Abstract
Glucagon-like peptide-1 receptor (GLP-1R) activation in the ventral tegmental area (VTA) is physiologically relevant for the control of palatable food intake. Here, we tested whether the food intake-suppressive effects of VTA GLP-1R activation are mediated by glutamatergic signaling within the VTA. Intra-VTA injections of the GLP-1R agonist exendin-4 (Ex-4) reduced palatable high-fat food intake in rats primarily by reducing meal size; these effects were mediated in part via glutamatergic AMPA/kainate but not NMDA receptor signaling. Additional behavioral data indicated that GLP-1R expressed specifically within the VTA can partially mediate the intake- and body weight-suppressive effects of systemically administered Ex-4, offering the intriguing possibility that this receptor population may be clinically relevant for food intake control. Intra-VTA Ex-4 rapidly increased tyrosine hydroxylase levels within the VTA, suggesting that GLP-1R activation modulates VTA dopaminergic signaling. Further evidence for this hypothesis was provided by electrophysiological data showing that Ex-4 increased the frequency of AMPA-mediated currents and reduced the paired/pulse ratio in VTA dopamine neurons. Together, these data provide novel mechanisms by which GLP-1R agonists in the mesolimbic reward system control for palatable food intake.
Keywords: 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid, obesity, glucagon-like peptide-1, glutamate, dopamine, reward, diabetes
glucagon-like peptide-1 (GLP-1) is an incretin hormone produced by the L cells of the distal small intestine and by neurons in the nucleus tractus solitarius (NTS) (23). GLP-1 and GLP-1 receptor (GLP-1R) agonists such as exendin-4 (Ex-4) potently suppress food intake and body weight when administered peripherally or centrally (20, 21, 33), although the intake-suppressive effects of peripherally administered GLP-1R agonists are mediated partially by direct activation of central GLP-1R (24). Because GLP-1R agonists are FDA approved for the treatment of type 2 diabetes mellitus (12) and are in clinical trials for obesity treatment (4), it is crucial to gain a fuller understanding of the central mechanisms mediating the food intake-suppressive effects of GLP-1R signaling.
Recently, NTS GLP-1-producing neurons were shown to project monosynaptically to mesolimbic reward system (MRS) nuclei, including the ventral tegmental area (VTA) (3) and nucleus accumbens (3, 14). These connections are physiologically relevant for the control of palatable food intake, as VTA GLP-1R blockade increases intake of palatable high-fat diet (HFD) (3). Although these previous reports have expanded our knowledge of central nervous system (CNS) GLP-1R-expressing nuclei that regulate food intake and body weight, the behavioral and neuronal mechanisms by which GLP-1R signaling in the MRS controls for food intake are less understood. Therefore, current data provide novel complementary in vivo behavioral and ex vivo molecular and electrophysiological evidence that GLP-1R signaling in the VTA controls for food intake via presynaptic modulation of glutamatergic excitation of dopamine neurons via 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid (AMPA)/kainate but not NMDA receptors.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed individually in hanging wire mesh cages in a temperature- and humidity-controlled environment (20–24°C; 41–68% humidity). Rats were maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All experimental procedures received approval from the University of Pennsylvania Institutional Animal Care and Use Committee.
Drugs
Ex-4 (American Peptide, Sunnyvale, CA), exendin-(9–39) (Ex-9; American Peptide), CNQX (Tocris Bioscience, Minneapolis, MN), and MK-801 (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) for central injections. Ex-4 was dissolved in sterile 0.9% NaCl for peripheral intraperitoneal (ip) injections and in sterile aCSF for electrophysiological studies. Both CNQX and MK-801 have been used extensively to probe the effects of glutamatergic signaling on food intake (8, 11, 17, 22, 27). Doses for drugs were selected from the literature (1, 3, 17, 21, 32).
Surgery
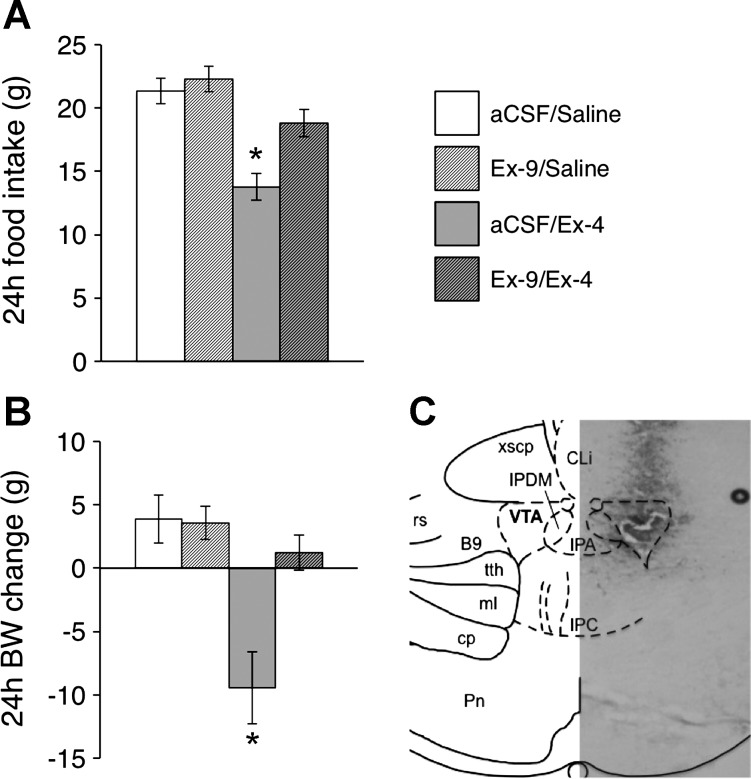
Rats were anesthetized using a mixture of ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) and placed into a stereotaxic apparatus. For behavioral experiments, a 26-gauge bilateral guide cannula (Plastics One, Roanoke, VA) directed at the VTA was implanted (guide cannulae coordinates: ±0.5 mm lateral to midline, 6.8 mm posterior to bregma, and 6.6 mm ventral to skull; internal cannula aimed 8.6 mm ventral to skull) and affixed to the skull with bone screws and dental cement. For all surgeries, analgesia was provided (2 mg/kg meloxicam). Animals were allowed to recover for ≥1 wk prior to the start of behavioral testing. VTA cannulae placements were verified histologically postmortem with injections of pontamine sky blue (100 nl). A representative image of a VTA cannula placement with 100-nl ink injection is shown in Fig. 1C (40-μm section).
