Abstract
Purpose
To report a case of sequential bilateral central retinal vein occlusions in a cystic fibrosis patient with hyperhomocysteinemia and hypergamma-globulinemia over 6 years of follow up.
Methods
Observational case report of one patient.
Results
A 31 year-old male with a history of cystic fibrosis presented with a central retinal vein occlusion (CRVO) in his left eye, followed by a CRVO in his right eye 4 years later. His medical workup was significant for elevated levels of homocysteine and gamma-globulins, which coincided with initiation of intravenous immunoglobulin (IVIG) proceeding his second CRVO.
Conclusions
We describe a case of sequential bilateral central retinal vein occlusions in a cystic fibrosis patient with hyperhomocysteinemia and hypergamma-globulinemia over 6 years of follow up and discuss the important role of these risk factors in retinal venous occlusive disease.
Keywords: cystic fibrosis, central retinal vein occlusion, homocysteine, gamma-globulin, IVIG
We report a case of a 31 year-old male with cystic fibrosis (CF) who presented with a central retinal vein occlusion (CRVO) and 4 years later developed a contralateral CRVO. Workup for an underlying cause revealed elevated levels of homocysteine and gamma-globulins.
Case Report
A 31-year-old male with a history of CF presented in May 2004 complaining of blurry spots and floaters in his left eye for 3 weeks. His past medical history included extensive complications from cystic fibrosis: bronchiectasis, chronic pulmonary pseudomonas infections, allergic bronchopulmonary aspergillosis, bronchiolitis obliterans syndrome, Barrett’s esophagitis, Clostridium difficile