Abstract
Background
Colorectal cancer represents a significant cause of morbidity and mortality, particularly in Appalachia where high mortality from colorectal cancer is more prevalent. Adherence to treatment guidelines leads to improved survival. This paper examines determinants of guideline concordance for colorectal cancer.
Methods
Colorectal cancer patients diagnosed in 2006-2008 from 4 cancer registries (Kentucky, Ohio, Pennsylvania, and North Carolina) were linked to Medicare claims (2005-2009.) Final sample size after exclusions was 2932 stage I - III colon, and 184 stage III rectal cancer patients. The 3 measures of guideline concordance include adjuvant chemotherapy (stage III colon cancer, <80 years), ≥ 12 lymph nodes assessed (resected stage I – III colon cancer), and radiation therapy (stage III rectal cancer, <80 years). Bivariate and multivariate analyses with clinical, sociodemographic, and service provider covariates were estimated for each of the measures.
Results
Rates of chemotherapy, lymph node assessment, and radiation were 62.9%, 66.3%, and 56.0%, respectively. Older patients had lower rates of chemotherapy and radiation. Five comorbidities were significantly associated with lower concordance in the bivariate analyses: myocardial infarction, congestive heart failure, respiratory diseases, and dementia with chemotherapy; and diabetes with adequate lymph node assessment. Patients treated by hospitals with no Commission on Cancer (COC) designation or lower surgical volumes had lower odds of adequate lymph node assessment.
Conclusions
Clinical, sociodemographic, and service provider characteristics are significant determinants of the variation in guideline concordance rates of 3 colorectal cancer measures.
Keywords: cancer, demography, epidemiology, health disparities, Medicare
Appalachian residents have a higher cancer burden and mortality than the rest of the country.1 Factors include more poverty and geographic isolation,2 and fewer health care resources, including most counties classified as health professional shortage areas.3 The result is cancer disparities.4
While access to health care resources is clearly important, so is quality of care. The Commission on Cancer (COC) of the American College of Surgeons, in coordination with the National Quality Forum, has endorsed evidence-based guidelines of colorectal cancer treatment. We know little about the extent of guideline concordant colorectal cancer care in Appalachia. Population-based studies are either uncommon, or focused on different guidelines, making comparisons difficult. Surveillance Epidemiology & End Results (SEER) studies of post-operative care find variability across guidelines and geographic regions.5-6
This study focuses exclusively on Appalachia. Although this region is usually characterized as remote, poor, and elderly, it is actually quite heterogeneous along many dimensions— urbanicity, health care accessibility, poverty, and socioeconomic status. We do not compare Appalachia to non-Appalachian regions; however, what we learn from Appalachia can doubtless apply to other parts of the country, eg, areas of concentrated poverty or in economic decline, areas with large universities/college towns, areas with a high percent of elderly who are aging in place, areas bypassed by the federal highway system, and areas lacking health infrastructure.
In this paper, we examine the clinical, sociodemographic and provider determinants of variation in concordance with widely accepted treatment guidelines for colorectal cancer patients.7-10 Although we expect some variation in clinical practice based on such factors as provider and patient choice, other determinants such as age,11 comorbidities or functional status,11 provider capacity,12 sociodemographics,11,13-16 and location13,16 are expected to influence treatment. We expect the larger and COC-designated hospitals with high surgical volumes, particularly in metropolitan areas, to have higher guideline concordance as well as those with radiation or chemotherapy units. We also expect higher concordance among younger white patients, those treated by physicians with higher volumes of care, and those with fewer comorbid illnesses.
METHODS
Defining the Sample
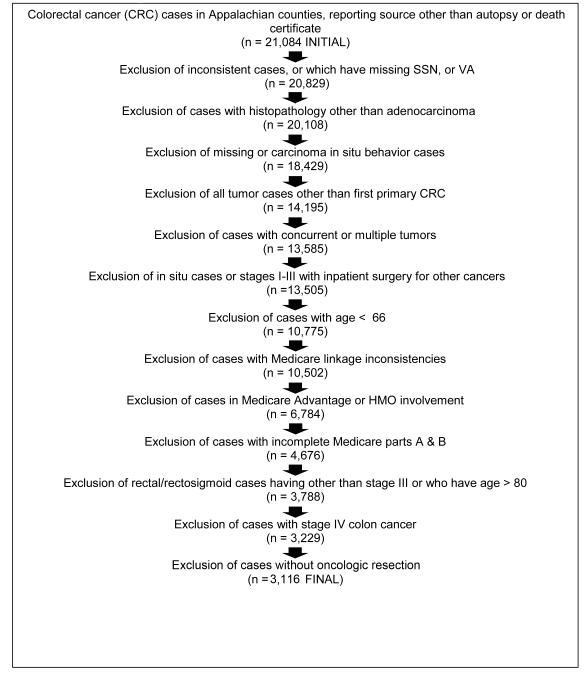
We collected unique patient identifiers from 4 state cancer registries (Kentucky, Ohio, Pennsylvania, and North Carolina) for colorectal cancer patients diagnosed during the 3-year period 2006-2008 from Appalachian counties (see Figure 1). Individual identifiers were submitted to the Centers for Medicare and Medicaid Services (CMS) to obtain Medicare claims for the years 2005-2009. Figure 2 shows the total number of cases and exclusion criteria. The final sample consisted of 2,932 stage I – III colon cancer cases and 184 stage III rectal cancer cases, for an overall total of 3,116 cases.
Figure 1.
Appalachian Counties in Pennsylvania, Kentucky,Ohioand North Carolina
Figure 2.
Study participarts and exclusion criteria, colorectal concer patients living in Appalachian counties in four states, 2006-2008
Measures
We measured guideline concordance across 3 dimensions of colorectal cancer care based on guidelines promulgated by the COC17 and the National Quality Forum:18 (1) adjuvant chemotherapy for stage III colorectal cancer; (2) removal and assessment of ≥ 12 lymph nodes in resected colorectal cancer.19 Additionally, the COC, with the American Society of Clinical Oncology and National Comprehensive Cancer Network, endorsed our third guideline: (3) radiation therapy for stage III rectal cancer receiving surgical resection, either pre- or post-operatively.20 We also considered oncologic resection of stages I-III colon cancer but since over 97.5% of patients received this guideline-concordant surgical resection, we decided against this guideline due to the uniformly high rates of resection and the small variabilities observed.