Fig. 1.
Twenty-four-hour high-fat diet intake (A) and body weight gain (B) after intra-ventral tegmental area (VTA) pretreatment of the glucagon-like peptide-1 receptor (GLP-1R) antagonist exendin-(9–39) (Ex-9; 10 μg) or its vehicle [200 nl of artificial cerebrospinal fluid (aCSF)] in combination with intraperitoneal (ip) injection of the GLP-1R agonist Ex-4 (3 μg/kg) or its vehicle (1 ml/kg 0.9% NaCl). The reductions in food intake and body weight gain were attenuated by intra-VTA pretreatment with Ex-9. *P < 0.05 compared with aCSF/saline. All data are shown as means ± SE. C: a representative histological section showing verification of VTA cannula placement with pontamine sky blue ink injection (100 nl) is shown.
Behavioral Experiments
Nonobese rats (381.7 ± 7.3 g, 11–14 wk of age on the 1st day of experimental testing) were maintained on HFD (60% kcal from fat; Research Diets, New Brunswick, NJ) during and for 2 wk prior to testing. HFD was selected because intra-VTA GLP-1R activation selectively reduces intake of HFD but not chow (3). All behavioral tests were run using within-subject counterbalanced designs, and all injections occurred just before onset of the dark period. For all behavioral studies, experimental treatments were separated by a minimum of 48 h.
To test whether the VTA is a direct site of action for peripheral GLP-1R agonist administration, rats received a unilateral intra-VTA infusion of either Ex-9 (10 μg) or its vehicle (200 nl of aCSF), followed by an ip injection of either Ex-4 (3 μg/kg) or its vehicle (1 ml/kg saline). Food intake and body weight were recorded manually 24 h following drug administration.
For glutamatergic receptor antagonist studies, rats were housed in a custom-made, automated feedometer system. Feedometers consisted of a hanging wire mesh cage with a small access hole leading to a food cup resting on an electronic scale. Weights of the rats' food cups were recorded by computer software (LabView) every 10 s for 24 h. Rats received two unilateral intra-VTA injections; the first injection consisted of a glutamatergic receptor antagonist [in one experiment, AMPA/kainate antagonist: CNQX (0.3 μg); in a separate experiment, NMDA antagonist: MK-801 (0.05 μg)] or its vehicle (100 nl of aCSF for both experiments), and the second injection consisted of Ex-4 (0.05 μg) or its vehicle (100 nl of aCSF). Cumulative food intake and meal patterns were analyzed, with a meal being defined as ingestion of ≥0.25 g of food, with a minimum of 10 min between feeding bouts.
Immunoblot Studies
Nonobese rats (361.3 ± 6.2 g, 11–12 wk of age) maintained on a HFD had food removed 30 min before dark onset. Shortly after the onset of the dark phase, each rat received a unilateral intra-VTA injection of Ex-4 (0.05 μg) or vehicle (100 nl of aCSF). Fifteen minutes after injection, rats were euthanized by decapitation; brains were rapidly removed from the crania and flash-frozen in −70°C isopentane and stored at −80°C until processing. Frozen whole brains were mounted in a cryostat and sliced caudal to rostral until the VTA was reached (−6.80 mm from bregma) (30). Bilateral 1.0 × 1.0 mm VTA samples from each brain were collected by micropunching the brain with a 1.0 mm Harris Uni-Core tool (Sigma-Aldrich). Samples were subsequently homogenized in NP-40 lysis buffer containing protease and phosphatase inhibitors. The homogenized tissue was then subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes for immunoblot analysis. Total tyrosine hydroxylase (TH) was measured using TH rabbit monoclonal antibody normalized to β-actin (TH, 1:1,000; β-actin, 1:5,000; Cell Signaling Technology).
