Abstract
The epithelial sodium channel (ENaC) is comprised of three homologous subunits. Channels composed solely of α- and β-subunits (αβ-channels) exhibit a very high open probability (Po) and reduced sensitivity to amiloride, in contrast to channels composed of α- and γ-subunits or of all three subunits (i.e., αγ- and αβγ-channels). A mutant channel comprised of α- and β-subunits, and a chimeric γ-subunit where the region immediately preceding (β12 and wrist) and encompassing the second transmembrane domain (TM2) was replaced with the corresponding region of the β-subunit (γ-βTM2), displayed characteristics reminiscent of αβ-channels, including a reduced amiloride potency of block and a loss of Na+ self-inhibition (reflecting an increased Po). Substitutions at key pore-lining residues of the γ-βTM2 chimera enhanced the Na+ self-inhibition response, whereas key γ-subunit substitutions reduced the response. Furthermore, multiple sites within the TM2 domain of the γ-subunit were required to confer high amiloride potency. In summary, we have identified novel pore-lining residues of the γ-subunit of ENaC that are important for proper channel gating and its interaction with amiloride.
Keywords: ENaC, amiloride, open probability, sodium, subunit
the epithelial na+ channel (ENaC)/degenerin family encodes a group of structurally related ion channels that are highly selective for Na+ and sensitive to amiloride and its derivatives (16, 21). Members of the ENaC/degenerin family are involved in many fundamental biological processes, including Na+ absorption and volume regulation (ENaCs), mechanosensation (ENaCs and Caenorhabditis elegans degenerins), and nociception [acid-sensing ion channels (ASICs); Refs. 11, 16, 44]. ENaCs are heterotrimeric channels composed of three homologous subunits, termed α, β, and γ. The second transmembrane helix from each subunit contributes to the channel pore where the gate, selectivity filter, and amiloride binding site likely reside (16, 32, 37, 43).
ENaCs with different subunit compositions have been reconstituted in heterologous expression systems (4, 12, 14, 27). Previous studies by Fyfe and Canessa (12) and by McNicholas and Canessa (27) have shown that rat ENaCs composed solely of α- and β-subunits exhibit functional properties that differ from channels including the γ-subunit (i.e., αβγ-channels or αγ-channels). When expressed in Xenopus oocytes, αβ-channels were more permeable to Na+ than Li+ and less sensitive to amiloride and related analogs (27). At the single channel level, the αβ-channel displayed a smaller conductance and higher open probability (Po; Ref. 12). These observations suggest that the γ-subunit has roles in restraining ENaC activity and enhancing the potency of amiloride block on channel activity. However, the sites within the γ-subunit that account for distinct properties of αβγ- and αγ-channels, compared with αβ-channels, have not been elucidated.
The functional differences between αβ- and αβγ-channels are relevant for a mice model where overexpression of the β-subunit results in a lung phenotype similar to that observed in humans with cystic fibrosis, with reduced airway surface liquid volume due to increased ENaC activity (25). The authors suggested that the presence of αβ-channels in airways contributed to this phenotype, reflecting a high baseline Po and a lack of channel inhibition by the cystic fibrosis transmembrane conductance regulator (26).
In the current study, we generated and examined the properties of channels bearing γ-β-chimeric subunits, as well as channels with mutations at specific sites, to define sites within the γ-subunit that are responsible for conferring the high amiloride potency and the moderate channel Po seen in channels containing a γ-subunit, compared with αβ-channels. We found that the pore-forming region of the γ-subunit (S529 - I556) contains key sites that contribute to the high amiloride potency and the moderate channel Po.
EXPERIMENTAL PROCEDURES
ENaC subunit domain swap and site-directed mutagenesis.
Wild-type β- and γ-subunits of mouse ENaC in pBluescript SK− vector were used as templates to generate a series of five chimeric γ-subunits by replacing specific regions of the γ-subunit with the corresponding regions of the β-subunit: 1) the NH2 terminus and the first transmembrane domain (TM1) of the β-subunit were inserted into the γ-subunit [γ-βNTM1 (residues β1–78 and γ81–655)], 2) the β-subunit extracellular domain (ECD) was inserted into the γ-subunit [γ-βECD (residues γ1–80, β79–505, and γ523–655)], 3) the second transmembrane domain (TM2, and the region just preceding TM2) and COOH terminus of the β-subunit were inserted into the γ-subunit [γ-βTM2C (residues γ1–522 and β506–638)], 4) the TM2 (and the region just preceding TM2) of the β-subunit was inserted into the γ-subunit [γ-βTM2 (residues γ1–522, β506–546 and γ565–655)], and 5) the COOH terminus of the β-subunit was inserted into the γ-subunit [γ-βC (residues γ1–564 and β547–638)]. Point mutations of sites within the γ-βTM2 chimera or the wild-type γ-subunit were generated using a QuikChange II XL kit. DNA sequencing was performed to confirm the desired domain swap or targeted point mutations.
ENaC expression in Xenopus oocytes.
cRNAs were synthesized with a T3 mMessage mMachine kit (Ambion). Wild-type and mutant ENaCs were expressed in stage V-VI Xenopus oocytes as previously described (41). After injection, oocytes were maintained at 18°C in modified Barth's saline [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 15 mM HEPES, 0.3 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamycin sulfate, pH adjusted to 7.4 with NaOH]. All experiments were performed at room temperature 20–28 h following cRNA injection.
Na+ self-inhibition.
The Na+ self-inhibition response was measured as previously described (41). Oocytes were perfused with a high Na+ concentration ([Na+]) bath solution (NaCl-110, containing 110 mM NaCl, 2 mM KCl, 1.6 mM CaCl2, and 10 mM HEPES, pH 7.4) for the first 60 s and then with a low [Na+] bath solution (NaCl-1, containing 1 mM NaCl, 109 mM N-methyl-d-glucamine, 2 mM KCl, 1.6 mM CaCl2, and 10 mM HEPES, pH adjusted to 7.4 with HCl) for another 60 s. To initiate Na+ self-inhibition, the NaCl-1 solution was rapidly replaced by the NaCl-110 solution while whole cell Na+ currents were continuously recorded. At the end of each experiment, the oocyte was perfused with 10 μM amiloride prepared with the NaCl-110 solution to determine the amiloride-insensitive component of the whole cell current. Oocytes expressing channels with reduced amiloride sensitivity (e.g., αβγ-βTM2 and αβγ-βTM2C) were perfused with 10 μM benzamil at the end of the experiment. To determine the rate constant of the Na+ self-inhibition response (τ-value), the first 40 s of current decay following the switch to the NaCl-110 solution was fit with an exponential equation using Clampfit 10.2 (Molecular Devices). The maximal inward current immediately after the switch from NaCl-1 to NaCl-110 (Ipeak) and the steady-state current (Iss) measured 40 s after the Ipeak were obtained. The ratio of the amiloride-sensitive Iss to the amiloride-sensitive Ipeak was used to assess the extent of Na+ self-inhibition.
