Abstract
Hydroxyurea is currently the only FDA-approved drug that ameliorates the pathophysiology of sickle cell anemia. Unfortunately, substantial interpatient variability in the pharmacokinetics (PK) of hydroxyurea may result in variation of the drug's efficacy. However, little is known about mechanisms that modulate hydroxyurea PK. Recent in vitro studies identifying hydroxyurea as a substrate for organic anion transporting polypeptide (OATP1B) transporters prompted the current investigation assessing the role of OATP1B transporters in modulating hydroxyurea PK. Using wild-type and Oatp1b knockout (Oatp1b−/−) mice, hydroxyurea PK was analyzed in vivo by measuring [14C]hydroxyurea distribution in plasma, kidney, liver, urine, or the exhaled 14CO2 metabolite. Plasma levels were significantly reduced by 20% in Oatp1b−/− mice compared with wild-type (area under the curve of 38.64 or 48.45 μg·h−1·ml−1, respectively) after oral administration, whereas no difference was observed between groups following intravenous administration. Accumulation in the kidney was significantly decreased by twofold in Oatp1b−/− mice (356.9 vs. 748.1 pmol/g), which correlated with a significant decrease in urinary excretion. Hydroxyurea accumulation in the liver was also decreased (136.6 vs. 107.3 pmol/g in wild-type or Oatp1b−/− mice, respectively) correlating with a decrease in exhaled 14CO2. These findings illustrate that deficiency of Oatp1b transporters alters the absorption, distribution, and elimination of hydroxyurea thus providing the first in vivo evidence that cell membrane transporters may play a significant role in modulating hydroxyurea PK. Future studies to investigate other transporters and their role in hydroxyurea disposition are warranted for understanding the sources of variation in hydroxyurea's PK.
Keywords: sickle cell, cell membrane transporters, hydroxyurea, pharmacokinetics, OATP1B
hydroxyurea is presently the only FDA-approved drug for the treatment of sickle cell anemia (SCA) that modifies the disease pathophysiology. The clinical benefits of hydroxyurea for SCA have been in large part attributed the drug's ability to increase fetal hemoglobin (3, 18, 23). Maximum benefits of the drug are thought to occur when the patient reaches a maximum tolerated dose, which is based on mild marrow suppression, typically neutropenia (31). Clinical studies have shown that the degree to which fetal hemoglobin is increased as well as the final maximum tolerated dose are highly variable among individuals with SCA (32). The etiology of this variation is poorly understood but is likely to be linked to the pharmacokinetics (PK) of hydroxyurea.
Hydroxyurea is a ribonucleotide reductase inhibitor that was initially developed and used as an antineoplastic agent. It is rapidly absorbed after oral administration with a reported bioavailability of 108% after an 80 mg/kg dose in patients with solid malignancies (26). Hydroxyurea is widely distributed and is excreted primarily in the urine as the parent compound (9). A fraction of hydroxyurea may undergo hepatic elimination. Possible metabolites that have been identified include carbon dioxide (CO2), nitric oxide (NO), and urea (1, 5, 11). However, the proportion of drug that is eliminated via the liver and extent of in vivo hydroxyurea metabolism are unclear (9). Great variation in hydroxyurea PK has been documented in populations of sickle cell patients showing 25–45% variation in area under the curve (AUC), maximum concentration, and clearance (8, 26, 32). Recent population PK analysis and modeling of hydroxyurea in adults with SCA reported that exposure varied by fivefold and the variation in hydroxyurea response was related in part to the PK (22). Because little is known about mechanisms that modulate the absorption, distribution, metabolism, and excretion of hydroxyurea, the potential sources of variation in hydroxyurea PK remain incompletely defined.
Various solute carrier (SLC) cell membrane transporters have been identified as key modulators of xenobiotic PK (12). Previously, an in vitro screen of hydroxyurea uptake by SLC transporter-overexpressing cells identified specific human SLC transporters that mediate the transmembrane movement of hydroxyurea (30). The human organic anion transporting polypeptide 1B (OATP1) family of transporters was among the transporters identified. In humans, the OATP1B family of transporters consists of OATP1B1 and OATP1B3 transporters that are encoded by SLCO1B genes (10). Predominantly expressed in the liver on the sinusoidal membrane of hepatocytes (15), these transporters are important for regulating the elimination of endogenous substrates such as bilirubin and can impact the disposition of drugs such as methotrexate and various statins (14, 16, 19, 21, 27). The rodent ortholog of the human OATP1B transporters is Oatp1b2 (4). Substrates for Oatp1b2 as well as the function of the Oatp1b2 are similar to those of the human transporters (6). Mouse models deficient for Oatp1b2, or deficient for both Oatp1b2 and Oatp1a transporters, have become useful models for predicting the impact of OATP1B transporters on the disposition of various drugs and substrates including methotrexate and bilirubin (13). In these models when the Oatp1b2 is absent, there is an increase in systemic docetaxel and bilirubin levels (7, 34). The established role of OATP1B transporters in modulating PK of various drugs and the fact that hydroxyurea is substrate for these transporters led to the hypothesis that Oatp1b transporters may modulate hydroxyurea PK.
