Abstract
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease (ESRD). The inhibitors of renin-angiotensin-aldosterone system (RAAS) can alleviate some of the symptoms of DN but fail to stop the progression to ESRD. Our previous studies demonstrate renoprotective action of nitro-oleic acid (OA-NO2) in several rodent models of renal disease. Here we examined the therapeutic potential and the underlying mechanism of combination of losartan and OA-NO2 in db/db mice. OA-NO2 was infused at 5 mg·kg−1·day−1 via osmotic minipump, and losartan was incorporated into diet at 10 mg·kg−1·day−1, each administered alone or in combination for 2 wk. Diabetic db/db mice developed progressive albuminuria and glomerulosclerosis, accompanied by podocytes loss, increased indexes of renal fibrosis, oxidative stress, and inflammation. Treatment of the diabetic mice with OA-NO2 or losartan alone moderately ameliorated kidney injury; however, the combined treatment remarkably reduced albuminuria, restored glomerular filtration barrier structure, and attenuated glomerulosclerosis, accompanied with significant suppression of renal oxidative stress and inflammation. These data demonstrate that combination of losartan and OA-NO2 effectively reverses renal injury in DN.
Keywords: diabetic nephropathy, losartan, nitro-oleic acid, oxidative stress
type 2 diabetes mellitus is an increasingly prevalent disease around the globe. Diabetic nephropathy (DN) is one of the most common complications in diabetes and the major cause of end-stage kidney disease (ESRD) with high mortality (33). Hyperglycemia is the main determinant for diabetic nephropathy, which promotes glomerular injury through multiple pathways, including stimulation of profibrotic and proinflammatory factors and increase of oxidative stress. Early alterations in DN include glomerular hyperfiltration, glomerular and tubular epithelial hypertrophy, glomerular basement membrane (GBM) thickening, and development of microalbuminuria, followed by progressive accumulation of extracellular matrix (ECM) proteins in the mesangium and overt proteinuria, eventually leading to glomerulosclerosis and ESRD (9). Despite progress in the prevention and treatment of DN, reversal and even stabilization of the development of DN are still difficult to achieve. The renin-angiotensin system (RAS) is an important mediator of progressive renal injury in DN. Inhibitors of the RAS including angiotensin (ANG)-converting enzyme inhibitors and ANG II type 1 receptor blockers (ARBs) have been shown to attenuate glomerulosclerosis, tubulointerstitial fibrosis, and proteinuria (2, 6, 26). However, these treatments often fail to stop the disease progression to ESRD. New approaches that would broaden the spectrum of available treatments for DN are needed to improve prognosis in diabetic patients.
Recently, nitrated free fatty acids (NO2-FA), notably nitroalkene derivatives of linoleic acid [nitrolinoleic acid (LNO2)] and oleic acid (OA-NO2) are found to be endogenous molecules with several attractive signaling properties (4, 35). Nitro-fatty acid derivatives (NO2-FA) are electrophilic lipid signaling mediators generated endogenously by nitric oxide (NO) and nitrite (NO2−)-derived reactive species during oxidative stress (17). NO2-FA serves as ligands for peroxisome proliferator-activated receptors that regulate the expression of cell differentiation, development, and inflammation (34). NO2-FA S-alkylates critical thiols of Keap-1, inducing the release of Nrf2 and activation of antioxidant response element-dependent gene products such as heme oxygenase (HO)-1 (5, 22–24, 42, 45). NO2-FA covalently bind the p65 subunit of nuclear factor-κB, inhibiting DNA binding activity, repressing nuclear factor κB-dependent gene expression, and suppressing downstream proinflammatory reactions such as macrophage cytokine and nitric oxide synthase-2 expression (10).
Our recent studies have provided evidence that OA-NO2 protects against the renal injuries caused by kidney ischemia/reperfusion or lipopolysaccharide-induced sepsis (28, 44). We also demonstrated that OA-NO2 treatment reduced proteinuria in obese Zucker rats (43). However, there is no experimental study to test the effect of OA-NO2 on DN. The aim of the present study was to investigate the therapeutic potential and the underlying mechanism of administration of OA-NO2 alone or in combination with an ARB for treatment of DN in diabetic db/db mice.
MATERIALS AND METHODS
Materials.
OA-NO2 was provided by Dr. Bruce A. Freeman (University of Pitssburgh) as a gift. Losartan potassium was purchased from TEVA Pharmaceuticals. All other reagents were purchased from Sigma-Aldrich unless otherwise specified.