colitis, paroxysmal supraventricular tachycardia, hypertension, and neuropathy. His past surgical history was also extensive, and of major significance he underwent a double lung transplant in July 2004 and a repeat double lung transplant in June 2007. He was on numerous medications including: tacrolimus, prednisone, mycophenolate, esomeprazole, pancrelipase, duloxetine, azithromycin, bactrim, valacyclovir, losartan, amlodipine, carvedilol, and gabapentin.
The day of presentation to our service, his best-corrected visual acuity was 20/20 OD and 20/40−1 OS. Intraocular pressures were 12 mm Hg OU by applanation tonometry. Anterior segments were normal bilaterally. Dilated funduscopic examination was normal OD and showed signs of papillophlebitis OS.
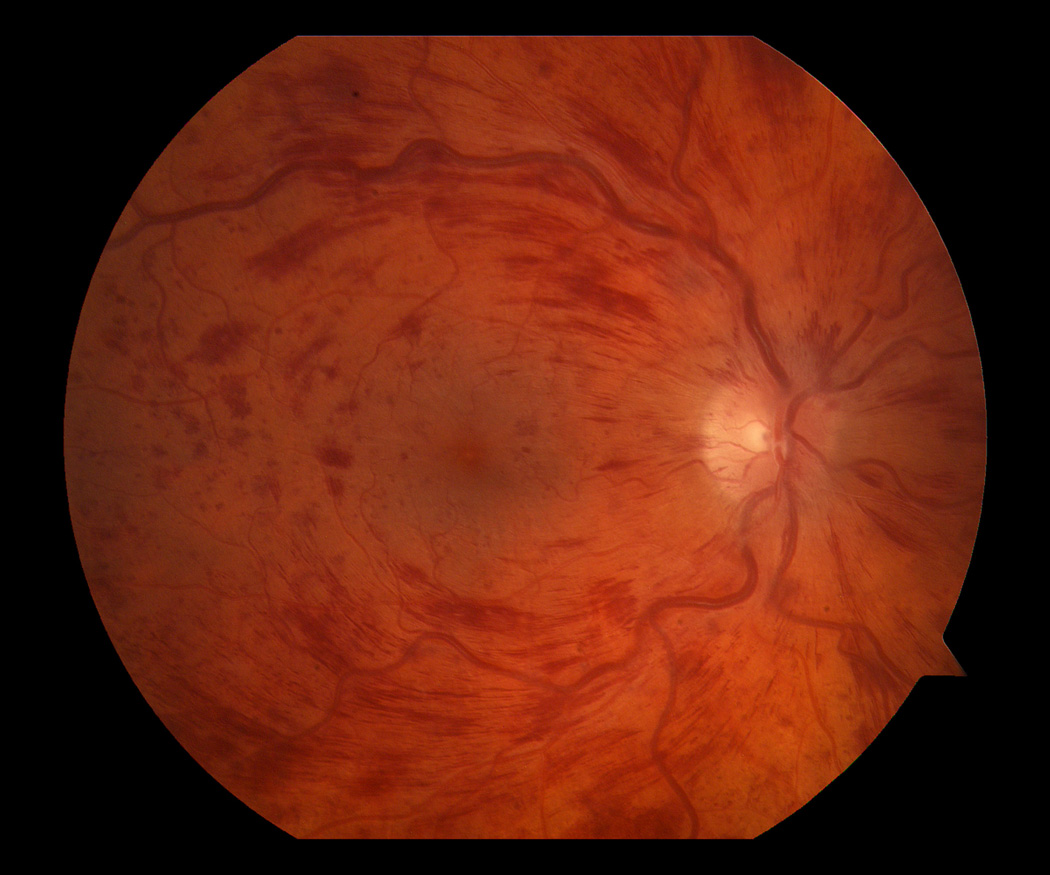
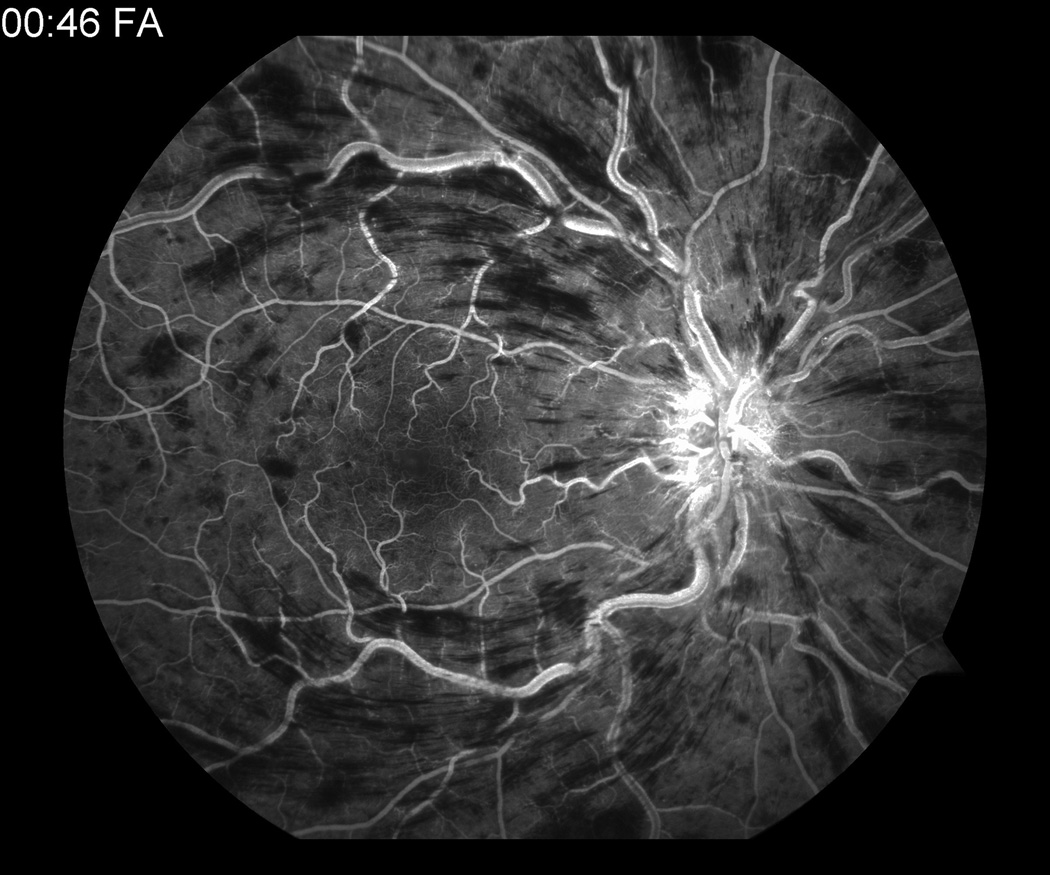
When he returned 3 days later for follow up, visual acuity worsened to counting fingers and the exam was consistent with a CRVO (figure 1). Fluorescein angiography confirmed the diagnosis (figure 2). A workup to find an underlying etiology showed unremarkable hematologic studies (CBC, coagulation profile, protein C and S activity, anti-cardiolipin antibodies, lupus anticoagulant, and anti-thrombin III). FTA-ABS, RPR, ANA, and hemoglobin electrophoresis were negative. Carotid dopplers, cardiac echography, and CT of the orbits were normal. A homocysteine level was not evaluated. The patient did not have any indwelling catheters, was afebrile and did not have symptoms or signs of infection.
Figure 1.
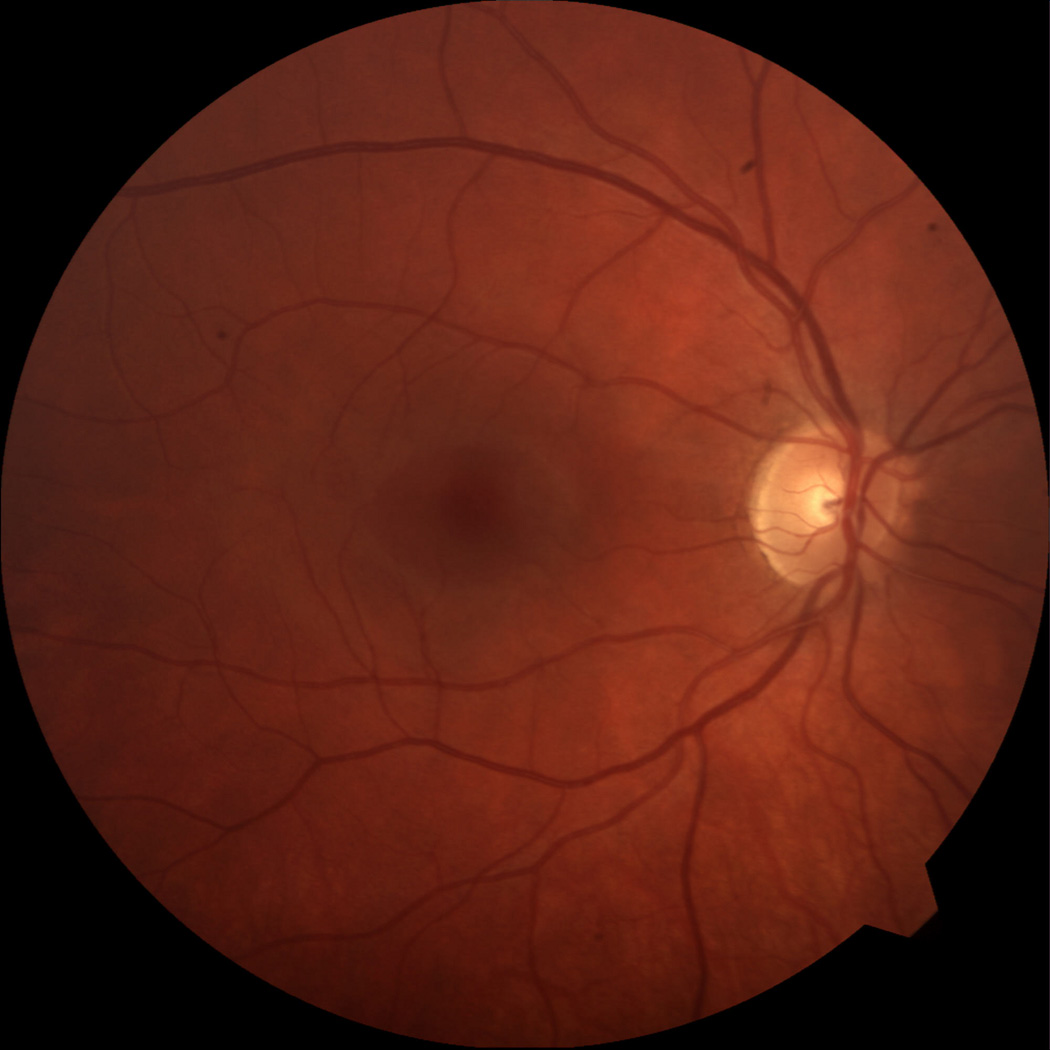
Color photographs from presentation showing (a) normal fundus OD and (b) CRVO OS.
Figure 2.
