors with less apoptotic and more proliferating cells relative to the tumors expressing c-myc alone. C-myc may suppress cyclin D1 expression and NF-κB activity whereas TGFα relieves the repression (Liao and Dickson, 2000; Cavin et al., 2005), which may be a mechanism behind the synergy between c-myc and TGFα. In one clinical study, the better prognosis was seen in c-myc overexpressing colorectal carcinomas but it was offset when there was concomitant p53 mutations (Smith and Goh, 1996). Collectively, these data suggest that c-myc expression may not always need to be silenced for cancer cells to acquire more survival ability in advanced stages. Concomitant expression of some survival genes such as TGFa or cyclin D1 or silencing of some pro-apoptotic genes such as p53 may also render a survival advantage via mechanisms that remain to be fully elucidated.
Apoptosis may be an important part of the mechanism for c-myc-induced carcinogenesis
Most proto-oncogenes have a function to drive cell proliferation, but few, if any, of them are as potent as c-myc in induction of apoptosis, although some of them (such as Ras and E2F1) have been shown to have certain ability of inducing apoptosis as well (Cox and Der, 2003; Bell and Ryan, 2004). A second trait that distinguishes c-myc from most other proto-oncogenes is its potent carcinogenicity in transgenic mouse models. For most proto-oncogenes (not viral oncogenes), each alone either fails to induce cancer in transgenic models or induces cancer only at a very low frequency with a long latent period, which is likely because of the need of alterations in a second gene. Besides the K-ras gene, which can also induce apoptosis, c-myc seems to be the only proto-oncogene that can induce cancer formation in most transgenic mouse models established to date, some of which yield tumors at 100% penetrance with a relatively short latent period. Tumors from most, if not all, of these transgenic models show pronounced apoptosis with distinctive morphology that is coined by us as “dead cell island”(Liao and Dickson, 2003). Therefore, it is possible that c-myc can efficiently induce carcinogenesis not only because it can drive cell proliferation, which many other proto-oncogenes can do as well, but also because it is the most potent apoptosis inducer among known proto-oncogenes.
There are two major types of cell proliferation, i.e. compensatory and hyperplastic proliferation, in solid organs such as the liver, pancreas and kidney (Ledda-Columbano et al., 1993; Columbano and Shinozuka, 1996). Compensatory proliferation is actually regeneration, usually triggered by tissue loss from surgical removal of part of the organ, chemical- or physical-induced cell death, etc. The proliferation ceases when the organ has restored its physiological size and number of cells. If the cell loss continues, such as in a situation of continuous exposure to chemical hazard or constant expression of apoptosis-inducing genes like c-myc, the regenerative proliferation also becomes constant. In contrast, hyperplastic proliferation is usually trigged by direct growth stimuli, such as treatment with or expression of growth factors (Ledda-Columbano et al., 1993; Columbano and Shinozuka, 1996). This type of cell proliferation results in redundancy of the cells and enlargement of the organ; the proliferating cells usually undergo apoptosis, presumably because the organ attempts to eliminate the redundant cells and retain its physiologic size. While initiation of carcinogenesis requires at least one round of cell replication to fix mutations of critical genes into the daughter cells before the mutations are repaired, promotion step of carcinogenesis requires many rounds of cell replication to propagate the initiated cells and to undergo malignant transformation. Ample animal studies have demonstrated that only compensatory proliferation can efficiently facilitate initiation and promotion of cancer formation, whereas hyperplastic proliferation is a very poor stimulus for these steps of carcinogenesis (Ledda-Columbano et al., 1993; Farber, 1995, 1996a,b; Columbano and Shinozuka, 1996; Laconi et al., 2001), presumably because the proliferating cells will undergo apoptosis (Laconi et al., 2001). It is likely that c-myc-induced apoptosis provides the organ or tissue a constant need for compensatory proliferation, which in turn drives those non-apoptotic cells to proliferate continuously while undergoing malignant transformation.
It is completely unknown that in the target organ of a c-myc transgenic mouse model, which c-myc expressing cells will undergo apoptosis and which cells will undergo proliferation and malignant transformation. Recent studies with transgenic drosophila have shown that this selection may not be a random event. Neighboring cells are shown to compete with each other on their c-myc levels, and the losers, i.e. the cells with lower c-myc activity, are actively eliminated via apoptotic death, while the winners in this so-called “cell competition” undergo compensatory proliferation (de la et al., 2004; Donaldson and Duronio, 2004; Moreno and Basler, 2004; Gallant, 2005). These results again suggest that c-myc-induced apoptosis and ensuing compensatory proliferation are of importance for carcinogenesis (Donaldson and Duronio, 2004; Gallant, 2005), and thus the c-myc transgenic animal model of PC is a good model but has certain limited value as discussed below.
Different animal models of pancreatic carcinogenesis have different strengths and weaknesses
Pancreatic carcinogenesis can be induced by chemical carcinogens in animals, as summarized in several reviews (Hotz et al., 2000; Standop et al., 2001; Wei et al., 2003; Leach, 2004). Of the animal models, hamster PCs induced by N-nitros-bis-(2oxopropyl) amine most resemble human PC (Schneider et al., 2001; Wei et al., 2003) because the tumors are ductal phenotypes with obvious fibrosis, progress rapidly with strong invasion potential, and metastasize to the liver (Hotz et al., 2000; Wei et al., 2003). Moreover, the hamster tumors also show high frequency of K-ras mutations as seen in human PC (Konishi et al., 1998; Hotz et al., 2000; Wei et al., 2003). In contrast, pancreatic tumors induced by the same chemical or other carcinogens in rats and mice are usually acinar cell carcinomas (Hotz et al., 2000; Wei et al., 2003) and rarely show K-ras mutations (Hotz et al., 2000; Wei et al., 2003). These species differences become the first intrinsic weakness of chemical-induced models in rodents since it raises a concern on the human relevance of these models and chemicals. Second, the carcinogens are used at high doses and for long periods of time, which does not occur in humans and thus has little relevance to humans in the context of etiology of human PC. Third, the chemicals cause complex biochemical, genetic and toxic alterations, and therefore it is almost impossible to pinpoint the critical molecular and cellular events that are crucial for cancer formation.
As reviewed recently by Hruban et al. (2006a), several transgenic mouse models have been established to study the excocrine pancreatic cancers (Ornitz et al., 1987; Quaife et al., 1987; Jhappan et al., 1990; Hotz et al., 2000; Bardeesy et al., 2002; Aguirre et al., 2003; Brembeck et al., 2003; Hingorani et al., 2003; Lewis et al., 2003; Lowy, 2003; Wei et al., 2003; Grippo and Sandgren, 2004; Leach, 2004; Hingorani et al., 2005; Maitra and Hruban, 2005; Tuveson and Hingorani, 2005; Tuveson et al., 2006) in addition to several other transgenic models of endocrine pancreatic tumors (Pelengaris and Khan, 2001). Because the majority of PC in humans are ductal adenocarcinomas but currently no gene promoter/enhancer has been known to be specific for pancreatic ductal cells (Grippo and Sandgren, 2004), all these transgenic mouse models share a common deficiency: If the transgene is controlled by a pancreas-specific promoter, such as elastase-1 gene (Ela) promoter, it is dominantly expressed in the acinar cells. Conversely, if the transgene is driven by a promoter specific for ductal cells, its expression is not pancreas-specific. For instance, the metallothionin-1 gene (MT) promoter targets the transgene to the mammary gland, liver and pancreas (Jhappan et al., 1990), while cytokeratin 19 gene promoter targets the transgene to the stomach, pancreas and other organs (Brembeck et al., 2003); both promoters are much weaker than the Ela-promoter.