Animals and treatments.
Leprdb/db(db/db) and Leprdb/m (db/m) mice in C57BLKS/J background were purchased from the Jackson Laboratories (Bar Harbor, ME). Twelve-week-old male db/db mice were randomized to receive the ethanol vehicle, losartan, OA-NO2, or the combination of losartan and OA-NO2 for 2 wk. Losartan was administered at 10 mg·kg−1·day−1 via diet. OA-NO2 was dissolved in ethanol and infused at 5 mg·kg−1·day−1 via osmotic minipump (DURECT, Cupertino, CA). The db/m mice served as the nondiabetic control. Food intake and water intake were determined every week; body weight, food intake, and 24-h urine collections were determined at the end of the experiments. Animals were fasted from 6:00 PM to 9:00 AM before blood sampling. Twenty-four-hour urine was collected using metabolic cages. All mice were killed 2 wk after treatments, and the kidneys were immediately harvested for protein or RNA extraction or for histological analyses. All protocols employing mice were conducted in accordance with the principles and guidance of the University of Utah Institutional Animal Care and Committee. Blood glucose levels were monitored with CONTOUR blood glucose monitoring system (Bayer Healthcare, Mishawaka, IN). Hematocrit was determined as previously described (46).
Histology and immunohistochemistry.
While the animals were under anesthesia, kidneys were removed and fixed with 4% paraformaldehyde. The tissues were subsequently embedded in paraffin, and 3-μm sections were cut and stained with periodic acid-Schiff (PAS). Semiquantitative scoring of glomerular sclerosis in PAS-stained slides was performed by a renal pathologist (Y. Hu) using a five-grade method described previously (12, 41, 48): 0, normal glomerulus; 1, sclerosis <25% of glomerular surface; 2, sclerosis between 25 and 50%; 3, sclerosis between 50 and 75%; and 4, sclerosis >75% of glomerular surface. Semiquantitative mesangial area was measured using computer software ImageJ (National Institutes of Health, Bethesda, MD). Thirty glomeruli were randomly selected for the quantitative analyses. Immunohistochemical staining was performed using EnVision TM FLEX Mini Kit (Dako, Carpinteria, CA) according to the manufacturer's instructions. Anti-WT1 antibody was purchased from Dako (Mob437, Dako).
Quantitative RT-PCR.
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and first-strand cDNAs were synthesized from 2 μg of total RNAs in 20 ml reaction using SuperScript (Invitrogen). The first-strand cDNAs served as the template for quantitative PCR performed in Applied Biosystems 7900 Real Time PCR System using SYBR green PCR reagent (Applied Biosystems, Foster City, CA). The amplification was carried out for 40 cycles with conditions of 15-s denaturation at 95°C and 1-min annealing at 60°C. The sequence of oligonucleotides used for quantitative PCR is listed as follows: GAPDH sense: 5′-GTCTTCACTACCATGGAGAAGG-3′ and antisense: 5′-TCATGGATGACCTTGGCCAG-3′; fibronectin (FN) sense: 5′-CGTGGAGCAAGAAGGACAA-3′ and antisense: 5′-GTGAGTCTGCGGTTGGTAAA-3′; plasminogen activator inhibitor-1 (PAI-1) sense: 5′-TGGTGAACGCCCTCTATTTC-3′ and antisense: 5′- GAGGGGCACATCTTTTTCAA-3′; smooth muscle actin-α (SMAα) sense: 5′-CCCTGAAGAGCATCCGACA-3′ and antisense: 5′-CCAGAGTCCAGCACAATACC-3′; transforming growth factor-β (TGF-β) sense: 5′-TACGCCTGAGTGGCTGTCTT-3′ and antisense: 5′-CGTGGAGTTTGTTATCTTTGCT-3′; WT1 sense: 5′-AGTTCCCCAACCATTCCTTC-3′ and antisense: 5′-ATTCAAGCTGGGAGGTCATTT-3′; p47phox sense: 5′-GTCGTGGAGAAGAGCGAGAG-3′ and antisense: 5′-CGCTTTGATGGTTACATACGG-3′; and NOX4 sense: 5′-AACCAGACATCATCCCAGAA-3′ and antisense: 5′-GGTCCAGAAATCCAAATCCA-3′.
Immunoblotting.