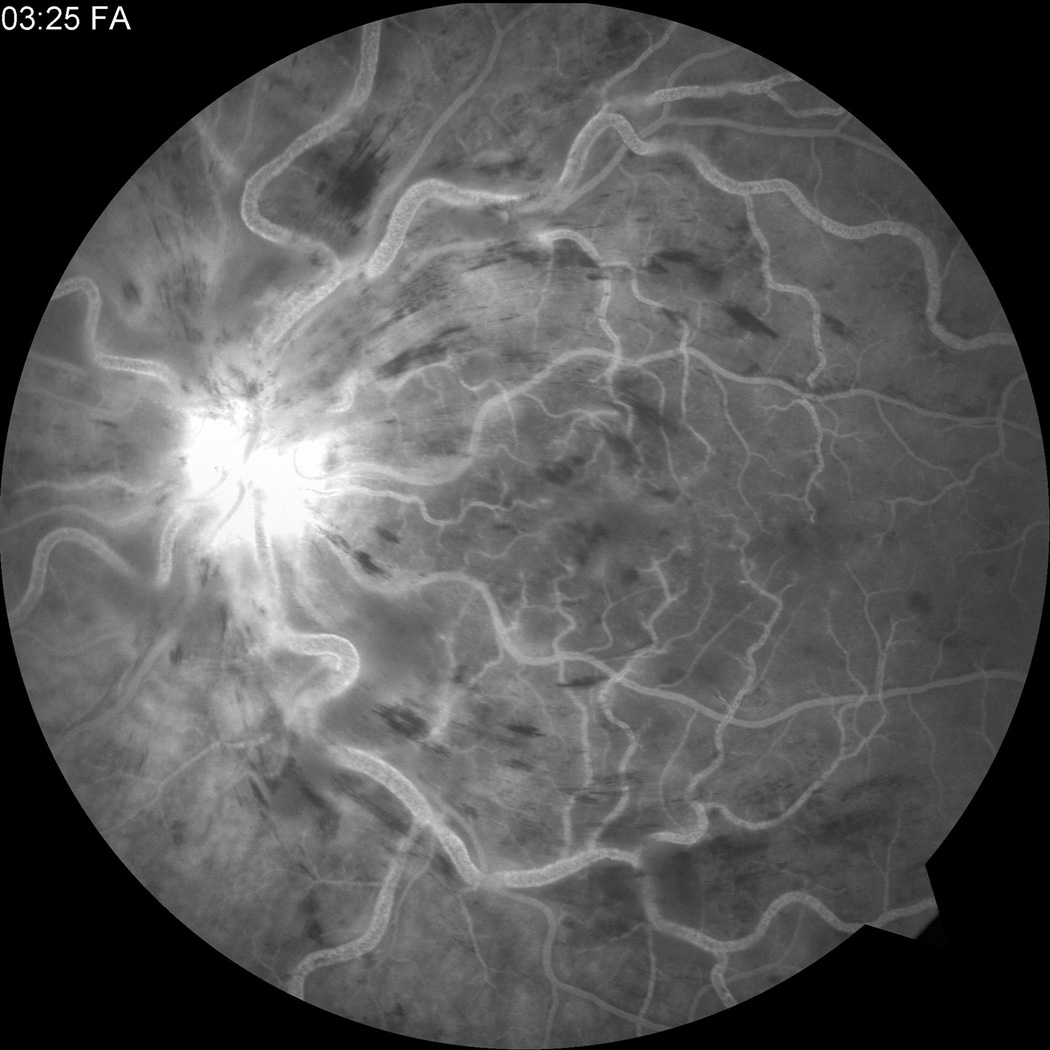
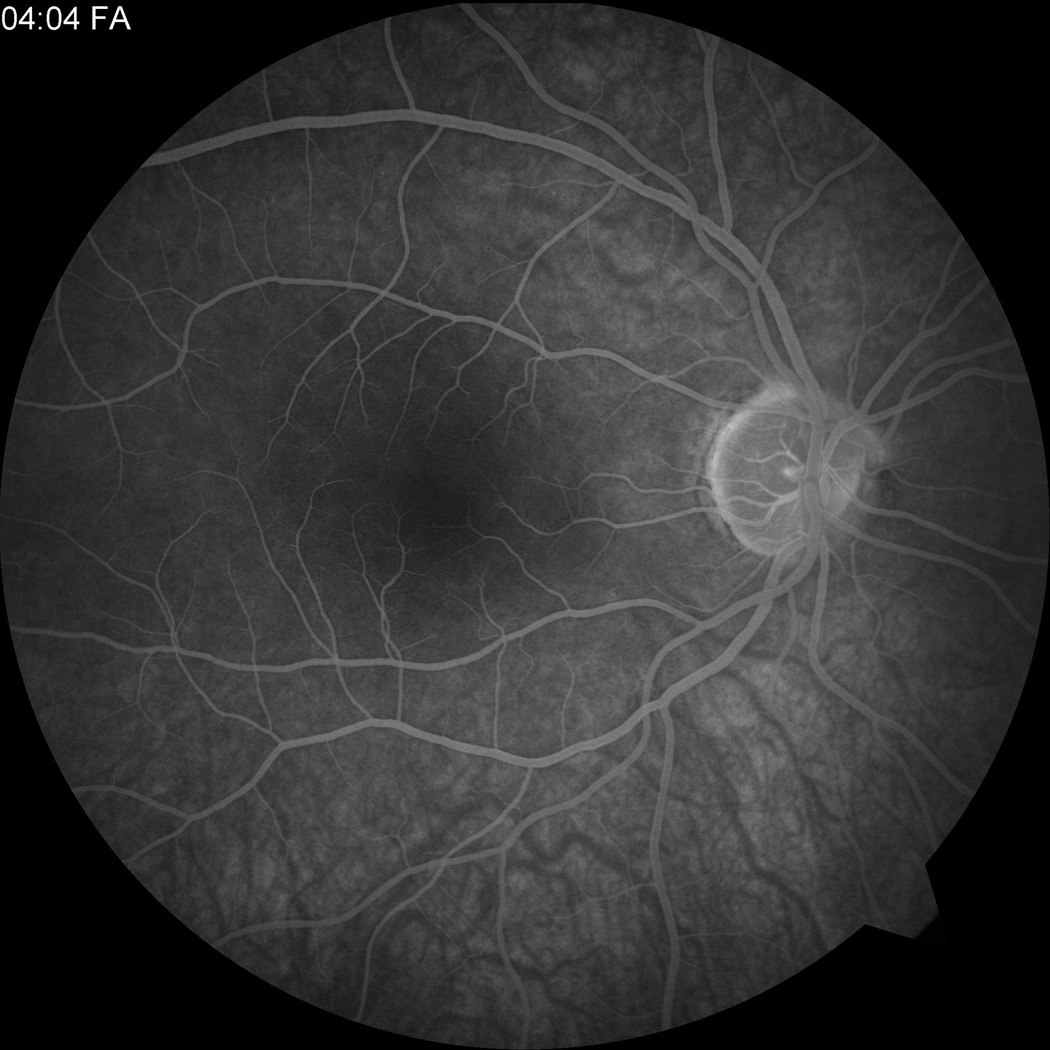
Fluorescein angiogram at presentation showing (a) CRVO OS and (b) normal angiogram OD.