Electrophysiological Studies
Rats maintained on chow were put under isoflurane anesthesia and decapitated. Their brains were rapidly removed, and coronal slices (300 μm) containing the VTA were cut using a Vibratome (VT1000S; Leica Microsystems, Buffalo Grove, IL) in an ice-cold aCSF solution in which NaCl was replaced with an equiosmolar concentration of sucrose. aCSF contained 130 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, 1 mM MgCl2, and 2 mM CaCl2 (pH 7.2–7.4 when saturated with 95% O2 and 5% CO2). Slices were incubated in aCSF at 32–34°C for 45 min and kept at 22–25°C thereafter until transfer to the recording chamber. All solutions had an osmolarity between 305 and 315 mOsm. Slices were viewed under an upright microscope (Eclipse FN1; Nikon Instruments, Melville, NY) with infrared differential interference contrast optics and a ×40 water immersion objective. For recordings, the chamber was perfused continuously at a rate of 1–2 ml/min with oxygenated aCSF heated to 32 ± 1°C using an automated temperature controller (Warner Instruments, Hamden, CT). Picrotoxin (100 μM) was also added to all solutions to block the GABAA receptors. Recording pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) to a resistance of 4–7 MΩ when filled with the intracellular solution. The intracellular solution contained 145 mM potassium gluconate, 2 mM MgCl2, 2.5 mM KCl, 2.5 mM NaCl, 0.1 mM BAPTA, 10 mM HEPES, 2 mM Mg-ATP, 0.5 mM GTP-Tris, and 1 mM QX-314 (pH 7.2–7.3 with KOH, osmolarity 280–290 mOsm). VTA dopamine neurons were identified morphologically by large soma sizes as well as the use of a twofold electrophysiological criterion (10, 15). Following the injection of a depolarizing current, the neuron had to exhibit a slow depolarizing shoulder preceding an action potential as well as exhibit an Ih current of >50 pA after a hyperpolarizing step to −120 mV or action potential half-width of >1.3 ms. Ten of 17 recorded neurons passed these criteria and had a mean Ih of 145 ± 30 pA and mean AP half-width of 1.64 ± 0.24 ms. Seven neurons were excluded from analyses. Spontaneous excitatory postsynaptic current (sEPSC) recordings were conducted in whole cell voltage clamp mode (Vh = −70 mV) using a Multi-Clamp700B amplifier (Molecular Devices, Sunnyvale, CA). Ex-4 (1 μM) was applied via the Y-tube perfusion system modified for optimal solution exchange in brain slices. sEPSCs were analyzed after a minimum of 4 min of Ex-4 exposure. Currents were low-pass filtered at 2 kHz and digitized at 20 kHz using a Digidata 1440A acquisition board (Molecular Devices) and pClamp10 software (Molecular Devices). Access resistance (10–30 MΩ) was monitored during recordings by injection of 10 mV hyperpolarizing pulses; data were discarded if access resistance changed by >25% over the course of data collection. For paired/pulse ratio (PPR) experiments, evoked responses with an interstimulus interval of 100 ms were triggered by 100-μs constant-current pulses generated by an A310 Accupulser (World Precision Instruments) and delivered at 0.2 Hz via a bipolar tungsten stimulation electrode positioned within 100 μm of the recorded cell.
Data Analysis and Statistics
Data were analyzed using Statistica (version 7; StatSoft), StatView for Windows (version 5.0.1; SAS), or Microsoft Excel. The α-level for all tests was set at P ≤ 0.05.
Behavioral studies.
Data from each time bin for food intake, meal size, and meal number were analyzed using separate mixed-design ANOVA tests to account for the within-subjects design of the experiments while testing for between-subjects effects of drug treatment(s). Statistically significant main effects and interactions were probed using Student-Newman-Keuls post hoc analyses.
Immunoblot studies.
Normalized data were analyzed by one-way ANOVA, with drug condition as a between-subjects factor.
Electrophysiological studies.
All analyses of electrophysiological recordings were completed using Clampfit 10 (Molecular Devices). The time constant of decay was based on a monoexponential fit to the decay phase of an average sEPSC trace computed from a minimum of 50 individual sEPSCs. Mean sEPSC frequencies were analyzed from 20-s trace segments. PPRs were calculated by averaging five to 10 responses and dividing the peak amplitude of the second evoked EPSC by the peak amplitude of the first evoked EPSC. Neurons from a total of five animals were analyzed. Statistical comparisons were done using two-tailed paired Student t-tests.
RESULTS
VTA GLP-1Rs are Activated by a Peripherally Administered GLP-1R Agonist to Control Food Intake
Given that the VTA is a physiologically relevant site of action for palatable food intake (3), this raises the possibility that VTA GLP-1R may also directly mediate the intake-suppressive effects of peripherally administered GLP-1R agonists. To test this possibility, HFD-maintained rats (n = 15) received an intra-VTA infusion of the GLP-1R antagonist Ex-9 (10 μg) or vehicle (200 nl of aCSF), followed by an ip injection of Ex-4 (3 μg/kg) or vehicle (1 ml/kg 0.9% NaCl), and 24-h food intake was measured. This dose of Ex-9 was selected because it has no effect on 24-h food intake or body weight gain when delivered to the VTA (3). Here, intra-VTA GLP-1R blockade with Ex-9 attenuated the anorectic effect of ip Ex-4 (F1,14 = 4.69, P = 0.049; aCSF/Ex-4 vs. Ex-9/Ex-4, P < 0.01; Fig. 1A). Additionally, VTA Ex-9 attenuated the reduction in 24-h body weight gain produced by ip Ex-4 (F1,14=5.58, P = 0.03; aCSF/Ex-4 vs. Ex-9/Ex-4, P < 0.01; Fig. 1B). Together, these data suggest that VTA GLP-1Rs partially mediate the intake- and body weight-suppressive effects of systemic Ex-4.