For this study, we made some restrictions. We focused on patients age < 80 for the chemotherapy and radiation measures because the supportive randomized trials did not include patients of advanced age. Moreover, the guidelines refer to the evaluation for, not receipt of these therapies. Therefore, we assume that patients strong enough to be resected are robust enough for these therapies; ie, receipt is a proxy for evaluation. Finally, our evaluation of the lymph node assessment was restricted to resected colorectal cancer patients (stage I-III) so as to limit our analysis to cases treated with curative intent, since it is impossible to distinguish curative from palliative intent in stage IV patients.
The claims-based ACE-27 was selected as a measure of comorbidity burden21 and is based on 26 different comorbidities, most with 4 levels of severity: none, mild, moderate, and severe. We calculated 2 volume of care variables from all 2008 national Medicare claims associated with facilities in our database. For these variables, we examined all claims with procedure codes for colorectal cancer removal/resection, and summed all non-duplicate unique claims for the facility in which each patient was treated. Similarly, we calculated the volume of each surgical provider using all 2008 national claims with surgery codes associated with the provider for all oncological colorectal resection procedures (colectomies, proctosigmoidectomies, protectomies, colostomies/ileostomies) defined in our study. We categorized these variables into volume quartiles. Patient-level sociodemographic variables (age, gender, race), and clinical variables (stage, tumor size, grade) were derived from registry data. Treatment information was based on registry and claims data combined using a study-specific algorithm (available upon request). Metro/non-metro location was a county-level variable based on patient address at time of diagnosis and the 2003 USDA rural-urban continuum codes.22 Facility characteristics were obtained from the Provider of Services file from CMS, and COC status was derived from the COC website facility locator using 2011 information.23 Surgical provider characteristics, including surgical graduation year, were obtained from 3 sources: Medicare Physician Identification and Eligibility Registry (MPIER)24 and National Provider Identification File, both from CMS, and Internet sources.25-26
Statistical Analysis
We conducted bivariate and multivariate analyses of sociodemographic and service provider characteristics with each of the 3 dimensions of colorectal cancer care using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). We assessed statistically significant differences with each variable and guideline concordance for each dimension of CRC using Chi-square statistics (t-tests for continuous variables), but comparing to Fisher exact tests and Wald tests from a logistic regression with firth correction for borderline cases. For the multivariate models, a multiple imputation procedure incorporated cases with missing data into the analysis, followed by a generalized linear mixed model. For each concordance sample, 5 data sets with different imputations were created using the SAS procedure PROC MI and results for each dataset were then combined using the SAS procedure PROC MIANALYZE according to the formulas used in multiple imputation.27 The Markov chain Monte Carlo (MCMC) method, which assumes multivariate normality, was used to impute all missing values, with the EM algorithm used to find the initial starting values.28 All candidate factors were included as part of the imputation model, including the outcomes in the multivariate models.29,30 Categorical variables were included as dummy variables and in cases where the variable was missing, the imputations were left unrounded.31-32 Selected covariates had missing data only for the lymph node concordance samples (N = 2932). These covariates included gender (0.2% missing), grade (3.7%), specialty (4.1%), log tumor size (8.2%), COC status (5.0 %), radiation treatment offered by facility (5.2%), facility surgical volume (5.2%), physician specialty (4.1%), graduation year (4.5%), and number of beds in facility (5.0%). A generalized linear mixed model33 was then used to examine the associations between concordance and predictors. The model took the form of a logistic regression with random intercept, where the random intercept captured a random effect at the county level and fixed effects included significant predictors from the bivariate analysis. Finally, the SAS procedure PROC GLIMMIX was used to calculate parameter estimates with the default estimation method (Pseudo-likelihood Estimation based on linearization). This estimation method conditions on the random effects, so that the parameter estimates from the model are interpreted as the slopes controlling for potential latent county-level confounders.
Covariate adjusted rates were also calculated (but not reported in tables) where the predicted concordance and non-concordance probabilities from the logistic model are calculated for each patient in the sample using their corresponding covariates and then the expected value over the covariate mix, or equivalently, the average of the predicted probabilities over the sample, is reported.27,28
RESULTS
The descriptive statistics of our colon (n=2932) and rectal cancer (n=184) samples are summarized in Table 1. Our sample contained proportionately more women with colon cancer (58.4%) but less with rectal cancer (43.2%). Both colon and rectal cancer patients were predominately white (97%), with about half (53.5%) living in Pennsylvania; note 30% of these lived in the 7-county Pittsburgh Metropolitan Statistical Area. Among patients without missing tumor size or grade, most colon and rectal tumors were >2 cm in size (88.8% and 89.0%, respectively) with moderately differentiated grade (71.6% and 71.9%, respectively). All 3 stages were well represented in the colon cancer sample, with stage II disease being the modal category. Rectal cancer cases were all stage III according to protocol. Comorbidity burden among these patients was substantial; 88.1% of the colon cancer patients and 80% of the rectal cancer patients had at least some level of burden, and nearly 30% and 20%, respectively, had “severe” comorbidity burden. Of the colon cancer patients, 23.6% received chemotherapy, although not all patients would qualify or be expected to receive such treatment. Almost 60% of the rectal cancer patients received radiation.
Table 1.
