Abstract
Deficient leptin signaling causes infertility via reduced activity of GnRH neurons, causing a hypogonadal state in both rodents and humans. Because GnRH neurons do not express leptin receptors, leptin's effect on GnRH neurons must be indirect. Neurons within the hypothalamic arcuate nucleus that coexpress AGRP and NPY are considered to be important intermediate neurons involved in leptin regulation of GnRH neurons. Previously, we reported that the absence of AGRP and haploinsufficiency of MC4R in leptin receptor mutant (Leprdb/db) females result in restoration of fertility and lactation despite the persistence of obesity and insulin resistance. The overarching hypothesis in the present study is that the absence or reduction of leptin's inhibition of AGRP/NPY neurons leads to suppression of GnRH release in cases of leptin signaling deficiency. Since TAC2 (NKB)-TAC3R signaling plays a role in puberty maturation and is modulated by metabolic status, the other aim of this study is to test whether TAC2/NKB neurons in ARC regulated by melanocortinergic signals herein affect leptin's action on puberty and reproduction. Our data showed that AGRP deficiency in Leprdb/db females restores normal timing of vaginal opening and estrous cycling, although uterine weight gain and mammary gland development are morphologically delayed. Nonetheless, Agrp−/− Leprdb/db females are fertile and sustain adequate nutrition of pups with lactation to weaning age. AGRP deficiency results in advanced vaginal opening in wild-type female mice. The postpubertal increase in hypothalamic TAC2 mRNA was not observed in Leprdb/db females, whereas AGRP deficiency restored it in Leprdb/db females. Additionally, MC4R activation with MTII induced FOS expression in TAC2 neurons, supporting the concept of melanocortinergic regulation of TAC2 neurons. These studies suggest that AGRP imposes an inhibitory effect on puberty and that TAC2 neurons may transmit melanocortinergic inhibition of GnRH neurons.
Keywords: agouti-related peptide, leptin, puberty, reproduction
the onset of puberty is the culmination of a subtle shift in balance of stimulatory and inhibitory neurotransmission within a complex neural network. Whereas the intrinsic pulsatility of GnRH neurons points toward a cell-autonomous function of gonadotropin-releasing hormone (GnRH) secretion, extrinsic signals are likely to be required to trigger activity in quiescent GnRH neurons. There is undoubtedly a link between metabolic status and GnRH activity (34). In the context of normal leptin signaling, a role has been implicated on the basis of a direct correlation between puberty onset and body weight (8, 16). Additionally, cases of defective leptin signaling in both humans and rodents result in hypogonadotropic hypogonadism (13). In a case report of a girl with leptin deficiency, treatment with leptin appeared to reverse the hypogonadal state (12). In cases of lipodystrophy with attendant hypoleptinemia, leptin therapy normalized pituitary hormone release (29) and restored menstrual cycles to all treated female patients.
The obese (Lepob/ob) and diabetes (Leprdb/db) mice are models of mutations in leptin and leptin receptor (LEPR) (6), respectively, that cause obesity, insulin resistance, and infertility (3, 17). Beyond infertility, these models are also characterized by lactational incompetence (18, 35). There is no direct input of leptin on GnRH neurons (33), although there is now strong evidence that leptin-regulated neurons from the arcuate nucleus (ARC) and the ventral premammilary nucleus provide afferent inputs that regulate GnRH neurons (10, 19, 23). Two of leptin's known mediators are agouti gene-related peptide (AGRP) and neuropeptide Y (NPY), neuropeptides that are coexpressed with γ-aminobutyric acid in neurons located medially within the hypothalamic ARC nucleus. Both neuropeptides are orexigenic and inhibitory to their respective receptors, whereas their expression is suppressed by leptin. NPY has been studied previously in the context of fertility; the absence of NPY restores fertility to leptin-deficient mice (11, 35). The transgenic mouse with Lepob/ob and NPY receptor Y4 knockout displayed restored fertility but persistent lactational incompetence (28, 35). Additionally, transgenic female mice with combined LEPR and AGRP deletion characterized by our group, the Leprdb/db Agrp−/− mouse, exhibit a normal fertility phenotype despite persistence of much of the metabolic phenotype (19). We set out to define the role of AGRP as a regulator of puberty and development of reproductive function.
Accumulating evidence showed that the subpopulation neurons located in ARC, named KNDy neurons, coexpress kisspeptin, neurokinin B [NKB; also known as tachykinin 2 (TAC2)], and dynorphin and send projections to the GnRH neurons in the preoptic area (21, 38). In particular, many lines of rodents and human studies have documented potent stimulatory effects of NKB on puberty onset (21, 40) and reproduction (7, 15). Both male and female pubertal failure cases resulting from NKB mutation are responsive to pulsatile administration of GnRH (42). However, the only link that has been found between TAC2/NKB and energy balance is in the context of lactation, which is characterized by decreased leptin, reduced POMC, increased NPY, and AGRP in the ARC, resulting in decreased melanocortin signaling (38). Specifically, during lactation there is suppression of kisspeptin and NKB concomitant with GnRH suppression (38). Despite the fact that kisspeptin can modulate proopiomelanocortin (POMC) neurons and NPY/AGRP neurons (14), the possibility of melanocortinergic signals such as AGRP/melanocortin-4 receptor (MC4R) modulating TAC2/NKB neurons remains virtually unexplored. Furthermore, it remains to be determined whether TAC2/NKB neuropeptide release from KNDy neurons acts as the intermediary of leptin's and AGRP's actions on puberty and reproductive development.
Pubertal development affects multiple end organs, principally via changes in hormonal output. In female mice, pubertal onset is commonly characterized by vaginal opening (4), which results from estrogenic effects on the vaginal epithelium (25). Estrous cyclicity reflects the ovarian hormonal environment created by periodic changes in the pattern of GnRH pulsing and gonadotropin release in postpubertal female mice (5). Mammary growth is a complex and important part of female development. Rudimentary ductal elongation of the mammary gland begins postnatally. Critical growth and differentiation subsequently occurs throughout puberty, with further maturation occurring during pregnancy. Another marker of puberty is uterine weight, as the majority of growth is estrogen driven and independent of acquired body weight (25, 30). In an effort to define the role of AGRP on pubertal development, we assessed all of these aspects in wild-type and obese (Leprdb/db) females. Finally, we observed Tac2 mRNA expression during pubertal development in different genotype mice and tested whether MC4R agonist melanotan II (MTII) activates TAC2/NKB neurons. We provide herein the first evidence that TAC2/NKB neurons may be a node in the leptin-regulated network controlling female reproduction.
MATERIALS AND METHODS
Animals.
All animals were housed at 22°C in barrier facilities on a 12:12-h light-dark cycle with ad libitum access to irradiated 5058 PicoLab Mouse Diet 20 chow and sterilized water. Mice were weaned at 21 days of age, at which time ear clippings were performed. Genotyping was performed as described previously (20, 32).