The Ela-SV40TAg, Ela-PyMT, and Ela-H-ras mutant transgenic mice develop mainly acinar cell carcinomas (Ornitz et al., 1987; Quaife et al., 1987; Lewis et al., 2003). However, Ueda et al. recently established a transgenic rat in which human H-ras mutant (G12V) is regulated by the Cre/lox system (Ueda et al., 2006). In these rats, injection of Cre carrying adenovirus into the pancreatic ducts and acini through the common bile duct can target expression of H-ras mutant to pancreatic ducts and acini and induce PanIN-like lesions and carcinomas. In contrast, K-ras-mutant mice with Ela- or cytokeratin-19 gene promoter develop only hyperplasia (early mouse PanIN lesions) without formation of frank adenocarcinomas (Brembeck et al., 2003; Wei et al., 2003; Grippo and Sandgren, 2004; Leach, 2004). K-ras mutant targeted to the pancreas using Pdx1-Cre system induces proliferating ductal lesions that histologically closely resemble human PanIN lesions. While this model appears to be the best thus far in creating mPanINs, unfortunately, progression to frank tumor occurs at very low frequency (2 out of 29 animals) (Hingorani et al., 2003) thus rendering it highly impractical for the purpose of cancer research in chemotherapy and chemoprevention. The time point for activation of the K-ras mutant is probably also important since Mist1-driven expression of the K-rasG12D mutant has recently been shown to induce invasive and metastatic hepatocellular carcinomas and pancreatic tumors of mixed cell types (Tuveson et al., 2006). Because Mist1 is a transcription factor controlling pancreatic development at an early stage, this may be a model of embryonic carcinogenesis that recapitulates childhood tumors in humans, such as retinoblastoma. If so, it may have little relevance to human PC.
It is likely that K-ras mutation alone is not sufficient to complete the whole carcinogenic process and alterations in other genes are required. Consistent with this thought, K-ras mutation in combination with deficiency of the p16-Ink4α, p19-Arf, or p53 tumor suppressor gene, achieved by crossing different transgenic lines, led to ductal adenocarcinomas that show propensity to metastasize to the liver (Bardeesy et al., 2002; Aguirre et al., 2003; Hingorani et al., 2005). While the Pdx1-Cre/LSL- K-rasG12D model (Hingorani et al., 2003; Tuveson and Hingorani, 2005) mostly recapitulate human PanIN lesions, the one simultaneously deficient with p53 (Hingorani et al., 2005; Tuveson and Hingorani, 2005) recapitulates human PC and its liver metastasis; both models are thus excellent for studying different stages of pancreatic carcinogenesis. Deficiency in PTEN tumor suppressor gene alone also induces ductal metaplasia, but the lesions progress to invasive ductal adenocarcinomas only at a low frequency (Maitra and Hruban, 2005). A weakness of those models that involve both transgenic and knockout systems is the requirement of a mouse concomitantly to bear four transgene alleles. For instance, the K-ras mutant/Ink4a/Arf deficient mouse needs to bear Pdx1-Cre, LSL- K-rasG12D and homozygous Ink4a/Arf lox/lox (Aguirre et al., 2003). Thus, this type of animal models involve huge amount of work for animal breeding and genotyping because only a small percent (12.5%) of the total pups will bear all four transgene alleles according to Mendelian inheritance. A few pre-bred animals may be obtained as breeders from the establishers of these models to help decrease the huge animal work. However, it will only help a little because one round of breeding procedure will segregate the transgenic alleles and the second round of breeding needs to start from heterozygous breeders again, which limits the broader usefulness of these animal models for prevention and/or therapeutic studies.
Female MT-TGFα transgenic mice of the MT100 line develop ductal adenocarcinomas at low frequency
Besides the above-described transgenic models, several lines of TGFα transgenic mice have been generated using Ela- or MT-promoter. The Ela-TGFα transgenic mice, which were generated by Drs. E. Sandgren and D.C. Lee et al (Sandgren et al., 1990) but studied extensively by Schmid and Wagner et al. (Schmid et al., 1999; Greten et al., 2001; Schmid, 2002), develop prominent fibrosis at an early age and show acinar-ductal transformation at four months of age (Wagner et al., 1998, 2001). At the age of one year or older, about one-fourth to one-third of the animals develop pancreatic tumors, mainly acinar cell carcinomas. Pronounced metaplasia from acinar to ductal cells appears in this model before tumor formation, but the meaning of this morphological trait is unclear since the majority of the tumors are acinar cell type. Genetic alterations of these Ela-TGFα transgenic pancreatic tumors resemble those seen in human PC (Wagner et al., 1998, 2001). Recently, Garbe et al. reported that the Ela-TGFα mice with deficiency of p53 gene (p53-/-), created by crossing Ela-TGFα mouse with p53 knockout mouse, developed growing tumors within 120 days of age (Garbe et al., 2006), although the morphological details of the tumors were not described. The shorter latent period indicate a synergistic effect of TGFα overexpression and p53 deficiency in pancreatic carcinogenesis.
Of the MT-TGFα mice available, the MT100 and MT42 lines developed by Drs. C. Jhappan and G. T. Merlino et al. (Jhappan et al., 1990) and the line developed by Drs. E. Sandgren and David C. Lee et al. (Sandgren et al., 1990), thus coined herein as MT-TGFα-SE, are widely used by many investigators. However, these mice are mainly studied for carcinogenesis of the mammary gland (Liao et al., 2000; Liao and Dickson, 2000) and liver (Thorgeirsson et al., 2000) but rarely for the pancreas. During our studies of mammary carcinogenesis with the MT100 line, we accidentally found that the female mice live only for 6-8 months of age whereas males live well up to 14 months of age (males older than this age were not followed). This female predominance may be one of the reasons why it is less studied for pancreatic lesions by other investigators who are engaged in using male animals. Currently, our laboratory is the only one that still keeps live animals of MT100 line (although Jackson Lab has cryo-preserved stock) and uses it to address pancreatic carcinogenesis in a systematic manner.
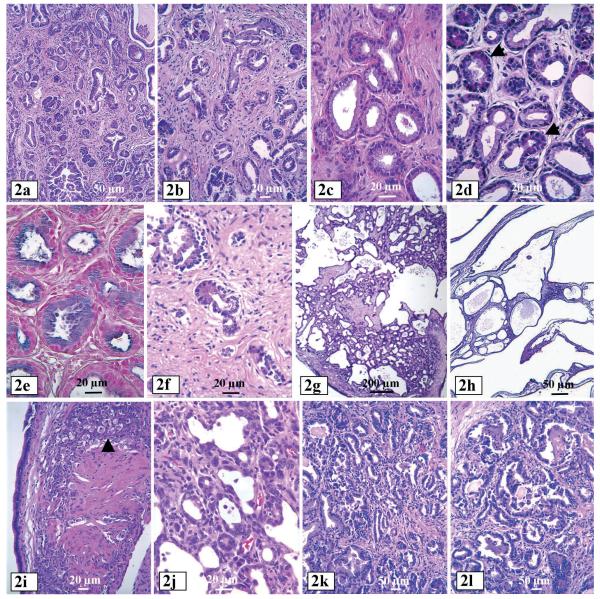
Gross examination of dead or moribund females at 6-8 months of age revealed that the pancreas in most (about 70%) of the females became severely atrophic, weighing only 10-20% of the pancreas from age-matched wild type littermates. Histological examinations of the pancreas from females showed that at two months of age the pancreatic lesions (Liao et al., 2006) were characterized by ductal proliferation with acinar-to-ductal metaplasia (Fig. 2c,d), acinar cell death, and obvious fibrosis. In proliferating ducts, the mucin was predominantly blue with both alcian blue and high iron diamine (acidic sialomucin, also regarded as the “neoplastic” mucin in the pancreas) (Fig. 2e) and, in rare foci, it was black with high iron diamine. All lesions became severe with increasing age. At four months of age, the mucinous ducts began to efface the pancreas, separated by a subtle fibrous stroma, and acini became scattered due to cell death and fibrosis. Severely proliferated ducts showed focally positive staining for CA19-9 and CEA, which are known to be more commonly expressed in neoplatically transformed ductal cells. At about five months of age, the proliferating ducts changed their characteristics, becoming haphazardly distributed and exhibited irregular contours. The cells became less mucinous and more cuboidal, suggestive of an evolving adenocarcinoma. In the areas without proliferating ducts, acini disappeared and were replaced by stroma (Fig. 2f). The dramatic disappearance of acinar cells may be the reason for the smaller pancreas and the death of the animals.