Characteristics of Stage I – III Colorectal Cancer Patients Living in Appalachian Counties in 4 States, 2006-2008
| Characteristics | Stages I-III Colon Cancer (n=2932) |
Rectal Cancer (n=184) | ||
|---|---|---|---|---|
|
|
||||
| Frequency | Percent | Frequency | Percent | |
| Age | ||||
| 66-73 | 883 | 30.2 | 97 | 52.7 |
| 74-80 | 962 | 32.8 | 87 | 47.3 |
| 81+ | 1087 | 37.1 | 0 | 0 |
|
| ||||
| Gender1 | ||||
| Male | 1220 | 41.6 | 104 | 56.5 |
| Female | 1712 | 58.4 | 80 | 43.5 |
|
| ||||
| Race/Ethnicity | ||||
| White | 2842 | 96.9 | 178 | 96.7 |
| Black | 60 | 2.0 | 5 | 2.7 |
| Hispanic | 14 | 0.5 | 1 | 0.5 |
| Other | 11 | 0.4 | 0 | 0 |
| Unknown | 5 | 0.2 | 0 | 0 |
|
| ||||
| Registry | ||||
| Kentucky | 347 | 11.8 | 25 | 13.6 |
| North Carolina | 439 | 15.0 | 28 | 15.2 |
| Ohio | 577 | 19.7 | 39 | 21.2 |
| Pennsylvania | 1569 | 53.5 | 92 | 50.0 |
|
| ||||
| Tumor Size (241 missing) | ||||
| <0.5 cm | 29 | 1.1 | 1 | 0.6 |
| 0.5–< 2 cm | 266 | 9.9 | 17 | 10.5 |
| 2–<4 cm | 900 | 33.4 | 63 | 38.9 |
| 4+ | 1496 | 55.6 | 81 | 50.0 |
|
| ||||
| Stage | ||||
| I | 864 | 29.5 | 0 | 0 |
| II | 1186 | 40.5 | 0 | 0 |
| III | 882 | 30.1 | 184 | 100.0 |
|
| ||||
| Grade (109 missing) | ||||
| Well differentiated | 277 | 9.8 | 12 | 6.8 |
| Moderately differentiated | 2020 | 71.6 | 127 | 71.8 |
| Poorly differentiated/undifferentiated | 526 | 18.6 | 38 | 21.5 |
|
| ||||
| Histologic Subtype | ||||
| Adenocarcinoma, arising from a polyp | 438 | 14.9 | 23 | 12.5 |
| Adenocarcinoma, mucinous | 345 | 11.8 | 144 | 78.3 |
| Adenocarcinoma, not otherwise specified | 2123 | 72.4 | 16 | 8.7 |
| Signet Ring Carcinoma | 26 | 0.9 | 1 | 0.5 |
|
| ||||
| Comorbidity | ||||
| None | 350 | 11.9 | 37 | 20.0 |
| Mild | 1208 | 41.2 | 92 | 49.7 |
| Moderate | 507 | 17.3 | 19 | 10.3 |
| Severe | 867 | 29.6 | 36 | 19.5 |
|
| ||||
| Treatment | ||||
| Oncologic resection | 2932 | 100.0 | 184 | 100.0 |
| Chemotherapy | 693 | 23.6 | 153 | 83.2 |
| Radiation | 62 | 2.1 | 108 | 58.7 |
We report the bivariate analyses of sociodemographic and service provider characteristics (Table 2) and comorbidity burden (Table 3) for the dimensions of guideline-concordant colorectal cancer care; the multivariate analyses are summarized in Table 4. A sensitivity analysis using smaller samples and no imputed data generated similar results (available upon request).
Table 2.
Bivariate Association of Sociodemographic or Service Provider Characteristics With Guideline Concordance for Stage I – III Colorectal Cancer Patients Living in Appalachian Counties in 4 States, 2006-2008d
| Sociodemographic and spatial characteristics |
Chemotherapy for stage III colon cancer (n=561) |
›12 lymph nodes stages I – III colon cancer (n=2932) |
Radiation therapy for Stage III rectal cancer (n=184) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| N | %yes | N | %yes | N | %yes | |
| Total | 561 | 62.9 | 2932 | 66.3 | 184 | 56.0 |
| Age | ||||||
| Ȁ66-73 | 277 | 69.7a | 883 | 68.3 | 97 | 66.0a |
| 74-80 | 284 | 56.3 | 962 | 65.3 | 87 | 44.8 |
| 81+ | 0 | -- | 1087 | 65.5 | 0 | -- |
|
| ||||||
| Race/Ethnicitye | ||||||
| White | 539 | 63.6 | 2842 | 66.7a | 178 | 57.8c |
| Black | 19 | 47.4 | 60 | 56.7 | 5 | 0.0 |
| Hispanic | 2 | 50.0 | 14 | 50.0 | 1 | 0.0 |
| Other | 1 | 0.0 | 11 | 54.5 | 0 | -- |
|
| ||||||
| Gender | ||||||
| Male | 263 | 63.9 | 1261 | 61.6a | 104 | 59.6 |
| Female | 298 | 62.1 | 1746 | 66.9 | 80 | 51.3 |
|
| ||||||
| Derived Stage | ||||||
| Stage I | 0 | -- | 846 | 53.6a | 0 | -- |
| Stage II | 0 | -- | 1186 | 70.6 | 0 | -- |
| Stage III | 561 | 62.9 | 882 | 73.1 | 184 | 56.0 |
|
| ||||||
| Grade | ||||||
| Well differentiated | 12 | 59.0 | 277 | 63.9a | 12 | 75.0 |
| Moderately differentiated | 359 | 59.0 | 2020 | 63.0a | 12 | 750 |
| Poorly diff/undifferentiated | 146 | 63.0 | 526 | 74.9 | 38 | 50.0 |
|
| ||||||
| Hospital size | ||||||
| <50 beds | 24 | 58.3 | 94 | 63.8a | 4 | 50.0 |
| 50 – 100 beds | 34 | 61.8 | 204 | 56.4 | 10 | 50.0 |
| 100-200 beds | 10 7 | 57.0 | 583 | 55.6 | 34 | 52.9 |
| 200 - 500 beds | 241 | 69.3 | 1233 | 66.3 | 69 | 60.9 |
| 500+ beds | 131 | 58.0 | 670 | 78.7 | 53 | 56.6 |
|
| ||||||
| Hospital COC designation | ||||||
| Hospital Cancer Pgm | 97 | 66.0 | 457 | 67.6a | 30 | 63.3b |
| Comprehensive Cancer Pgm | 170 | 63.5 | 925 | 73.2 | 49 | 65.3 |
| Teaching Hospital Cancer Pgm | 52 | 71.2 | 314 | 76.8 | 30 | 43.3 |
| NCI designated Cancer Pgm | 18 | 50.0 | 105 | 82.9 | 15 | 80.0 |
| Network Cancer Pgm | 5 | 100.0 | 31 | 74.2 | 1 | 0.0 |
| No designation | 195 | 59.5 | 952 | 53.3 | 45 | 46.7 |
|
| ||||||
| Hospital Ownership | ||||||
| Not-for profit | 473 | 63.2 | 2507 | 66.2 | 153 | 57.5 |
| For profit | 24 | 66.7 | 97 | 65.0 | 3 | 33.3 |