Intra-VTA GLP-1R Activation Reduces Palatable High-Fat Diet Intake By Suppressing Meal Size, Effects That Are Mediated By VTA AMPA/Kainate Receptors
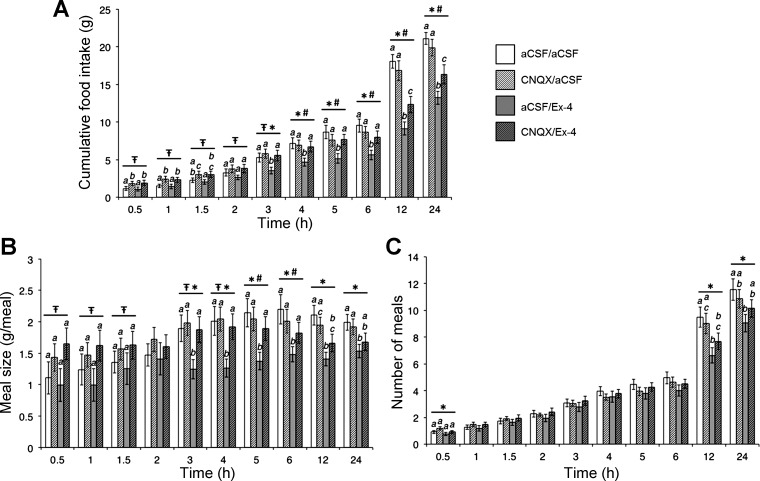
The VTA receives afferent glutamatergic projections from other nuclei involved in the control of food intake and other motivated behaviors (16, 29). Importantly, glutamatergic signaling through AMPA/kainate but not NMDA receptors in the MRS reduces feeding (27), leading to the hypothesis that VTA GLP-1R activation reduces food intake via modulation of glutamatergic signaling. Consistent with our hypothesis and our previous results (3), intra-VTA Ex-4 (0.05 μg/100 nl) reduced HFD intake beginning at 3 h postinjection and persisting throughout the remainder of the 24-h measurement period (n = 22; all ANOVAs F1,21 ≥ 6.17, P ≤ 0.02; aCSF/aCSF vs. aCSF/Ex-4, all P < 0.02; Fig. 2A). Statistically significant interactions between CNQX and Ex-4 were observed for cumulative food intake beginning at 4 h and lasting until 24 h (all ANOVAs F1,21 ≥ 4.89, p≤0.04), demonstrating that intra-VTA pretreatment with the AMPA/kainate receptor antagonist CNQX (0.3 μg/100 nl) attenuated the intake-suppressive effect of intra-VTA Ex-4 (aCSF/Ex-4 vs. CNQX/Ex-4, all P < 0.04). These data suggest that VTA GLP-1R activation reduces food intake in part through glutamatergic AMPA/kainate receptor signaling. Interestingly, intra-VTA administration of CNQX alone increased food intake at early times (vehicle/vehicle vs. CNQX/vehicle, different at 30 min-1.5 h; all main effects of CNQX F1,21 ≥ 5.20, P ≤ 0.04), but this hyperphagic effect subsided by the time that a statistically significant interaction between CNQX and Ex-4 was observed at 4 h, suggesting that the AMPA/kainate-mediated attenuation of Ex-4-induced suppression of food intake is not simply the result of competing bidirectional effects. Meal pattern analyses revealed that intra-VTA Ex-4 reduces intake of a palatable high-fat food by decreasing meal size (from 3 to 24 h, all ANOVAs F1,21 ≥ 6.11, P ≤ 0.03; aCSF/aCSF vs. aCSF/Ex-4, all P < 0.03; Fig. 2B), with minimal effects on meal frequency (main effect of Ex-4 at 30 min, 12 h, and 24 h; all ANOVAs F1,21 ≥ 4.46, P ≤ 0.05; post hoc comparison of aCSF/aCSF vs. aCSF/Ex-4, P < 0.03 at 12 and 24 h only; Fig. 2C). Together with previous reports (3, 13), these findings suggest that VTA GLP-1R signaling potentially controls for food intake by modulating the rewarding value of the ongoing meal while having fewer effects on between-meal satiety processes. Pretreatment with intra-VTA CNQX attenuated the meal size-suppressive effect of VTA Ex-4 from 3 to 6 h (planned post hoc comparisons of aCSF/Ex-4 vs. CNQX/Ex-4, all P < 0.05; Fig. 2B), suggesting that this meal size-suppressive effect of VTA GLP-1R activation is also mediated partly by AMPA/kainate receptors.
Fig. 2.