In the present study, the ability of OATP1B transporters to modulate hydroxyurea PK was evaluated in OATP transporter-deficient mice. Here we demonstrate for the first time that the absence of these drug transporters can significantly decrease systemic exposure, tissue accumulation, and elimination of hydroxyurea. This study highlights the importance of examining the role of transporters as sources of inter-patient variability in hydroxyurea PK.1
METHODS
In vitro analysis of hydroxyurea accumulation and protein binding assay.
Hydroxyurea accumulation was measured in oocytes injected with rodent Oatp1b2 transporter cRNA or water (BD Biosciences). Oocytes were incubated in transportocyte sodium buffer (pH 7.4; BD Biosciences) containing 50 μM [14C]hydroxyurea (American Radiolabeled Chemicals, St. Louis, MO) and washed four times in cold buffer. Individual oocytes were lysed in 10% SDS, and radioactivity was measured by liquid scintillation counter. For inhibition studies, 1 mM naringin (Sigma, St. Louis, MO) or rifampin (Sigma, St Louis, MO) dissolved in 1% DMSO solution or 1 mM methotrexate (Sigma, St Louis, MO) dissolved in water was added to hydroxyurea uptake medium. Inhibition is expressed as a percent of accumulation in the water-injected control oocytes.
Unbound fraction of hydroxyurea in mouse and human plasma was determined in vitro using an equilibrium dialysis procedure. Deidentified samples of human plasma were obtained from Blood Bank at St. Jude Children's Research Hospital and mouse plasma was obtained from wild-type mice. Human or mouse plasma containing [14C]hydroxyurea at concentrations ranging from 1.5 μM to 500 μM was incubated at 37°C in an equilibrium dialysis plate containing a dialysis membrane with a molecular mass cutoff of 5 kDa (Harvard Apparatus, Holliston, MA). PBS was added to the opposite side of the dialysis membrane. After a 60-h incubation period, an aliquot was taken from each side of the dialysis membrane, and the radioactivity was measured using a liquid scintillation counter. The unbound fraction of hydroxyurea was calculated as the percent [14C]hydroxyurea measured in 1× PBS compartment relative to the initial amount in the plasma compartment. The final reported unbound fraction is the average percentage measured in four replicates of three different samples of mouse or human plasma.
Hydroxyurea PK in mouse models.
Hydroxyurea PK were assessed in 8- to 12-wk-old female Oatp1b2 knockout mice (Oatp1b2−/−) (34) and age-matched wild-type mice on a DBA1/lacJ background (DBA WT), and 8- to 12-wk-old female Oatp1a/1b knockout mice (Oatp1a/1b−/−) and age-matched wild-type mice on an FVB background (FVB WT) (Taconic Farms, Germantown, NY). All mice were housed in a temperature-controlled environment with a 12-h light cycle and given a standard diet and water ad libitum. Experiments were approved by the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital. For PK experiments, all mice received a dose of 50 mg/kg hydroxyurea spiked with [14C]hydroxyurea via tail vein injection or oral gavage. Blood samples were collected at 5, 15, 30, 60, 90, and 120 min after drug administration from each animal. Approximately 50 μl blood samples were obtained from the facial vein, retro orbital plexus, and terminal cardiac puncture. At the terminal 120-min time point, liver and kidneys were excised from each mouse. Plasma from the blood samples was isolated, and radioactivity in each sample was measured using a liquid scintillation counter. Tissues were homogenized in homogenization buffer containing 100 mM Tris-Base, 100 mM potassium chloride, 1 mM EDTA, and 20 μM butylated hydroxytoluene. Radioactivity was then measured in the tissue samples by liquid scintillation counter. To determine urinary concentration of hydroxyurea, mice were given a single 50 mg/kg oral dose of hydroxyurea and placed in a metabolic cage for 72 h for urine collection. Cumulative urine concentration was calculated on the basis of radioactivity measurements taken at 5, 24, 48, and 72 h after drug administration. Analytical analysis confirmed that radioactivity measured in tissues and plasma represented the parent compound and not the metabolites of hydroxyurea. Student's t-tests comparing hydroxyurea levels in knockout mice and wild-type mice were performed to detect statistically significant differences.
[14C]hydroxyurea breath test.
The amount of exhaled CO2 metabolite of hydroxyurea was measured using a breath test as described elsewhere (16, 33). Briefly, the mice received a tail vein injection of 50 mg/kg hydroxyurea containing [14C]hydroxyurea (1 μCi/100 g; American Radiolabeled Chemicals) in saline. Mice were then placed in a water-sealed polyurethane breath chamber with air continuously drawn through a vapor trap (acetone and dry ice), bubbled through an acidic methanol solution, and finally through three gas-trapping washes containing 30 ml of gas-trapping solution composed of 27% (vol/vol) methanol, 41% toluene, 5% Emulsifier-Safe, and 27% phenethylamine. Breath collection was performed at 5, 15, 30, 60, 90, and 120 min. Samples were analyzed using liquid scintillation counting. The values obtained were used to calculate 14CO2 exhaled during the collection period and are reported as the percentage of dose given to the mice. The experiments were performed in triplicate on six separate occasions, and statistical significance was determined by two-tailed paired t-test comparing the DBA WT to Oatp1b2−/−.