The kidney lysates were stored at −80°C until assayed. Protein concentrations were determined using Coomassie reagent. An equal amount of the whole tissue protein was denatured at 100°C for 10 min, separated by SDS-PAGE, and transferred onto nitrocellulose membranes. The blots were blocked overnight with 5% nonfat dry milk in TBS, followed by incubation for 1 h with primary antibody. The blots were washed with TBS followed by incubation with horseradish peroxidase-conjugated secondary antibody. Immune complexes were detected using ECL methods. The immunoreactive bands were quantified using the Gel and Graph Digitizing System (Silk Scientific, Tustin, CA). The antibodies used are listed as follows: anti-fibronectin antibody (F3648; Sigma-Aldrich); anti-PAI-1 antibody (WH0005054M1; Sigma-Aldrich); anti-β-actin antibody (A1978, Sigma-Aldrich); Anti-p47phox antibody (610355; BD Biosciences, San Jose, CA); anti-NOX-4 antibody (NB110; Novus, Littleton, CO); and anti-HO-1 antibody (ADI-SPA-896-D; Enzo Life Sciences, Farmingdale, NY).
Measurement of thiobarbituric acid-reactive substances.
The measurement of thiobarbituric acid-reactive substances (TBARS) in the mouse kidney was based on the formation of malondialdehyde by using a commercially kit (catalog no. 10009055; Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Enzyme immunoassay.
Enzyme immunoassay was performed to measure the concentration of PGE2 (Cayman Chemical), albumin (EXOCELL, Philadelphia, PA), and TNF-α (BD Bioscience) in the biological fluid or tissue homogenates according to the manufacturers' instructions.
Statistical analysis.
Values shown represent means ± SE. Data were analyzed using unpaired t-test or ANOVA followed by a Bonferroni posttest. A P value <0.05 was considered significant.
RESULTS
Body weight, kidney weight, blood glucose concentrations, and hematocrit.
The db/db mice on C57BLKS/J background were chosen in the present study since they develop detectable DN (19, 20); 12-wk-old male db/db mice were randomized to receive treatment with vehicle, losartan, OA-NO2, or the combination of losartan and OA-NO2 for 2 wk (43) with nonobese db/m mice as the nondiabetic control. As shown in Table 1, body weight and blood glucose were significantly greater in 12-wk-old db/db mice than those of db/m mice, and these parameters were not significantly affected by treatment with losartan, OA-NO2, or the combination of the two agents. The ratio of kidney to body weight was a little lower in the combination treatment group than the vehicle group.
Table 1.
Body weight, kidney weight, blood glucose, and hematocrit in db/db mice after drug treatment
| Lean (n = 3∼6) | Vehicle (n = 7∼12) | Losartan (n = 7∼9) | OA-NO2 (n = 7∼10) | Losartan + OA-NO2 (n = 7∼11) | |
|---|---|---|---|---|---|
| Body weight, g | |||||
| Before treatment | 46.95 ± 0.78 | 46.34 ± 0.81 | 47.23 ± 0.60 | 45.78 ± 0.59 | |
| After treatment | 29.97 ± 1.09* | 44.64 ± 1.28 | 42.82 ± 1.28 | 46.46 ± 0.99 | 43.01 ± 0.75 |
| Kidney weight, g | |||||
| After treatment | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.02 | 0.25 ± 0.02 | 0.23 ± 0.01 |
| Kidney weight/body weight, % | |||||
| After treatment | 0.75 ± 0.02* | 0.54 ± 0.02 | 0.60 ± 0.03 | 0.53 ± 0.03 | 0.52 ± 0.02 |
| Blood glucose, mg/dl | |||||
| Before treatment | 431.14 ± 41.22 | 458.43 ± 51.02 | 455.43 ± 47.37 | 450.43 ± 38.84 | |
| After treatment | 146.67 ± 8.95* | 467.67 ± 39.96 | 411.42 ± 37.10 | 455.57 ± 42.48 | 401.85 ± 57.99 |
| Hematocrit, % | |||||
| Before treatment | 57.43 ± 0.03 | 57.96 ± 0.75 | 60.92 ± 1.10 | 57.77 ± 2.70 | |
| After treatment | 58.44 ± 3.75 | 51.01 ± 3.56 | 55.35 ± 0.49 | 53.56 ± 1.44 | 52.26 ± 0.85 |
Data are means ± SE. OA-NO2, nitro-oleic acid.
P < 0.05 vs. vehicle.
Effects of the combination of losartan and OA-NO2 on albuminuria and glomerulosclerosis.