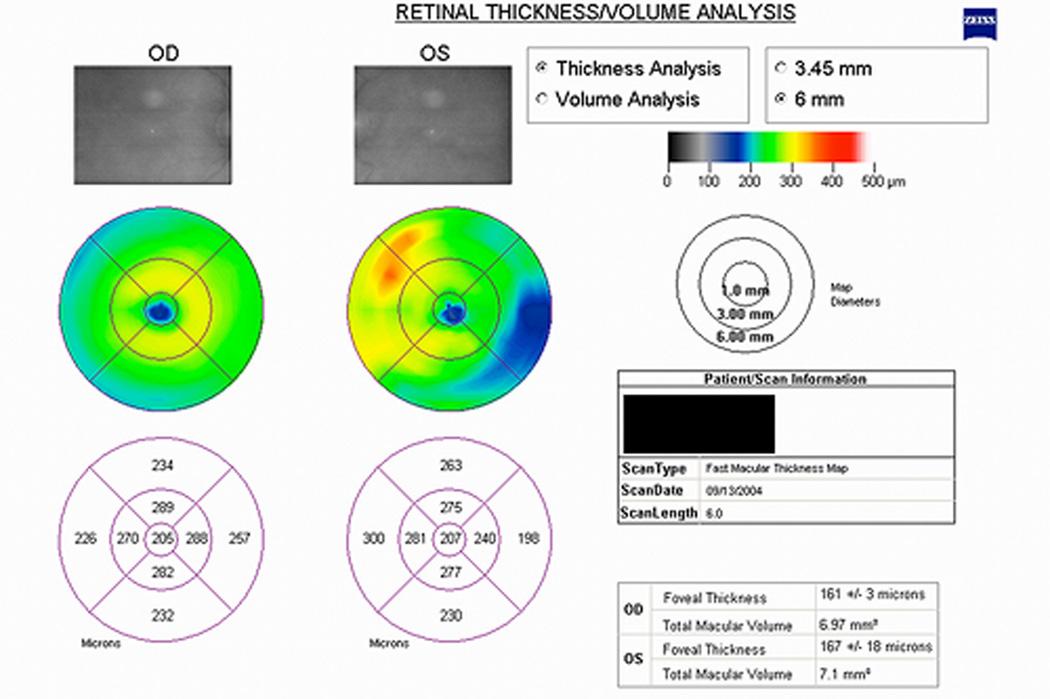
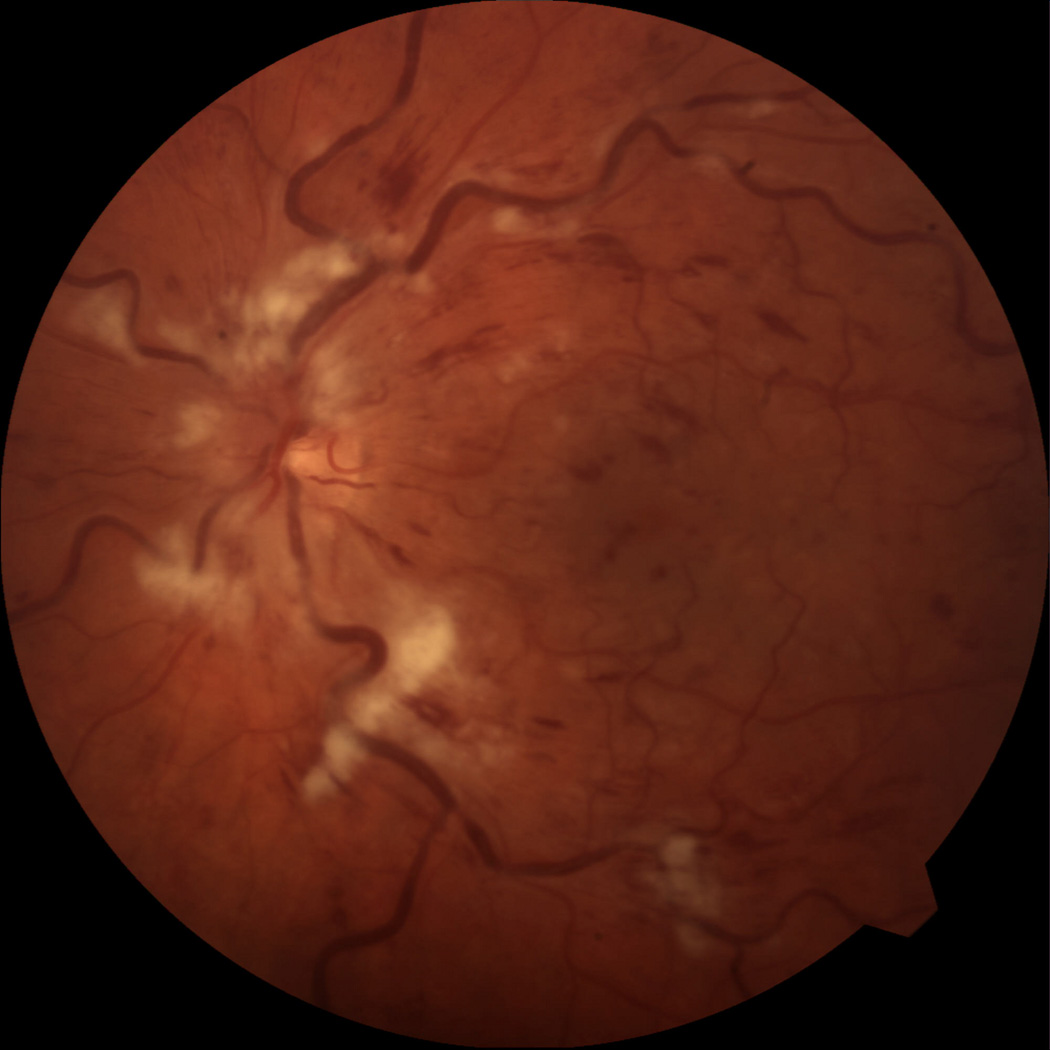
At one-month follow up, the macula remained edematous and an intravitreal triamcinolone injection was administered. By 4 months, the macula was much less edematous with decreased exudation and hemorrhages (figure 3). Visual acuity was 20/100 and OCT confirmed decreased macular edema (figure 4).