RNA isolation and gene expression analysis.
Organs for RNA analysis were harvested from DBA-WT and Oatp1b−/− mice following euthanasia, exsanguination, and perfusion with 60 ml saline. The stomach and the duodenum (first 1.5 cm of small intestine) were excised and placed in RNA later (Invitrogen, Carlsbad, CA). RNA was isolated using an RNeasy Kit (Qiagen, Valencia, CA) according to manufacturer protocol and quantified by Nanodrop (Thermo Scientific, Waltham, MA) spectrophotometry. The quality of the mRNA was determined by using Affymetrix quality control methods, and gene expression was assessed using Affymetrix GeneChip Mouse Gene 1.0 ST. Gene expression was normalized across samples using Robust multiarray analysis. Student's t-test of unequal variance was performed to identify genes that were differentially expressed between DBA-WT and Oatp1b2−/− mice.
Real-time PCR analysis was performed to evaluate relative expression of Slc14A2 in the kidneys of wild-type and Oatp1b−/− mice. Kidneys were harvested from untreated mice and RNA was isolated using an RNeasy extraction kit (Qiagen). mRNA was reverse transcribed into cDNA using High Capacity RNA-to-cDNA Reverse Transcription Kit (Invitrogen). Taqman primers Assay no. Mm01261839_m1 (Invitrogen) were used in real-time PCR. For analysis, data were normalized to β-actin levels and relative quantity was determined by comparative CT method (ΔΔCT).
RESULTS
In vitro hydroxyurea accumulation by Oatp1b2.
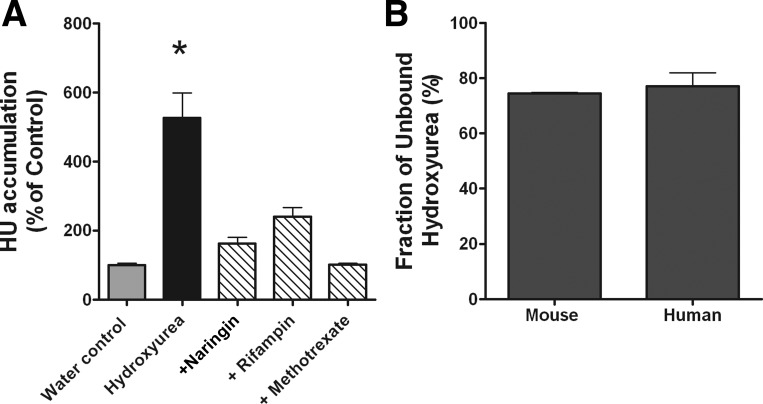
Our recent studies have shown that hydroxyurea is a substrate for the human OATP1B family of transporters (30). In vitro uptake studies were conducted to determine whether hydroxyurea is a substrate for rodent Oatp1b2 (rOatp1b2) transporter, the homolog of the human OATP1B1 and 1B3 transporters. In oocyte uptake experiments, hydroxyurea accumulation was significantly increased by fivefold in oocytes that overexpress rOatp1b2 transporter compared with control (P = 0.0001). Hydroxyurea accumulation in Oatp1b2 oocytes was inhibited during coincubation with other substrates of the transporter, and the accumulation was not different from control oocytes that did not express the rOatp1b2 transporter (Fig. 1A). These data indicate that hydroxyurea is a strong substrate for Oatp1b2 transporter. Prior to conducting in vivo PK studies, protein binding of hydroxyurea in mouse and human serum was determined in vitro. Greater than 75% of hydroxyurea remains unbound to serum proteins in either mouse or human serum (Fig. 1B).
Fig. 1.
Hydroxyurea (HU) cellular accumulation and protein binding in vitro. A: intracellular accumulation of hydroxyurea in ooctyes that overexpress rodent organic anion transporting polypeptide 1B (Oatp1b2) transporters or water-injected controls after 30 min of incubation in the presence or absence of Oatp1b2 inhibitors (n = 8–12; *P = 0.0001 compared with all other conditions). B: fraction of unbound hydroxyurea in mouse or human plasma as determined by equilibrium dialysis (n = 3).
Oatp1b2 dependent absorption and systemic exposure in vivo.