Channel activation by laminar shear stress.
To measure the effects of laminar shear stress (LSS), oocytes were clamped at a holding potential of −60 mV while whole cell Na+ currents were continuously recorded. LSS stimulation was initiated through a vertical pipette (1.8-mm internal diameter) placed near the surface of the oocyte. The flow rate of the vertical perfusion was adjusted to 1.6 ml/min, corresponding to 0.137 dyn/cm2 of shear stress as previously described (7). At the end of each experiment, 10 μM benzamil was delivered to the chamber to determine the ENaC specific current. To assess the magnitude of the LSS response, we determined the benzamil-sensitive current just before the initiation of LSS (Ibasal), and the benzamil-sensitive current measured 40 s following the initiation of LSS (ILSS). The ILSS/Ibasal value was calculated for each recording. The time constant for channel activation (τ value) was determined by fitting the first 40 s of current activation measured following the initiation of the vertical perfusion with a single-component exponential equation using Clampfit 10.2 (Molecular Devices) as previously described (7).
Potency of amiloride block.
Oocytes were clamped at −100 mV while whole cell currents were continuously recorded. In the first 60 s, oocytes were perfused with NaCl-110 to measure basal currents (I0) in the absence of amiloride. An amiloride stock solution (0.1 M) prepared in DMSO was diluted to a series of concentrations (10−11, 10−9, 10−8.5, 10−8, 10−7.5, 10−7, 10−6.5, 10−6, 10−5.5, 10−5, 10−4.5, 10−4, 10−3.5, and 10−3 M) with NaCl-110 and delivered to oocytes in sequence. Oocytes were perfused with each concentration for 20 s. Normalized whole cell currents (I/I0 values) were plotted as a function of amiloride concentrations (M), and IC50 values of amiloride block were estimated by fitting the equation:
where X is the concentration of amiloride (M). The IC50 is defined as the concentration of amiloride that inhibits 50% of the whole cell Na+ current (y).
Statistical analyses.
Data were expressed as the means ± SE. Given the variability in the response of channels to extracellular Na+ or amiloride between different batches of oocytes, experiments were repeated using a minimum of two batches of oocytes obtained from different frogs. Electrophysiological data were analyzed with Clampfit 10.2 (Molecular Devices). Statistical comparisons were obtained with the Student's t-test or one-way ANOVA followed by a Bonferroni test. P values of <0.05 were considered significantly different.
RESULTS
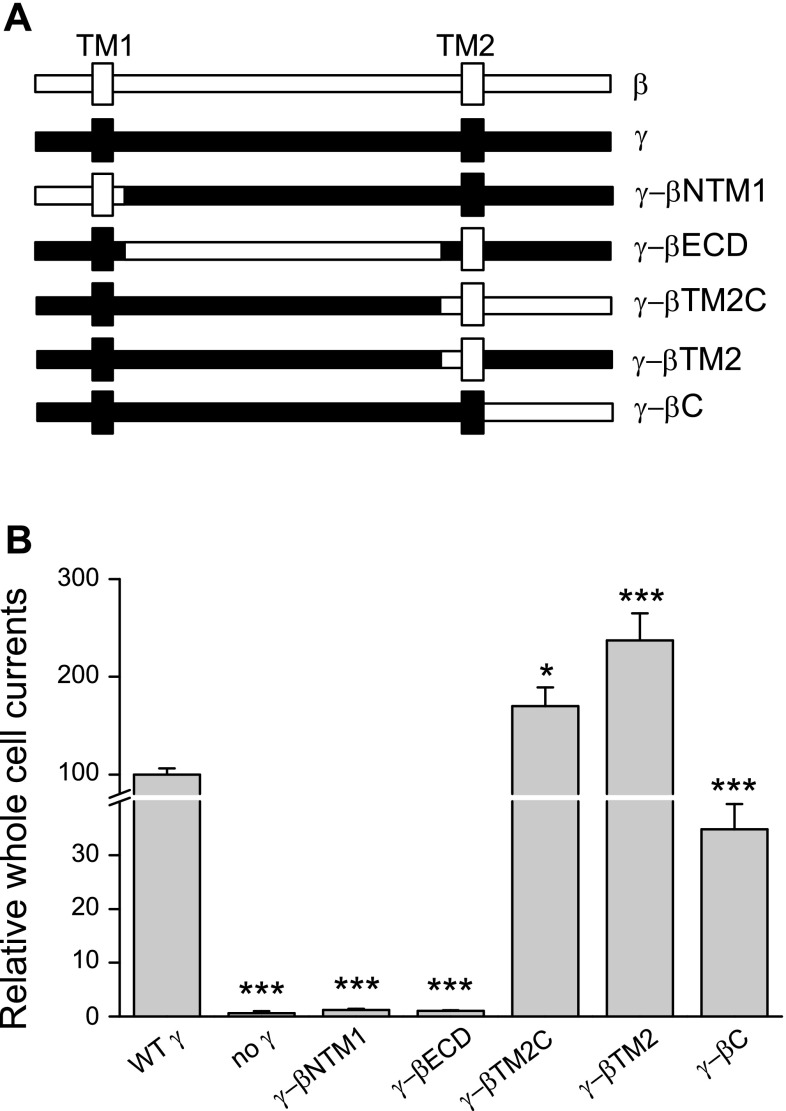
Channels that are comprised solely of α- and β-subunits (αβ-channels) exhibit properties that differ from αγ- and αβγ-channels, including a high Po and a reduced sensitivity to amiloride (12, 20). To determine which region of the γ-subunit is responsible for restraining channel activity and enhancing amiloride sensitivity, a series of γ-β-chimeras were generated by replacing the specific regions in the γ-subunit with the corresponding β-subunit regions: γ-βNTM1 (β1–78 and γ81–566), γ-βECD (γ1–80, β79–505, and γ523–655), and γ-βTM2C (γ1–522 and β506–638; see experimental procedures and Fig. 1A). γ-βNTM1 and γ-βECD generated minimal currents when coexpressed with wild-type α- and β-subunits, similar to what we observed in oocytes expressing only α- and β-subunits. In contrast, αβγ-βTM2C channels exhibited larger amiloride sensitive Na+ currents than αβγ-channels (Fig. 1B).