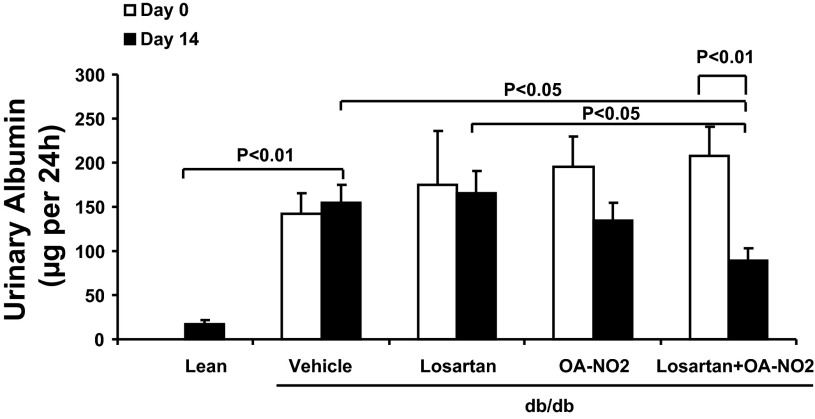
As shown in Fig. 1, there was overt albuminuria in vehicle-treated db/db mice compared with db/m mice. Albuminuria in db/db mice was unaffected by losartan and was only modestly reduced by OA-NO2. In contrast, the combination of losartan and OA-NO2 produced a significant reduction of albuminuria (P < 0.01).
Fig. 1.
Effect of the drug treatments on the development of albuminuria in db/db mice. Urinary albumin was detected before and after 2 wk infusion with vehicle, losartan, nitro-oleic acid (OA-NO2), and losartan + OA-NO2. Lean mice with vehicle treatment were used as controls. Lean: n = 4; vehicle: n = 12; losartan: n = 8; OA-NO2: n = 10; losartan + OA-NO2: n = 11. Data are means ± SE.
To correlate the changes in albuminuria with glomerular structure, glomerulosclerosis was assessed by periodic acid-Schiff (PAS) staining. Consistent with the data on albuminuria, vehicle-treated db/db mice showed marked glomerulosclerosis as evidenced by expanded mesangium consisting of increased accumulation of ECM compared with db/m mice (Fig. 2). The losartan or OA-NO2 alone did not significantly reduce glomerulosclerosis in db/db mice, but the combination of the two agents produced a marked improvement of the glomerular structure. This observation was confirmed by a semiquantitative glomerulosclerotic index of kidney sections.
Fig. 2.
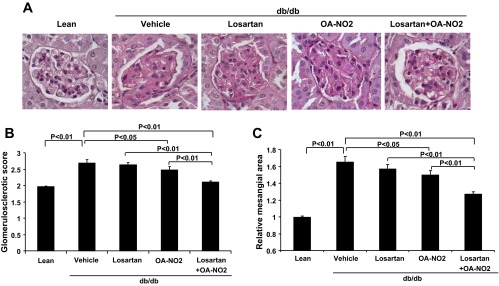
Effect of the drug treatments on the development of glomerulosclerosis in db/db mice. A: representative glomerular morphology after 2 wk infusion with vehicle, losartan, OA-NO2, and losartan + OA-NO2. Lean mice with vehicle treatment were used as controls. The sections were stained with periodic acid-Schiff (PAS). B: semiquantitative glomerulosclerotic scores. C: relative mesangial areas. Lean: n = 5; vehicle: n = 8; losartan: n = 6; OA-NO2: n = 7; losartan + OA-NO2: n = 8. Data are means ± SE.
The extent of glomerulosclerosis was further evaluated at molecular level, e.g., the determination of expression of FN, PAI-1, SMAα, collagen IV, and TGF-β in the kidney. Quantitative RT-PCR demonstrated increases in FN and PAI-1 mRNA expression in vehicle-treated db/db mice relative to the nondiabetic control (Fig. 3, A and B). Similar results were obtained by immunoblotting (Fig. 3, F, G, and H). The combination of losartan and OA-NO2 reversed the upregulation of FN and PAI-1 in the diabetic mice (P < 0.01). In addition, the mRNA expression of several other fibrosis/sclerosis-related genes in the kidney was upregulated in the vehicle-treated db/db mice, including SMAα (Fig. 3C), collagen-IV (Fig. 3D), and TGF-β (Fig. 3E). The combination of losartan and OA-NO2 treatment induced a dramatic suppression of these genes in the kidney. These data are consistent with the antisclerotic effect of losartan and OA-NO2 treatment.
Fig. 3.