Figure 3.
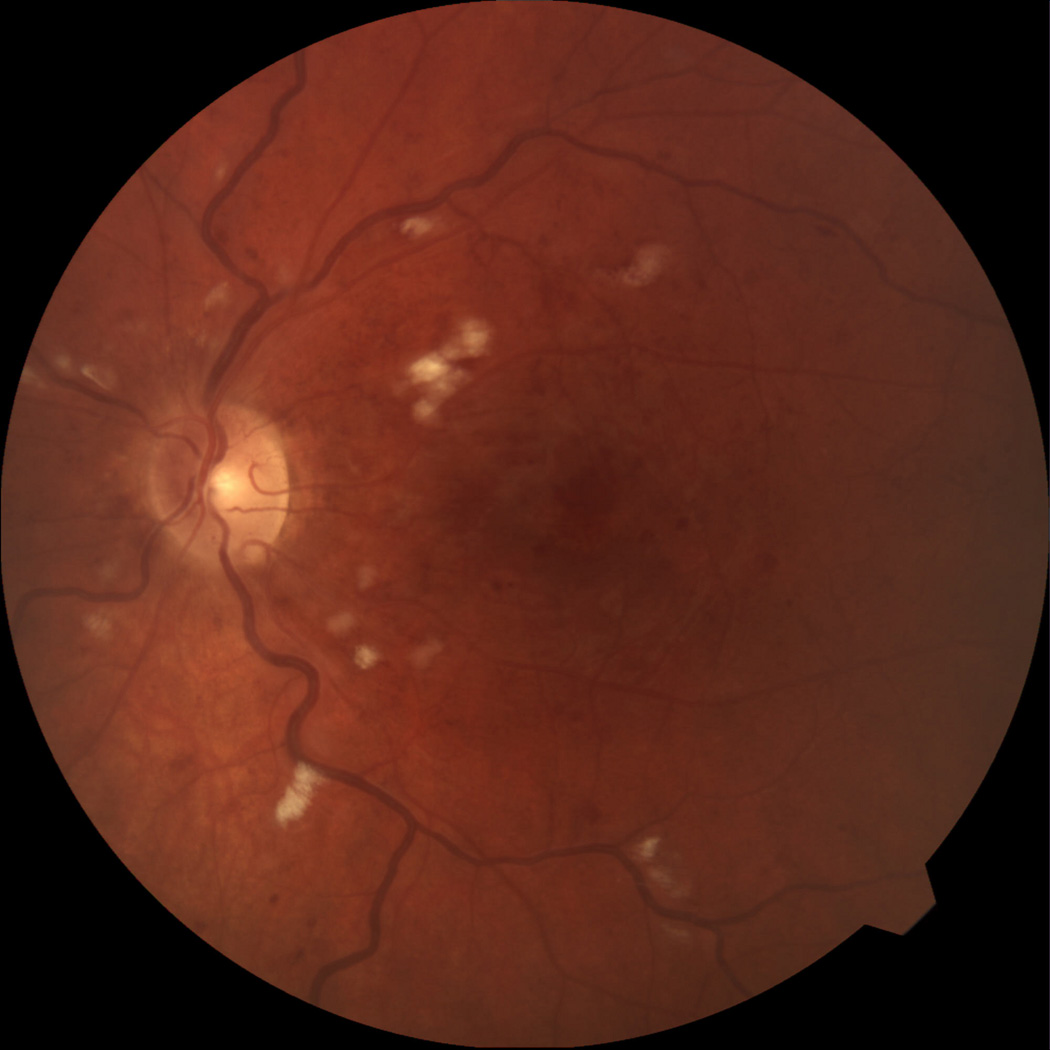
Color photograph at 4 months follow up showing less macular edema, exudates, and hemorrhages.
Figure 4.
Time domain OCT (a) retinal thickness map and (b) horizontal line scan through the fovea OS showing decreased macular edema at 4 months follow up.
By 6 months, visual acuity dropped to 20/200 and the macular edema worsened. He then received a second intravitreal triamcinolone injection. Three weeks later, the macular edema improved but gonioscopy showed neovascularization of the angle. He underwent prompt panretinal laser photocoagulation and the neovascularization regressed. At the 10-month follow up visit, the macular edema worsened although visual acuity was stable at 20/200.
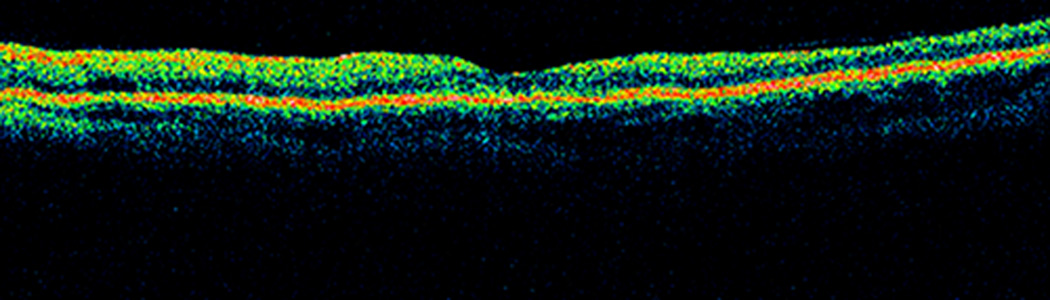
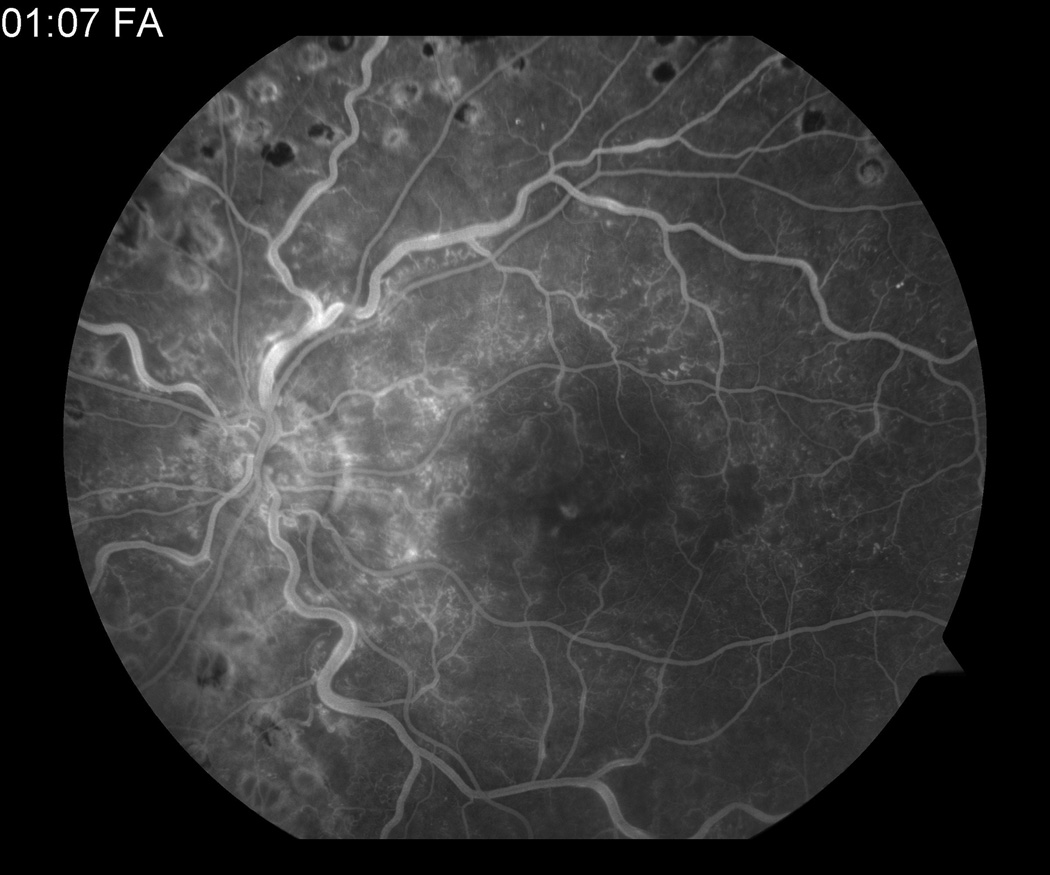
The patient was subsequently lost to follow up over the next 3.5 years. He returned in September 2009 complaining of blurry vision in his previously unaffected right eye. Visual acuity was 20/30 OD with an intraocular pressure of 10 mm Hg. Anterior segment examination was normal. Dilated fundus examination revealed a CRVO OD, confirmed with fluorescein angiography (figure 5). Optical coherence tomography showed macular edema OU, worse in the right eye, and retinal atrophy OS (figure 6).
Figure 5.
