Abstract
Refractory wounds in diabetic patients present a significant clinical problem. Sonic hedgehog (SHH), a morphogenic protein central to wound repair, is deficient in diabetes. Regulation of SHH in wound healing is poorly understood. We hypothesize that thrombospondin-1 (TSP-1), through its receptor CD36, contributes to the SHH signaling defect in bone marrow-derived angiogenic cells (BMACs) in type 1 diabetic mice. Isolated BMACs from TSP-1-knockout mice demonstrated improved tube formation, migration, and adhesion in parallel with active SHH signaling. BMACs from STZ-induced type 1 diabetic mice showed significantly impaired Matrigel tube formation (n = 5; P < 0.05 vs. control), which was rescued by TSP-1 depletion (n = 5; P < 0.05 STZ-TSP-1−/− vs. STZ-WT) or exogenous SHH (20 mg/l, 24 h, n = 4; P < 0.05 vs. STZ-control). The expression of CD36 was elevated in BMACs from STZ mice (n = 4; P < 0.05). SHH signaling was significantly higher in BMACs from TSP-1−/− mice and TSP-1 receptor CD36-knockout mice (n = 6; P < 0.05 vs. WT) but not CD47-knockout mice (n = 3; P > 0.05 vs. WT). The impairment of recombinant human TSP-1 (2.2 nM, 24 h) on BMAC Matrigel tube formation was delayed significantly by CD36 deletion (n = 5; P < 0.05). CD36−/− BMACs demonstrated better tube formation under both normal and diabetic conditions with active SHH signaling (n = 4; P < 0.05 vs. WT BMACs). In conclusion, The TSP-1/CD36 pathway contributes to the SHH signaling defect, resulting in BMAC dysfunction in type 1 diabetic mice.
Keywords: bone marrow-derived angiogenic cells, Sonic hedgehog, thrombospondin-1, type 1 diabetes
impaired angiogenesis is a major clinical problem and affects tissue repair, especially in diabetic patients (3, 22). Endothelial progenitor cells (EPCs) are a group of bone marrow-derived endothelial precursors that home to sites of neovascularization and neoendothelialization and differentiate into endothelial cells (ECs) in situ, maintaining endothelial integrity and promoting angiogenesis. Because of their essential role in vascular homeostasis, recent controversy has arisen regarding the identification, characterization, and exact role of EPCs. Some early EPCs are heterogenous with the potential to facilitate angiogenesis, and therefore, they are better termed bone marrow-derived angiogenic cells (BMACs). However, despite the various EPC phenotypes, strong evidence supports EPCs as an individual's endogenous potential for vascular homeostasis and angiogenesis. Unfortunately, in patients with type 1 (28) or type 2 diabetes (43), EPCs are impaired either in quantity or in quality, directly affecting their therapeutic potential and limiting the eventual success of autologous cell therapies in the clinic. The complicated factors contributing to EPC dysfunction in diabetes are poorly understood to date.
Sonic hedgehog (SHH) is the ligand of the hedgehog signaling pathway, a morphogenic pathway critical in governing embryonic development and adult tissue homeostasis (32). Canonical SHH signaling in mammals is composed of SHH, its immediate binding protein patched (Ptc), the membrane receptor smoothened (Smo), and the transcriptional factor Gli. Hedgehog-bound Ptc dissociates Smo, resulting in the entry of Gli into the nuclei, which induces the expression of downstream target molecules (40). SHH signaling has been found to be central in controlling vascular development in the embryo, but it is also reactivated during adult repair processes (25, 35, 38). Reports from our laboratory and others demonstrate that deficient SHH signaling results in diminished EC angiogenesis and delayed tissue repair in type 1 diabetic mice (2, 29). However, despite the important role of SHH in regeneration, little information exists regarding endogenous regulation of the SHH pathway in diabetes.