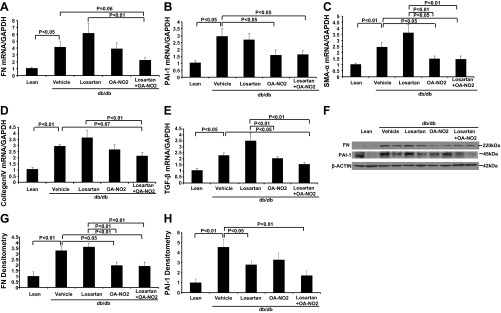
Effect of the drug treatments on fibrosis/scl-associated factors in db/db mice. A: renal fibronectin (FN) mRNA expression. B: renal plasminogen activator inhibitor-1 (PAI-1) mRNA expression. C: renal smooth muscle actin-α (SMAα) mRNA expression. D: renal collagen IV mRNA expression. E: renal transforming growth factor-β (TGF-β) mRNA expression. F: renal FN and PAI-1 protein expression. G: FN densitometry. H: PAI-1 densitometry. Lean: n = 5; vehicle: n = 8∼10; losartan: n = 6∼8; OA-NO2: n = 8∼10; losartan + OA-NO2: n = 8∼10. Data are means ± SE.
Effects of losartan and OA-NO2 combination on podocyte loss in db/db mice.
We surveyed podocytes in glomeruli by immunostaining with anti-WT1 antibody, a podocyte-specific antibody. As shown in Fig. 4, there was a marked reduction in glomerular WT1-positive cells in vehicle-treated db/db mice relative to db/m mice, and losartan and OA-NO2 combination treatment effectively reversed the decline of WT1-positive cell number in db/db mice (Fig. 4, A and B). Consistently, mRNA levels of WT1 were significantly reduced in vehicle-treated db/db mice relative to the lean controls, and treatment with losartan and OA-NO2 significantly reversed the reduction of WT1 mRNA expression (Fig. 4C).
Fig. 4.
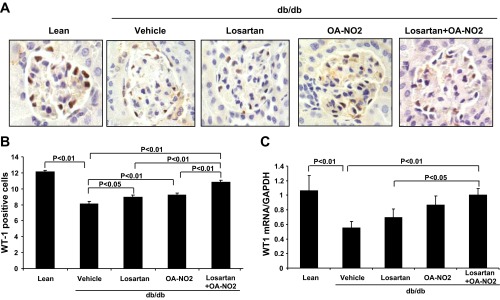
Effect of the drug treatments on glomerular podocytes injury and WT1 mRNA expression in db/db mice. A: immunostaining of kidney slides with anti-WT1 antibody after 2 wk infusion with vehicle, losartan, OA-NO2, and losartan + OA-NO2. Lean mice with vehicle treatment were used as controls. B: average WT1-positive podocytes per glomerulus. C: WT1 mRNA expression. Lean: n = 5; vehicle: n = 8∼10; losartan: n = 8; OA-NO2: n = 7∼8; losartan + OA-NO2: n = 8∼10. Data are means ± SE.
Effects of losartan and OA-NO2 combination on renal oxidative stress in db/db mice.
To investigate whether combination of losartan and OA-NO2 had antioxidative effects in db/db mice, we analyzed the levels of TBARS in the serum, kidney, and urine. The vehicle group had a marked increase in urinary (Fig. 5A), renal (Fig. 5B) and serum TBARS (Fig. 5C) compared with lean controls. Treatment with either losartan or OA-NO2 alone was without an effect on this parameter. In contrast, the concurrent administration of losartan and OA-NO2 markedly attenuated the diabetes-induced increase in renal and urinary TBARS compared with vehicle-treated db/db mice. However, serum TBARS was unaffected by the combined treatment.
Fig. 5.
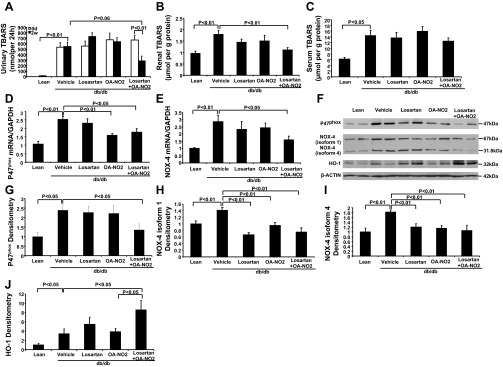
Effect of the drug treatments on oxidative stress in db/db mice. A: urinary thiobarbituric acid-reactive substances (TBARS) before and after 2 wk infusion with vehicle, losartan, OA-NO2, and losartan + OA-NO2. Lean mice with vehicle treatment were used as controls. B: renal TBARS after 2 wk infusion. C: serum TBARS. D: renal P47phox mRNA expression. E: renal NOX-4 mRNA expression. F: renal P47phox, NOX-4 (isoform 1 and 4), and heme oxygenase (HO)-1 protein expression. G: P47phox densitometry. H: NOX-4 isoform 1 densitometry. I: NOX-4 isoform 4 densitometry. J: HO-1 densitometry. Lean: n = 5; vehicle: n = 8∼10; losartan: n = 8; OA-NO2: n = 8∼10; losartan + OA-NO2: n = 8∼10. Data are means ± SE.