A: VTA administration of the 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid (AMPA)/kainate receptor antagonist CNQX (0.3 μg; vehicle, 100 nl of aCSF) attenuates the anorectic effect of VTA GLP-1R activation by exendin-4 (Ex-4; 0.05 μg; vehicle, 100 nl of aCSF). This effect occurs via suppression of meal size (B), with only minimal effects on meal frequency (C). *Main effect of Ex-4 (P < 0.05);  main effect of CNQX (P < 0.05); #interaction between CNQX and Ex-4 (P < 0.05). Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
main effect of CNQX (P < 0.05); #interaction between CNQX and Ex-4 (P < 0.05). Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
VTA NMDA Receptors are not Required to Mediate the Food Intake-Suppressive Effects of Intra-VTA GLP-1R Activation
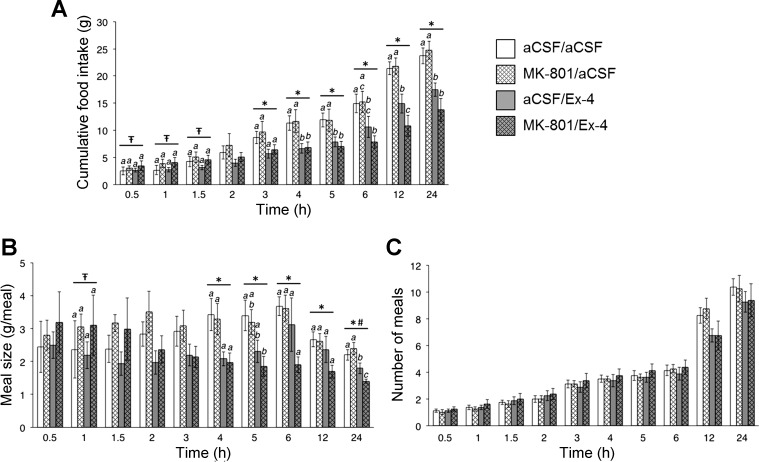
In contrast to the findings for CNQX, intra-VTA pretreatment with the NMDA receptor antagonist MK-801 (0.05 μg/100 nl) did not attenuate the food intake-suppressive effects of intra-VTA Ex-4 [n = 8; significant main effect of Ex-4 from 3 to 24 h, all ANOVAs F1,7 ≥ 8.40, P < 0.03; no statistically significant interaction between MK-801 and Ex-4 at any time (all ANOVAs F1,7 ≤ 3.38, P > 0.05; Fig. 3A)]. Although VTA Ex-4 again reduced meal size in this experiment (significant main effect of Ex-4 from 4 to 24 h, all ANOVAs F1,7 ≥ 6.66, P < 0.05; Fig. 3B), MK-801 pretreatment did not attenuate this effect. Importantly, the Ex-4-induced meal pattern effects observed in this experiment were comparable with those observed in the CNQX experiment, with Ex-4-induced suppression of food intake being driven predominantly by meal size reductions, with no significant effects on meal frequency. Worth noting is that the dose of MK-801 used here had a statistically significant main effect at early times for cumulative food intake (30–90 min, all ANOVAs F1,7 ≥ 5.75, P < 0.05) and meal size (60 min, F1,7 = 12.04, P = 0.01), suggesting that the dose was appropriately selected from previous literature examining NMDA-mediated effects on food intake (17). However, in the current experiments, blockade of NMDA receptors by MK-801 did not reverse or attenuate the suppression of food intake by VTA Ex-4 administration. Collectively, these results indicate that the food intake- and meal size-suppressive effects of GLP-1R activation in the VTA are mediated by glutamatergic signaling through AMPA/kainate but not NMDA receptors.
Fig. 3.
A: VTA administration of the NMDA receptor antagonist MK-801 (0.05 μg; vehicle, 100 nl of aCSF) does not attenuate the intake-suppressive effect of VTA GLP-1R activation by Ex-4 (0.05 μg; vehicle, 100 nl of aCSF). B: VTA MK-801 does not attenuate VTA Ex-4-induced reductions in meal size. C: no effects on meal frequency were observed (all P > 0.05). *Main effect of Ex-4 (P < 0.05);  main effect of MK-801 (P < 0.05); #statistically significant interaction between MK-801 and Ex-4. Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
main effect of MK-801 (P < 0.05); #statistically significant interaction between MK-801 and Ex-4. Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
Intra-VTA GLP-1R Activation Increases VTA Tyrosine Hydroxylase Levels
Given the present findings and the role of MRS dopamine signaling in the control of food intake (7, 28, 35), we hypothesized that GLP-1R activation in the VTA modulates dopaminergic signaling. Because TH is the rate-limiting step in dopamine synthesis, we tested whether VTA GLP-1R activation would alter TH levels within the VTA. Compared with vehicle treatment, intra-VTA administration of Ex-4 (0.05 μg/100 nl) increased VTA TH levels at 15 min postinjection (n = 11, F1,9 = 5.12, P = 0.050; Fig. 4).
Fig. 4.
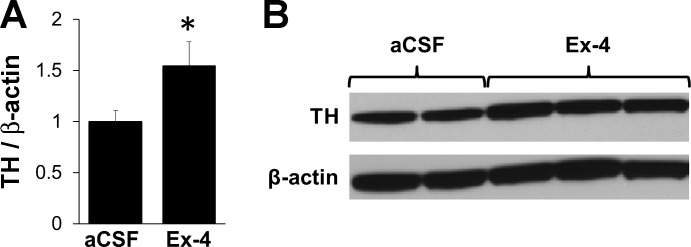
A: intra-VTA injection of Ex-4 (0.05 μg; vehicle, 100 nl of aCSF) increased VTA tyrosine hydrolase (TH; normalized to β-actin) 15 min after drug administration. B: representative immunoblots for TH and β-actin are shown. *P ≤ 0.05 compared with aCSF. Data are shown as means ± SE.