Fig. 1.
γ-β-chimeric constructs. A: schematic representations of the wild-type (WT) β (open)- and γ (closed)-subunits and a panel of chimeras where specific regions within the γ-subunit were replaced by the corresponding β residues. TM1 and TM2 are noted. B: oocytes that express αβ-channels, αβγ-channels, or channels comprising of α- and β-subunits and a set of γ-β-chimeric constructs were clamped at −100 mV to measure whole cell Na+ currents. After basal currents were obtained, oocytes were perfused with 10 μM benzamil to determine the benzamil-insensitive component of the whole cell current. Data were gathered from multiple batches of oocytes from different frogs (n = 16–51 for each group). Statistical significance was determined with one-way ANOVA followed by a Bonferroni test. *P < 0.05; ***P < 0.001, values that were significantly different from those of the αβγ-channel.
The γ-βTM2C construct contains β-subunit residues starting in the region just preceding TM2 (β12 and wrist), extending through TM2 and cytoplasmic COOH terminus (Fig. 1A). Multiple lines of evidence suggest that the outer mouth of the channel pore contains residues from the TM2 domains that have key roles in channel gating and amiloride binding (16, 32, 36, 43). Two additional chimeras, γ-βTM2 (γ1–522, β506–546, and γ565–655) and γ-βC (γ1–564 and β547–638) were generated and analyzed (see experimental procedures and Fig. 1A). Amiloride-sensitive Na+ currents were readily detected in oocytes expressing αβγ-βTM2 channels or αβγ-βC channels, suggesting that both γ-βTM2 and γ-βC were components of functional channel complexes. Interestingly, αβγ-βTM2 channels exhibited increased channel activity (higher Na+ currents) compared with wild-type αβγ-channels. In contrast, αβγ-βC channels exhibited reduced channel activity compared with wild-type channels (Fig. 1B).
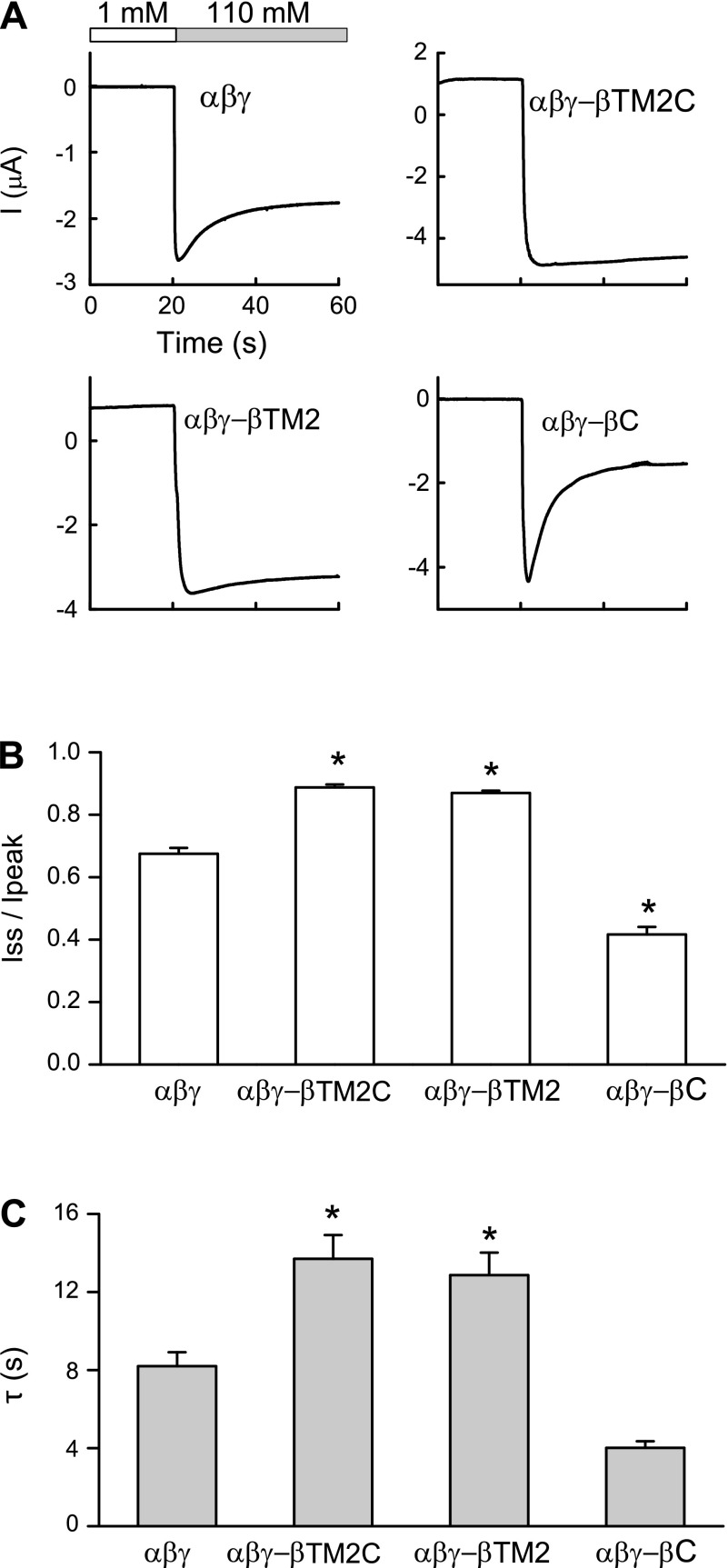
ENaCs are inhibited by external Na+, a process referred to as Na+ self-inhibition that reflects a reduction in channel Po (10, 16, 17, 24, 34). When assessing the Po and the Na+ self-inhibition response of a series of ENaC mutants, we observed a linear correlation between the magnitude of the Na+ self-inhibition response and Po (24). We used Na+ self-inhibition as a surrogate for channel Po. Na+ self-inhibition was assessed by initially bathing oocytes in a low [Na+] bath and rapidly changing to high [Na+] bath while measuring ENaC currents (16, 33). This leads to a rapid increase in current due to the chemical driving force, which then falls to a steady state as the channel is inhibited by external Na+ (33) (Fig. 2). Previous studies suggest that channels with a high intrinsic Po are less sensitive to extracellular Na+ (9, 24, 31, 45) and stimulation by mechanical forces (7, 8). As shown in Fig. 2, we observed that the Na+ self-inhibition response was largely diminished with αβγ-βTM2C as well as αβγ-βTM2 channels, suggesting that these channels have a high Po, when compared with αβγ-channels. In contrast, αβγ-βC channels exhibited an enhanced Na+ self-inhibition response suggesting that they reside in a lower Po state.