Furthermore, we examined the expression of renal NAD(P)H oxidase components. As shown in Fig. 5, D and E, compared with db/m mice, db/db mice exhibited increased renal mRNA expression of p47phox and NOX4 (P < 0.01). The combination of losartan with OA-NO2 conferred great reduction of p47phox and NOX4 mRNA expression in db/db mice (P < 0.05). Western blotting analysis confirmed that the protein levels for p47phox and NOX4 were significantly increased in the diabetic kidney (Fig. 3, F, G, H, and I), and this increase was reversed by losartan and OA-NO2 combination treatment.
Because previous studies have shown that HO-1 expression is induced in response to oxidative insults under pathophysiological conditions, we also assessed the renal HO-1 expression (Fig. 5, F and J). Induction of diabetes significantly increased renal HO-1 expression in db/db mice compared with the lean control (P < 0.05). However, the HO-1 protein levels were much higher after losartan and OA-NO2 combination treatment compared with the vehicle mice (P < 0.05).
Effects of losartan and OA-NO2 combination on renal inflammation in db/db mice.
As shown in Fig. 6A, ELISA analysis showed significantly increased protein levels of TNF-α in the kidney of vehicle-treated db/db mice compared with the lean controls (P <0.01). Compared with vehicle mice, this increase was reduced in the combination treatment animals.
Fig. 6.
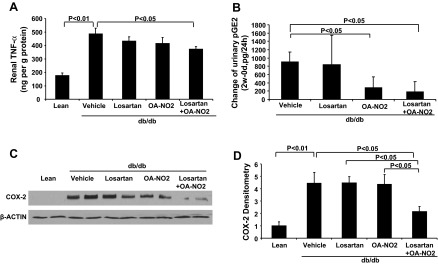
Effect of the drug treatments on inflammatory response in db/db mice. A: renal TNF-α protein contents after 2 wk infusion with vehicle, losartan, OA-NO2, and losartan + OA-NO2. Lean mice with vehicle treatment were used as controls. B: change of urinary pGE2 contents after a 2-wk infusion. C: COX-2 protein expression. G: COX-2 densitometry. Lean: n = 4∼5; vehicle: n = 8∼11; losartan: n = 4∼8; OA-NO2: n = 7∼8; losartan + OA-NO2: n = 8∼9. Data are means ± SE.
Like TNF-α, COX-2 is highly inducible by proinflammatory stimuli and is another important marker of inflammatory responses. In the present study, renal COX-2 protein level was much greater in db/db mice vs. lean control mice (Fig. 6, C and D); losartan and OA-NO2 combination treatment attenuated the induction of COX-2 in db/db mice. Consistently, urinary excretion of PGE2, a major product of COX-2, exhibited a marked decrease after treatment with losartan and OA-NO2 (Fig. 6B).
DISCUSSION
The current study developed a new strategy for type 2 diabetic nephropathy treatment by using OA-NO2 in combination with losartan. The comprehensive examination performed in this study defines that the combination strategy produced effective therapeutic synergism in the reversion of renal injury in type 2 diabetic db/db mice, an experimental diabetes model.
Our data show that short-term (2 wk) treatment with either low-dose losartan or OA-NO2 alone was not able to significantly attenuate diabetic renal injury. The combination of losartan and OA-NO2 had much better therapeutic effects against renal injury than losartan or OA-NO2 alone. The cotreatment markedly reversed albuminuria, which is regarded as a major index of progression of DN. Although the mechanism of albuminuria in diabetes remains to be elucidated, the pathogenesis of albuminuria is thought to be mainly attributed to the alterations of glomerular filtration barrier (39). In this study, the development of albuminuria in the vehicle-treated diabetic mice was accompanied by glomerulosclerosis and decrease in podocyte number. The glomerulosclerosis seen in the diabetic mice was manifested by increased production of ECM proteins in the mesangium. Most of these pathological changes were reversed by the combination therapy.