Several separate breeding models were used to assess fertility as well as reproductive physiology. Females heterozygous for LEPR mutation (Leprdb/+) were crossed with Leprdb/+ males. Additionally, Leprdb/+ females homozygous for Agrp knockout (Agrp−/−) were crossed with Leprdb/+ Agrp−/− males. The groups selected from these matings were 1) wild-type females (+/+ for both genes), 2) homozygous LEPR mutant females (Leprdb/db), 3) homozygous Agrp knockout LEPR mutant females (Leprdb/db Agrp −/−), and 4) females homozygous for only Agrp knockout (Agrp−/−). To assess innate fertility, transgenic females from the four genotypes were mated with wild-type males. Experiments were done at the ages of 3, 4, 5, and 8 wk. All animals were of a mixed genetic background of C57BL/6J × FVB/NJ × 129 (19). TAC2-enhanced green fluorescent protein (GFP) mice (37) were kindly provided by Dr. Martin Myers.
In experiments that required the harvesting of organs, animals were euthanized by carbon dioxide asphyxiation. Blood was collected by cardiac puncture. Blood specimens for metabolic studies were attained by tail nick bleeds. The Albert Einstein College of Medicine Institute's Animal Care and Use Committee approved all protocols and procedures.
Metabolic studies.
Animals were fasted for 16–20 h prior to body composition and fasting glucose and insulin studies. Body composition, specifically fat mass and lean mass measurements, was attained using an EchoMRI Whole Body Composition Analyzer from Echo Medical Systems (Houston, TX). Total body weight was measured on a scale to 0.1 g. Blood glucose levels were measured with a Bayer Ascensia Elite (Tarrytown, NY) glucose meter. Insulin levels were assessed using the Ultrasensitive Mouse EIA kit from Alpco Diagnostics (Salem, NH).
Puberty onset observational measurements.
Vaginal opening was assessed by observation starting from 21 days of life. After vaginal opening, cycle phase was determined by daily vaginal lavage using glass pipettes and normal saline and continued for 3 wk or until euthanization. Vaginal smears for cycle phase were viewed under ×10 and ×40 magnification using an Olympus CX31 microscope (5).
To assess fertility, all breeding experiments were done by mating 8- to 12-wk-old females from the four genotypes with a proven fertile wild-type male. The females were assessed for pregnancy, with weight determined weekly. The litter size was documented immediately after birth and followed until weaning for survival. Each pair was permitted ≤6 wk of mating for assessment of fertility.
Tissue harvest for evidence of pubertal development.
Female mice from each of the groups were euthanized as described at 4, 5, and 8 wk. Uteri were resected, and after careful removal of adhered fat, uteri were weighed on a scale to 0.0001 g. Ovaries were resected and placed in Z-FIX (Anatech, Battle Creek, MI) for 1–2 days and subsequently embedded in paraffin. Hypothalami were removed by macroscopic dissection, immediately placed in a specimen tube on dry ice, and then stored at −80°C (19).
Mammary gland development.
Female mice from each of the groups were euthanized as described at 3, 4, 5, and 8 wk. Four of five right inguinal mammary fat pads were removed, placed and spread on a glass slide (31), fixed with Carnoy's, and stained with carmine alum. Mammary fat pads were dehydrated sequentially in ethanol and finally in xylene. The slides were mounted with Permount and imaged digitally with a Nikon D70 camera with a 60-mm 1:2.8 macro lens.
Quantitative PCR of hypothalami.
Hypothalami were collected at 4 and 8 wk of age from each group, and total RNA was extracted using the RNeasy Lipid Tissue Mini kit (Qiagen, Venlo, The Netherlands). Presence and quality of RNA were confirmed by intact 18S and 28S bands following electrophoresis on a native agarose gel and by spectrophotometry using a NanoDrop UV-Vis Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). cDNA was synthesized by use of the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed on the Roche LightCycler 480 Real-Time PCR System using the LightCycler 480 SYBR Green Master I kit (Roche Diagnostics, Mannheim, Germany). Two sets of samples were run in triplicate on the same plate; one set had TAC2 primer (forward, 5′-AGGGAGGGAGGCTCAGTAAG-3′; reverse, 5′-GGCGGCTGTCGTAGAGTC-3′), and the second set had β-actin primer (forward, 5′-GCGCAAGTACTCTGTGTGGA-3′; reverse, 5′-GAAAGGGTGTAAAACGCAGC-3′) as the housekeeping gene. The PCR protocol for the amplification was 95°C for 10 s → 60°C for 10 s → 72°C for 10 s × 45 cycles. Fold change relative to the mean of wild-type controls was calculated with 2−ΔΔCT (24).
Intraperitoneal injection of MTII and FOS immunohistochemistry in TAC2-GFP mice.
Diestrus female TAC2-GFP mice (8 wk old) were injected intraperitoneally (ip) with 10 mg/kg body wt of MTII or saline (n = 4). Animals were perfused with 4% paraformaldehyde in phosphate buffer (pH 7.0) ∼1.5 h after the injection. The brains were postfixed in 4% paraformaldehyde overnight at 4°C and then placed in 30% sucrose until they sank. Five coronal sections (30 μm) of ARC were collected from each animal and stored in cryoprotectant at −20°C until they were processed for immunolabeling.
Tissue sections were rinsed in phosphate-buffered saline (PBS; 1×) to remove cryoprotectant, incubated in 3% H2O2 for 10 min to block endogenous peroxidase activity, and incubated in PBS plus 0.04% Triton X-100 (PBS-Tx) and 1% bovine serum albumin (BSA) for 1 h at room temperature. Sections were then incubated in goat anti-c-fos antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) in PBS-Tx and 1% BSA for 48 h at 4°C. The sections were then incubated in biotinylated anti-goat immunoglobulin G (1:600; Vector Laboratories, Burlingame, CA) in PBS-Tx for 1 h at room temperature, rinsed, and incubated for 1 h in avidin biotin complex (“Elite” ABC kit; Vector Laboratories). After being rinsed in PBS and 0.175 M sodium acetate, the sections were stained in nickel sulfate (25 mg/ml) and diaminobenzidine-HCl (0.2 mg/ml) in 0.175 M sodium acetate containing 30% H2O2 for 10 min, followed by a final rinse in PBS and sodium acetate. FOS immunoreactivity was visualized as blue-black in the nuclei of neurons. Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). After drying overnight, the sections were dehydrated with ascending alcohol concentrations, cleared with xylenes, and coverslipped. A series of tissue sections were treated identically, except that primary antibody was omitted from the incubation to control for antibody specificity.
To quantify TAC2-GFP and FOS-immunoreactive (ir) neurons, five sections of ARC were viewed under a Leica SP2 AOBS scanning confocal microscope (Heidelberg, Germany). Cells were considered TAC2-GFP FOS-ir positive if they had blue-black nuclear staining with distinct nuclear boundaries in TAC2-GFP cells. Total TAC2-GFP and FOS-ir neurons as well as the percentage of TAC2 neurons expressing FOS were calculated.