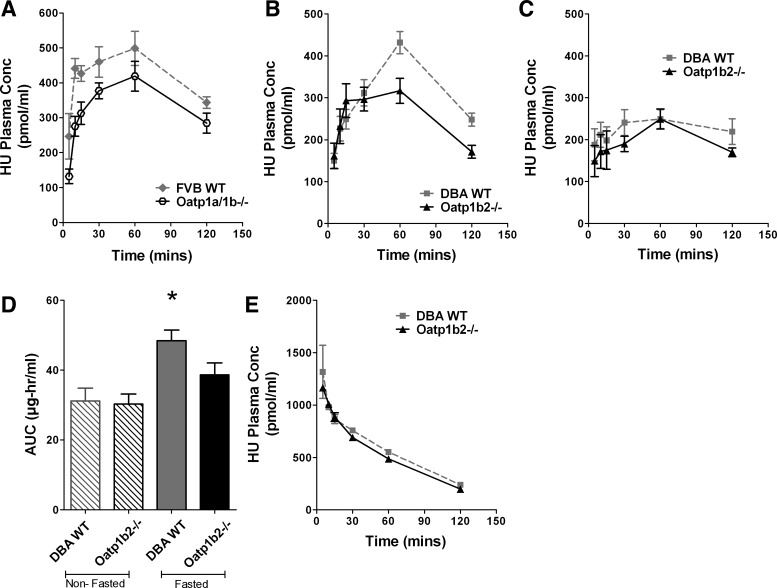
To examine the impact of the Oatp1b2 transporter on the disposition of hydroxyurea in vivo, systemic exposure (AUC) of hydroxyurea was evaluated in Oatp1a/1b−/− and Oatp1b−/− mice. Because OATP transporters are highly expressed in the GI tract and may have a role in absorption, we examined hydroxyurea PK in fasted FVB mice that were deficient in all Oatp1a and Oatp1b transporters. Plasma concentration of hydroxyurea was measured in mice after administration of 50 mg/kg hydroxyurea following a 3-h fast. In this model, AUC of hydroxyurea was significantly decreased by 22% in the Oatp1a/1b−/− mice (50.45 μg·h−1·ml−1) compared with the FVB WT mice (64.43 μg·h−1·ml−1) (P = 0.0185; Fig. 2A). To determine the impact of the Oatp1b transporters specifically, similar experiments were performed in mice with a targeted deletion of only the Oatp1b2. Following oral administration, AUC of hydroxyurea was significantly decreased by 20% in Oatp1b−/− mice (38.64 μg·h−1·ml−1) compared with DBA WT (48.45 μg·h−1·ml−1; P = 0.04), and there was a significant reduction in maximum concentration in Oatp1b−/− mice (25.06 μg/ml) compared with DBA WT (33.02 μg/ml; P = 0.021; Fig. 2B). Interestingly, when mice were not fasted prior to the PK studies, the differences in the systematic exposure between DBA WT and Oatp1b−/− mice were negated (Fig. 2C). The AUC of hydroxyurea in DBA WT decreased to 31.17 μg·h−1·ml−1 in nonfasted DBA WT mice and was comparable to AUC measured in Oatp1b−/− mice (Fig. 2D). Following intravenous injection of 50 mg/kg hydroxyurea, plasma levels detected in Oatp1b−/− mice were no different from plasma levels in DBA WT (Fig. 2E).
Fig. 2.
Plasma pharmacokinetics (PK) of hydroxyurea in vivo. A: plasma concentration (Conc) of hydroxyurea in Oatp1a/1b−/− mice (black open circle) compared with FVB wild-type (gray diamond) mice following oral administration (n = 4). B and C: plasma concentration of hydroxyurea in Oatp1b−/− mice (black triangle) compared with wild-type mice on a DBA1/lacJ background (DBA WT; gray square) following oral administration after a 3-h food fast (n = 9–12) (B) and after nonfasting conditions (C). D: systemic exposure in DBA wild-type and Oatp1b−/− mice with and without fasting (*P = 0.0009 compared with all other conditions). AUC, area under the curve. E: plasma concentration of hydroxyurea in DBA wild-type and Oatp1b−/− mice following intravenous administration.
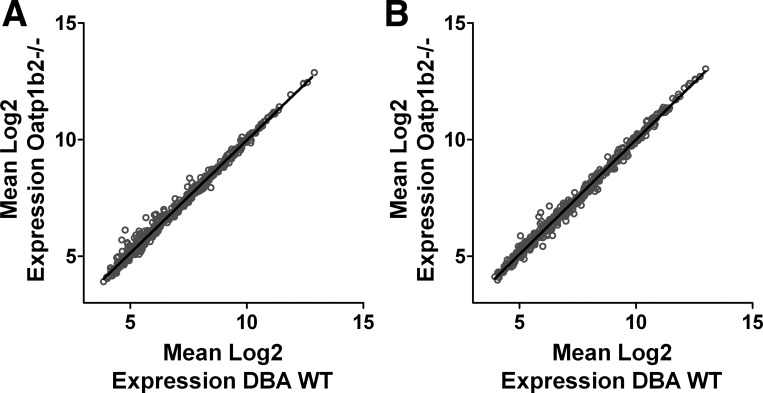
Microarray analysis was conducted to evaluate possible compensatory expression of other SLC or ATP-binding cassette (ABC) transporter genes as well as cytochrome-P450 (CYP) and UDP glucuronosyltransferases (UGT) families of metabolic enzyme genes. Analysis of this gene subset demonstrated a near perfect linear correlation between gene expression of Oatp1b−/− and DBA WT mice in both the stomach and duodenum, with r-squared values of greater than 0.99 in both tissues (Fig. 3, A and B). This means that gene expression for SLC, ABC, CYP, and UGT families of genes was not different between the Oatp1b−/− and DBA WT mice. Real-time PCR analysis of gene expression in the kidney of the mice confirmed similar expression of Slc14A2, with mean relative expression of 9.937 and 9.908 in DBA WT and Oatp1b−/− mice, respectively. Together, these results indicate that the Oatp1b2 transporter may play an important role in the disposition of hydroxyurea and suggest that the absence of Oatp1b transporters may impact hydroxyurea absorption.