| Government | 40 | 60.0 | 177 | 67.8 | 14 | 57.1 |
|
| ||||||
| Metropolitan location | 289 | 61.9 | 1608 | 69.2* | 96 | 58.3 |
| Nonmetropolitan location | 272 | 64.0 | 1324 | 62.9 | 88 | 53.4 |
|
| ||||||
| Surg facility Volume | ||||||
| Quartile 1 (lowest volume) | 135 | 65.9 | 687 | 56.7a | 29 | 48.3 |
| Quartile 2 | 142 | 62.0 | 690 | 55.5 | 32 | 53.1 |
| Quartile 3 | 132 | 66.7 | 707 | 72.4 | 47 | 61.7 |
| Quartile 4 (highest volume) | 126 | 57.9 | 696 | 80.0 | 61 | 59.0 |
|
| ||||||
| Surg Provider Volume | ||||||
| Quartile 1 (lowest volume) | 131 | 58.8 | 658 | 61.9a | 33 | 48.5 |
| Quartile 2 | 123 | 67.5 | 749 | 65.4 | 37 | 46.0 |
| Quartile 3 | 135 | 67.4 | 663 | 64.4 | 34 | 64.7 |
| Quartile 4 (highest volume) | 150 | 59.3 | 742 | 74.4 | 58 | 56.9 |
|
| ||||||
| Facility Radiation Treatment | ||||||
| Yes | 415 | 62.3 | 2151 | 68.7a | 135 | 59.3 |
| No | 122 | 55.7 | 629 | 57.7 | 35 | 48.6 |
|
| ||||||
| Facility Chemotherapy Treatment | ||||||
| Yes | 155 | 60.7 | 919 | 67.9 | 38 | 63.2 |
| No | 382 | 64.1 | 1861 | 65.4 | 132 | 55.3 |
|
| ||||||
| Provider Specialty | ||||||
| Colorectal Surgery | 71 | 56.3 | 310 | 75.2a | 25 | 60.0 |
| Other | 468 | 64.1 | 2501 | 65.7 | 137 | 53.3 |
|
| ||||||
| Year of diagnosis | ||||||
| 2006 | 205 | 61.0 | 1038 | 58.8a | 64 | 57.8a |
| 2007 | 193 | 66.3 | 995 | 69.5 | 62 | 66.1 |
| 2008 | 163 | 61.4 | 899 | 71.6 | 58 | 43.1 |
|
| ||||||
| Continuous Variables | No | Yes | No | Yes | No | Yes |
| Age | 74.5(4.3)a | 73.0(4.2) | 78.0(6.8) | 77.8(7.0) | 73.9(4.3) | 72.0(4.0)a |
| Provider Graduation year | 1984(10) | 1983(11) | 1982(11) | 1984(10)a | 1984(11) | 1984(9) |
| Log Tumor size | 3.8(0.6) | 3.8(0.5) | 3.4(0.7) | 3.7(0.6)a | 3.6(0.5) | 3.6(0.5) |
Chi-square or t-test, P < .05
For COC status vs radiation, there is only 1 facility with “Network Cancer Program” designation (Fisher exact = .0473. When this facility is grouped into another category, “Community Hospital Comprehensive Program,” (Fisher exact P = .0609.). Due to very small sample size, results are not as robust and classified as statistically insignificant.
Fishers exact test P< .05.
Missing cases not included in bivariate comparisons; total sample size may change by each comparison.
Race statistical comparisons compared White vs Other only.
Table 3.
Bivariate Association of Comorbidity Burden With Guideline Concordance for Stage I - III Colorectal Cancer Patients Living in Appalachian Counties in 4 States, 2006- 2008
| Comorbidity Burden | Chemotherapy for stage III colon cancer (n=561) |
›12 lymph nodes harvested stages I – III colon cancer (n=2932) |
Radiation therapy for Stage III rectal cancer (n=184) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| N | %yes | N | %yes | N | %yes | |
|
| ||||||
| Total | 561 | 62.9 | 2932 | 66.3 | 184 | 56.0 |
| Comorbidity Severity | ||||||
| none | 72 | 65.3a | 350 | 69.7 | 37 | 64.9 |
| mild | 247 | 70.0 | 1208 | 66.0 | 92 | 52.2 |
| moderate | 79 | 60.8 | 507 | 67.9 | 19 | 52.6 |
| Severe | 163 | 52.1 | 867 | 64.6 | 36 | 58.3 |
|
| ||||||
| Comorbidities | ||||||
| AMI | 40 | 45.0a | 193 | 62.2 | 8 | 25.0 |
| CAD | 34 | 58.8 | 163 | 63.8 | 6 | 66.7 |
| CHF | 67 | 44.8a | 442 | 63.1 | 15 | 46.7 |
| Arrhythmia | 68 | 57.4 | 514 | 66.1 | 18 | 50.0 |
| Hypertension | 396 | 63.6 | 2114 | 66.0 | 120 | 52.5 |
| Venous Disease | 14 | 64.3 | 66 | 63.6 | 2 | 50.0 |
| PAD | 16 | 62.5 | 81 | 60.5 | 1 | 100.0 |
| Respiratory diseases | 132 | 52.2a | 823 | 64.6 | 45 | 44.4 |
| Hepatic | 8 | 37.5 | 37 | 70.3 | 2 | 100.0 |
| Stomach or Intestine | 41 | 58.5 | 281 | 68.0 | 15 | 60.0 |
| Pancreas | 1 | 100.0 | 8 | 62.5 | 0 | -- |
| Renal System | 27 | 55.5 | 180 | 60.0 | 7 | 28.6 |
| Diabetes | 189 | 61.9 | 883 | 62.5a | 51 | 58.8 |
| Stroke or CVA | 4 | 25.0 | 65 | 58.5 | 3 | 33.3 |
| Dementia | 8 | 12.5a | 63 | 60.3 | 2 | 0.0 |
| Paralysis | 1 | 0.0 | 24 | 70.8 | 0 | -- |
| Neuromuscular | 12 | 50.0 | 103 | 62.1 | 2 | 50.0 |
| Psychiatric | 1 | 0.0 | 7 | 71.4 | 0 | -- |
| Rheumatologic | 3 | 66.6 | 10 | 50.0 | 0 | -- |
| AIDS | 0 | -- | 0 | -- | 0 | -- |
| Solid Tumor | 145 | 66.9 | 565 | 66.7 | 24 | 41.7 |
| Leukemia | 10 | 60.0 | 33 | 66.7 | 0 | -- |
| Lymphoma | 0 | -- | 1 | 0.0 | 0 | -- |
| Alcohol Abuse | 4 | 25.0 | 11 | 54.5 | 1 | 100.0 |
| Illicit Drugs | 0 | -- | 0 | -- | 0 | -- |
| Obesity | 12 | 58.3 | 32 | 81.3 | 2 | 50.0 |
|
| ||||||
| Multimorbidity Count | ||||||
| 0 morbidities | 72 | 65.3 | 350 | 69.7 | 37 | 64.9 |
| 1 comorbidity | 155 | 69.7 | 742 | 66.8 | 56 | 55.4 |
| 2-3 comorbidities | 223 | 61.0 | 1221 | 66.4 | 70 | 55.7 |
| 4-5 comorbidities | 86 | 57.0 | 464 | 65.7 | 17 | 52.9 |
| 6+ comorbidities | 25 | 52.0 | 155 | 57.4 | 4 | 0.0 |
Chi-square, P < .05 for colon cancer resection.