Statistics.
All statistical analyses were performed using GraphPad Prism software version 5.04. ANOVAs were used for within-genotype, multiple-age comparisons and for a given age group between genotype comparisons. Repeated-measures ANOVA was used when appropriate. Posttest analysis was performed using the Bonferroni method of correction for the designated multiple comparisons. Occurrence of vaginal opening and presence of cyclicity were analyzed by chi-square tests. Unpaired t-test was used to analyze group differences in TAC2 and FOS-ir cell numbers and percentage of TAC2 neurons expressing FOS. For all statistical analyses, P < 0.05 was the criteria for statistical significance.
RESULTS
Metabolic phenotype of Leprdb/db and Leprdb/db Agrp−/− mice.
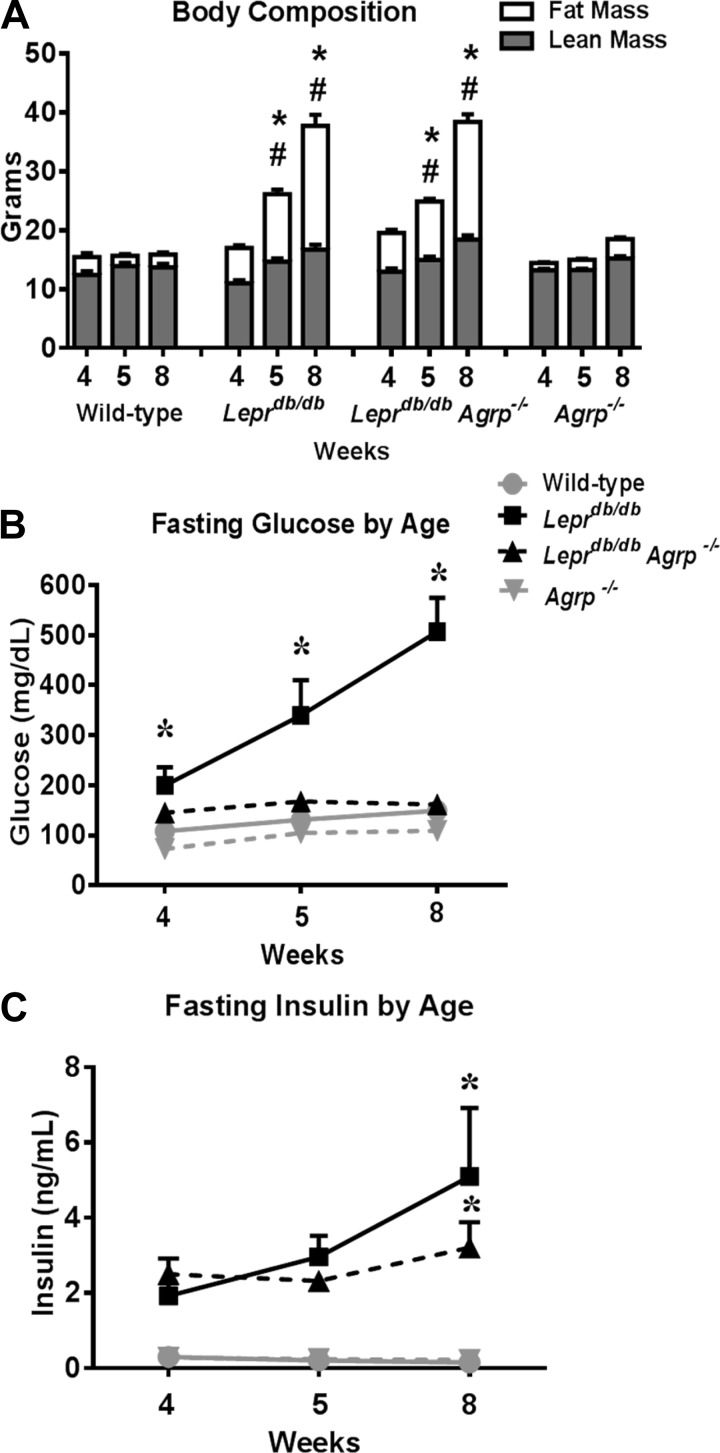
By 8 wk of age, the Leprdb/db mice developed the expected phenotype of severe obesity, hyperinsulinism, and hyperglycemia. Moreover, body weight reached more than double and percent fat mass more than tripled in Leprdb/db mice compared with wild-type mice (Fig. 1A). The Lepr db/db Agrp −/− group was comparable with Leprdb/db in both weight and percent fat mass in all age groups (Fig. 1A).
Fig. 1.
Metabolic parameters of pubertal and postpubertal female mice lacking agouti gene-related peptide (AGRP) and/or leptin receptor (LEPR)-B. A: weight gain and body composition of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice (n = 6–9). B: fasting glucose levels of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice at 4, 5, and 8 wk of age (n = 5–8). C: fasting insulin levels of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice. Data are presented as means ± SE. *P < 0.05 compared with wild-type; #P < 0.05 compared with genotype-matched preceding age group.
Hyperglycemia, a known feature of the Leprdb/db phenotype, occurred gradually in Leprdb/db mice. Compared with wild-type controls, 4-wk-old Leprdb/db mice were euglycemic, although mild hyperglycemia was present at 5 wk of age and became more severe by 8 wk of age (Fig. 1B). In contrast, hyperglycemia was not observed in Leprdb/db Agrp−/− mice (Fig. 1B). Hyperinsulinemia was evident in both Leprdb/db and Leprdb/db Agrp−/− female mice by 8 wk of age (Fig. 1C). The pattern of glucose and insulin levels observed in response to a glucose challenge suggests a significant resistance to insulin in the Leprdb/db compared with Leprdb/db Agrp−/− mice.
Body weight, fat mass, serum glucose, and insulin levels in Agrp−/− mice were comparable with wild-type mice (Fig. 1, A–C).