Fig. 2.
Na+ self-inhibition is reduced in αβγ-βTM2C and αβγ-βTM2 channels A: representative recordings of the Na+ self-inhibition response of wild-type or mutant channels are shown. At t = 20 s, bath perfusion with 1 mM Na+ (open bar) was switched to 110 mM Na+ (grey bar) to initiate the Na+ self-inhibition response. B: extent of channel inhibition by Na+ (Iss/Ipeak) is illustrated. The peak current (Ipeak) was the maximal inward current immediately after bath exchange. The steady-state current (Iss) was measured 40 s after Ipeak. C: time constants of current decay (τ-values) were estimated as described in the experimental procedures. A total of 42 recordings of wild-type channels and 14–40 recordings of mutant channels were collected. *P < 0.05, statistical significance between αβγ-channels and mutant channels, as determined by one-way ANOVA followed by a Bonferroni test.
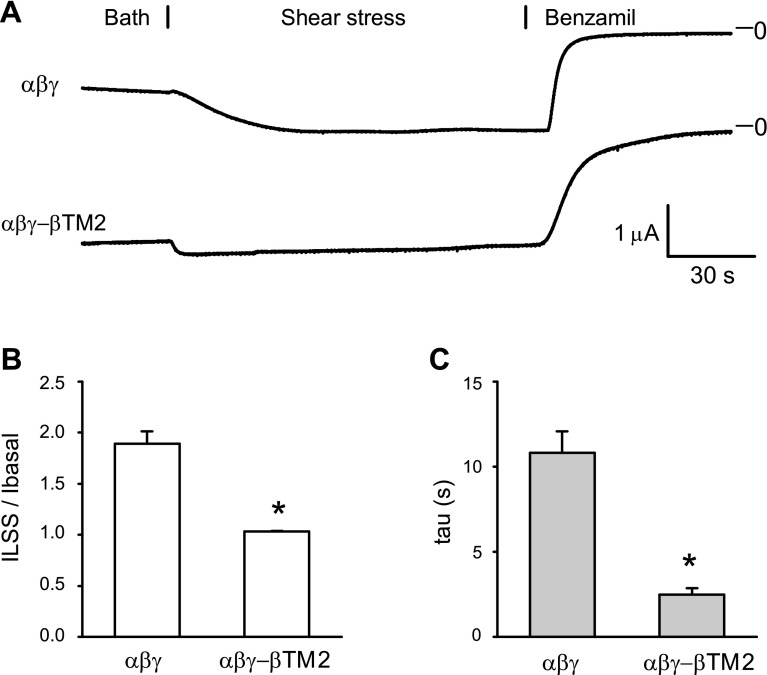
We have previously shown that LSS activates ENaC by increasing channel Po and that channels with mutations that result in a high intrinsic Po have a blunted response to LSS (7, 8). We observed that the activation of αβγ-βTM2 channels by LSS was largely blunted when compared with wild-type channels (Fig. 3), suggesting that αβγ-βTM2 channels reside at a high Po state. These changes in Na+ self-inhibition and LSS responses observed with αβγ-βTM2 channels suggest that the TM2 region in the γ-subunit dampens channel Po, preventing channels from residing in a high Po state.
Fig. 3.
αβγ-βTM2 channels display a reduced response to shear stress. A: laminar shear stress (LSS)-mediated activation of wild-type (top) or αβγ-βTM2 channels (bottom). Vertical flow was initiated at t = 30 s. At the end of experiment, 5 μM benzamil were added to the bath. (B) The magnitude of channel activation by LSS was assessed as the benzamil-sensitive Na+ current (iLSS)/basal benzamil-sensitive current (Ibasal) value. The peak response of the iLSS following the initiation of LSS was normalized to the Ibasal before initiation of LSS. C: time constants of current increase (τ-values) were obtained as previously described (41). τ-Values were determined by fitting the first 40 s of current increase following the initiation of LSS. *P < 0.001, statistically significant differences between αβγ-channels and αβγ-βTM2 channels, as determined by Student's t-test (n = 11 for each group).
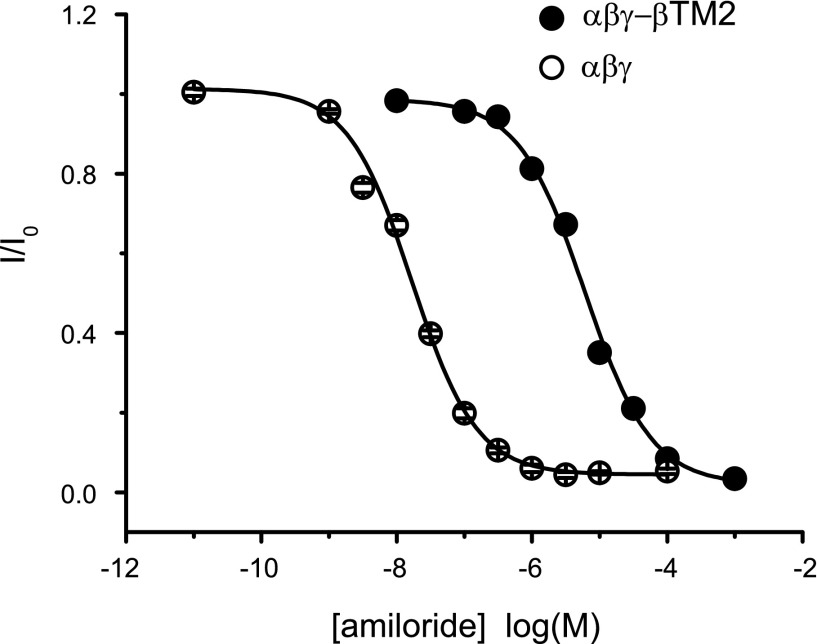
In addition, the apparent amiloride binding affinity was reduced in αβγ-βTM2 channels, as shown by a large rightward shift in the amiloride dose-response curves (Fig. 4). The IC50 of amiloride inhibition for αβγ-βTM2 channels was 6.6 ± 0.7 μM, compared with a value of 0.07 ± 0.01 μM for αβγ-channels. These properties, a presumed high Po and a reduced sensitivity to amiloride, have been observed in heterologously expressed αβ-channels (12). Our results suggest that the pore-lining region of the γ-subunit has a key role in restraining channel activity (i.e., reducing channel Po) and enhancing amiloride potency.