GLP-1R Expressed on Presynaptic Glutamatergic Terminals in the VTA Modulate the Activity of VTA Dopamine Neurons
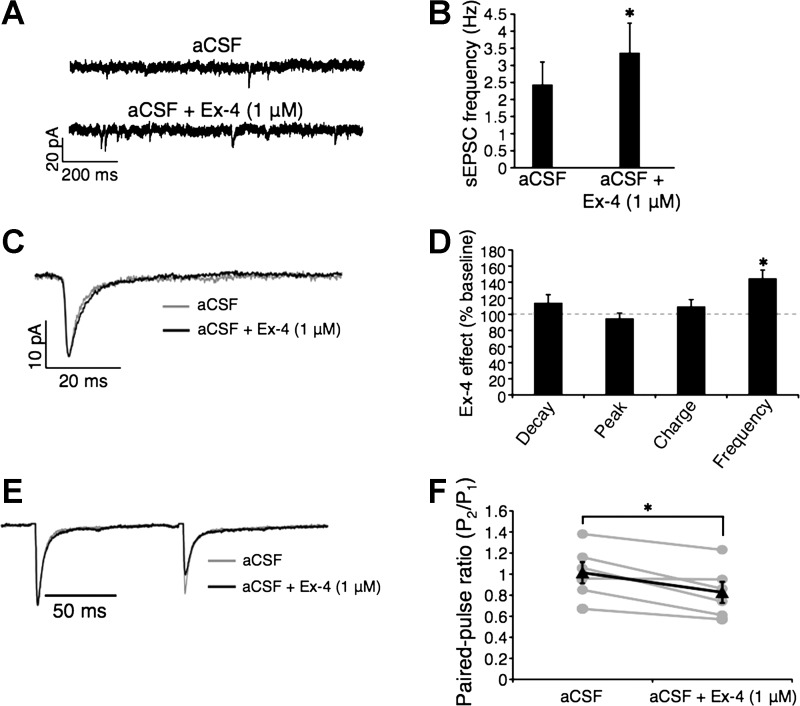
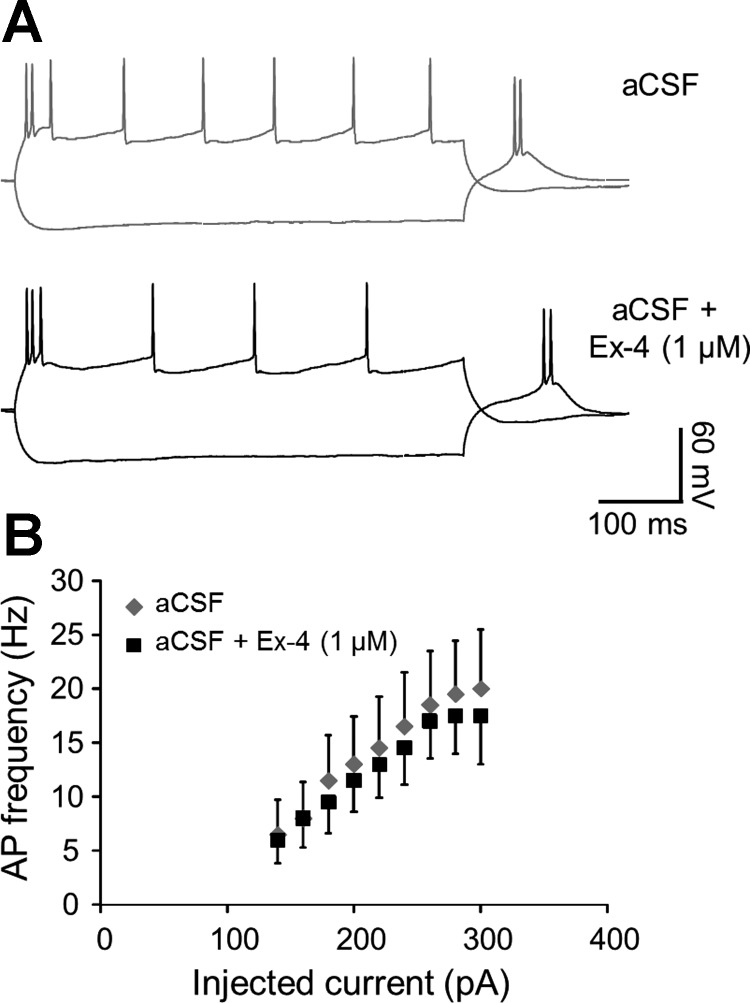
The finding that VTA GLP-1R activation increased TH levels in the VTA, together with our behavioral studies indicating a glutamatergic AMPA/kainate-mediated attenuation of VTA Ex-4-induced suppression of food intake, led us to hypothesize that activation of GLP-1R modulates the activity of VTA dopaminergic neurons via AMPA/kainate receptors. Thus, using patch clamp electrophysiology, we recorded pharmacologically isolated AMPA/kainate receptor-mediated sEPSCs in VTA dopamine neurons before and during bath application of Ex-4 (1 μM). Ex-4 increased sEPSC frequency significantly in VTA dopamine neurons [Fig. 5, A, B, and D; n = 10; t(9) = 3.08, P = 0.01], suggesting a presynaptic effect of GLP-1R activation. sEPSC decay, time, peak amplitude, and charge transfer were unchanged by Ex-4 (Fig. 5, C and D), arguing against postsynaptic effects of GLP-1R activation. To confirm the presynaptic locus of GLP-1R effects on VTA dopamine neurons, we analyzed the PPR before and during Ex-4 bath application. Corroborating the sEPSC frequency findings, Ex-4 reduced the PPR [n = 6; t(5) = 3.77, P = 0.01; Fig. 5, E and F], consistent with a presynaptic mechanism. To examine a possible effect of GLP-1R activation on VTA dopamine neuronal firing (1), we analyzed the relationship between action potential frequency and injected current (f-I). Ex-4 induced a slight but nonsignificant reduction in the mean f-I relationship (n = 4, P > 0.05; Fig. 6). Together, these results show that activation of presynaptic GLP-1R, expressed putatively on glutamatergic terminals, increases synaptic excitation of VTA dopamine neurons via AMPA/kainate receptors.