Table 4.
Multivariate Logistic Regression of the Impact of Sociodemographic, Hospital, Distance, and Contextual Characteristics on the Receipt of Guideline Concordant Treatment for Stage I - III Colorectal Cancer Patients Living in Appalachian Counties in 4 States, 2006- 2008
| Chemotherapy (n=561) |
Nodal Assessment (n=2932) |
Radiation (n=184)b | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Age in years | 0.918* | 0.879 – 0.959 | 0.90* | 0.841 – 0.974 | ||
| Gender: Female vs. Male | 1.207a | 1.016 – 1.435 | ||||
| Race: White vs. Other | 1.637a | 1.004 – 2.668 | ||||
|
| ||||||
| Grade Diff: Well vs. Poor | 0.990 | 0.687 – 1.425 | ||||
| Grade: Diff. Moderate. vs. Poor | 0.824 | 0.647 – 1.050 | ||||
|
| ||||||
| Stage: I vs. III | 0.505a | 0.398 – 0.641 | ||||
| Stage: II vs. III | 0.784a | 0.633 – 0.971 | ||||
|
| ||||||
| Comorbidity Index (vs. None) | ||||||
| Mild | 1.442 | 0.812 – 2.559 | ||||
| Moderate | 1.091 | 0.539 – 2.209 | ||||
| Severe | 0.902 | 0.469 – 1.737 | ||||
|
| ||||||
| ACE-27 Comorbidities | ||||||
| AMI | 0.658 | 0.323 – 1.339 | ||||
| Congestive heart failure | 0.699 | 0.384 – 1.270 | ||||
| Dementia | 0.107* | 0.013 – 0.898 | ||||
| Respiratory Diseases | 0.713 | 0.453 – 1.121 | ||||
| Diabetes | 0.780a | 0.649 – 0.938 | ||||
| Log Tumorsize | 1.872a | 1.608 – 2.179 | ||||
|
| ||||||
| Hospital Bedsize Category (vs. 500+) | ||||||
| <50 | 1.700 | 0.863 – 3.347 | ||||
| 50 - 100 | 1.427 | 0.829 – 2.459 | ||||
| 100 - 200 | 1.036 | 0.664 – 1.618 | ||||
| 200 - 500 | 0.814 | 0.575 – 1.152 | ||||
|
| ||||||
| Hospital COC designation (vs. None) | ||||||
| Hospital Cancer Program | 2.405a | 1.763 – 3.281 | ||||
| Comprehensive Cancer Program | 2.136a | 1.593 – 2.864 | ||||
| NCI designated Cancer Program | 2.292a | 1.198 – 4.383 | ||||
| Network Cancer Program | 1.794 | 0.691 – 4.661 | ||||
| Teaching Hospital | 2.069a | 1.332 – 3.212 | ||||
| Facility Radiation (yes vs. no) | 1.026 | 0.789 – 1.335 | ||||
|
| ||||||
| Facility Surgical Volume (versus Q4) | ||||||
| Quartile 1 | 0.344a | 0.223 – 0.530 | ||||
| Quartile 2 | 0.345a | 0.232 – 0.512 | ||||
| Quartile 3 | 0.667a | 0.474 – 0.939 | ||||
| Other MD Specialty (vs. Colorectal Surgery) | 0.917 | 0.652 – 1.292 | ||||
|
| ||||||
| Surgery Provider Volume (vs. Q4) | ||||||
| Quartile 1 (lowest) | 0.963 | 0.716 – 1.295 | ||||
| Quartile 2 | 0.985 | 0.744 – 1.304 | ||||
| Quartile 3 | 0.867 | 0.648 – 1.160 | ||||
|
| ||||||
| Provider Graduation Year | 1.010a | 1.001 – 1.019 | ||||
| Metropolitan vs. non-metropolitan | 0.980 | 0.756 – 1.270 | ||||
| Year of diagnosis | ||||||
| 2007 | 1.668a | 1.361 - 2.045 | 1.281 | 0.609 – 2.694 | ||
| 2008 | 1.902a | 1.532 - 2.361 | 0.561 | 0.269 – 1.169 | ||
F-statistic, P < .05
Race and COC designation removed from analysis because of excessively unbalanced distributions and otherreasons.
The bivariate analyses with sociodemographic characteristics are reported in Table 2. The table also shows overall rates of chemotherapy (for stage III colon cancer), adequate lymph node assessment (for stage I-III colon cancer), and radiation therapy (for stage III rectal cancer) of 62.9%, 66.9%, and 56.0%, respectively. There were significantly lower rates of chemotherapy among the 561 stage III colon cancer patients who were older rather than younger (56.3% and 69.7%, respectively). Among the 2932 stages I-III colon cancer patients there were significantly lower rates of adequate lymph node assessment among males versus females (61.6% and 66.9%, respectively), among blacks, Hispanics, and other races versus whites, among those with stage I disease, those treated in smaller hospitals, facilities with no COC designation, and among patients treated in either a surgical facility or by a provider with a relatively low volume of cases. Adequate lymph node assessment rates were also lower among patients treated in hospitals in non-metropolitan areas (62.9%), with no radiation therapy units (57.7%), or by other than colorectal cancer surgeons (65.7%). The radiation therapy analyses among the stage III rectal cancer cases were limited by small sample size (n=184) with age, race (only whites), and hospital COC designation being significant. Concordance rates of 66% versus 45.5% among those aged 66-73 versus 74-80 were statistically different (P < .05.)