Fig. 2.
Pancreatic lesions from female MT100 mice. a and b: Ductal proliferation and fibrosis from mice at two months of age. c: Ducts lined by tall columnar mucinous cells. Some of the ducts are lined by acinar cells, an evidence of acinar to duct transformation (transdifferentiation). d: acinar cells in ductal-acinar glandular loops, indicating acinar-ductal metaplasia. e: High iron diamine stains blue mucin in the ducts. f: Prominent fibrosis and cell death of acinar-ductal cells. g and h: Cystic ductal lesions. i: A focus of epithelial cells with features resembling luteal cells (arrow) on the wall of a multilocular cystic tumor in a female mouse. j: The septae of cystic tumor show a small ductular proliferation with morphologic features of ductal adenocarcinoma with cytological atypia, marked nuclear pleomorphism and mitotic figures. k and l: ductal adenocarcinomas.
In the remaining 30% of the female mice, the pancreas was dramatically enlarged, as opposed to the shrinking pancreas described above, due to the development of multi-locular cystic neoplasms. Histologically, the pancreas manifested all of the above described ductal proliferation, acinar-to-ductal metaplasia, acinar cell death and fibrosis. In addition, however, there were multilocular cysts (Fig. 2g), which became evident at two months of age and developed to multi-locular cystic masses (1.2 and 1.0 cm) with 11 to 16 loculi in each cystic neoplasm (Fig. 2h) at the age of 4-5 months. Size of the loculi ranged from 0.5 to 5 mm. The largest cystic neoplasm with colloidal liquid obtained was 2.2 cm in diameter from a mouse at 6 months of age. The septae were thin and contained loose mesenchymal tissue reminiscent of ovarian stroma, manifested by clusters of epithelioid cells with features suggestive of luteal cells (Fig. 2i). The lining epithelium was mucinous and focally positive for B72.3, CA19-9 and CEA. The septae sometimes contained small foci of ductal proliferation with all the morphologic features of an adenocarcinoma, including severe cytological atypia, marked nuclear pleomorphism and mitotic figures (Fig. 2j). Ductal adenocarcinomas (Fig. 2k,l) were found in roughly 10% of the total females.
Bardeesy et al. also observed similar cystic lesions in MT100 mice that were Ink4a and/or p53 heterozygous, but not in MT100 mice with Ink4a or p53 wild type (Bardeesy et al., 2002). The reason for this slight discrepancy is unknown, but it could be partly because their study involved only 25 mice without information of animal gender, since multi-locular neoplasms appeared in only 30% of the females in our laboratory. Moreover, Muller-Decker et al. also reported recently similar cystic neoplasms in association with serous cystadenomas and dysplasia in cyclooxygenase-2 (COX2) transgenic mice driven by keratin-5 promoter (Muller-Decker et al., 2006). In our laboratory, pancreatic lesions in male MT100 mice were much less severe and do not progress to cystic lesions and cancer. The reason for the female predilection is unknown. The MT promoter is probably more potent in female pancreas, like in the female mammary gland. The preferential occurrence in female mice also raised the analogy with mucinous cystic neoplasm of the pancreas in humans, which occur almost exclusively in females and is characterized by ovarian like stroma. It was difficult to ascertain the nature of the hypercellular mesenchyme in these female mice; however, the similarities were striking.
Immunohistochemical staining showed that the TGFα transgene was mainly expressed in the proliferating ductal cells of MT100 mice. Even in the same acinar-to-ductal glandular loop (acinar transdifferentiation), the ductal cells showed much stronger staining for TGFα than the neighboring acinar cells (Liao et al., 2006). This feature indicates that the MT-promoter is active mainly in the ductal epithelial cells, which makes the MT100 mouse a good model for studies of pancreatic carcinogenesis of ductal cell origin. At this moment when the morphology of ductal lesions from the aforementioned kartin-5 driven COX2 mice have not yet been described detailed enough (Muller-Decker et al., 2006), the MT100 line may be the only single-transgene model established to date that produces a series of premalignant and malignant ductal lesions. Although in only 10% of the females there were frank adenocarcinomas, the MT100 line may still be a cost-effective model for ductal adenocarcinomas compared with the aforementioned models that involve expression of K-ras mutant and simultaneous knockout of a tumor suppressor gene because the MT100 line requires less animal work, animal housing and genotyping. On the other hand, the model itself may have some imperfections such as lack of typical mPanINs.
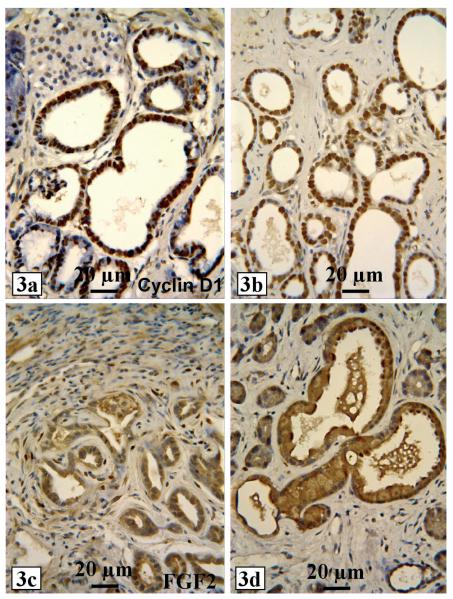
As indicated earlier that there may be a cross-talk between c-myc, TGFα/EGFR and cyclin D1, cyclin D1 expression was found to be colocalized with TGFα to the proliferating ducts in this animal model (Fig. 3a), together with the expression of proliferating cell nuclear antigen (PCNA) (Fig. 3b) and Ki67 staining. Therefore, TGFα-induced proliferation of ductal cells may be mediated by cyclin D1, a well known downstream effector of TGFα/EGFR signaling pathway. On the other hand, immunohistochemical staining for fibroblast growth factor-2 (FGF2) showed that proliferating ductal cells were strongly positive in the cytoplasm, whereas fibroblasts in the adjacent stroma were positive in the nuclei (Fig. 3c,d). A logical explanation for these results is that TGFα may induce expression of FGF2 in ductal cells, which is later secreted and taken up by stromal cells leading to the induction of proliferation of stromal fibroblasts resulting in fibrosis as frequently seen in human PC. Interestingly, Ela-Smad7 transgenic mice, in which the TGFß inhibitor Smad7 was presumably targeted to acinar cells by Ela-promoter, developed ductal change (acinar to ductal metaplasia) and fibrosis at 6 months of age (Kuang et al., 2006). It deserves further investigation whether the Smad7 transgene is mainly expressed in acini in this model and, if so, whether inhibition of TGFß in acini causes the ductal proliferation and fibrosis via an epithelial-stromal interaction.
Fig. 3.