Fig. 5.
A: representative spontaneous excitatory postsynaptic current (sEPSC) traces from VTA dopamine neurons before and during Ex-4 application. B: Ex-4 increases sEPSC frequency. Effect of Ex-4 on sEPSC averages (C) is expressed as %difference from baseline recorded before Ex-4 application (D). E: Ex-4 reduces paired/pulse ratio (PPR). F: quantified PPR results (gray lines represent individual neuron responses before and during Ex-4 application; black line is the mean). Quantified data are shown as means ± SE. *P < 0.05.
Fig. 6.
A: voltage responses of a VTA dopamine neuron to a −140-pA and a +140-pA current step illustrate a slight decrease in action potential (AP) firing during Ex-4 treatment. B: quantified frequency vs. injected current (f-I) data (means ± SE) shows that the effect of Ex-4 on the f-I relationship is not significant (P > 0.05).
DISCUSSION
The present data support the hypothesis that VTA GLP-1R signaling regulates the control of palatable high-fat food intake. Behavioral data presented here indicate that VTA GLP-1R activation reduces intake of palatable high-fat diet primarily by reducing meal size, with minimal and inconsistent effects on meal frequency. Additionally, the results demonstrate that the anorectic effects of intra-VTA GLP-1R activation are mediated in part by glutamatergic AMPA/kainate but not NMDA receptor signaling. The intake-suppressive effect of Ex-4 was observed from 3 to 24 h postinjection, and this hypophagic response was attenuated by the AMPA/kainate receptor antagonist CNQX at each of these times. The Ex-4-induced hypophagia was not completely blocked by AMPA/kainate receptor antagonism, highlighting the possibility that the feeding effects of intra-VTA GLP-1R activation are also mediated by additional nonglutamatergic mechanisms that persist regardless of the presence of glutamatergic receptor antagonists. Although the independent contributions of AMPA receptors vs. kainate receptors cannot be elucidated by these studies, the finding that AMPA/kainate receptors rather than NMDA receptors mediate the food intake- and meal size-suppressive effects of VTA GLP-1R activation is consistent with previous results demonstrating a role for AMPA/kainate but not NMDA receptors in MRS-mediated feeding (27).
Immunoblot results indicate that intra-VTA Ex-4 increases levels of TH within the VTA. This finding suggests an upregulation of dopamine production in the VTA as a result of VTA GLP-1R activation. Further support for this idea is revealed by electrophysiological data demonstrating that presynaptic GLP-1R activation increases AMPA receptor-mediated glutamatergic transmission onto the VTA dopamine neurons. The fact that Ex-4 increased VTA dopamine neuron activity is interesting given that intra-VTA GLP-1R activation potently suppresses food intake, an effect often associated with reduced dopaminergic signaling in the MRS. There are several hypothetical possibilities for these seemingly discrepant data. One potential explanation is that GLP-1R excitation of VTA dopaminergic neurons may lead to dopamine-induced activation of D2 autoreceptors within the VTA (2), having the subsequent net effect of decreasing dopamine signaling in the MRS. Another possibility is that GLP-1R signaling in the VTA somehow replaces the hedonic within-meal-promoting signals of continued ingestion of a palatable food, thus reducing the likelihood of further feeding. This would be consistent with evidence demonstrating that VTA GLP-1R activation reduces rats' motivation to obtain a palatable food in an operant paradigm (13) as well as present findings showing suppression in meal size by VTA GLP-1R activation. Finally, it is possible that the GLP-1-producing preproglucagon neurons in the hindbrain coexpress glutamate, and thus cotransmission of GLP-1 and glutamatergic signals may occur in the VTA from the same axon.