Fig. 3.
Microarray analysis of mRNA expression in stomach and duodenum of mice. Comparison of sickle cell anemia (SCA), ATP-binding cassette (ABC), cytochrome-P450 (CYP), and UDP glucuronosyltransferases (UGT) gene expression in the stomach (A) and duodenum (B) of DBA wild-type and Oatp1b−/− mice shows near perfect correlation in all genes (r2 > 0.99).
Elimination of hydroxyurea in the Oatp1b2 knockout mouse model.
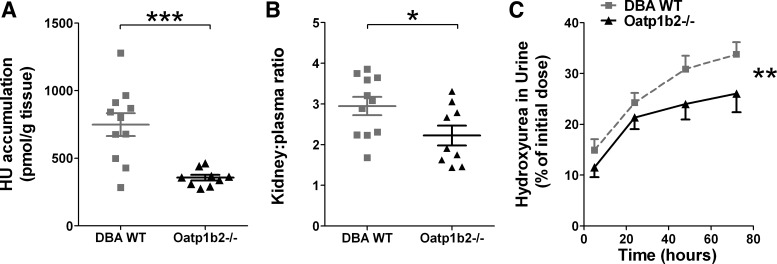
The Oatp1b2 transporter is expressed in both the kidney and the liver of mice and may play a role in the elimination of hydroxyurea. Therefore, the contribution of Oatp1b2 to renal and hepatic elimination of hydroxyurea was evaluated in Oatp1b−/− mice. Reports that a substantial fraction of orally absorbed hydroxyurea is excreted primarily through the urine prompted the study of renal elimination, in which the accumulation of hydroxyurea in kidney and urine of mice was measured. Two hours after oral administration, renal accumulation of hydroxyurea was significantly less in Oatp1b−/− mice compared with DBA WT mice (P = 0.0006), with mean levels measuring 356.9 and 748.1 pmol/g, respectively (Fig. 4A). Calculation of kidney-to-plasma ratio resulted in Oatp1b−/− mice having a mean ratio of 2.2 while DBA WT mice had a significantly higher ratio of 3.0 (P = 0.04; Fig. 4B). While both ratios are greater than 1, which suggests that a large portion of hydroxyurea accumulates in the kidney compared with plasma, the difference in ratios of Oatp1b−/− compared with DBA WT mice indicates that decreased renal accumulation is not merely a result of decreased circulating levels of hydroxyurea. There may also be Oatp1b−/−-dependent mechanisms in the kidney that accounts for the twofold decrease of renal hydroxyurea accumulation. As expected based on renal accumulation differences, a significant decrease in hydroxyurea accumulation in the urine of Oatp1b−/− mice was detected (P = 0.02; Fig. 4C).
Fig. 4.
Renal accumulation and urinary excretion of hydroxyurea. A: hydroxyurea accumulation in the kidneys of DBA wild-type and Oatp1b−/− mice (***P = 0.0006). B: kidney-to-plasma ratio of hydroxyurea levels in DBA wild-type and Oatp1b−/− mice (*P = 0.04). C: hydroxyurea accumulation in the urine of DBA wild-type (gray squares) and Oatp1b−/− (black triangles) mice (n = 5; **P = 0.02).
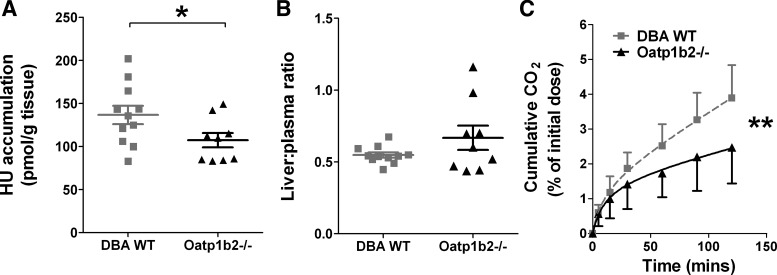
Hepatic elimination was examined by measuring hydroxyurea accumulation in the liver as well as the production of 14CO2 metabolite. Accumulation in the livers of the Oatp1b2−/− mice was slightly less than accumulation in DBA WT mice, which measured 107.3 and 136.6 pmol/g, respectively (Fig. 5A). This modest difference was statistically significant (P = 0.05), but the liver-to-plasma ratio was not different between the two groups, with mean ratios <1 (Fig. 5B). Because hydroxyurea may be metabolized by liver enzymes to form CO2 metabolite (1), the amount of exhaled 14CO2 was measured after [14C]hydroxyurea administration using a breath test assay. Less than 5% of the administered dose of hydroxyurea was exhaled as the 14CO2 metabolite over a 2-h period in the DBA WT mice, and this amount metabolite was decreased in the Oatp1b−/− mice (P = 0.035; Fig. 5C). These studies of hydroxyurea elimination indicate that Oatp1b2 transporters are involved in the renal and hepatic processes of excretion and/or metabolism of hydroxyurea.