In recent years, oxidative stress has emerged as an important pathogenic factor in the development of diabetic nephropathy (7, 15). Notably, in this study, we showed that combination therapy with losartan and OA-NO2 ameliorated albuminuria concomitantly with a reduction of urinary and renal tissue TBARS. Therefore, the protective effect of losartan and OA-NO2 against DN is associated with the suppression of oxidative stress. To elucidate source of ROS generation, we examined the effect of combination of losartan and OA-NO2 on NADPH oxidase subunits expressed in the kidney. Interestingly, we found that cotreatment with losartan and OA-NO2 significantly attenuated mRNA and protein expression for NADPH oxidase subunit NOX4 and p47phox in db/db mice. NADPH oxidase system is a major superoxide-generating system; NOX4 and p47phox are of particular importance as the former contains the catalytic domain and the latter is necessary for cytosolic subunit translocation and for initiation of NADPH oxidase assembly in kidney (3, 18, 36). These results showed that the beneficial effects of the combination therapy may be mediated by downregulation of NOX4 and p47phox expression. In addition, HO-1 is found to be significantly induced in response to the cotreatment of losartan and OA-NO2 in db/db mice. HO-1 plays a crucial role in protection against oxidative insult in DN (1, 13, 25), and modulating NADPH oxidase activity in the kidney seems to be an important mechanism of the renal protective actions of HO-1 (11). Consistent with previous reports, our data support that HO-1 induction inhibits renal NADPH oxidase system, which may represent an important mechanism underlying the protective effect of losartan and OA-NO2 combination against oxidative damage in db/db mice.
Recently attention has been drawn to the correlation between inflammatory activity and diabetic complications. In 1991, Hasegawa et al. (21) suggested for the first time that the proinflammatory cytokines TNF-α and interleukin-1 (IL-1) could significantly contribute to the pathogenesis of DN. After this initial study, accumulating evidence indicates that DN exhibits signs of inflammation and renal inflammation plays an important role in the pathogenesis of DN (16, 27, 29, 30, 40). It was demonstrated that diabetes increased circulating (8, 14), renal (21, 31), and urinary (32) levels of proinflammatory cytokine TNF-α renal production. Our results revealed excessive renal production of TNF-α in the early stage of DN. Administration of losartan and OA-NO2 significantly inhibited TNF-α elevation in the diabetic kidneys. COX-2 is a cytokine-inducible cyclooxygenase generating proinflammatory prostaglandins under inflammatory conditions (27, 29, 37, 38). In the present study, diabetes-induced renal COX-2 expression and PGE2 production were significantly suppressed by losartan and OA-NO2 combination treatment. Therefore, our data suggest that the anti-inflammatory action of losartan and OA-NO2 combination treatment may be attributable to their ability to suppress diverse inflammatory pathways mediated by TNF-α and COX-2. Future studies are needed to determine a causal relationship between the altered proinflammatory signals and the renoprotective action of the combination therapy.
Administration of 8 mg/kg OA-NO2 via osmotic minipump over a period of 4 wk almost normalized blood glucose levels in obese ob/ob mice, an effect comparable to that of rosiglitazone; the antiglycemic effect was observed within 4 days of minipump implantation (34). However, in the present study, a 2-wk treatment of 5 mg/kg OA-NO2 was without an effect on blood glucose levels in db/db mice. It is unclear whether the differences in the dosage or the mouse strain accounts for the discrepancy between the two studies. In the present study, the metabolic parameters including body weight and glycemia were not different among the experimental groups, suggesting that the beneficial effect of combined OA-NO2 and losartan treatment is likely a result of direct renal action rather than being secondary to overall improvement of diabetes.
The present study has a number of limitations. For example, we did not determine the mechanism for the synergistic effect between OA-NO2 and losartan in mice with DN. It is known that the ARBs selectively interfere with ANG II binding at the AT1. Zhang et al. (47) showed OA-NO2 had negative regulating effect on ANG II system. OA-NO2, but not OA, specifically binds to the AT1R, reduces heterotrimeric G-protein coupling, and inhibits inositol-1,4,5-trisphosphate and calcium mobilization, without inhibiting ANG II binding to the receptor. Taken together, these findings suggest that the synergy between OA-NO2 and ARB may lie in their different targeting sites in the ANG II signaling pathway. The present study is also limited in a short-term study in which mice were treated for only for 2 wk. Since DN is a chronic disease, it is important to determine the long-term effect of this therapy in future studies.