Proliferating ducts from female MT100 mice at two months of age show strong cyclin D1 immunohistochemical staining (a), whereas many proliferating ductal cells and some stromal cells show strong PCNA staining (b). FGF2 immunohistochemical staining is mainly localized in the cytoplasm of proliferating ductal cells but in the nuclei of stromal fibroblasts (c and d).
Ela-myc transgenic mice develop mixed acinar and ductal adenocarcinomas
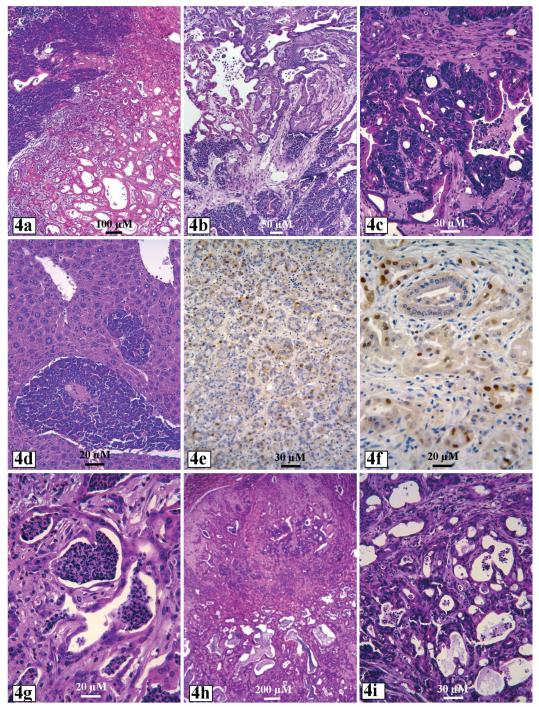
Ela-myc transgenic mice were generated by Drs. E. Sandgren and Brinster et al. (Sandgren et al., 1991). These mice develop pancreatic tumors at 100% penetrance at 2-7 months of age without obvious sex predilection. One-half of the tumors are acinar cell carcinomas, while the remaining one-half are mixed ductal adenocarcinomas and acinar carcinomas (Sandgren et al., 1991; Schaeffer et al., 1994; Aguilar et al., 2004; Liao et al., 2006). The mixture of ductal and acinar tumors manifests in different ways. It can be pure acinar cell tumors in some areas whereas pure ductal adenocarcinomas in other areas (Fig. 4a,b). It is also observed that acinar tumor cells are mingled with ductal tumor cells in the same tumor area and even in the same glandular loops (Fig. 4c), which is probably related to the continuation of acinar-to-ductal metaplasia (acinar cell transdifferentiation). Ductal tumor areas usually show obvious fibrosis, a morphologic trait of human PC. According to our observation (Liao et al., 2006), the acinar tumors manifested many “dead cell islands”, a feature coined by us to define c-myc-induced programmed cell death in tumors from c-myc transgenic mice (Liao and Dickson, 2000, 2003). Many tumor cells express cyclin D1 (Fig. 4e,f), which is somewhat inconsistent with the observations that c-myc suppresses cyclin D1 expression in fibroblasts, MMTV-c-myc transgenic mammary tumors and mouse pancreatic cancer cell lines (Jansen-Durr et al., 1993; Philipp et al., 1994; Marhin et al., 1996; Liao et al., 2000). It remains to be determined whether those cyclin D1 positive cells have lost expression of the c-myc transgene, as seen in some focal lesions in MMTVc-myc mammary tumors (Liao et al., 2000) or show activation of some growth factor signaling pathways such as Ras or TGFα that activate cyclin D1 signaling.
Fig. 4.
Ela-myc pancreatic tumors contain acinar cells in one area but ductal cells in another area (a and b), or show mixture of acinar and ductal tumor cells in the same area or even the same glandular loops (c). A liver shows metastatic acinar cell carcinoma (d). Acinar (e) and ductal (f) tumor cells show positive cyclin D1 immunohistochemical staining. MT100/Ela-myc double transgenic pancreatic tumors show ductal elements, prominent cell death (g), cystic alteration (h) and area of mixed ductal and acinar tumor cells (i).
In about 20% of the Ela-myc mice, the pancreatic tumors metastasize to the liver (Fig. 4d) (Liao et al., 2006), which was not reported in the original study by Sandgren et al. (1991). The reason for the metastasis uniquely observed in our animals is unclear, but it is probably because we changed the genetic background of the mice from C57BL/6xSJL to a mixture of FVBxC57BL/6xSJL after we received a breeder mouse from Dr. Sandgren. To our knowledge, this Ela-myc mouse is the first and the only single-transgene model that yields the highest frequency of malignant ductal lesions, although associated with acinar cell lesions, and develop frank tumors in the shortest latency period compared with other single-transgene models (Ornitz et al., 1987; Quaife et al., 1987; Lowy, 2003; Wei et al., 2003; Grippo and Sandgren, 2004; Leach, 2004; Hruban et al., 2006a). This transgenic line is also one of the very few transgenic models that produce liver metastasis.
MT-TGFα/Ela-myc double transgenic mice develop various ductal lesions and tumors with a higher frequency of liver metastasis
Sandgren et al. reported that Ela-TGFα/Ela-myc or MT-TGFα-ES/Ela-myc double transgenic mice, generated by crossing an Ela-myc mouse with an Ela-TGFα or MT-TGFα-ES mouse, developed pancreatic tumors at an earlier age than Ela-myc mice, and the tumors manifested more malignant histology, i.e. less differentiation than Ela-myc tumors (Sandgren et al., 1993). We crossed MT100 with Ela-myc mice to create MT-TGFα/Ela-myc mice, considering that the appearance of various ductal lesions in the Ela-myc pancreas indicates that the Ela-myc transgene is also expressed in the ductal cells, although at a lower level than in the acinar cells, and thus may synergize with the MT-TGFα transgene to induce ductal tumors. We found that the pancreatic tumors from MT100/Ela-myc mice were more malignant than the Ela-myc tumors, especially in females (Liao et al., 2006). Apoptotic and necrotic cell death is common in the double transgenic tumors (Fig. 4g). We also observed various ductal lesions, cysts and adenocarcinomas (Fig. 4g,h) in the double transgenic pancreas (Liao et al., 2006), in addition to mixed tumors (Fig. 4i) and pure acinar cell tumors as seen in Ela-myc mice. These findings are surprising because Sandgren et al. did not observe ductal elements in the pancreas of their Ela-TGFα/Ela-myc or MT-TGFα-ES/Ela-myc double transgenic mice (Sandgren et al., 1993). Another of our novel findings was that the MT100/Ela-myc tumors metastasized to the liver at a slightly higher frequency (30%) than the Ela-myc pancreatic tumors (Liao et al., 2006), which was, again, not observed by Sandgren et al (Sandgren et al., 1991, 1993). A possible explanation for these discrepancies is that their double transgenic mice were probably mainly Ela-TGFα/Ela-myc and less MT-TGFα-ES/Ela-myc. Moreover, according to our observation, the ductal lesions in MT-TGFα-ES mice are less severe and progressed much slower than those in the MT100 line we used (Liao et al., 2006). In addition, Sandgren et al. found primary liver tumors in their MT-TGFα-ES mice with C57BL/6xSJL background at a high frequency (16 of 27 animals) and in their wild type mice at a low frequency (1 of 20 animals) (Sandgren et al., 1993), whereas we did not find any primary liver tumor in any of our mice with FVBxC57BL/6xSJL background (Liao et al., 2006). Therefore, different genetic backgrounds may partly explain the discrepancy.