Fig. 5.
Hepatic accumulation and exhaled CO2 metabolite of hydroxyurea. A: hydroxyurea accumulation in liver of DBA wild-type and Oatp1b−/− mice (*P = 0.05). B: liver-to-plasma ratio of hydroxyurea in DBA wild-type and Oatp1b−/− mice. C: cumulative amount of 14CO2 exhaled by DBA wild-type (gray squares) and Oatp1b−/− (black triangles) mice measured by breath test (n = 5–10; **P = 0.035).
DISCUSSION
Numerous recent studies have demonstrated that transporters play an integral role in modulating the PK of endogenous substrates as well as xenobiotics. Previously, our in vitro studies were the first to identify specific SLC transporters that mediate the transmembrane movement of hydroxyurea (30). Here, it was hypothesized that SLC transporters may be important for determining hydroxyurea PK in vivo. Results of the present study support this hypothesis, showing that mice deficient in the Oatp1b transporter have an altered disposition of hydroxyurea, specifically differences in systemic exposure and excretion. These PK studies provide the first evidence that transmembrane transporters may be important modulators of hydroxyurea PK in vivo and could help explain interindividual PK differences.
The modulators of hydroxyurea PK have not been elucidated to date, but the present study provides compelling evidence that OATP1B transporters may be involved. OATP1A and OATP1B transporters were found to mediate hydroxyurea intracellular accumulation in previous studies. Here, hydroxyurea PK was also evaluated in both Oatp1a/1b−/− and Oatp1b−/− mice. In both mice genotypes, a significant decrease in systemic exposure was observed compared with wild-type mice (Fig. 2). In contrast to the Oatp1b2−/−, larger differences in hydroxyurea concentrations between Oatp1a/1b−/− and wild-type mice were noted at the earlier time points. The exaggerated differences at the early time points suggest a contribution of the Oatp1a transporters in addition to the Oatp1b2 transporters for affecting hydroxyurea PK. Because of the differences noted at early time points and the fact that Oatp1a transporters are predominantly found in the small intestine, it is most likely these transporters contribute to absorption of hydroxyurea (13). During in vivo experiments to study a specific transporter, compensatory transporters can sometimes mask the effects of the transporter of interests. In Oatp1b−/− mice, after confirming the lack of compensatory expression of Oatp1a and other SLC transporters in the stomach and duodenum, we conclude that the decrease in hydroxyurea AUC in Oatp1b−/− is at least partially mediated by Oatp1b2 transporters. Despite the possibility of increased function or affinity of other transporters, a 20% decrease in hydroxyurea AUC in the absence of Oatp1b2 was observed, suggesting that this transporter has an influential role in determining the PK of hydroxyurea.
The findings of this study are likely relevant in identifying modulators of hydroxyurea disposition in humans. Because of the functional homology that has been demonstrated between the rodent Oatp1b2 transporters and human OATP1B1 and 1B3 transporters (29), the Oatp1b knockout mouse provides a suitable model for investigating the PK of hydroxyurea. Based on what is currently known, this mouse model recapitulates the PK of hydroxyurea. Our data confirm that hydroxyurea is rapidly absorbed and excreted primarily through the kidneys, with some evidence of hepatic elimination including the excretion of CO2 metabolite (1, 9). In this study, the PK profiles of the mice demonstrated rapid absorbance with detectable levels of hydroxyurea in plasma as early as 5 min and concentrations peaking around 60 min after an oral dose (Fig. 2). It was also observed that the primary route of excretion was through renal elimination as indicated by the high kidney-to-plasma ratio and the high percentage of hydroxyurea measured in the urine (Fig. 4). Furthermore, a small degree of hepatic elimination of hydroxyurea was measured in these murine models as noted by liver accumulation and excretion of the CO2 metabolite (Fig. 5). The absence of the Oatp1b2 created a notable change in the distribution and elimination of hydroxyurea in this model. Because the model recapitulates key aspects of human hydroxyurea disposition, the results presented here suggest that Oatp1b transporters may be modulators of hydroxyurea PK in humans.
The results from this study also indicate a potential role for OATP1B transporters beyond their function in hepatic uptake and elimination. OATP1B transporters are highly expressed on the sinusoidal membrane of hepatocytes and are generally thought to be liver specific. These transporters have been implicated in the hepatic elimination of bilirubin and various xenobiotics. The absence of the Oatp1a/1b transporters in a mouse model resulted in the increase in plasma and urinary levels of bilirubin. There is also a significant increase in the plasma and decrease in the liver and intestine of methotrexate and paclitaxel and (28, 29). In the present study, hepatic accumulation of hydroxyurea was found to be minimal, yet there was a significant change in systemic exposure. Analysis of mRNA expression resulted in the detection of the transcript at low levels in both the duodenum and the stomach, which is consistent with other studies (20). Microarray analysis verified that no other transporter transcripts were increased or decreased in Oatp1b−/− compared with the DBA WT mice in the stomach and duodenum (Fig. 3). However, the change occurred only after oral administration and not after intravenous injection, indicating a potential role in for the Oatp1b transporters in gastrointestinal drug absorption.