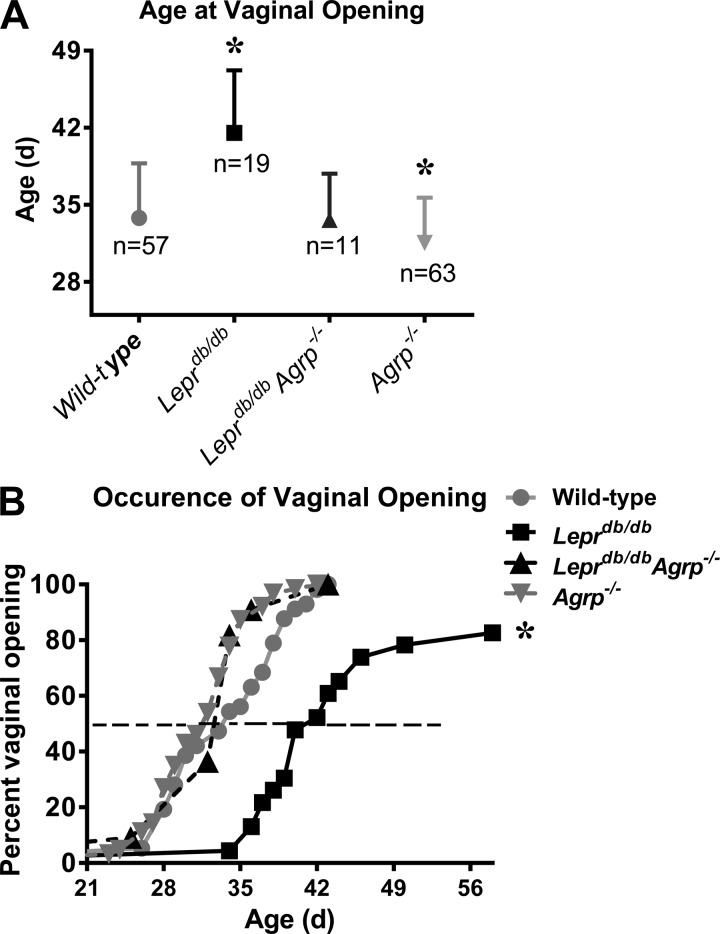
Loss of AGRP affects the timing of vaginal opening.
The average age of vaginal opening in mice ranges from 26 to 35 days, depending on the strain (5, 27, 25, 26). Consistent with other reports, the mean age of vaginal opening in wild-type mice was 34 days (Fig. 2A). Vaginal opening in Leprdb/db Agrp−/− mice was similar to that of wild-type. Agrp−/− females had earlier vaginal opening (32 days) than wild types (Fig. 2A).
Fig. 2.
Loss of Agrp affects the timing of vaginal opening. A: mean age at the time of vaginal opening in wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice. B: cumulative percentage of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice with vaginal opening is presented relative to age. Data are presented as means ± SE (n = 11–63). *P < 0.05 compared with all other groups, respectively.
Approximately 80% of Leprdb/db females showed vaginal opening by 8 wk of age (Fig. 2B). Of those with vaginal opening, the average age was delayed by >1 wk, with some females exhibiting vaginal opening as late as 58 days of age (Fig. 2B).
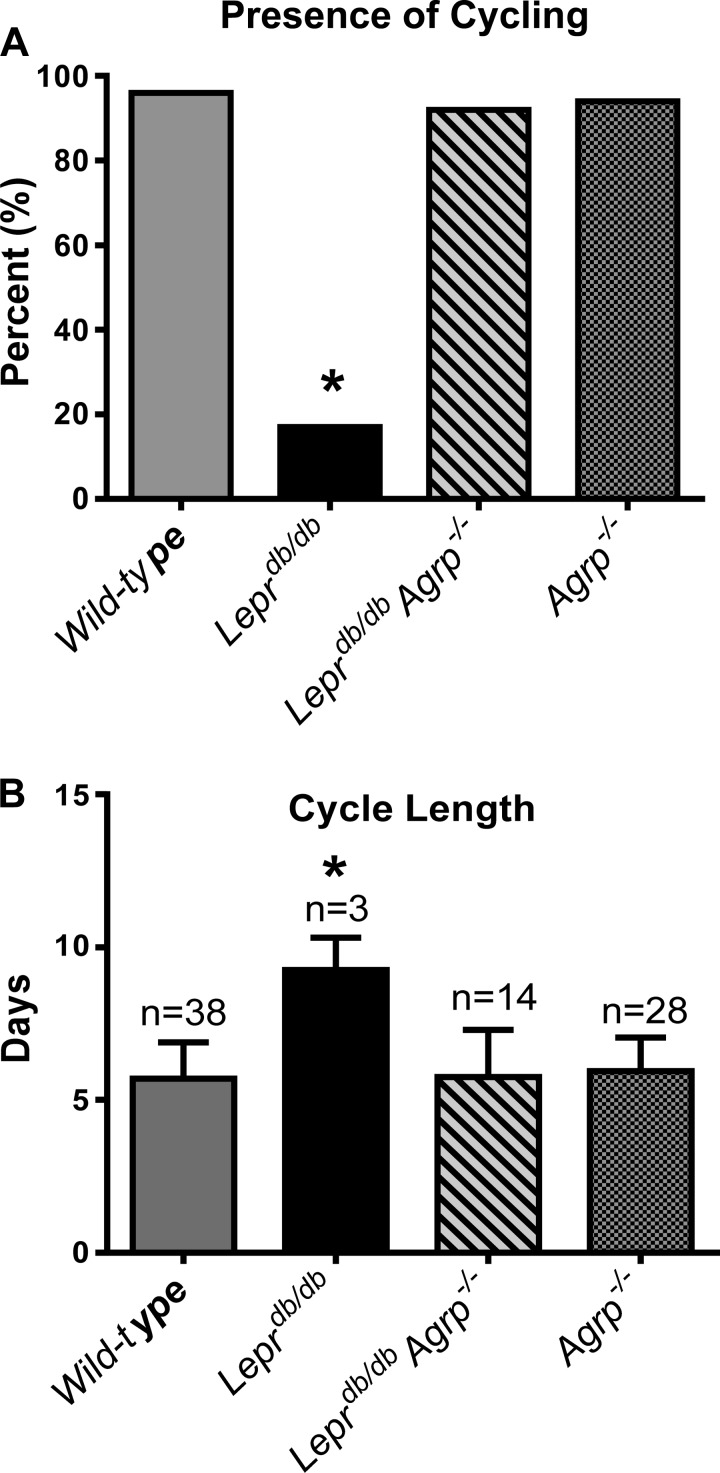
Normal estrous cycling and fertility is fully restored in db/db Agrp−/− females.
Estrous cyclicity and length was monitored after vaginal opening. More than 90% of wild-type, Leprdb/db Agrp−/−, and Agrp−/− mice had regular estrous cycles (Fig. 3, A and B). In contrast, <17% of Leprdb/db mice exhibited regular estrous cycles. Instead, Leprdb/db mice had estrous cycles that were typically 50% longer than wild-type control females (Fig. 3, A and B).
Fig. 3.
Loss of Agrp in Leprdb/db mice fully restored normal estrous cycling and fertility. A: percentage of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice exhibiting estrous cycles (n = 12–26). B: the estrus cycle length of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice (n = 3–38 cycles).
To assess fertility, 8- to 12-wk-old Leprdb/db and Leprdb/db Agrp−/− females were mated with proven fertile males. Pregnancy and lactation in the Leprdb/db Agrp−/− females was normal (Table 1). Conversely, only one Leprdb/db female achieved pregnancy despite all breeding pairs being permitted as much as 6 wk for mating. This Leprdb/db female pregnancy delivered only one pup that was not successfully sustained after birth (Table 1).