Different animal models bear different disadvantages with regard to their use for testing drug efficacy
Xenograft models, in which human cancer cell lines are inoculated at a subcutaneous site of immunodeficient mice, are frequently used in studies of cancer chemotherapy. Sometimes, the human cancer cells are not implanted at a subcutaneous site but at a specific organ of the mouse, such as the pancreas; in this case it is called orthotopic model. Both xenograft and orthotopic models are the most commonly used tools in studies of testing new therapeutic or preventive approaches for cancer. However, these in vivo methods have several weaknesses: 1) Cancer cells retain certain features of the adjacent normal cells, which make it difficult for the tested agent to kill just the cancer cells without hurting normal tissue. Human cells differ from mouse cells in genome and thus in many parameters; the sharp contrast between the two species makes it easier for a drug to differentially kill cancer cells in these models. 2) Many human cancer cell lines cannot grow in immunodeficient mice, usually because they are not malignant enough. Thus, these models actually favor those that are more malignant, faster growing cells, which are more sensitive to chemotherapy. 3) Because in xenograft or orthotopic models the tumors do not actually develop from host (recipient) mice in a chronic process, the host mice are in a better health condition than most patients with advanced cancer and, thus, better tolerate the treatment. 4) The deficiency of immune function in the host mouse may affect the efficacy of the tested agent, especially considering that inflammatory response is common in human cancer. In addition to these weaknesses, xenograft and orthotopic models cannot be used in the studies of cancer prevention since the implanted cells are already malignant.
Chemical-induced models of carcinogenesis are better than xenograft or orthotopic models because the tumor develops spontaneously in the animals and thus are superior for studies of new therapeutic approaches for cancer. In addition, these models can be used for studies of cancer prevention. However, in most of these models, chemical carcinogens are used at high doses for a relatively long period which cause toxicity in the liver, kidney, immune system and probably other organs. These toxic effects likely affect the metabolism of the tested drugs and weaken the general health of the animals. Therefore, the animals actually receive double drugs, i.e. the chemical carcinogen and the tested agent, although the two agents may not be administered simultaneously. Moreover, chemical-induced carcinogenesis is a long-time process, usually in months, which makes it difficult to determine when the treatment with the to-be-tested agent should be started.
Transgenic models eliminate the deficiencies of xenograft and orthotopic models when used for studies of new therapeutic or preventive approaches. However, when transgenic mice are used for these purposes, special concerns should be considered regarding the homozygous or heterozygous status of transgene carriers. Researchers frequently breed animals by mating a heterozygous male with a heterozygous female to increase the frequency of transgenic pups. This breeding procedure produces a mixture of homozygous and heterozygous pups. Technically, southern blot with radioactive probe is the common method to distinguish homozygous from heterozygous pups, although it still has certain difficulties because the homozygote shows only one-fold higher signal. It is not practical, although possible, to perform Southern blot to genotype a large number of pups. Because the mice bearing two alleles of the transgene may show different sensitivity to the agent to be tested, compared with those bearing only one allele, animals bred this way cannot be used for the purpose of testing drug efficacy, although they may still be used for studies of carcinogenic mechanisms.
Theoretically, one can use homozygous male and homozygous female as breeders because the pups bred in this way will be 100% homozygote. If homozygous animals are not overly sensitive to the to-be-tested agent, they can be used in chemotherapeutic or chemopreventive studies. Unfortunately, this breeding method is usually not an option because homozygous transgene carriers are often infertile or do not nurse the pup. Because many publications of transgenic models do not provide information on the animal’s fertility, it is difficult to assess which of the currently available transgenic models can be propagated this way. Ideally, transgenic mice to be used for drug testing should be bred by mating a heterozygous mouse with a wild type animal. This procedure should produce 50% of heterozygous pups according to Mendelian inheritance, but the actual frequency may be lower, especially when the phenotype is severe, probably because of the natural selection of relatively healthy embryos.
The above-described special concern of the breeding method further increases the difficulty in animal propagation and limits the use of transgenic models that require a single mouse bear several transgene alleles in the studies of chemotherapy and chemoprevention. After taking all the aforementioned limitations of transgenic models, it seems that the MT100 and MT100/Ela-myc mice may be superior to other currently available transgenic models for testing the effects of chemopreventive and/or chemotherapeutic agents and will likely provide data that could be easily translated for the prevention and/or treatment of human PC.
Perspectives
Compared with other types of malignancies, pancreatic cancer has hitherto received much less attention with studies on its mechanism, treatment and prevention, although it is the 4th leading cause of cancer deaths, now higher than prostate cancer. Several transgenic mouse models have been established thus far. While some of these models are excellent for mechanistic studies of pancreatic carcinogenesis, none of them are ideal for therapeutic studies. Studies of cancer therapeutics usually require a large number of animals that are the same gender and age because gender and age affect drug metabolism. Moreover, either heterozygous or homozygous, but not both, transgenic animals should be used in therapeutic studies. Single-transgene models are the best to meet these special requirements, but, unfortunately, no one single-transgene model established hitherto produces mainly ductal adenocarcinomas at a high penetrance. Pertaining to the studies of cancer prevention, the MT100 line of TGFα transgenic mice may be one of the best alternatives available thus far since the females yield ductal proliferation with 100% penetrance at several months of age. In future studies, more efforts should be assigned to identification of gene promoters or enhancers that are specific for ductal cells of the pancreas and are highly active in vivo, and also practical with high-yield so that the model can be utilized in testing potential molecules for chemoprevention and chemotherapy.
Acknowledgements
This work was supported by an NIH grant (CA100864) and a Susan G. Komen Breast Cancer Foundation grant (BCTR0201648) to Dr. D.J. Liao. We thank Dr. Sandgren at University of Wisconsin-Madison for generously providing us the original Ela-myc and MT-TGFα-ES mice.