Table 3 summarizes the bivariate analyses of the 3 guidelines and comorbidity burden. Of the 561 colon cancer patients, 62.9% received chemotherapy, but those with myocardial infarction, congestive heart failure, respiratory diseases, or dementia had statistically significantly lower rates (45.0%, 44.8%, 52.2%, and 12.8%, respectively). Among the 2932 stage I-III colon cancer patients, only those with diabetes had somewhat lower rates of adequate lymph node assessment (62.5%) compared to an overall rate of 66.3% among all colon cancer patients. There were no significant determinants of radiation for stage III rectal cancer.
The multivariate analyses (Table 4) summarize the statistically significant predictors of each of the 3 guidelines. Older patients had lower odds of chemotherapy (one-third lower odds for every 5 years increase in age using the parameter estimates), and those with dementia had one-tenth the odds of chemotherapy, although the prevalence of patients with dementia was extremely small. Among the 2932 stage I-III colon cancer patients, whites and females were associated with higher odds (OR = 1.6 with adjusted rates 67% whites versus 57% nonwhites, and OR = 1.2 with adjusted rates 68% females versus 64% males) of adequate lymph node assessment. Patients with stage I vs III had half the odds of adequate nodal assessment (adjusted rates of 59% versus 72%). Stage II was also significantly different from Stage III (OR=0.78 with adjusted rates of 68% vs 72%). Those who had diabetes had a 22% lower odds of adequate nodal assessment (adjusted rates of 63% vs 68%). Log tumor size was associated with higher nodal assessment (OR = 1.87), which results in adjusted rates at 20mm, 40mm, and 60mm of 59%, 68%, and 73%, respectively. Physician graduation year was also associated with higher nodal assessment (OR = 1.01), which results in adjusted rates at 1975, 1985, and 1995 of 65%, 67%,69%, respectively. Patients treated in hospitals with some COC designation had 2 to 3 times the odds of receiving adequate lymph node assessment. Patients with lower surgical resection volumes were less likely to receive adequate nodal assessment than those with higher volumes (q1 = 58%, q2 = 58%, q3 = 72%, vs q4 = 78%). Among the 184 stage III rectal cancer patients, there was a 10% lower odds of radiation therapy for every year increase in age (41% lower odds for every 5 years using parameter estimates), with adjusted rates for 65, 70, and 75 years being 73%, 63%, and 51%, respectively. We interpret the rectal cancer results with caution because of the low sample size.
DISCUSSION
It is estimated that 143,460 new cases of colorectal cancer will be diagnosed in the US in 2012, and 51,690 Americans will die from colorectal cancer.34 Colorectal cancer is the number 2 cause of cancer death in the US and the fourth most prevalent cancer. Within Appalachia, colorectal cancer represents a significant cause of morbidity and mortality, yet treatment guidelines are associated with improved survival.7-10
Among the colorectal cancer patients in our study, the rates of guideline-concordant chemotherapy, adequate lymph node assessment, and radiation (for rectal cancer) were 65.3%, 66.3%, and 56.0%, respectively. Our results in Appalachia were consistently lower than a recent NewYork study35 of Medicaid and Medicare stage III colorectal cancer patients diagnosed between 2004-2006, which reported 79.4% and 71.8% chemotherapy concordance rates, respectively, for the 2 payer groups. Concordance rates for adjuvant radiation therapy among Medicaid and Medicare stage IIB and stage III rectal cancer patients were 72.3% and 66.9%, respectively. Concordance in Appalachia was also lower than one Veterans Administration study36 that reported 73.5% chemotherapy concordance among stage III colorectal cancer patients, lower than another study among 8 Comprehensive Cancer Network centers with chemotherapy concordance rates of 90%,37 and lower than a recent SEER study38 where 73.6% of patients undergoing radical (curative intent) colon resection had at least 12 lymph nodes harvested. On the other hand, our results were similar to a CDC Patterns of Care study39 among 7 states where 67% of stage III colorectal cancer patients received chemotherapy.
Our study identified specific sociodemographic, clinical, comorbidity, and service provider characteristics that were related to the rates of guideline concordance among 4 measures. Older Medicare beneficiaries had lower rates of both chemotherapy and radiation, and whites had nearly double the odds of adequate lymph node assessment compared with other races. Clinical characteristics (stage, grade, and tumor size) determined the odds of adequate lymph node assessment. The evidence of a dose-response relationship in the bivariate analysis, lower rates of concordance for chemotherapy associated with higher levels of comorbidity burden, did not hold up in the multivariate analysis, with comorbidity burden probably confounded by age. Patients treated in hospitals with no COC designation or lower surgical volumes had lower odds of lymph node assessment. Hsu and colleagues40 reported that depth of tumor invasion, poor versus well differentiated grade, and tumor length and localization influenced whether patients had at least 12 lymph nodes assessed. Baxter et al41 used SEER data to show that rates of adequate lymph node assessment were higher among whites, those with more advanced disease (Stage II or III versus I) and those with moderate or well grade differentiation.
Some of the results from the study are intuitive. It is both logical and clinically sound that older patients would have lower rates of both chemotherapy and radiation therapy because of the higher risk of complications from these treatments. In fact, the guidelines for adjuvant chemotherapy in colorectal cancer specify that consideration of chemotherapy is only required in those less than 80 years of age. Most papers in the literature have shown lower rates of these 2 therapies with age.42,43 Additionally, patients with dementia are less likely to receive a meaningful clinical benefit from aggressive adjuvant therapy.