Table 1.
Agrp KO restores pregnancy and lactation in Leprdb/db mice
| Genotype | Days From Mating to Birth of Litter | Pups Born | Pups Weaned |
|---|---|---|---|
| Leprdb/dbAgrp−/− | 22 ± 2* (11 litters) | 5.3 (4–8 pups)* | 5.3 (4–8 pups)* |
| Leprdb/db | 38 (1 litter) | 1 pup | 0 |
Lepr, leptin receptor; KO, knockout. Breeding tests revealed that Agrp−/− Leprdb/db females have successful pregnancy, parturition, and lactation (n = 12).
P < 0.05 compared with Leprdb/db.
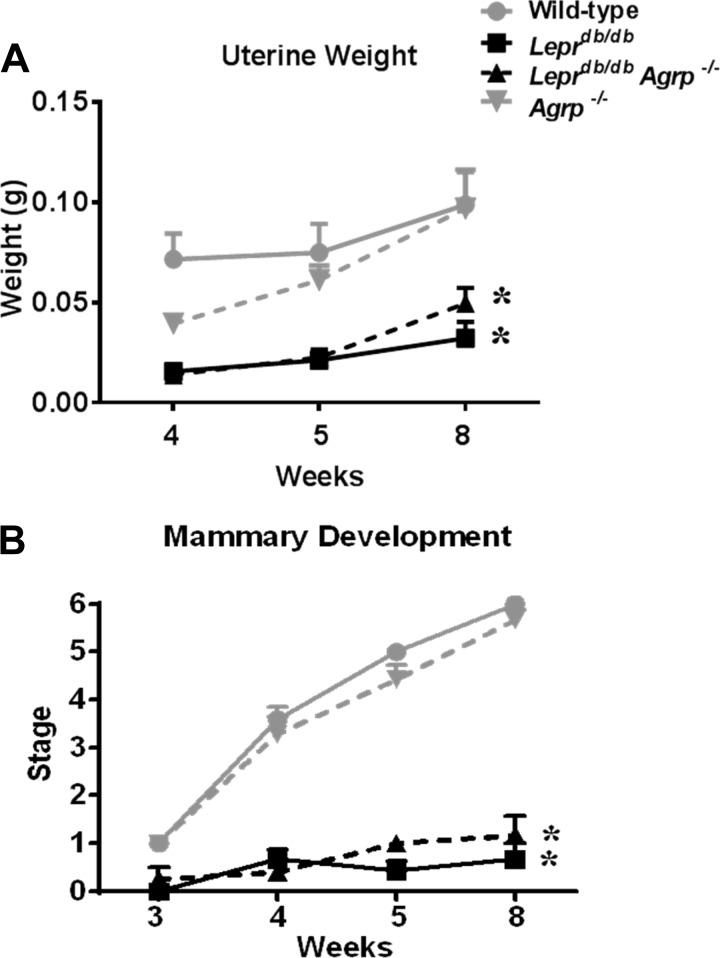
Uterine weight is decreased in both Leprdb/db and Leprdb/db Agrp−/− mice.
Although there is variability concomitant with estrous cyclicity related to fluctuating hormone levels, there is an overall trend for uterine growth with age in correlation with pubertal development. Thus we evaluated uterine weight in the 4-, 5-, and 8-wk-old mice in a sampling of our four mouse genotypes as a surrogate of pubertal development independent of estrous cycle phase at time of harvesting. As expected, Leprdb/db females had significantly lower uterine weights at all ages, whereas Agrp−/− mice were comparable to wild types (Fig. 4A). Similarly, Leprdb/db Agrp−/− mice showed significantly lower uterine weights than age-matched wild-type mice at all ages, and these mice did not present significant improvement in uterine weight compared with Leprdb/db mice (Fig. 4A). Surprisingly, these uteri of Leprdb/db Agrp−/− females are able to support implantation and maintain a pregnancy, albeit with a smaller litter size on average (Table 1).
Fig. 4.
Loss of Agrp did not alter uterine weight or rescue morphological changes in mammary development in Leprdb/db mice. A: uterine weights of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice cycling at 4, 5, and 8 wk of age (n = 6–9). B: stage of mammary development of wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice cycling at 3, 4, 5, and 8 wk of age (n = 3–9). Data are shown as means ± SE. *P < 0.05 compared with age-matched wild-type mice.
Mammary development is delayed in both Leprdb/db and Leprdb/db Agrp−/− female mice.
In an effort to quantify and thus compare mammary ductal development with the ability to perform statistical analysis, we used a staging system adapted from Alston-Mills et al. (1). Stages are defined by ductal elongation and extent of invasion through the fat pad as well as presence of terminal end buds (TEBs). We used a method that would account for both elongation and TEBs such that it would reflect pubertal change and control for isometric growth as well as large differences in size of fat pad.
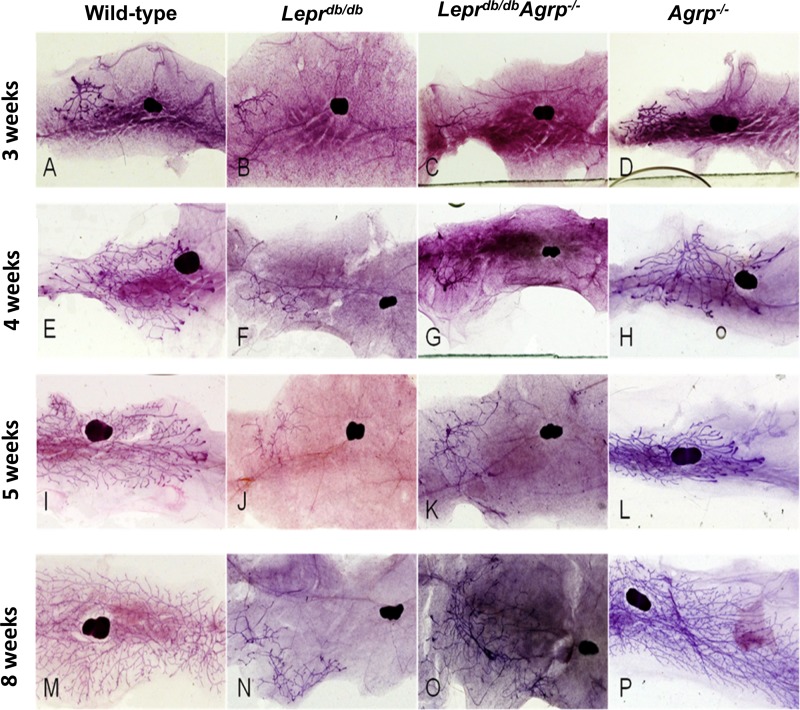
As it pertains to mammary development, Leprdb/db mice had either no pubertal development, defined by the presence of only rudimentary glandular tissue, or minimal pubertal development, which was characterized by the presence of a few TEBs (Figs. 4B and 5). This phenotype is consistent with the single Leprdb/db female that was unable to lactate and sustain nutritional support for one live pup (Table 1).