Fig. 4.
Amiloride dose-response curves. Whole cell Na+ currents were measured in oocytes expressing wild-type (○) or αβγ-βTM2 (●) channels in the presence of increasing concentrations of amiloride. Currents were normalized to the basal current in the absence of amiloride to obtain normalized whole cell currents (I/I0) values. Each data point represents the mean value from 7 to 18 measurements ± SE. Data were fit to the equation described in experimental procedures. Error bars are within symbols.
Mutations of γ-subunit pore-lining residues alter the channel response to external Na+.
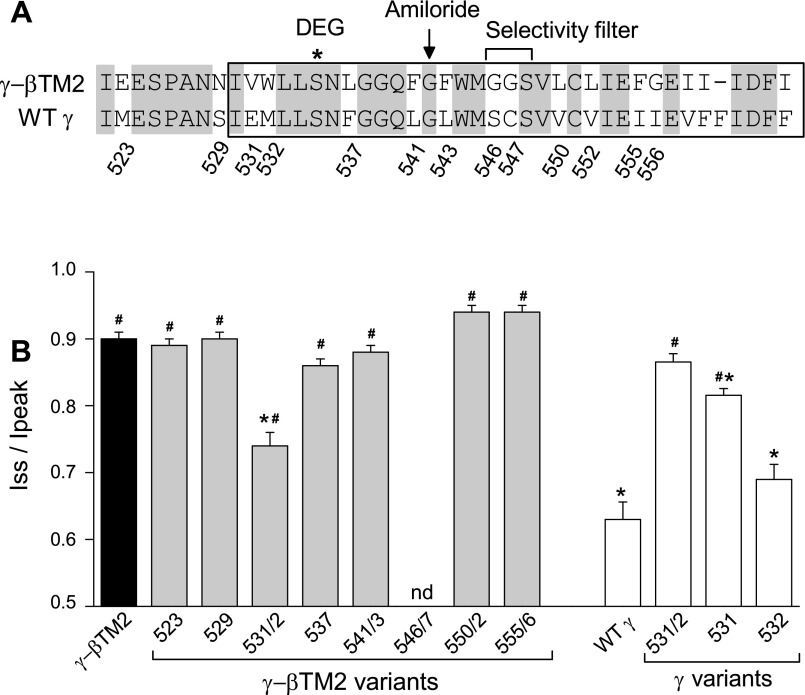
The pore-forming regions, primarily comprised of the TM2 domains, are highly conserved between β and γ-subunits (see Fig. 5A). While αβγ-βTM2 channels displayed characteristics reminiscent of αβ-channels, residues known to have important roles in channel gating (i.e., the degenerin site; Refs. 5, 16, 19, 36, 42) and amiloride block (i.e., the amiloride binding site; Refs. 18, 20, 32) are identical in the β- and γ-subunits (Fig. 5A). Thus, we hypothesize that other residues within the pore of the γ-subunit influence channel gating (as assessed by Na+ self-inhibition) and amiloride block. To identify these residues, we examined a panel of γ-βTM2 chimeras where sites within the TM2 region of the γ-βTM2 construct were replaced with the corresponding γ-subunit residues. Sites were identified by their residue number in the linear γ-subunit sequence (see Fig. 5A). One construct, γ-βTM2–546/7 (G546S/G547C), failed to generate amiloride-sensitive Na+ currents when coexpressed with wild-type α- and β-subunits. Most of the constructs exhibited a marked reduction in the Na+ self-inhibition response compared with αβγ-channels, similar to that seen with αβγ-βTM2 channels (Fig. 5B). However, the αβγ-βTM2–531/2 (V531E/W532M) channel exhibited a moderate Na+ self-inhibition response, with an Iss/Ipeak value significantly lower than that of the αβγ-βTM2 channel (Fig. 5B).
Fig. 5.
γE531 restrains the activity of wild-type channels. A: sequence alignments of the swapped regions within γ-βTM2 and the wild-type γ-subunit. Identical residues are indicated with a grey background. TM2 residues are within the box. The degenerin (DEG) site, putative amiloride binding site, and selectivity filter are noted. B: amiloride-sensitive Iss/Ipeak values of channels with γ-βTM2, γ-βTM2 mutants, a wild-type γ-subunit, or γ-subunit mutants are shown (n = 9 to 24 for each group from a minimal of 2 batches of oocytes; nd, not determined). *P < 0.05, channels with an Iss/Ipeak value significantly different than the αβγ-βTM2 channel. #P < 0.05, channels with an Iss/Ipeak value significantly different than the αβγ-channel. Statistical significance was determined by one-way ANOVA followed by a Bonferroni test.
If these two sites in the γ-subunit, E531 and M532, have important roles in dampening channel Po, mutating these residues in the γ-subunit to their corresponding β-subunit residues (i.e., γE531V/M532W) should result in a loss of Na+ self-inhibition. Indeed, this is what we observed. The Na+ self-inhibition response of αβγ531/2 (E531V/M532W) channels was largely abolished, compared with αβγ-channels, but was similar to that of αβγ-βTM2 channels (Fig. 5B). We generated two additional mutants, γE531V and γM532W, and compared their response to external Na+ to that of the wild-type channel. The channel Na+ self-inhibition response was significantly reduced in αβγE531V channels but not in αβγM532W channels (Fig. 5B). These results suggest that γE531 has an important role in reducing channel Po in response to external Na+.