Some of our findings are less easy to explain, and may reflect provider biases or choices. Several studies have shown that rates of ≥12 lymph nodes harvested have increased dramatically since the late-1990s.44 Nevertheless, there are several possible explanations for fewer than 12 lymph nodes being sampled in an oncologic resection. Because node-positive patients are stage III, and the more nodes that are sampled, the less likely it is for every lymph node to be negative, some of the patients are probably falsely staged as stage I due to undersampling. Similarly, diabetic patients may have smaller surgical resections because of concerns about the patient’s ability to recover from the surgery. Physicians who graduated more recently may be more likely to excise at least 12 nodes because they were trained in an era when the guidelines were being promulgated. Finally, the quality of the pathologic examination is closely tied with the institution. Large hospital size and hospital COC designation are proxies for provider education and awareness of appropriate guidelines, and one would expect higher concordance with increased awareness among providers. The effects may also relate to increased scrutiny of cancer outcomes and process measures at these larger and COC-designated facilities. The fact that whites had nearly double the odds of adequate lymph nodes assessed, even while controlling for hospital size and hospital COC designation, implies that there may be unmeasured socioeconomic, cultural, or behavioral disparities among races that affect guideline concordance for this measure. This racial disparity deserves further scrutiny, particularly in areas such as southern Appalachia where the number of minority cases is much higher.
Our study has both weaknesses and strengths. Sample size is limited in some analyses, particularly among rectal cancer patients, and claims data limitations are well-known and reported45-54 We focused on elderly (66+) colorectal cancer patients from 4 Appalachian states by design, and we needed to have at least 1 year of Medicare claims prior to cancer diagnosis. Although one could certainly argue that generalizability is limited to this age group and region, we suggested earlier that some characteristics of Appalachia, eg, economic decline and lack of health infrastructure, are shared by other regions of the United.States. Thus, the importance of COC designation certainly reaches beyond Appalachia. The breadth of our study is notable, and a significant strength, because we draw colorectal cancer cases from the Appalachian population of 4 different states. The linkage of registry to Medicare claims is ideal for this kind of research because it combines the strengths of both central cancer registries and Medicare claims. Cancer registries are among the best source of cancer case identification for population research,55,56 and Medicare claims provides more reliable and complete information on treatment.
Future research could be aimed at confirming our results by expanding the scope of our analysis to non-Appalachian urban and rural populations. Also, since COC status and surgical volume are significant in this study, we could investigate whether smaller centers that are affiliates of larger ones excel compared to similarly sized non-affiliated centers. If there is a difference in guideline concordance, we could design an intervention that provides more avenues of linking smaller centers to experts.
Acknowledgments
The Patterns of Patient Care in Appalachia Study was supported by the National Cancer Institute (1R01CA140335). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute. Additional support has been provided by the Population Research Institute which receives core funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Award R24- HD41025.
Footnotes
None of the authors have any financial disclosures to report.
REFERENCES
- 1.Lengerich EJ, Tucker TC, Powell RK, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. Journal of Rural Health. 2005;21:39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 2.Appalachian Regional Commission [accessed 02/23/12];The Appalachian Region. 2012 http://www.arc.gov/appalachian_region/TheAppalachianRegion.asp (website.
- 3.Appalachian Regional Commission [accessed 03/21/12];An analysis of disparities in health status and access to health care in the Appalachian region. 2004 http://www.etsu.edu/health/index_files/halversonreport.pdf (website.
- 4.Armstrong LR, Thompson T, Hall HI, et al. Colorectal carcinoma mortality among Appalachian men and women, 1969-1999. Cancer. 2004;101:2851–2858. doi: 10.1002/cncr.20667. [DOI] [PubMed] [Google Scholar]
- 5.Cooper GS, Kou TD, Reynolds HL. Receipt of guideline-recommended follow-up in older colorectal cancer survivors. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 6.Chung-Yuan H, Delclos GL, Chan W, Du XL. Post-treatment surveillance in a large cohort of patients with colon cancer. American Journal of Managed Care. 2011;17:329–336. [PubMed] [Google Scholar]
- 7.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Nat Canc Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 8.Sargent D, Sobrero A, Grothey A, et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camma C, Giunta M, Fiorica F. Preoperative Radiotherapy for Resectable Rectal Cance A Meta-analysis. J Am Med Assoc. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 10.Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant Therapy of colorectal Cancer: why we still don’t know. J Am Med Assoc. 1988;259:3571–3578. [PubMed] [Google Scholar]
- 11.Chagpar R, Xing Y, Chiang Y, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clinical Oncology. 2012;30:972–979. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas JS, Brawarsky P, Iyer A, Fitzmaurice GM, Neville BA, Earle C. Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality. Cancer. 2011;117:4267–4276. doi: 10.1002/cncr.26034. [DOI] [PubMed] [Google Scholar]
- 13.Hao Y, Landrine H, Jemal A, et al. Race, neighborhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiology Community Health. 2011;65:211–217. doi: 10.1136/jech.2009.096008. [DOI] [PubMed] [Google Scholar]
- 14.Cueto CV, Szeja S, Wertheim BC, Ong ES, Tsikitis VL. Disparities in treatment and survival of white and Native American patients with colorectal cancer: a SEER analysis. J Am Coll Surg. 2011;213:469–474. doi: 10.1016/j.jamcollsurg.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. J Rural Health. 2012;28:296–305. doi: 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 16.Esnaola NF, Gebregziabher M, Finney C, Ford ME. Underuse of surgical resection in black patients with nonmetastatic colorectal cancer. Annals of Surgery. 2009;250:549–557. doi: 10.1097/SLA.0b013e3181b732a5. [DOI] [PubMed] [Google Scholar]
- 17.Commission on Cancer . The Rapid Quality Reporting System (RQRS) Chicago, Illinois: [Accessed May 9, 2013]. 2011. Available at: http://www.facs.org/cancer/ncdb/rqrs.html. [Google Scholar]
- 18.National Quality Forum . National Voluntary Consensus Standards for Quality of Cancer Care: A concensus report. Washington, DC: [Accessed May 9, 2013]. 2009. Available at: http://www.qualityforum.org/Publications/2009/05/National_Voluntary_Consensus_Standard s_for_Quality_of_Cancer_Care.aspx. [Google Scholar]
- 19. [Accessed May 9, 2013];Colon cancer measures submitted by the CoC to the National Quality Forum (NQF) and endorsed by the NQF in April 2007. Available at: http://www.facs.org/cancer/ncdb/colonmeasures.pdf.