Fig. 5.
Images of representative mammary gland whole mounts. Ages and genotypes are as labeled. Images were selected to represent the mean stage of each age and genotype group as follows. A, E, I, and M: representative mammary glands for wild-type females at 3, 4, 5, and 8 wk of age. B, F, J, and N: representative mammary glands for Leprdb/db females at 3, 4, 5, and 8 wk of age. C, G, K, and O: representative mammary glands for Leprdb/db Agrp−/− females at 3, 4, 5, and 8 wk of age. D, H, L, and P: representative mammary glands for Agrp−/− females at 3, 4, 5, and 8 wk of age.
In parallel with the uterine weights, the Leprdb/db Agrp−/− females had mammary development similar to that of the Leprdb/db group (Figs. 4B and 5). However, this group had sufficient ductal development for lactation and to sustain a full litter of pups until weaning.
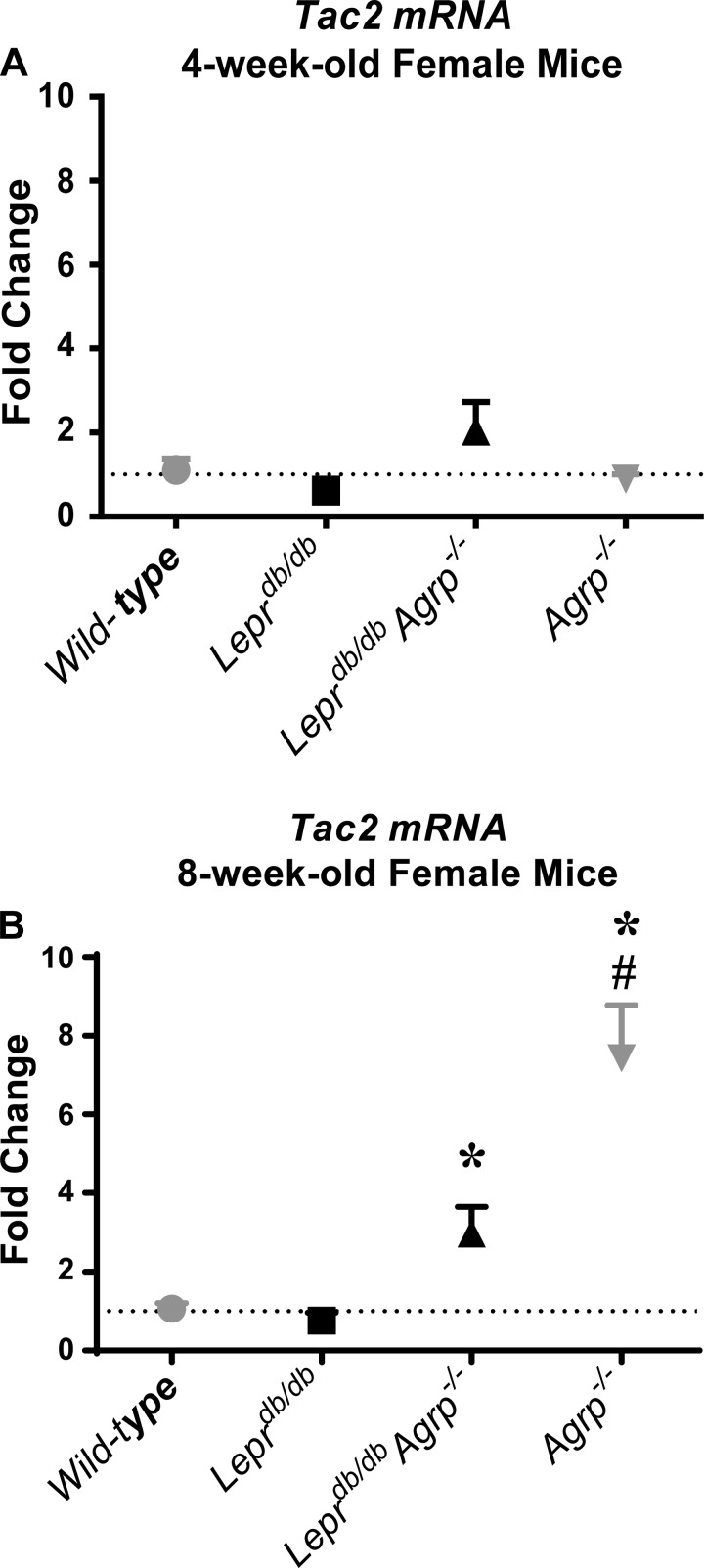
Tac2 is upregulated in the absence of AGRP postpuberty.
To evaluate the role of TAC2 on puberty, we studied both peripubertal (4 wk) and postpubertal (8 wk) Tac2 mRNA expression in the hypothalami of wild-type, Leprdb/db, Agrp−/−, and Leprdb/db Agrp−/− mice. In the peripubertal group, there was no difference in Tac2 mRNA expression among the four genotypes (Fig. 6A). Similarly, Tac2 mRNA was not increased in postpubertal wild-type or Leprdb/db females. In contrast, postpubertal hypothalamic Tac2 mRNA results were significantly increased in Agrp−/− and Leprdb/db Agrp−/− mice compared with wild-type controls. Relative to wild-type controls, Agrp−/− and Leprdb/db Agrp−/− females had a greater than three- and 7.4-fold increase, respectively, in Tac2 mRNA levels (Fig. 6B).
Fig. 6.
Loss of Agrp upregulated hypothalamic tachykinin 2 (Tac2) gene expression in postpubertal mice. A: fold change in Tac2 transcript expression in hypothalami harvested from wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice at 4 wk of age. B: fold change in Tac2 transcript expression in hypothalami harvested from wild-type controls and Leprdb/db, Leprdb/db Agrp−/−, and Agrp−/− mice at 8 wk of age. All results are presented as mean fold change ± SE. Fold changes were calculated by the 2−ΔΔCT method, using the 4-wk-old wild-type mean as the comparison group. *P < 0.05 compared with age-matched wild-type mice; #P < 0.05 compared with control and genotype-matched 4-wk-old mice.