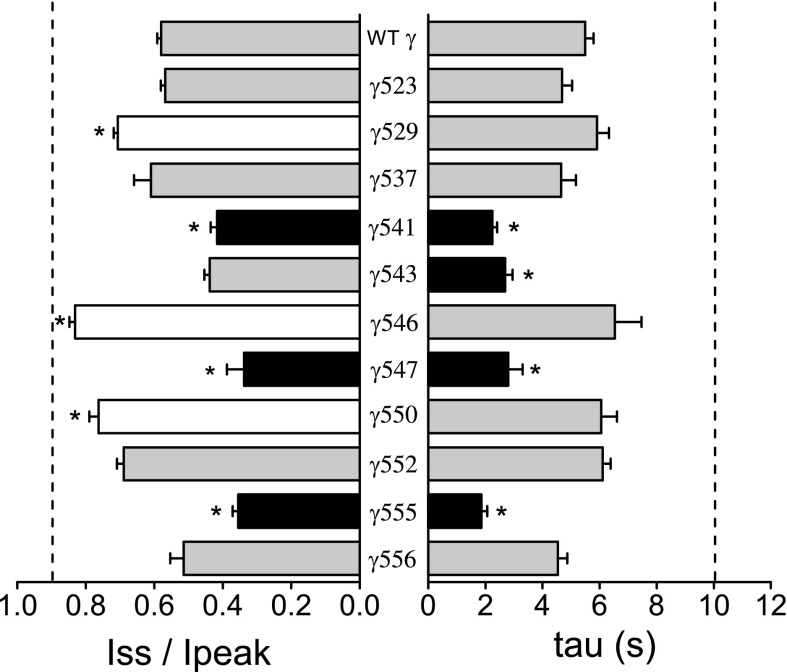
We then systemically examined the role of the nonconserved γ-subunit pore-lining residues in the channel's response to external Na+. A series of mutants were generated by replacing nonconserved γ-subunit TM2 residues with their corresponding β-subunit residues (Fig. 6). The Na+ self-inhibition response of each mutant was examined. We found that the magnitude and/or time constant of the Na+ self-inhibition response was altered with several mutants. The Na+ self-inhibition response was reduced in four mutants (αβγS529N, αβγE531V, αβγS546G, and αβγV550L) but was enhanced in another three mutant channels αβγL541F, αβγC547G, and αβγI555F (Figs. 5 and 6). Interestingly, these sites are scattered throughout the region in the γ-subunit immediately preceding and encompassing TM2. γS529 and γE531 precede the degenerin site (γS535). Two Leu residues (γL541 and γL543) flank the putative amiloride binding site (γG542). γS546 and γC547 are within the selective filter. γV550 and γI555 are in the distal part of TM2.
Fig. 6.
Multiple residues within the γ-subunit contribute to the channel's response to external Na+. A: amiloride-sensitive Iss/Ipeak ratios (left) and time constants of current decay (τ, right) of wild-type channels or channels with selected β-subunit residues substituted into the γ-subunit (at the indicated sites) were determined. Dashed lines represent the average values of αβγ-βTM2 channels from 45 observations (oocytes harvested from nine frogs). The average values of wild-type channels are from 31 observations (9 batches of oocytes), whereas mutant data are from 9 to 14 observations from 2 or 3 batches of oocytes. Values that were significantly different from that of the wild-type channel (*P < 0.05, determined by one-way ANOVA followed by a Bonferroni test) are shown as black (decreased value) or white (increased value) bars.
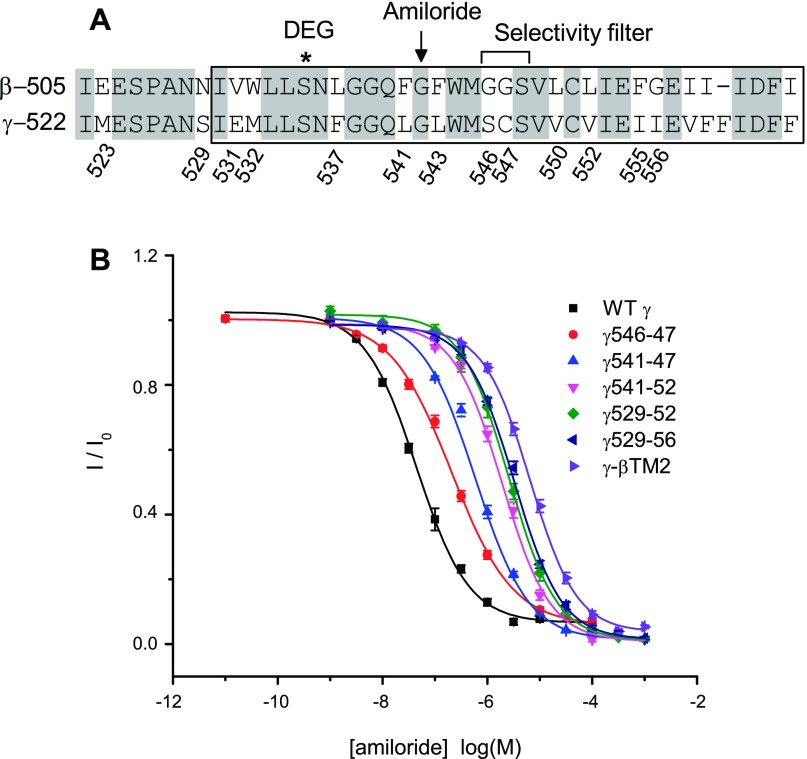
Multiple sites within γTM2 contribute to the high sensitivity to amiloride seen in αβγ-channels.
The amiloride IC50 value of αβγ-βTM2 channels (6.6 μM) is ∼100-fold higher than that of αβγ-channels (0.07 μM, see Table 1 and Fig. 4). A conserved Gly residue in β- and γ-subunits was previously shown to have a key role in the amiloride-dependent block of ENaC activity (18, 20, 32, 35). We examined whether other sites within the γ-subunit TM2 domain were required for the high potency of amiloride observed with αβγ-channels. We generated a panel of γ-subunit mutants where nonconserved sites within γTM2 were replaced with their corresponding β-subunit residues (see Table 1). We found that substitutions at one or two neighboring sites within γTM2 had only modest effects on amiloride potency, whereas the introduction of multiple substitutions had a cumulative effect on the amiloride IC50 (Fig. 7 and Table 1). When 12 γ-subunit residues spanning S529 through I556 were mutated to their corresponding β-subunit residues (γ529-56), the amiloride IC50 value of the mutant channel, αβγ529-56 (3.3 μM) was comparable to that of the αβγ-βTM2 channel (6.6 μM, Table 1 and Fig. 7). Our results suggest that, in addition to the key Gly residue (γG542), amiloride potency is determined by multiple sites within γTM2.