- 20. [Accessed May 9, 2013];Rectal cancer measure developed collaboratively by the CoC and ASCO/NCCN. Available at: http://www.facs.org/cancer/ncdb/rectalmeasure.pdf.
- 21.Fleming ST, Sabatino SA, Kimmick G, et al. for the Patterns of Care Study Group Developing a claim-based version of the ACE-27 comorbidity index: a comparison with medical record review. Medical Care. 2011;49:752–760. doi: 10.1097/MLR.0b013e318215d7dd. [DOI] [PubMed] [Google Scholar]
- 22.USDA [Accessed May 9, 2013];Rural-Urban Continuum Codes. 2000 Available at: http://www.ers.usda.gov/Briefing/Rurality/RuralUrbanCommutingAreas/
- 23. http://www.facs.org/cancerprogram/index.html.
- 24. https://nppes.cms.hhs.gov/NPPESRegistry/NPIRegistryHome.do.
- 25. www.healthgrades.com/
- 26. http://upin.ecare.com/
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; New York: 1987. [Google Scholar]
- 28.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall; New York: 1997. [Google Scholar]
- 29.Jonathan W, Forst C, Carpenter JR. Multiple imputation models should incorporate the outcome in the model of interest. Brain. 2011;134:e189. doi: 10.1093/brain/awr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moons KG, Donders RA, Stijnen T, et al. Using the outcome for imputation of missing predictor values was preferred. Journal of Clinical Epidemiology. 2006;59:1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Ake CF. Rounding after multiple imputation with non-binary categorical covariates; Paper presented at the annual meeting of the SAS Users Group International; Philadelphia, PA. 2005. [Google Scholar]
- 32.Allison PD. Imputation of categorical variables with PROC MI; Paper presented at the annual meeting of the Sas Users Group International; Philadelphia, PA. 2005. [Google Scholar]
- 33.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. Journal of the American Statistical Association. 1993;88:9–25. doi:10.2307/2290687. JSTOR 2290687. [Google Scholar]
- 34.American Cancer Society . Cancer Facts and Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 35.Sinclair AH, Schymura MJ, Boscoe FP, et al. Measuring colorectal cancer care quality for the publicly insured in New York State. Cancer Medicine. 2012;1(3):363–371. doi: 10.1002/cam4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. Journal of Clinical Oncology. 2010;28:3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanus D, Weiser MR, Skibber JM, et al. Concordance with NCCN Colorectal Cancer Guidelines and ASCO/NCCN Quality Measures: an NCCN institutional analysis. Journal of the National Comprehensive Cancer Network. 2009;7:895–904. doi: 10.6004/jnccn.2009.0059. [DOI] [PubMed] [Google Scholar]
- 38.Parsons HM, Tuttle TM, Kuntz KM, et al. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306:1089–1097. doi: 10.1001/jama.2011.1285. [DOI] [PubMed] [Google Scholar]
- 39.Cress RD, Sabatino SA, Wu X, et al. Adjuvant chemotherapy for patients with stage III colon cancer: results from a CDC-NPCR Patterns of Care Study. Clinical Medicine: Oncology. 2009;3:107–119. doi: 10.4137/cmo.s2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu CW, Lin CH, Wang JH, Wang HT, Ou WC, King TM. Factors that influence 12 or more harvested lymph nodes in early-stage colorectal cancer. World Journal of Surgery. 2009;33:333–9. doi: 10.1007/s00268-008-9850-z. [DOI] [PubMed] [Google Scholar]
- 41.Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. Journal of the National Cancer Institute. 2005;97(3):219–25. doi: 10.1093/jnci/dji020. [DOI] [PubMed] [Google Scholar]
- 42.Hu CY, Delclos GL, Chan W, et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 43.Chang GJ, Skibber JM, Feig BW, et al. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg. 2007;246:215–221. doi: 10.1097/SLA.0b013e318070838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou JF, Row D, Gonen M, et al. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. 2010;116:2560–2570. doi: 10.1002/cncr.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano P, Mark D. Bias in the coding of hospital discharge data and it implications for quality assessment. Medical Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Steinwachs D, Stuart M, Scholle S, Starfield B, Fox M, Weiner J. A comparison of ambulatory medicaid claims to medical records: a reliability assessment. Am J Med Qual. 1998;13:63–69. doi: 10.1177/106286069801300203. [DOI] [PubMed] [Google Scholar]
- 47.Dismuke C. Underreporting of computed tomography and magnetic resonance imaging procedures in inpatient claims data. Medical Care. 2005;43:713–17. doi: 10.1097/01.mlr.0000167175.72130.a7. [DOI] [PubMed] [Google Scholar]
- 48.Bright R, Avorn J, Everitt D. Medicaid data as a resource for epidemiologic studies: strengths and weaknesses. J Clin Epidemiol. 1989;42:937–45. doi: 10.1016/0895-4356(89)90158-3. [DOI] [PubMed] [Google Scholar]
- 49.Quan H, Parsons G, Ghali W. Validity of procedure codes in international classification of diseases, 9th revision, clinical modification administrative data. Medical Care. 2004;42:801–9. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 50.Romano P. Using administrative data to identify associations between implanted medical devices and chronic diseases. Ann Epidemiol. 2000;10:197–9. doi: 10.1016/s1047-2797(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 51.Tyree P, Lind B, Lafferty W. Challenges of using medical insurance claims data for utilization analysis. Am J Med Qual. 2006;21:269–75. doi: 10.1177/1062860606288774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper G, Yuan Z, Stange K, Dennis L, Amini S, Rimm A. Agreement of medicare claims and tumor registry data for assessment of cancer-related treatment. Medical Care. 2000;38:411–21. doi: 10.1097/00005650-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Pinfold P, Goel V, Sawka C. Quality of hospital discharge and physician data for type of breast cancer surgery. Medical Care. 2000;38:99–107. doi: 10.1097/00005650-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Warren JL, Harlan LC. Can cancer registry data be used to study cancer treatment? MedicalCare. 2003;41:1003–1005. doi: 10.1097/01.MLR.0000086827.00805.B5. [DOI] [PubMed] [Google Scholar]
- 56.Cress RD, Zaslavsky AM, West DW, et al. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Medical Care. 2003;41:1006–1012. doi: 10.1097/01.MLR.0000083740.12949.88. [DOI] [PubMed] [Google Scholar]