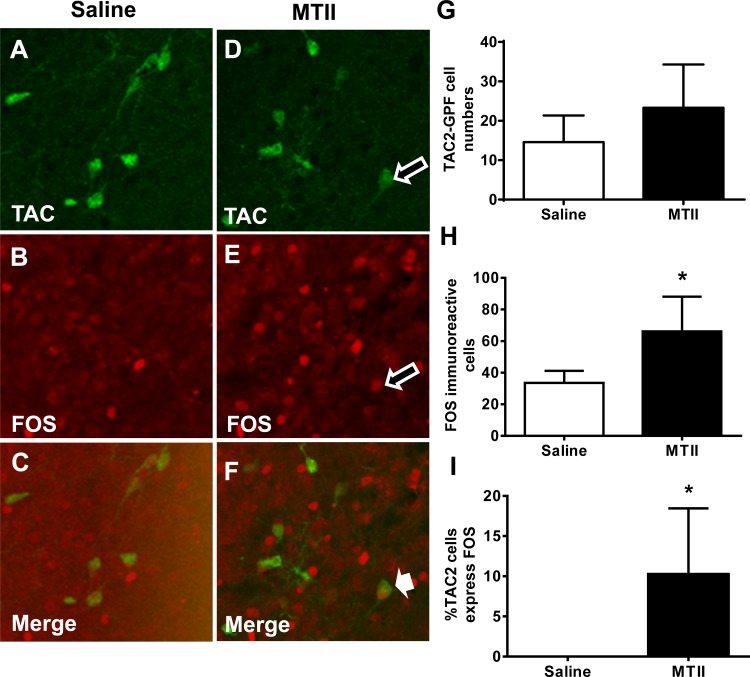
MC4R agonist activates TAC2 neurons.
To determine whether melanocortins affect TAC2 neurons, we injected MTII, a synthetic MC3/4 receptor agonist, into TAC2-GFP mice and used immunohistochemistry to identify FOS-ir in TAC2 neurons as a marker of TAC2 neuron activation. We observed low FOS-ir in TAC2 neurons of saline-treated mice (Fig. 7C). Notably, compared with saline-injected diestrus females, the MC4R agonist MTII significantly induced FOS expression in TAC2 neurons in ARC (Fig. 7, F and I). The overall number of FOS-ir cells in MTII-treated mice was doubled compared with saline-injected females (Fig. 7, E and H). MTII injection did not significantly alter the number of TAC2-GFP neurons (Fig. 7, D and G).
Fig. 7.
Agonizing melanocortin 4 receptors stimulates TAC2 neurons in the ARC. Eight-week-old diestrus TAC2-green fluorescent protein (GFP) females were injected intraperitoneally with 10 mg/kg body wt saline or melanotan II (MTII; n = 4) and perfused 1.5 h after the injection. Representative sections of immunohistochemistry (×40 magnification) showing TAC2-GFP neurons (green; A and D), FOS-immunoreactive (ir) cells (red; B and E), and TAC2-GFP neurons coexpressing FOS (C and F) in mice infused with saline (A–C) and MTII (D–F). For FOS-ir images (B and E), the grayscale images were processed to mimic red fluorescence and merged with the green channel for the TAC2-GFP neurons. Open arrows and indicate TAC2-GFP neurons and FOS-ir cells, respectively. Solid arrow indicates neurons with colocalized TAC2 and FOS. G: total no. of TAC2-GFP neurons in the arcuate nuclei (ARC) of mice injected with saline or MTII. H: total no. of FOS-ir cells in the ARC of mice injected with saline or MTII. I: %TAC2 neurons coexpressing FOS in mice injected with saline or MTII. Bar data represent means ± SE. *P < 0.05 vs. mice infused with saline.
DISCUSSION
Ablation of AGRP in Leprdb/db female mice completely rescues fertility and lactation potentially via modulation of melanocortin receptors on GnRH neurons (19). The present study demonstrates that reduced inhibitory tone in Agrp−/− db/db females rescues pubertal onset, adult reproductive physiology, and fertility through mechanisms that may involve Tac2 upregulation. Although Agrp−/− Leprdb/db females exhibit delayed mammary gland development, unlike Leprdb/db females they do have the capacity to lactate and sustain adequate nutrition for pup survival and development after pregnancy. These results suggest that AGRP imposes an inhibitory signal that modulates GnRH neurons and KNDy neurons, acting as a mediator of leptin's effects on puberty and reproductive physiology. AGRP deficiency upregulated Tac2 gene expression on postpubertal rather than peripubertal Leprdb/db mice, which suggests that TAC2 is unlikely to be involved in the onset of puberty in this model. These data are consistent with a prior study suggesting that genetic ablation of AGRP in female Lepob/ob mice restored fertility (41). Recently, a study in rats showed that blockade of MC4R blunted the LH surge in ovariectomized, steroid-primed female rats (36).
The reproductive phenotype of db/db females is variable in the severity of the hypogonadotropic hypogonadism and nature of the pubertal defect. Nonetheless, puberty is typically severely delayed or absent in all Leprdb/db mice. Deletion of AGRP in the Leprdb/db Agrp−/− mouse model rescues puberty as well as fertility. Abnormal mammary development and lactational insufficiency is one of the most severe defects found in Leprdb/db females. The lack of mammary development in leptin-deficient mouse models is described elsewhere, and transplant studies suggest that the defect is not an end-organ defect (39). Similarly, mice with mutation in STAT3, a transcription factor involved in the leptin-mediated signaling pathway, do not exhibit mammary gland development, suggesting that the mammary defect does not reflect insufficient circulating estrogen levels or local IGF-I signaling (39). Although the ductal development is not significantly improved in the Leprdb/db Agrp−/− by 8 wk, the age by which female mice are normally postpubertal, there is compensation and catchup in mammary development during pregnancy sufficient enough for normal lactation (19). In this case, our data suggest that the mammary gland must be sufficiently primed in the nulliparous state to permit the appropriate response to the enhanced hormonal milieu of pregnancy (2).
Uterine growth is a marker of elevated circulating estrogen levels, as is normally observed during the normal pubertal transition (25, 30). Similar to the mammary gland, as determined by a lack of significant increase in uterine weight by 8 wk, the uteri of the Leprdb/db mice are underdeveloped. Although the uterine weights of the 8-wk-old Leprdb/db Agrp−/− mice are not significantly different from those of Leprdb/db mice, the uteri of the Leprdb/db Agrp−/− mice may have been primed by the absence of AGRP to permit implantation and placentation.