Traditionally, fluctuations in MRS dopamine signaling have been examined over a very short time scale (i.e., ms to h) with regard to influences on appetitive behaviors (18, 31, 36), whereas alterations on the scale of several hours to days have not been as carefully characterized. Together with our previous report (3), present data demonstrate that the food intake-suppressive effects of intra-VTA GLP-1R activation occur hours after drug administration for high-fat diet but within 20 min for a sucrose solution (3). Therefore, VTA GLP-1R modulation of food intake may add levels of complexity and depend in part on macronutrient composition and/or the physical properties (solid vs. liquid) of the palatable food being ingested. Another important question raised by the electrophysiological data presented here is the neuroanatomic location of the glutamatergic cell bodies that project to the VTA and putatively express GLP-1R on their presynaptic terminals. Several nuclei implicated in the control of food intake provide glutamatergic input to the VTA (e.g., prefrontal cortex, bed nucleus of the stria terminalis, lateral hypothalamus) [reviewed by Geisler and Wise (16)], and although the present data cannot determine which source(s) is relevant to the effects of GLP-1R in the VTA, further investigation is certainly warranted and will provide more insight into the mechanism of action of GLP-1R signaling in the VTA.
That the intake-suppressive effects of a peripherally administered GLP-1R agonist are attenuated by blockade of VTA GLP-1R highlights the intriguing possibility that VTA GLP-1Rs are not only physiologically relevant for the control of food intake (3) but may also be a clinically relevant site of action for peripherally administered GLP-1R ligands in humans. Interestingly, we observed that unilateral VTA administration of Ex-9 was sufficient to attenuate the food intake- and body weight-suppressive effects of systemically administered Ex-4. Although not statistically different, the mean 24-h food intake and body weight of the Ex-9/Ex-4 group were lower than that of the vehicle/vehicle group, suggesting that the contralateral VTA and other CNS GLP-1R-expressing nuclei are also mediating in part the 24-h anorectic effect produced by IP Ex-4 (see Ref. 19 for review). It is worth noting that whereas GLP-1R agonists can induce nausea/malaise when administered systemically (4, 9, 25, 26), GLP-1R activation in the VTA reduces food intake in the absence of nausea/malaise (3, 13). Therefore, the novel findings presented here, together with a growing body of basic science evidence, suggest that VTA GLP-1R may be a potential future clinical target for obesity pharmacotherapies to selectively reduce palatable food intake independent of nausea/malaise (3, 13).
In summary, the data presented here provide complementary behavioral, molecular, and electrophysiological evidence that AMPA/kainate receptor signaling in the VTA mediates at least in part the food intake-suppressive effects of VTA GLP-1R activation. Given that NTS GLP-1-producing neurons are activated by gastrointestinally derived, vagally mediated satiation signals (34) and NTS neurons project monosynaptically to the VTA (3), the present data provide a novel mechanism by which increased satiation signaling may decrease the rewarding value of an ongoing meal (5, 6).
GRANTS
This research was supported by DA-031747 (P. I. Ortinski), DA-22339 and DA-18678 (R. C. Pierce), DK-097954 (E. G. Mietlicki-Baase), and DK-096139 and DK-085435, the University of Pennsylvania Research Foundation, and the Diabetes Research Center (DK-19525) (M. R. Hayes).
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
E.G.M.-B., P.I.O., A.L.A., R.C.P., and M.R.H. contributed to the conception and design of the research; E.G.M.-B., P.I.O., L.E.R., D.R.O., A.L.A., and M.R.H. performed the experiments; E.G.M.-B., P.I.O., L.E.R., D.R.O., and M.R.H. analyzed the data; E.G.M.-B., P.I.O., and M.R.H. interpreted the results of the experiments; E.G.M.-B., P.I.O., and M.R.H. prepared the figures; E.G.M.-B., P.I.O., and M.R.H. drafted the manuscript; E.G.M.-B., P.I.O., L.E.R., D.R.O., A.L.A., R.C.P., and M.R.H. edited and revised the manuscript; E.G.M.-B., P.I.O., L.E.R., D.R.O., A.L.A., R.C.P., and M.R.H. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Valuable technical assistance was provided by Shaila Berlas, Lauren McGrath, Orianne Montaubin, David Reiner, Line Stensland, Frank Wang, and Derek Zimmer.
REFERENCES
- 1.Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci 24: 8141–8152, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev 28: 415–431, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology 203: 475–487, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 34: 1997–2006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav 56: 145–149, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374: 39–47, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Chieng B, Azriel Y, Mohammadi S, Christie MJ. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol 589: 3775–3787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covasa M, Hung CY, Ritter RC, Burns GA. Intracerebroventricular administration of MK-801 increases food intake through mechanisms independent of gastric emptying. Am J Physiol Regul Integr Comp Physiol 287: R1462–R1467, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Davidson MB, Bate G, Kirkpatrick P. Exenatide. Nat Rev Drug Discov 4: 713–714, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26: 2788–2797, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci 19: 227–244, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol 289: R1504–R1511, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav Neurosci 121: 1234–1242, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav 106: 413–416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hettes SR, Gonzaga J, Heyming TW, Perez S, Wolfsohn S, Stanley BG. Dual roles in feeding for AMPA/kainate receptors: receptor activation or inactivation within distinct hypothalamic regions elicits feeding behavior. Brain Res 992: 167–178, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15: 6779–6788, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol 31: 104–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci 30: 1239–1250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier Academic, 2005 [Google Scholar]
- 31.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24: 1265–1271, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30: 1358–1369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285: R470–R478, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2: 577–593, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev 2: 195–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]