Table 1.
Effect of substituting residues within the TM2 domain of the γ-subunit with equivalent β-residues on amiloride inhibition of channel activity
| Channels | γ-Residues | β-Residues | n | IC50, μM |
|---|---|---|---|---|
| αβγ | 15–30 | 0.07 ± 0.01 | ||
| αβγ529 | S | N | 7 | 0.07 ± 0.01 |
| αβγ531/32 | E/M | V/W | 7 | 0.06 ± 0.00 |
| αβγ541/43 | L/l | F/F | 8–25 | 0.15 ± 0.05 |
| αβγ546/47 | S/C | G/G | 7–19 | 0.29 ± 0.04 |
| αβγ550/52 | V/V | L/l | 7–19 | 0.10 ± 0.01 |
| αβγ555/56 | I/I | F/G | 8–21 | 0.13 ± 0.02 |
| αβγ541-47 | L/l/S/C | F/F/G/G | 6–11 | 0.68 ± 0.06 |
| αβγ541-52 | L/l/S/C/V/V | F/F/G/G/l/l | 5–11 | 1.9 ± 0.2* |
| αβγ529-52 | S/E/M/F/l/l/S/C/V/V | N/V/W/l/F/F/G/G/l/l | 7–8 | 2.8 ± 0.3† |
| αβγ529-56 | S/E/M/F/l/l/S/C/V/V/I/I | N/V/W/l/F/F/G/G/l/l/F/G | 7–14 | 3.3 ± 0.3† |
| αβγ-βTM2 | 9–22 | 6.6 ± 0.7† |
IC50 values of amiloride block of whole cell Na+ currents of αβγ-channels or noted mutant channels are expressed as means ± SE. Statistic significance was analyzed with one-way ANOVA followed by a Bonferroni's test (
P < 0.01;
Fig. 7.
Multiple sites within the TM2 domain of the γ-subunit facilitate high affinity amiloride block. A: sequence alignments of the pore-forming regions of β- and γ-subunits (see Fig. 5 legend). B: dose-response curves of amiloride block of αβγ- or mutant channels, where multiple sites within the γ-subunit were replaced by equivalent β-subunit residues (see Table 1). Na+ currents were normalized to the basal current in the absence of amiloride to obtain I/I0 values. Each data point represents the mean value from 5 to 25 measurements ± SE. Wild-type and αβγ-βTM2 dose-response data in this figure were obtained from different groups of oocytes that were used to generate the data presented in Fig. 4. Data were fit to the equation described in experimental procedures.
DISCUSSION
ENaC activity is regulated by a number of factors that affect either channel density at the cell surface, channel Po, or both (2, 16, 17, 39). Three homologous ENaC subunits have different roles in channel regulation by various factors. For example, the α- and γ-subunits are processed by proteases, and the release of embedded inhibitory tracts activates the channel by increasing its Po (3, 6, 13, 22, 29, 30). Palmitoylation of the β-subunit (βC43 and βC557) modulates channel gating, likely by facilitating its interaction with membrane lipids (28).
Prototypic channels composed of all three subunits exhibit a lower Po and higher sensitivity to amiloride compared with channels comprised solely of α- and β-subunits (12), suggesting that there are sites within the γ-subunit that dampen Po. Our analyses of a series of γ-subunit chimeras where regions of the γ-subunit were replaced with the corresponding regions of the β-subunit, using Na+ self-inhibition as a surrogate measurement of channel Po (24), suggested that the region immediately preceding and containing the γ-subunit TM2 domain was responsible for the differences in the Po and amiloride potency observed with αβ- and αβγ-channels. Channels with the γ-βTM2 construct exhibited a higher basal activity and a markedly blunted Na+ self-inhibition response and LSS response, consistent with a high baseline channel Po (Figs. 1–3). The loss of inhibition by extracellular Na+ seen with the αβγ-βTM2 channel was partially rescued by introducing a pair of γ-subunit residues, E531 and M532, into γ-βTM2 (Fig. 5). Furthermore, replacing these two sites in the γ-subunit with their corresponding β residues caused a loss of Na+ self-inhibition (Fig. 5), a characteristic observed with the αβγ-βTM2 channel. Both sites, γ531 and γ532, are located within the vicinity of the channel gate based on previous functional studies and the resolved ASIC1 structures (1, 15, 16, 19, 36, 43).
In assessing the roles of individual residues that were not conserved between β- and γ-subunits, residues at positions γ529, γ531, γ546, and γ550 appeared to have important roles in enhancing the Na+ self-inhibition response. Substitutions of the corresponding β-subunit residue at these sites led to a marked loss in Na+ self-inhibition (Figs. 5 and 6). Our observations suggest that the pore-forming region of the γ-subunit restrains the activity of αβγ-channels to a moderate Po state and are consistent with previous studies where mutations introduced in the TM2 regions of each of the three ENaC subunits have profound effects on channel activity (16, 19, 35, 36, 43). While the effects of mutations in the TM2 regions on Na+ self-inhibition have not previously been systematically addressed, we have noted modest effects with mutations introduced in the region immediately following TM1 and preceding TM2 on Na+ self-inhibition (40).