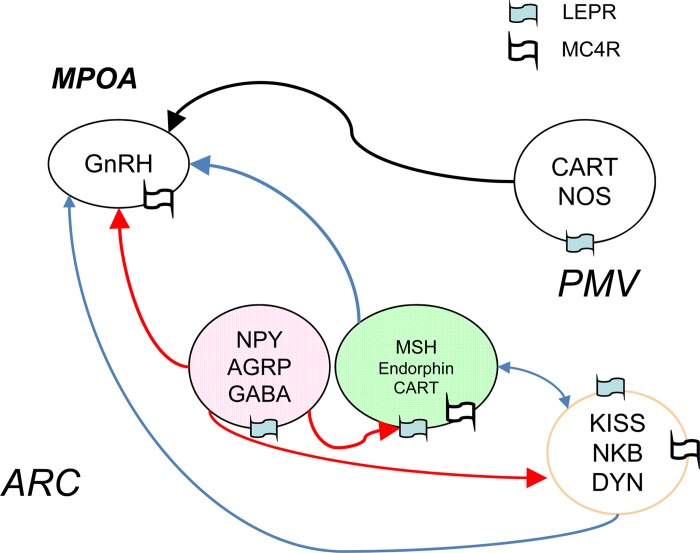
KNDy neurons in ARC contribute to GnRH release and pubertal development in humans and rodents (15, 22, 38). In the Leprdb/db mice model, we propose that KNDy inhibition by overactivity of AGRP may be a key factor responsible for puberty delay and reproductive failure. However, conditional deletion of LEPR-B in KNDy neurons of the Kiss1CRE Leprflox/flox mice (9) exhibits normal puberty, normal reproductive physiology, and fertility phenotypes, suggesting that direct leptin action in KNDy neurons is not required for appropriately timed puberty and reproduction. Surprisingly, Tac2 mRNA expression is dramatically upregulated in postpubertal females in our Leprdb/db Agrp−/− mouse model. This new finding suggests that melanocortinergic regulation of KNDy neurons, in addition to direct modulation of GnRH neurons, by AGRNP/NPY neurons is an important mediator of leptin's effects on reproductive physiology (Fig. 8).
Fig. 8.
A network of leptin-sensitive neurons modulates gonadotropin-releasing hormone (GnRH) neurons. AGRP/neuropeptide Y (NPY) neurons of the ARC send inhibitory signals (red arrows) to proopiomelanocortin, KNDy neurons, and GnRH neurons. Presence of LEPR and melanocortin receptor 4 (MC4R) expression is represented on the neurons. MPOA, medial preoptic area; CART, cocaine amphetamine-related transcript; NOS, nitric oxide synthase; PMV, ventral premamillary nucleus; KISS, kisspeptin; NKB, neurokinin B; DYN, dynorphin.
In summary, our data provide convincing evidence that AGRP acts as an inhibitory modulator of puberty and adult reproductive physiology independent of the obese phenotype. It is possible that TAC2 neurons are suppressed by AGRP overexpression in leptin-resistant states, and removal of AGRP's inhibitory effects may restore developmentally appropriate expression of Tac2 and pubertal development. These observations corroborate the putative role of TAC2 neurons as important intermediaries coordinating AGRP and leptin's effects on the female reproductive system.
GRANTS
This work was supported by National Institutes of Health Grants PO1-DK-026687 (S. C. Chua, Jr.), U54-HD-058155 (S. C. Chua, Jr., G. Neal-Perry), and P60-DK-020541 (S. C. Chua, Jr.).
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
S.S.-B., Y.S., D.D.I., and S.-M.L. performed the experiments; S.S.-B., Y.S., D.D.I., S.-M.L., G.S.N.-P., and S.C.C. analyzed the data; S.S.-B., Y.S., D.D.I., S.-M.L., G.S.N.-P., and S.C.C. interpreted the results of the experiments; S.S.-B. and Y.S. prepared the figures; S.S.-B., Y.S., D.D.I., G.S.N.-P., and S.C.C. edited and revised the manuscript; S.S.-B., Y.S., D.D.I., S.-M.L., G.S.N.-P., and S.C.C. approved the final version of the manuscript; Y.S., D.D.I., and S.C.C. contributed to the conception and design of theresearch; Y.S. drafted the manuscript.
REFERENCES
- 1.Alston-Mills B, Lepri JJ, Martin CA. Modulation of mammary gland development in pre-pubertal mice as affected by soya and milk protein supplements. Br J Nutr 106: 502–509, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Augustine RA, Ladyman SR, Grattan DR. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 586: 387–397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boquist L, Hellman B, Lernmark A, Taljedal IB. Influence of the mutation “diabetes” on insulin release and islet morphology in mice of different genetic backgrounds. J Cell Biol 62: 77–89, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, Sippell W, Abbott DH, Soto A, Tyl RW, Bourguignon JP, Skakkebaek NE, Swan SH, Golub MS, Wabitsch M, Toppari J, Euling SY. Environmental factors and puberty timing: expert panel research needs. Pediatrics 121, Suppl 3: S192–S207, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4: Appendix 4I, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience 146: 630–642, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics 111: 815–821, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121: 355–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato J, Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2: 57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274: 1704–1707, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30: 10205–10219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95: 2857–2867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res 49: 244–251, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966 [DOI] [PubMed] [Google Scholar]
- 18.Israel D, Chua S., Jr Leptin receptor modulation of adiposity and fertility. Trends Endocrinol Metab 21: 10–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israel DD, Sheffer-Babila S, de Luca C, Jo H, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC., Jr Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153: 2408–2419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50: 425–435, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: An update. Peptides 32: 1972–1978, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151: 3479–3489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29: 3138–3147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Meehan TP, Harmon BG, Overcast ME, Yu KK, Camper SA, Puett D, Narayan P. Gonadal defects and hormonal alterations in transgenic mice expressing a single chain human chorionic gonadotropin-lutropin receptor complex. J Mol Endocrinol 34: 489–503, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Michael SD, Kaplan SB, Macmillan BT. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J Reprod Fertil 59: 217–222, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Novaira HJ, Yates M, Diaczok D, Kim H, Wolfe A, Radovick S. The gonadotropin-releasing hormone cell-specific element is required for normal puberty and estrous cyclicity. J Neurosci 31: 3336–3343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278: 135–138, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Park JY, Chong AY, Cochran EK, Kleiner DE, Haller MJ, Schatz DA, Gorden P. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab 93: 26–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SE, Xu J, Frolova A, Liao L, O'Malley BW, Katzenellenbogen BS. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol 25: 1989–1999, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plante I, Stewart MK, Laird DW. Evaluation of mammary gland development and function in mouse models. J Vis Exp 21: 2828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 22: 5027–5035, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150: 2805–2812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roa J, García-Galiano D, Castellano JM, Gaytan F, Pinilla L, Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol 324: 87–94, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev 16: 1077–1088, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schioth HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport 12: 687–690, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt EF, Kus L, Gong S, Heintz N. BAC transgenic mice and the GENSAT database of engineered mouse strains. Cold Spring Harb Protoc 2013: pdb.top073692, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Smith MS, True C, Grove KL. The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin. Brain Res 1364: 139–152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn SR, Giesy SL, Myers MG, Jr, Boisclair YR. Mammary ductal growth is impaired in mice lacking leptin-dependent signal transducer and activator of transcription 3 signaling. Endocrinology 151: 3985–3995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topaloglu AK. Neurokinin B signaling in puberty: human and animal studies. Mol Cell Endocrinol 324: 64–69, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA 109: 3155–3160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95: 2287–2295, 2010 [DOI] [PubMed] [Google Scholar]