(a) Fundus photo OD at the time of presentation of second CRVO. Fluorescein angiogram demonstrating (b) new CRVO OD and (c) old CRVO OS.
Figure 6.
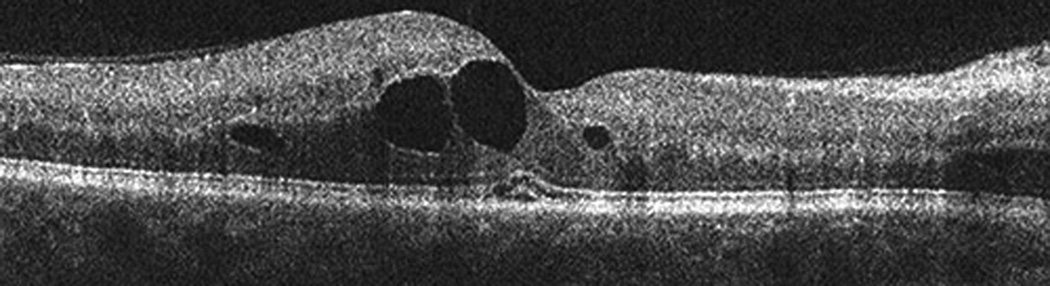
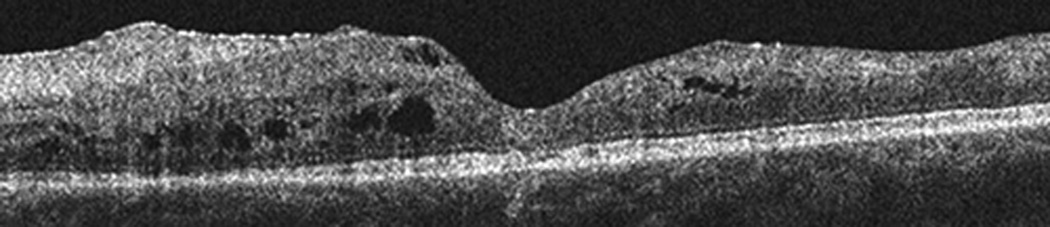
SD-OCT images obtained at the time of presentation of the second CRVO. Horizontal line scan through fovea (a) OD and (b) OS showing cystic edema OU and retinal atrophy OS.
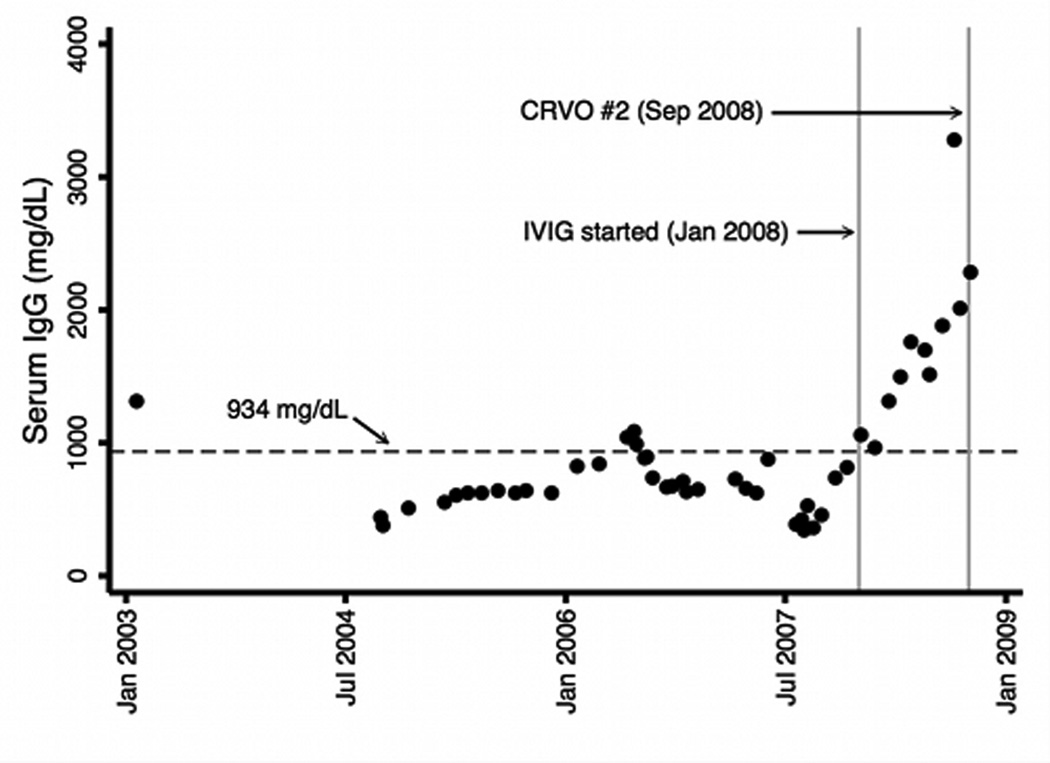
The previous laboratory tests were repeated with the addition of Factor V Leiden and prothrombin gene mutation, all which were unremarkable. The patient had a history of treated hypertension, but review of his medical record over a 6-year period showed he was relatively well controlled (figure 7). He had no indwelling catheters, was afebrile and did not have symptoms or signs of infection. His lipid profile over this period showed normal cholesterol and LDL levels, but elevated triglycerides (figure 7). His homocysteine level was elevated at 24.7 µM/L (lab reference range 5.6–13.4 µM/L). A serum protein electrophoresis showed presence of cryoglobulins and an elevated gamma fraction. Review of the medical record indicated that he started intravenous immunoglobulin (IVIG) 9 months prior to the second CRVO and there was a sharp rise in serum IgG preceding the second CRVO (figure 7).