References
- Adsay NV, Dergham ST, Koppitch FC, Dugan MC, Mohamed AN, Vaitkevicius VK, Sarkar FH. Utility of fluorescence in situ hybridization in pancreatic ductal adenocarcinoma. Pancreas. 1999;18:111–116. doi: 10.1097/00006676-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Corominas JM, Malats N, Pereira JA, Dufresne M, Real FX, Navarro P. Tissue plasminogen activator in murine exocrine pancreas cancer: selective expression in ductal tumors and contribution to cancer progression. Am. J. Pathol. 2004;165:1129–1139. doi: 10.1016/S0002-9440(10)63374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akervall J, Bockmuhl U, Petersen I, Yang K, Carey TE, Kurnit DM. The gene ratios c-MYC:cyclin-dependent kinase (CDK)N2A and CCND1:CDKN2A correlate with poor prognosis in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2003;9:1750–1755. [PubMed] [Google Scholar]
- American Cancer Society Cancer statistics. 2006:1–49. [Google Scholar]
- Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod. Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- Arango D, Corner GA, Wadler S, Catalano PJ, Augenlicht LH. c-myc/p53 interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res. 2001;61:4910–4915. [PubMed] [Google Scholar]
- Arango D, Mariadason JM, Wilson AJ, Yang W, Corner GA, Nicholas C, Aranes MJ, Augenlicht LH. c-Myc overexpression sensitises colon cancer cells to camptothecin-induced apoptosis. Br. J. Cancer. 2003;89:1757–1765. doi: 10.1038/sj.bjc.6601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Bachireddy P, Bendapudi PK, Felsher DW. Getting at MYC through RAS. Clin. Cancer Res. 2005;11:4278–4281. doi: 10.1158/1078-0432.CCR-05-0534. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M, Merlino G, DePinho RA. Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol. Cell Biol. 2002;22:635–643. doi: 10.1128/MCB.22.2.635-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J. Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–142. doi: 10.1038/sj.cdd.4401324. [DOI] [PubMed] [Google Scholar]
- Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–586. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Brackett DJ, Smith BJ, Lerner MR, Hanas JS, Postier RG. Gene activity associated with cancers treated by surgical oncologists. J. Okla. State Med. Assoc. 2003;96:485–494. [PubMed] [Google Scholar]
- Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, Grippo P, Stoffers DA, Silberg DG, Rustgi AK. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63:2005–2009. [PubMed] [Google Scholar]
- Calvo EL, Dusetti NJ, Cadenas MB, Dagorn JC, Iovanna JL. Changes in gene expression during pancreatic regeneration: activation of c-myc and H-ras oncogenes in the rat pancreas. Pancreas. 1991;6:150–156. doi: 10.1097/00006676-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Cavin LG, Wang F, Factor VM, Kaur S, Venkatraman M, Thorgeirsson SS, Arsura M. Transforming growth factor-alpha inhibits the intrinsic pathway of c-Myc-induced apoptosis through activation of nuclear factor-kappaB in murine hepatocellular carcinomas. Mol. Cancer Res. 2005;3:403–412. doi: 10.1158/1541-7786.MCR-04-0186. [DOI] [PubMed] [Google Scholar]
- Chana JS, Grover R, Wilson GD, Hudson DA, Forders M, Sanders R, Grobbelaar AO. The prognostic importance of c-myc oncogene expression in head and neck melanoma. Ann. Plast. Surg. 2001;47:172–177. doi: 10.1097/00000637-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Chana JS, Wilson GD, Cree IA, Alexander RA, Myatt N, Neale M, Foss AJ, Hungerford JL. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br. J. Ophthalmol. 1999;83:110–114. doi: 10.1136/bjo.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Kampalath B, Schultz C, Bunyi-Teopengco E, Logan B, Eshoa C, Dincer AP, Perkins SL. Expression of p53, c-Myc, or Bcl-6 suggests a poor prognosis in primary central nervous system diffuse large B-cell lymphoma among immunocompetent individuals. Arch. Pathol. Lab Med. 2003;127:208–212. doi: 10.5858/2003-127-208-EOPMOB. [DOI] [PubMed] [Google Scholar]
- Chuang LY, Hung WC, Yang ML, Chang CC, Tsai JF. Urinary epidermal growth factor receptor-binding growth factors in patients with cancers of the digestive tract. Clin. Biochem. 1994;27:485–489. doi: 10.1016/0009-9120(94)00053-x. [DOI] [PubMed] [Google Scholar]
- Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- Cubilla AL, Fitzgerald PJ. Classification of pancreatic cancer (nonendocrine) Mayo Clin. Proc. 1979;54:449–458. [PubMed] [Google Scholar]
- de la Cova CC, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Donaldson TD, Duronio RJ. Cancer cell biology: Myc wins the competition. Curr. Biol. 2004;14:R425–R427. doi: 10.1016/j.cub.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Donzelli M, Bernardi R, Negri C, Prosperi E, Padovan L, Lavialle C, Brison O, Scovassi AI. Apoptosis-prone phenotype of human colon carcinoma cells with a high level amplification of the c-myc gene. Oncogene. 1999;18:439–448. doi: 10.1038/sj.onc.1202309. [DOI] [PubMed] [Google Scholar]
- Estival A, Clerc P, Vaysse N, Tam JP, Clemente F. Decreased expression of transforming growth factor alpha during differentiation of human pancreatic cancer cells. Gastroenterology. 1992;103:1851–1859. doi: 10.1016/0016-5085(92)91444-9. [DOI] [PubMed] [Google Scholar]
- Farber E. Cell proliferation as a major risk factor for cancer: a concept of doubtful validity. Cancer Res. 1995;55:3759–3762. [PubMed] [Google Scholar]
- Farber E. Cell proliferation is not a major risk factor for cancer. Mod. Pathol. 1996a;9:606. [PubMed] [Google Scholar]
- Farber E. The step-by-step development of epithelial cancer: from phenotype to genotype. Adv. Cancer Res. 1996b;70:21–48. doi: 10.1016/s0065-230x(08)60870-2. [DOI] [PubMed] [Google Scholar]
- Friess H, Kleeff J, Korc M, Buchler MW. Molecular aspects of pancreatic cancer and future perspectives. Dig. Surg. 1999;16:281–290. doi: 10.1159/000018737. [DOI] [PubMed] [Google Scholar]
- Gallant P. Myc, cell competition, and compensatory proliferation. Cancer Res. 2005;65:6485–6487. doi: 10.1158/0008-5472.CAN-05-1101. [DOI] [PubMed] [Google Scholar]
- Garbe AI, Vermeer B, Gamrekelashvili J, von WR, Greten FR, Westendorf AM, Buer J, Schmid RM, Manns MP, Korangy F, Greten TF. Genetically induced pancreatic adenocarcinoma is highly immunogenic and causes spontaneous tumor-specific immune responses. Cancer Res. 2006;66:508–516. doi: 10.1158/0008-5472.CAN-05-2383. [DOI] [PubMed] [Google Scholar]
- Giraud AS. X. Trefoil peptide and EGF receptor/ligand transgenic mice. Am. J. Physiol Gastrointest. Liver Physiol. 2000;278:G501–G506. doi: 10.1152/ajpgi.2000.278.4.G501. [DOI] [PubMed] [Google Scholar]
- Greten FR, Wagner M, Weber CK, Zechner U, Adler G, Schmid RM. TGF alpha transgenic mice. A model of pancreatic cancer development. Pancreatology. 2001;1:363–368. doi: 10.1159/000055835. [DOI] [PubMed] [Google Scholar]
- Grippo PJ, Sandgren EP. Modeling pancreatic cancer in animals to address specific hypotheses. Methods Mol. Med. 2004;103:217–244. doi: 10.1385/1-59259-780-7:217. [DOI] [PubMed] [Google Scholar]
- Grover R, Pacifico MD, Wilson GD, Sanders R. Use of oncogene expression as an independent prognostic marker for primary melanoma. Ann. Plast. Surg. 2003;50:183–187. doi: 10.1097/01.SAP.0000032308.89737.EA. [DOI] [PubMed] [Google Scholar]
- Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Holzmann K, Kohlhammer H, Schwaenen C, Wessendorf S, Kestler HA, Schwoerer A, Rau B, Radlwimmer B, Dohner H, Lichter P, Gress T, Bentz M. Genomic DNA-chip hybridization reveals a higher incidence of genomic amplifications in pancreatic cancer than conventional comparative genomic hybridization and leads to the identification of novel candidate genes. Cancer Res. 2004;64:4428–4433. doi: 10.1158/0008-5472.CAN-04-0431. [DOI] [PubMed] [Google Scholar]