In summary, the present study is the first to evaluate the in vivo renoprotective action of OA-NO2 alone or in combination with losartan in db/db mice. The combination of the two agents remarkably attenuates albuminuria, glomerulosclerosis, and other indexes of kidney injury in this model, suggesting a novel intervention for DN. This study provides a strong rational for future clinical studies of the therapeutic regime in patients with type 2 diabetes.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-079162, National Basic Research Program of China 973 Program 2012CB517600 (No. 2012CB517602), Merit Review from the Department of Veterans Affairs, and an Established Investigator Award from the American Heart Association. T. Yang is a Research Career Scientist at the Salt Lake City Veterans Affairs Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., Z.J., S.L., M.D., and G.L. performed experiments; Y.L. and T.Y. analyzed data; Y.L. and T.Y. interpreted results of experiments; Y.L. prepared figures; Y.L. drafted manuscript; Y.D. and T.Y. edited and revised manuscript; T.Y. conception and design of research; T.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bruce A. Freeman (University of Pittsburgh) for providing OA-NO2 and Yongbin Hu (Central South University) for technical assistance.
REFERENCES
- 1.Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol 297: F1137–F1152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601–606, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Activation of the respiratory burst oxidase. Environ Health Perspect 102, Suppl 10: 53–56, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280: 42464–42475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker PR, Schopfer FJ, O'Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free Radic Biol Med 46: 989–1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cavallo MG, Pozzilli P, Bird C, Wadhwa M, Meager A, Visalli N, Gearing AJ, Andreani D, Thorpe R. Cytokines in sera from insulin-dependent diabetic patients at diagnosis. Clin Exp Immunol 86: 256–259, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 352: 213–219, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem 281: 35686–35698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension 50: 636–642, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int 77: 1000–1009, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Di Noia MA, Van Driesche S, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem 281: 15687–15693, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106: 2067–2072, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10–17, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem 283: 15515–15519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallin JI, Leto TL, Rotrosen D, Kwong CH, Malech HL. Delineation of the phagocyte NADPH oxidase through studies of chronic granulomatous diseases of childhood. Curr Opin Immunol 4: 53–56, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Garris DR. Ultrastructural analysis of progressive endometrial hypercytolipidemia induced by obese (ob/ob) and diabetes (db/db) genotype mutations: structural basis of female reproductive tract involution. Tissue Cell 36: 19–28, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Garris DR, Garris BL. Cytolipotoxicity-induced involution of the female reproductive tract following expression of obese (ob/ob) and diabetes (db/db) genotype mutations: progressive, hyperlipidemic transformation into adipocytic tissues. Reprod Toxicol 18: 81–91, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40: 1007–1012, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem 284: 33233–33241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol 10: 179–184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med 48: 230–239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol 14: S250–253, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012: 146154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Jia Z, Soodvilai S, Guan G, Wang MH, Dong Z, Symons JD, Yang T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am J Physiol Renal Physiol 295: F942–F949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luis-Rodriguez D, Martinez-Castelao A, Gorriz JL, De-Alvaro F, Navarro-Gonzalez JF. Pathophysiological role and therapeutic implications of inflammation in diabetic nephropathy. World J Diabetes 3: 7–18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes 42: 450–456, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Navarro JF, Milena FJ, Mora C, Leon C, Claverie F, Flores C, Garcia J. Tumor necrosis factor-alpha gene expression in diabetic nephropathy: relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int Suppl S98–102, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Navarro JF, Mora C, Rivero A, Gallego E, Chahin J, Macia M, Mendez ML, Garcia J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis 33: 458–463, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 34: 795–808, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem 285: 12321–12333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA 102: 2340–2345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor 4: 17–23, 1994 [PubMed] [Google Scholar]
- 38.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91: 12013–12017, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia 42: 878–886, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol 14: 968–980, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol 293: H770–H776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Liu H, Jia Z, Guan G, Yang T. Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese zucker rats. PPAR Res 2010: 601562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am J Physiol Renal Physiol 298: F754–F762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright MM, Kim J, Hock TD, Leitinger N, Freeman BA, Agarwal A. Human haem oxygenase-1 induction by nitro-linoleic acid is mediated by cAMP, AP-1 and E-box response element interactions. Biochem J 422: 353–361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 102: 9406–9411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res 107: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163–171, 2008 [DOI] [PubMed] [Google Scholar]