Figure 7.
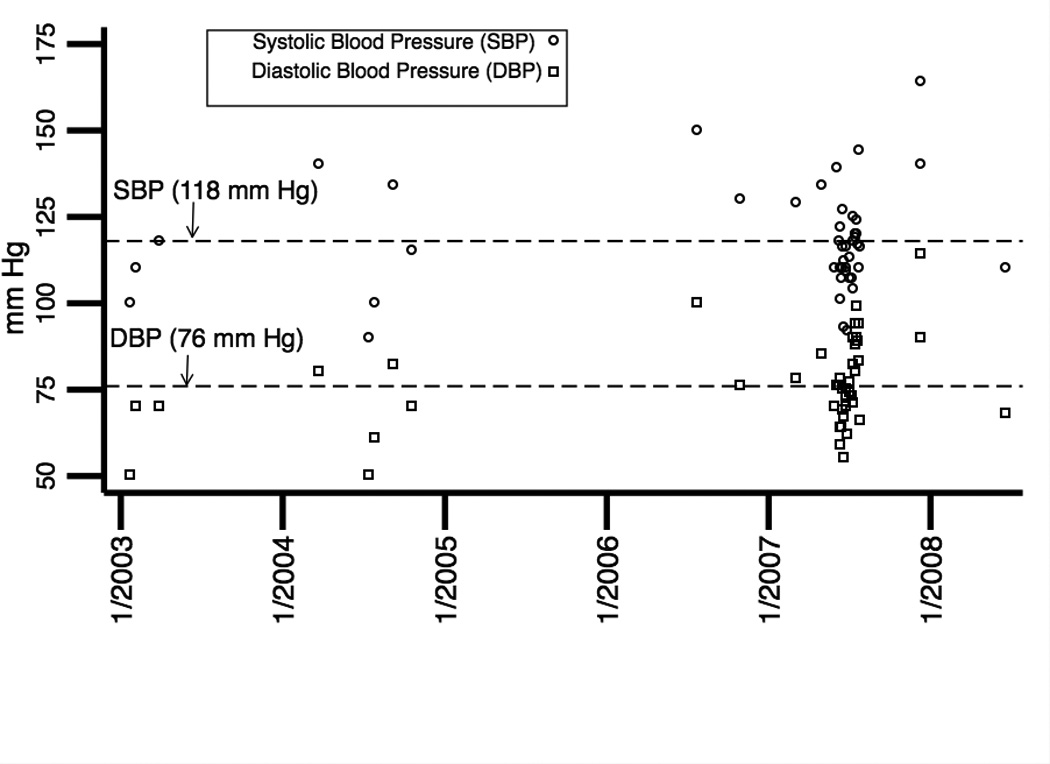
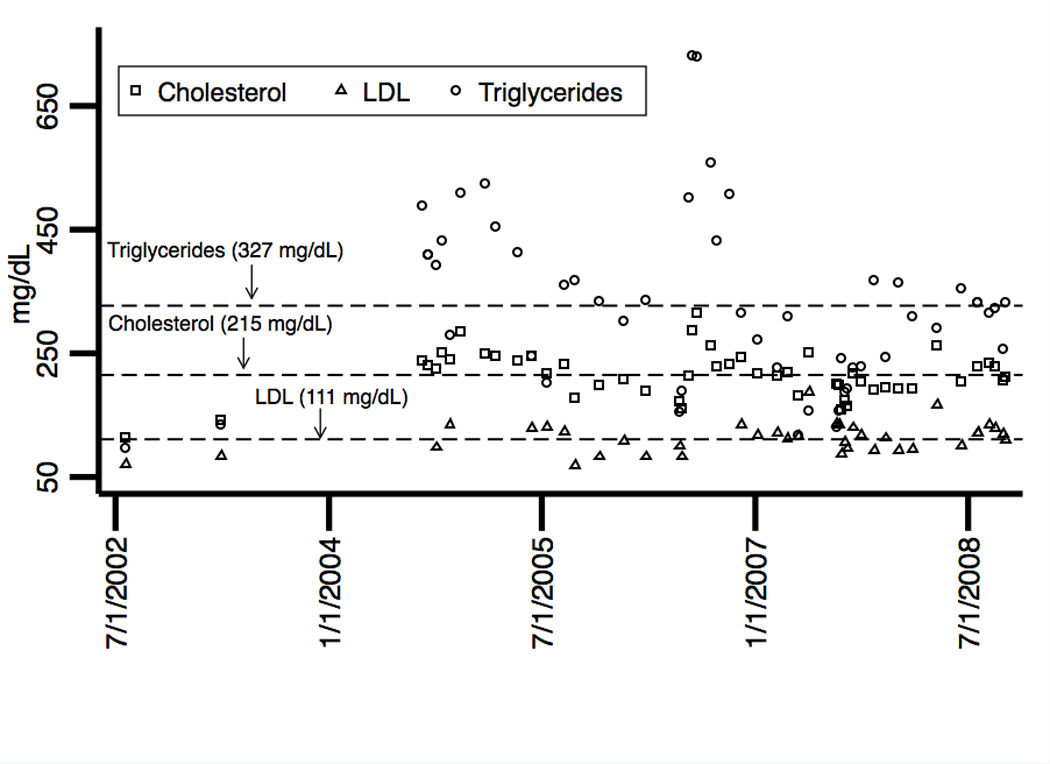
Plotted as a function of time are the patient’s: (a) systolic and diastolic blood pressures, (b) cholesterol, LDL, and triglyceride levels and (c) serum IgG levels. Mean levels over the time periods are indicated by horizontal dashed reference lines. Of significance, triglyceride levels were elevated. Intravenous immunoglobulin (IVIG) was initiated in January 2008 and serum IgG levels sharply increased preceding the second CRVO in September 2008.