A low level of Slco1b2 transcript has been previously detected in the kidney of DBA mice (20). Previous microarray analysis showed that expression of transporter genes in the kidneys of wild-type and Oatp1b−/− mice is similar with the exception of Slc14a2, which was increased in Oatp1b−/− mice (17). Because Slc14a2 transcript encodes urea transporter A (UTA), a protein that has been shown to mediate cellular entry of hydroxyurea (30), real-time analysis was conducted to evaluate the relative expression of this gene in wild-type and Oatp1b−/− mice. In these studies, differential expression of Slc14a2 or UTA protein was not detected by real-time PCR, suggesting that there was no compensatory upregulation of UTA in Oatp1b−/− mice. Since no changes in transporter or enzyme expression in the kidney were detected, the significant decrease in renal accumulation and urinary excretion observed in Oatp1b−/− mice indicate a potential role for Oatp1b2 transporters in the kidney. Taken together, these results suggest a potential role for the OATP1B transporter separate from its documented role in the liver. This finding is supported by studies by Ramsey and colleagues, which showed that the presence of functional variants of the OATP1B1 transporter was associated with changes in the clearance of methotrexate (24, 25), a drug that mostly is eliminated via urinary excretion (2).
The clinical translation of these results could potentially involve in-depth pharmacogenetic studies. Here, the OATP1B family of transporters was tested, yielding results that provide proof-of-concept that transporters may be important modulators of hydroxyurea PK. If these transporters and others are important for determining PK properties of hydroxyurea, then pharmacogenetic analysis of mutations and polymorphisms could help explain interpatient variability. It is important to investigate the role of relevant transporters and their transporter functional variants in modulating hydroxyurea PK. Pharmacogenetic studies related to cancer therapies have identified specific variants in OATP1B transporters that are associated with drug disposition. In addition, although urea transporter function in hydroxyurea PK has not been evaluated in vivo, hydroxyurea pharmacogenetic analysis in pediatric sickle cell patients has identified significant associations between genetic variants of the urea transporters and AUC and maximum concentration of hydroxyurea (32). Additional pharmacogenetic studies to investigate PK changes related to transporter variants are needed for evaluation of hydroxyurea use in patients with sickle cell anemia.
In conclusion, increased knowledge about mechanisms driving pharmacologic efficacy of hydroxyurea is needed to increase the effectiveness of hydroxyurea therapy for individuals with SCA. Because drug efficacy may be directly related to drug disposition, identification of mechanisms that modulate hydroxyurea PK will play an important role in understanding and improving hydroxyurea efficacy. Results from the present study suggest an influential role of transporters in mediating hydroxyurea PK. Though these studies do not fully elucidate how OATP1B transporters impact the specific processes of absorption, distribution, and renal excretion of hydroxyurea, these studies clearly demonstrate that the absence of functional OATP1B transporters can decrease systemic exposure of hydroxyurea. This knowledge is important for future studies seeking to understand the interindividual variability of hydroxyurea PK and efficacy, and it may contribute to strategies for improving hydroxyurea treatment for sickle cell patients.
GRANTS
This study was supported by the American Lebanese Syrian Associated Charities (ALSAC), National Cancer Institute Cancer Center Support Grant 3P30 CA-021765, and the Diggs Sickle Cell Scholar Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.L.W. and A.S. conception and design of research; A.L.W. and C.S.L. performed experiments; A.L.W. and D.F. analyzed data; A.L.W. and A.S. interpreted results of experiments; A.L.W. prepared figures; A.L.W. drafted manuscript; A.L.W., R.E.W., and A.S. edited and revised manuscript; A.L.W., C.S.L., D.F., R.E.W., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the Animal Resource Center and Hartwell Center for Bioinformatics at St. Jude Children's Research Hospital for assistance in these research studies. We thank Richard Kim and Jeffrey Stock for providing the Oatp1b−/− Oatp1b−/− mice.
Footnotes
This article is the topic of an Editorial Focus by Courtney D. Thornburg (26a).