One of the characteristic features of members of the ENaC/degenerin ion channel family is a high sensitivity to amiloride. A conserved site within the TM2 region of each subunit (αS583, βG525, and γG542 in mouse ENaC) has been proposed to bind amiloride, as specific mutations introduced at this site in any of the three subunits resulted in a profound loss in amiloride sensitivity (18, 32). We previously reported that selected mutations of residues in the selectivity filter of the α- or β-subunit were also associated with large increases in the amiloride IC50 (23, 38). While the key amiloride binding site in β- and γ-subunits has a conserved Gly residue (Fig. 7A), the amiloride IC50 of αβ-channels is significantly higher than that of αγ or αβγ-channels (12, 20, 27). We observed that the amiloride IC50 of αβγ-βTM2 was nearly two orders of magnitude higher than that of αβγ-channels (Table 1 and Fig. 4), similar to the value reported for αβ-channels (27). To identify sites in the γ-subunit that are involved in amiloride binding, in addition to γG542, we determined the amiloride IC50 for a panel of mutants where one or multiple sites within the γ-subunit TM2 domain were replaced with their corresponding β-subunit residues. Single substitutions within the γ-subunit TM2 did not reduce the amiloride potency to the extent seen with mutations at the putative amiloride binding site (γG542; Table 1). However, we observed a cumulative effect of substitutions on amiloride potency (Table 1). A mutant bearing substitutions at 12 sites within the γ-subunit, αβγ529-556, had an amiloride IC50 comparable to that of αβγ-βTM2 channels (Table 1 and Fig. 7). Our results are consistent with a previous work suggesting that differences in the amiloride IC50 of rat αβ- and αβγ-channels were conferred by residues in a stretch of 15 amino acids (residues 531 to 545) that are within γTM2 (27). These results suggest that, in addition to the well-characterized amiloride binding site, multiple residues within the TM2 domains of ENaC subunits influence amiloride potency (38).
The fact that αβγ-βTM2 channels exhibit a high Po and reduced amiloride potency, consistent with the behavior of αβ-channels, suggests that αβ channels have a subunit stoichiometry of one α- and two β-subunits. However, we cannot rule out a subunit stoichiometry of two α- and one β-subunits. In summary, analyses of αβγ and αβγ-βTM2 channels allowed us to identify key sites within the TM2 domain of the γ-subunit (γS529, γE531, γS546, and γV550) that restrain channel activity. Furthermore, in addition to the amiloride-binding site (γG542) there are multiple residues in the TM2 region of the γ-subunit that, as a group, contribute to the high amiloride potency of αβγ-channels. The TM2 regions within individual subunits are well conserved across species. As the TM2 regions of human and mouse γ-subunit are identical, it is likely that the sites we found in the mouse γ-subunit TM2 region that either restrain channel activity or contribute to amiloride potency will have similar roles in the human γ-subunit.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37-DK-051391, R01-DK-065161, and P30-DK-079307. S. Shi is supported by a postdoctoral fellowship award from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S. and T.R.K. conception and design of research; S.S. performed experiments; S.S. and T.R.K. analyzed data; S.S. and T.R.K. interpreted results of experiments; S.S. prepared figures; S.S. and T.R.K. drafted manuscript; S.S. and T.R.K. edited and revised manuscript; S.S. and T.R.K. approved final version of manuscript.
REFERENCES
- 1.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 489: 400–405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Carattino MD, Sheng S, Kleyman TR. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem 280: 4393–4401, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Kleyman TR, Sheng S. Gain-of-function variant of the human epithelial sodium channel. Am J Physiol Renal Physiol 304: F207–F213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120: 133–145, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastwood AL, Goodman MB. Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda) 27: 282–290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyfe GK, Canessa CM. Subunit composition determines the single channel kinetics of the epithelial sodium channel. J Gen Physiol 112: 423–432, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Ismailov II, Awayda MS, Berdiev BK, Bubien JK, Lucas JE, Fuller CM, Benos DJ. Triple-barrel organization of ENaC, a cloned epithelial Na+ channel. J Biol Chem 271: 807–816, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol 301: F684–F696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashlan OB, Sheng S, Kleyman TR. On the interaction between amiloride and its putative alpha-subunit epithelial Na+ channel binding site. J Biol Chem 280: 26206–26215, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channel ENaC. J Physiol 543: 413–424, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellenberger S, Gautschi I, Schild L. Mutations in the epithelial Na+ channel ENaC outer pore disrupt amiloride block by increasing its dissociation rate. Mol Pharmacol 64: 848–856, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Sheng S, Perry CJ, Kleyman TR. Asymmetric organization of the pore region of the epithelial sodium channel. J Biol Chem 278: 13867–13874, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, Boyd CR, Kleyman TR, Sheng S. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem 284: 7756–7765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O'Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol 109: 681–692, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller GM, Maarouf AB, Kinlough CL, Sheng N, Kashlan OB, Okumura S, Luthy S, Kleyman TR, Hughey RP. Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel. J Biol Chem 285: 30453–30462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 299: F854–F861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passero CJ, Mueller GM, Myerburg MM, Carattino MD, Hughey RP, Kleyman TR. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the gamma-subunit distal to the furin cleavage site. Am J Physiol Renal Physiol 302: F1–F8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauh R, Diakov A, Tzschoppe A, Korbmacher J, Azad AK, Cuppens H, Cassiman JJ, Dotsch J, Sticht H, Korbmacher C. A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition. J Physiol 588: 1211–1225, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng S, Bruns JB, Kleyman TR. Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J Biol Chem 279: 9743–9749, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. J Biol Chem 275: 8572–8581, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Sheng S, Li J, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial sodium channel pore region. Structure and role in gating. J Biol Chem 276: 1326–1334, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sheng S, McNulty KA, Harvey JM, Kleyman TR. Second transmembrane domains of ENaC subunits contribute to ion permeation and selectivity. J Biol Chem 276: 44091–44098, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sheng S, Perry CJ, Kashlan OB, Kleyman TR. Side chain orientation of residues lining the selectivity filter of epithelial Na+ channels. J Biol Chem 280: 8513–8522, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Shi S, Carattino MD, Hughey RP, Kleyman TR. ENaC regulation by proteases and shear stress. Curr Mol Pharmacol 6: 28–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi S, Carattino MD, Kleyman TR. Role of the wrist domain in the response of the epithelial sodium channel to external stimuli. J Biol Chem 287: 44027–44035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi S, Ghosh DD, Okumura S, Carattino MD, Kashlan OB, Sheng S, Kleyman TR. Base of the thumb domain modulates epithelial sodium channel gating. J Biol Chem 286: 14753–14761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder PM, Bucher DB, Olson DR. Gating induces a conformational change in the outer vestibule of ENaC. J Gen Physiol 116: 781–790, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na(+) channels. J Biol Chem 274: 28484–28490, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 14: 461–471, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winarski KL, Sheng N, Chen J, Kleyman TR, Sheng S. Extracellular allosteric regulatory subdomain within the gamma subunit of the epithelial Na+ channel. J Biol Chem 285: 26088–26096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]