- Hormi K, Lehy T. Developmental expression of transforming growth factor-alpha and epidermal growth factor receptor proteins in the human pancreas and digestive tract. Cell Tissue Res. 1994;278:439–450. doi: 10.1007/BF00331362. [DOI] [PubMed] [Google Scholar]
- Hotz HG, Hines OJ, Foitzik T, Reber HA. Animal models of exocrine pancreatic cancer. Int. J. Colorectal Dis. 2000;15:136–143. doi: 10.1007/s003840000229. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Bores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Wilentz RE, Maitra A. Identification and analysis of precursors to invasive pancreatic cancer. Methods Mol. Med. 2004;103:1–14. doi: 10.1385/1-59259-780-7:001. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Bores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, Kloppel G, Lauwers GY, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Perez-Gallego L, Redston M, Tuveson DA. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006a;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Rustgi AK, Brentnall TA, Tempero MA, Wright CV, Tuveson DA. Pancreatic cancer in mice and man: the Penn Workshop 2004. Cancer Res. 2006b;66:14–17. doi: 10.1158/0008-5472.CAN-05-3914. [DOI] [PubMed] [Google Scholar]
- Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- Jansen-Durr P, Meichle A, Steiner P, Pagano M, Finke K, Botz J, Wessbecher J, Draetta G, Eilers M. Differential modulation of cyclin gene expression by MYC. Proc. Natl. Acad. Sci. USA. 1993;90:3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Friess H, Berberat PO, Martignoni ME, Z’graggen K, Buchler MW. Pancreatic cancer--new aspects of molecular biology research. Swiss. Surg. 2000;6:231–234. doi: 10.1024/1023-9332.6.5.231. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Tsutsumi M, Tsujiuchi T. Mechanistic analysis of pancreatic ductal carcinogenesis in hamsters. Pancreas. 1998;16:300–306. doi: 10.1097/00006676-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Korc M. Growth factors and pancreatic cancer. Int. J. Pancreatol. 1991;9:87–91. doi: 10.1007/BF02925583. [DOI] [PubMed] [Google Scholar]
- Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J. Clin. Invest. 1992;90:1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang C, Xiao Y, Liu X, Stringfield TM, Zhang S, Wang Z, Chen Y. In vivo disruption of TGF-beta signaling by Smad7 leads to premalignant ductal lesions in the pancreas. Proc. Natl. Acad. Sci. USA. 2006;103:1858–1863. doi: 10.1073/pnas.0508977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullenberg B, Jansen C, Fredang N, Ohlsson B, Axelson J. Transforming growth factor alpha (TGF-alpha) increases cell number in a human pancreatic cancer cell line but not in normal mouse pancreas. Int. J. Pancreatol. 2000;28:199–205. doi: 10.1385/IJGC:28:3:199. [DOI] [PubMed] [Google Scholar]
- Laconi S, Pani P, Pillai S, Pasciu D, Sarma DS, Laconi E. A growth-constrained environment drives tumor progression invivo. Proc. Natl. Acad. Sci. USA. 2001;98:7806–7811. doi: 10.1073/pnas.131210498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach SD. Mouse models of pancreatic cancer: the fur is finally flying! Cancer Cell. 2004;5:7–11. doi: 10.1016/s1535-6108(03)00337-4. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Coni P, Simbula G, Zedda I, Columbano A. Compensatory regeneration, mitogen-induced liver growth, and multistage chemical carcinogenesis. Environ. Health Perspect. 1993;101(Suppl 5):163–168. doi: 10.1289/ehp.93101s5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003;17:3127–3138. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. c-Myc in breast cancer. Endocr. Relat. Cancer. 2000;7:143–164. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. Cell death in MMTV-c-myc transgenic mouse mammary tumors may not be typical apoptosis. Lab. Invest. 2003;83:1437–1449. doi: 10.1097/01.lab.0000090153.13977.ae. [DOI] [PubMed] [Google Scholar]
- Liao DJ, Natarajan G, Deming SL, Jamerson MH, Johnson M, Chepko G, Dickson RB. Cell cycle basis for the onset and progression of c-Myc-induced, TGFalpha-enhanced mouse mammary gland carcinogenesis. Oncogene. 2000;19:1307–1317. doi: 10.1038/sj.onc.1203430. [DOI] [PubMed] [Google Scholar]
- Liao DJ, Wang Y, Wu J-S, Adsay NV, Grignon D, Khanni F, Sarkar FH. Characterization of pancreatic lesions from MT-tgf-alpha, Ela-myc, and MT-tgf-alpha/Ela-myc signle and double transgenic mice. J. Carcinogenesis. 2006;5:1–14. doi: 10.1186/1477-3163-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Levens D. Making myc. Curr. Top. Microbiol. Immunol. 2006;302:1–32. doi: 10.1007/3-540-32952-8_1. [DOI] [PubMed] [Google Scholar]
- Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am. J. Pathol. 1998;153:263–269. doi: 10.1016/S0002-9440(10)65567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy AM. Transgenic models of pancreatic cancer. Int. J. Gastrointest. Cancer. 2003;33:71–78. doi: 10.1385/IJGC:33:1:71. [DOI] [PubMed] [Google Scholar]
- Mahlamaki EH, Barlund M, Tanner M, Gorunova L, Hoglund M, Karhu R, Kallioniemi A. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell. 2005;8:171–172. doi: 10.1016/j.ccr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best. Pract. Res. Clin. Gastroenterol. 2006;20:211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Marhin WW, Hei YJ, Chen S, Jiang Z, Gallie BL, Phillips RA, Penn LZ. Loss of Rb and Myc activation co-operate to suppress cyclin D1 and contribute to transformation. Oncogene. 1996;12:43–52. [PubMed] [Google Scholar]
- Miettinen PJ, Heikinheimo K. Transforming growth factor-alpha (TGF-alpha) and insulin gene expression in human fetal pancreas. Development. 1992;114:833–840. doi: 10.1242/dev.114.4.833. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, Yamasaki Y, Sandgren EP, Kawaguchi Y, Wright CV, Fujitani Y. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Fukumoto M, Bonavida B, Yoshida O. Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer. 1994;74:2546–2554. doi: 10.1002/1097-0142(19941101)74:9<2546::aid-cncr2820740924>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. cMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Morita Y, Moriai T, Takiyama Y, Makino I. Establishment and characterization of a new hamster pancreatic cancer cell line: the biological activity and the binding characteristics of EGF or TGF-alpha. Int. J. Pancreatol. 1998;23:41–50. doi: 10.1007/BF02787502. [DOI] [PubMed] [Google Scholar]
- Moskal TL, Huang S, Ellis LM, Fritsche HA, Jr., Chakrabarty S. Serum levels of transforming growth factor alpha in gastrointestinal cancer patients. Cancer Epidemiol. Biomarkers Prev. 1995;4:127–131. [PubMed] [Google Scholar]
- Muller-Decker K, Furstenberger G, Annan N, Kucher D, Pohl-Arnold A, Steinbauer B, Esposito I, Chiblak S, Friess H, Schirmacher P, Berger I. Preinvasive duct-derived neoplasms in pancreas of keratin 5-promoter cyclooxygenase-2 transgenic mice. Gastroenterology. 2006;130:2165–2178. doi: 10.1053/j.gastro.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Nagy A, Kozma L, Kiss I, Ember I, Takacs I, Hajdu J, Farid NR. Copy number of cancer genes predict tumor grade and survival of pancreatic cancer patients. Anticancer Res. 2001;21:1321–1325. [PubMed] [Google Scholar]