REFERENCES
- 1.Adamson RH, Ague SL, Hess SM, Davidson JD. The distribution, excretion, and metabolism of hydroxyurea-C14. J Pharmacol Exp Ther 150: 322–327, 1965 [PubMed] [Google Scholar]
- 2.Balis FM, Savitch JL, Bleyer WA. Pharmacokinetics of oral methotrexate in children. Cancer Res 43: 2342–2345, 1983 [PubMed] [Google Scholar]
- 3.Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, Milner PF, Orringer EP, Phillips G, Jr, Platt OS. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 79: 2555–2565, 1992 [PubMed] [Google Scholar]
- 4.Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (OATPS). Drug Metab Dispos 33: 1062–1073, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Colvin M, Bono VH., Jr. The enzymatic reduction of hydroxyurea to urea by mouse liver. Cancer Res 30: 1516–1519, 1970 [PubMed] [Google Scholar]
- 6.Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: studies in Oatp1b2-null mice. Hepatology 53: 272–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Graan AJM, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, de Bruijn P, Hu S, Gibson AA, Bruun GH, Corydon TJ, Mikkelsen TS, Walker AL, Du G, Loos WJ, van Schaik RHN, Baker SD, Mathijssen RHJ, Sparreboom A. Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res 18: 4433–4440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Montalembert M, Bachir D, Hulin A, Gimeno L, Mogenet A, Bresson JL, Macquin-Mavier I, Roudot-Thoraval F, Astier A, Galacteros F. Pharmacokinetics of hydroxyurea 1,000 mg coated breakable tablets and 500 mg capsules in pediatric and adult patients with sickle cell disease. Haematologica 91: 1685–1688, 2006 [PubMed] [Google Scholar]
- 9.Gwilt PR, Tracewell WG. Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin Pharmacokinet 34: 347–358, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hagenbuch B, Meier P. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch 447: 653–665, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Yakubu M, Kim-Shapiro DB, King SB. Rat liver-mediated metabolism of hydroxyurea to nitric oxide. Free Radic Biol Med 40: 1675–1681, 2006 [DOI] [PubMed] [Google Scholar]
- 12.International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KLR, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov 9: 215–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci 33: 100–108, 2012 [DOI] [PubMed] [Google Scholar]
- 14.König J. Uptake transporters of the human OATP family. In: Drug Transporters, edited by Fromm MF, Kim RB. Berlin: Springer, 2011, p. 1–28 [DOI] [PubMed] [Google Scholar]
- 15.König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275: 23161–23168, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Lancaster CS, Bruun GH, Peer CJ, Mikkelsen TS, Corydon TJ, Gibson AA, Hu S, Orwick SJ, Mathijssen RH, Figg WD, Baker SD, Sparreboom A. OATP1B1 polymorphism as a determinant of erythromycin disposition. Clin Pharmacol Ther 92: 642–650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther 12: 1537–1544, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebensburger JD, Pestina TI, Ware RE, Boyd KL, Persons DA. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica 95: 1599–1603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letschert K, Keppler D, Konig J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8). Pharmacogenetics 14: 441–452, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Meyer zu Schwabedissen HE, Ware JA, Tirona RG, Kim RB. Identification, expression, and functional characterization of full-length and splice variants of murine organic anion transporting polypeptide 1b2. Mol Pharm 6: 1790–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi T, Tamai I. Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet 27: 106–121, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Paule I, Sassi H, Habibi A, Pham K, Bachir D, Galacteros F, Girard P, Hulin A, Tod M. Population pharmacokinetics and pharmacodynamics of hydroxyurea in sickle cell anemia patients, a basis for optimizing the dosing regimen. Orphanet J Rare Dis 6: 30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest 74: 652–656, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey LB, Bruun GH, Yang W, Treviño LR, Vattathil S, Scheet P, Cheng C, Rosner GL, Giacomini KM, Fan Y, Sparreboom A, Mikkelsen TS, Corydon TJ, Pui CH, Evans WE, Relling MV. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res 22: 1–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, Martin PL, Cheng C, Devidas M, Pui CH, Evans WE, Hunger SP, Loh M, Relling MV. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood 121: 898–904, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez GI, Kuhn JG, Weiss GR, Hilsenbeck SG, Eckardt JR, Thurman A, Rinaldi DA, Hodges S, Von Hoff DD, Rowinsky EK. A bioavailability and pharmacokinetic study of Oral and intravenous hydroxyurea. Blood 91: 1533–1541, 1998 [PubMed] [Google Scholar]
- 26a.Thornburg CD. Sickle cell anemia: time for personalized prescription of hydroxyurea? Focus on “Organic anion transporting polypeptide 1B transporters modulate hydroxyurea pharmacokinetics.” Am J Physiol Cell Physiol (September 4, 2013). 10.1152/ajpcell.00268.2013 [DOI] [PubMed] [Google Scholar]
- 27.van de Steeg E, Stránecký V, Hartmannová H, Nosková L, Hřebíček M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, Sticová E, al-Edreesi M, Knisely AS, Kmoch S, Jirsa M, Schinkell AH. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest 122: 519–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Steeg E, van Esch A, Wagenaar E, Kenworthy KE, Schinkel AH. Influence of Human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin Cancer Res 19: 821–832, 2013 [DOI] [PubMed] [Google Scholar]
- 29.van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest 120: 2942–2952, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker AL, Franke RM, Sparreboom A, Ware RE. Transcellular movement of hydroxyurea is mediated by specific solute carrier transporters. Exp Hematol 39: 446–456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 115: 5300–5311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware RE, Despotovic JM, Mortier NA, Flanagan JM, He J, Smeltzer MP, Kimble AC, Aygun B, Wu S, Howard T, Sparreboom A. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 118: 4985–4991, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Invest 83: 688–697, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol 74: 320–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]