His hyperhomocysteinemia was due to dietary causes, and he was treated with folate and vitamin B12. Over the course of the next 16 months, the patient received 10 bevacizumab injections to his right eye with visual acuity improving to 20/20 at the best point, but declining to 20/150 with accumulation of the macular edema. Five weeks after his last visit, he was admitted to the intensive care unit for pseudomonas pneumonia. His clinical course worsened and he expired a few weeks later due to advanced chronic lung rejection and respiratory infection.
Discussion
This case report illustrates an unusual presentation of sequential bilateral CRVOs in a cystic fibrosis patient within a 4-year period. To our knowledge, there is no known association between cystic fibrosis and CRVO. Though our patient did have a history of hypertension, review of his medical record indicated that his hypertension was reasonably well controlled. His workup revealed two possible etiologies for the second CRVO: hyperhomocysteinemia and hypergamma-globulinemia.
Hyperhomocysteinemia is a well-known independent risk factor for vascular disease. Researchers have documented that patients with coronary artery disease (CAD) have elevated levels of homocysteine and that the relative risk of CAD in patients with hyperhomocysteinemia may be 24 times that of controls.1 Further work led to the findings that hyperhomocysteinemia confers an independent risk factor for myocardial infarcation.2 Investigators have found that hyperhomocysteinemia may confer up to a 3.4 fold risk of venous thrombosis.3
Associations between hyperhomocysteinemia and retinal vascular occlusion have been mostly limited to case reports or small series.4–6 In 2000, Vine published a case-control series of 74 patients who had unilateral or bilateral CRVOs.7 He found that the odds ratio for a CRVO in subjects with a homocysteine level greater than the 95th percentile compared with those less than this percentile was 6.53 (p=0.003). Most cases of elevated homocysteine can be readily reversed with folic acid supplementations, stressing the importance of testing. Moreover, studies in children with CF have found that choline phosphoglyceride excretion is increased and is associated with decreased plasma methionine and increased homocysteine and S-adenosylhomocysteine.8,9
The association between hypergamma-globulinemia and CRVO has not been well established in the literature. Oh et al. reported a case of a 17-year-old male with acute lymphocytic leukemia who developed bilateral CRVOs in association with high dose IVIG.10 Although our patient was not treated with IVIG prior to the first CRVO, he developed a contralateral CRVO within 9 months of initiating IVIG. Review of his medical record showed a sharp rise in gamma-globulin levels preceding the second CRVO. While we cannot establish cause and effect based on this single observation, we refer the reader to a manufacturer’s product label (GAMMAGARD, Baxter Healthcare Corporation, Deerfield, Il) which states “thrombotic events have been reported in association with IVIG” and that “baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triaglycerols (triglycerides), or monoclonal gammapathies.” We note that our patient had cyroglobulins and high levels of triglycerides.
In summary, we report an unusual case of a 31 year-old male with CF who developed sequential bilateral CRVOs within 4 years. His workup revealed hyperhomocysteinemia, an important etiology for CRVO, and hypergamma-globulinemia, a possibly important etiology for CRVO that has not been well established. Homocysteine levels may be increased in CF patients due to alterations in the methionine-homocysteine cycle and therefore homocysteine serves as an important risk factor for thrombosis in these patients. The compounding of hyperhomocysteinemia with hypergamma-globulinemia may have further increased the risk of thrombosis in our patient and emphasizes the importance of both risk factors.
Acknowledgments
Financial support: RG was supported by a Foundation Fighting Blindness Alan Laties Career Development Program Award.
Footnotes
Financial disclosure: none.
The authors of this manuscript do not have a proprietary interest.
References
- 1.Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777–1782. doi: 10.1161/01.cir.95.7.1777. [DOI] [PubMed] [Google Scholar]
- 4.Wenzler EM, Rademakers AJ, Boers GH, et al. Hyperhomocysteinemia in retinal artery and retinal vein occlusion. Am J Ophthalmol. 1993;115:162–167. doi: 10.1016/s0002-9394(14)73919-4. [DOI] [PubMed] [Google Scholar]
- 5.Biousse V, Newman NJ, Sternberg P Jr. Retinal vein occlusion and transient monocular visual loss associated with hyperhomocysteinemia. Am J Ophthalmol. 1997;124:257–260. doi: 10.1016/s0002-9394(14)70800-1. [DOI] [PubMed] [Google Scholar]
- 6.de Bruijne EL, Keulen-de Vos GH, Ouwendjik RJ. Ocular venous occlusion and hyperhomocysteinemia. Ann Intern Med. 1999;130:78. doi: 10.7326/0003-4819-130-1-199901050-00026. [DOI] [PubMed] [Google Scholar]
- 7.Vine AK. Hyperhomocysteinemia: a new risk factor for central vein occlusion. Trans Am Ophthalmol Soc. 2000;98:493–503. [PMC free article] [PubMed] [Google Scholar]
- 8.Innis SM, Davidson AGF, Chen A, et al. Increased plasma homocysteine and S-Adenosylhomocysteine and decreased methionine is associated with altered phosphatidylcholine and phosphatidylethanolamine in cystic fibrosis. J Pediatr. 2003;143:351–356. doi: 10.1067/S0022-3476(03)00326-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen AH, Innis SM, Davidson AGF, et al. Phosphatidylcholine and lysophosphatidylcholine excretion is increased in children with cystic fibrosis and is associated with plasma homocysteine, S-adenosylhomocysteine, and S-adenosylmethionine. Am J Clin Nutr. 2005;81:686–691. doi: 10.1093/ajcn/81.3.686. [DOI] [PubMed] [Google Scholar]
- 10.Oh KT, Boldt HC, Danis RP. Iatrogenic central retinal vein occlusion and hyperviscosity associated with high-dose intravenous immunoglobulin administration. Am J Ophthalmol. 1997;124:416–418. doi: 10.1016/s0002-9394(14)70844-x. [DOI] [PubMed] [Google Scholar]