- Nagy B, Lundan T, Larramendy ML, Aalto Y, Zhu Y, Niini T, Edgren H, Ferrer A, Vilpo J, Elonen E, Vettenranta K, Franssila K, Knuutila S. Abnormal expression of apoptosis-related genes in haematological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br. J. Haematol. 2003;120:434–441. doi: 10.1046/j.1365-2141.2003.04121.x. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Hammer RE, Messing A, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987;238:188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M. Oncogenic co-operation in beta-cell tumorigenesis. Endocr. Relat. Cancer. 2001;8:307–314. doi: 10.1677/erc.0.0080307. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Abouna S, Cheung L, Ifandi V, Zervou S, Khan M. Brief inactivation of c-Myc is not sufficient for sustained regression of c-Myc-induced tumours of pancreatic islets and skin epidermis. BMC Biol. 2004;2:1–14. doi: 10.1186/1741-7007-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp A, Schneider A, Vasrik I, Finke K, Xiong Y, Beach D, Alitalo K, Eilers M. Repression of cyclin D1: a novel function of MYC. Mol. Cell Biol. 1994;14:4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzielli R, Katz S, Barsyte-Lovejoy D, Penn LZ. Cancer therapeutics: targeting the dark side of Myc. Eur. J. Cancer. 2005;41:2485–2501. doi: 10.1016/j.ejca.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Pour PM, Pandey KK, Batra SK. What is the origin of pancreatic adenocarcinoma? Mol. Cancer. 2003;2:1–10. doi: 10.1186/1476-4598-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour PM, Standop J, Batra SK. Are islet cells the gatekeepers of the pancreas? Pancreatology. 2002;2:440–448. doi: 10.1159/000064718. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Pinkert CA, Ornitz DM, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987;48:1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Luetteke NC, Qiu TH, Palmiter RD, Brinster RL, Lee DC. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol. Cell Biol. 1993;13:320–330. doi: 10.1128/mcb.13.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc. Natl. Acad. Sci. USA. 1991;88:93–97. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer BK, Terhune PG, Longnecker DS. Pancreatic carcinomas of acinar and mixed acinar/ductal phenotypes in Ela-1-myc transgenic mice do not contain c-K-ras mutations. Am. J. Pathol. 1994;145:696–701. [PMC free article] [PubMed] [Google Scholar]
- Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod. Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J. Clin. Invest. 2002;109:1403–1404. doi: 10.1172/JCI15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RM, Kloppel G, Adler G, Wagner M. Acinar-ductal-carcinoma sequence in transforming growth factor-alpha transgenic mice. Ann. NY Acad. Sci. 1999;880:219–230. doi: 10.1111/j.1749-6632.1999.tb09526.x. [DOI] [PubMed] [Google Scholar]
- Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, Yang Q, Bishop JM, Contag CH, Felsher DW. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Silverman JA, Kuhlmann ET, Zurlo J, Yager JD, Longnecker DS. Expression of c-myc, c-raf-1, and c-Ki-ras in azaserine-induced pancreatic carcinomas and growing pancreas in rats. Mol. Carcinog. 1990;3:379–386. doi: 10.1002/mc.2940030610. [DOI] [PubMed] [Google Scholar]
- Sklar MD, Prochownik EV. Modulation of cis-platinum resistance in Friend erythroleukemia cells by c-myc. Cancer Res. 1991;51:2118–2123. [PubMed] [Google Scholar]
- Smith DR, Goh HS. Overexpression of the c-myc proto-oncogene in colorectal carcinoma is associated with a reduced mortality that is abrogated by point mutation of the p53 tumor suppressor gene. Clin. Cancer Res. 1996;2:1049–1053. [PubMed] [Google Scholar]
- Soh LT, Heng D, Lee IW, Ho TH, Hui KM. The relevance of oncogenes as prognostic markers in cervical cancer. Int. J. Gynecol. Cancer. 2002;12:465–474. doi: 10.1046/j.1525-1438.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Alexander J, Lomakin I, Dayal Y. Immunohistochemical expression of transforming growth factor alpha and epidermal growth factor receptor in pancreatic endocrine tumors. Hum. Pathol. 2001;32:1184–1189. doi: 10.1053/hupa.2001.28959. [DOI] [PubMed] [Google Scholar]
- Standop J, Schneider MB, Ulrich A, Pour PM. Experimental animal models in pancreatic carcinogenesis: lessons for human pancreatic cancer. Dig. Dis. 2001;19:24–31. doi: 10.1159/000050650. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Factor VM, Snyderwine EG. Transgenic mouse models in carcinogenesis research and testing. Toxicol. Lett. 2000;112-113:553–555. doi: 10.1016/s0378-4274(99)00224-6. [DOI] [PubMed] [Google Scholar]
- Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, Inokuchi S, Makuuchi H. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int. J. Mol. Med. 2003;11:305–309. [PubMed] [Google Scholar]
- Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb. Symp. Quant. Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R, Pin CL, Mitin NY, Taparowsky EJ, Gimotty PA, Hruban RH, Jacks T, Konieczny SF. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- Ueda S, Takasuka N, Takeshita F, Naito A, Matsuoka Y, Iigo M, Fukamachi K, Alexander DB, Moore MA, Saito I, Ochiya T, Tsuda H. Ductal origin of pancreatic adenocarcinomas induced by conditional activation of a human Ha-ras oncogene in rat pancreas. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl090. (In Press) [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Pascall JC, Brown KD. Tissue distribution of mRNA for heparin-binding epidermal growth factor. Biochem. J. 1992;287(Pt 3):681–684. doi: 10.1042/bj2870681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan TJ, James PS, Pascall JC, Brown KD. Molecular cloning and tissue distribution of pig transforming growth factor alpha. Biochem. J. 1993;296(Pt 3):837–842. doi: 10.1042/bj2960837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayalakshmi N, Selvaluxmi G, Mahji U, Rajkumar T. C-myc oncoprotein expression and prognosis in patients with carcinoma of the cervix: an immunohistochemical study. Eur. J. Gynaecol. Oncol. 2002;23:135–138. [PubMed] [Google Scholar]
- Visser CJ, Bruggink AH, Korc M, Kobrin MS, de Weger RA, Seifert-Bock I, van Blokland WT, van Garderen-Hoetmer A, Woutersen RA. Overexpression of transforming growth factor-alpha and epidermal growth factor receptor, but not epidermal growth factor, in exocrine pancreatic tumours in hamsters. Carcinogenesis. 1996;17:779–785. doi: 10.1093/carcin/17.4.779. [DOI] [PubMed] [Google Scholar]
- Visser CJ, Woutersen RA, Bruggink AH, van Garderen-Hoetmer A, Seifert-Bock I, Tilanus MG, de Weger RA. Transforming growth factor-alpha and epidermal growth factor expression in the exocrine pancreas of azaserine-treated rats: modulation by cholecystokinin or a low fat, high fiber (caloric restricted) diet. Carcinogenesis. 1995;16:2075–2082. doi: 10.1093/carcin/16.9.2075. [DOI] [PubMed] [Google Scholar]
- Vora HH, Shah NG, Patel DD, Trivedi TI, Chikhlikar PR. Prognostic significance of biomarkers in squamous cell carcinoma of the tongue: multivariate analysis. J. Surg. Oncol. 2003;82:34–50. doi: 10.1002/jso.10183. [DOI] [PubMed] [Google Scholar]
- Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254–1262. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- Wagner M, Greten FR, Weber CK, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid RM. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Xiong HQ, Abbruzzese JL, Xie K. Experimental animal models of pancreatic carcinogenesis and metastasis. Int. J. Gastrointest. Cancer. 2003;33:43–60. doi: 10.1385/IJGC:33:1:43. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sakamoto H, Taira M, Nishimura S, Shimosato Y, Terada M, Sugimura T. Amplifications of both c-Ki-ras with a point mutation and c-myc in a primary pancreatic cancer and its metastatic tumors in lymph nodes. Jpn. J. Cancer Res. 1986;77:370–375. [PubMed] [Google Scholar]
- Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- Yan YX, Nakagawa H, Lee MH, Rustgi AK. Transforming growth factor-alpha enhances cyclin D1 transcription through the binding of early growth response protein to a cis-regulatory element in the cyclin D1 promoter. J. Biol. Chem. 1997;272:33181–33190. doi: 10.1074/jbc.272.52.33181. [DOI] [PubMed] [Google Scholar]
- Yu Y, Dong W, Li X, Yu E, Zhou X, Li S. Significance of c-Myc and Bcl-2 protein expression in nasopharyngeal carcinoma. Arch. Otolaryngol. Head Neck Surg. 2003;129:1322–1326. doi: 10.1001/archotol.129.12.1322. [DOI] [PubMed] [Google Scholar]