Thrombospondin-1 (TSP-1) is a multifunctional glycoprotein with strong antiangiogenic potential (16, 18). It is secreted by a variety of cell types, including ECs (25). Evidence suggests that TSP-1 may represent a link between the pathology of diabetes and vascular complications, but the mechanisms remain largely unknown (4, 5, 42). The two major receptors for thrombospondins, CD47 and CD36, have shown diverse yet related effects on TSP-1-induced impairment in angiogenesis. Isenberg and colleagues (17, 19, 20) discovered that TSP-1 restricts tissue survival via CD47 under physical conditions. CD36 is better recognized as a scavenger receptor, as it mediates the uptake of oxidized low-density lipoproteins (LDL) by macrophages and the formation of foam cells during arterial atherogenesis (11, 14). However, reports show that the binding of TSP-1 to CD36 inhibits EC migration and induces cell apoptosis (9, 23). Although several signaling proteins have been implicated in TSP-1-induced EC dysfunction, such as VEGF-A-induced phosphorylation of VEGFR-2 and p38 MAPK (34), the precise signaling pathway has not been fully elucidated (33). Furthermore, no reports have demonstrated the possible participation of TSP-1/CD36 under diabetic conditions. Therefore, we investigated the role of the TSP-1/CD36 pathway in the regulation of SHH signaling in BMAC function in diabetes.
MATERIALS AND METHODS
Animals.
Eight-week-old adult male C57BL/6 mice were purchased from The Jackson Laboratory. Male adult TSP-1 global knockout mice (B6.129S2-Thbs1tm1Hyn/J, termed TSP-1−/− mice) on the C57BL/6 background were purchased from The Jackson Laboratory. Male adult homozygous CD36-knockout (CD36−/−) mice on the C57BL/6 background came from an in-house breeding colony (kindly provided by Dr. T. R. Billiar). Male adult homozygous CD47-knockout (CD47−/−) mice on the C57BL/6 background were kindly provided by Dr. J. S. Isenberg. Age- and sex-matched C57BL/6 mice served as wild-type (WT) controls. Diabetes was induced at 8 wk of age in male mice by five daily intraperitoneal injections of streptozotocin (STZ; Sigma), 45 mg/kg in citrate buffer (0.05 mol/l, pH 4.5), which is an established mouse model demonstrating multiple vascular dysfunctions and delayed wound healing similar to the conditions found in diabetic patients (29, 30, 44, 45). Control mice (C57BL/6) were treated with five daily injections of the same volume of citrate buffer. Whole blood glucose levels were measured by OneTouch meter (LifeScan) after the consecutive injections were completed. Mice with blood glucose levels >280 mg/dl were considered diabetic (29, 30, 44, 45). The animals were maintained under controlled environmental conditions (12:12-h light-dark cycle, temperature ∼25°C) and provided with standard laboratory food and water ad libitum. After 4–6 wk of hyperglycemia, mice were used for experiments. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
In vitro BMAC culture and characterization.
In vitro expansion of BMACs was performed as described in our recent reports (31, 49). Bone marrow mononuclear cells from the tibias and femurs of mice were plated on a culture flask coated with rat plasma vitronectin (Sigma-Aldrich) and maintained in endothelial growth medium (EGM-2; Lonza) at 37°C and 5% CO2. After 4 days in culture, nonadherent cells were washed away. New medium was applied. After 7 days in culture, to confirm the BMAC phenotype, attached cells were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate-labeled acetylated LDL (Dil-ac-LDL; 1 μg/ml) (Sigma) and fluorescein isothiocyanate (FITC)-labeled Ulex europeus agglutinin (Ulex-Lectin, 1 μg/ml) (Sigma) for 1 h. After nuclei staining by Hoechst 33258 (5 μg/ml; Invitrogen), the samples were viewed with an inverted fluorescent microscope (Nikon). Pictures were taken in ×200 high-power fields. The cells demonstrating double-positive fluorescence of Dil-ac-LDL and Ulex-Lectin were identified as differentiating BMACs (47). In addition, the expression of stem cell markers such as Sca-1 and CD34 and endothelial lineage markers such as Flk-1 and VE-cadherin (CD144), as well as the monocyte marker CD11b, was analyzed by flow cytometry and compared with freshly isolated bone marrow mononuclear cells. Adherent cells were gently detached using 5 mmol/l ethylenediaminetetraacetic acid (EDTA)-phosphate-buffered saline (PBS) at 37°C, washed, and incubated for 1 h on ice in PBS-0.5% (wt/vol) bovine serum albumin (BSA) together with FITC-labeled mouse antibodies (Abs) against Sca-1 (1 μg/1 × 106 cells; BD Biosciences), PE-labeled mouse Abs against Flk-1 (0.5 μg/1 × 106 cells; BD Biosciences), FITC-labeled mouse Abs against CD34 (1 μg/1 × 106 cells; BD Biosciences), PE-labeled mouse Abs against CD11b (0.5 μg/106 cells; BD Biosciences), or their corresponding isotype control Abs, respectively. To detect VE-cadherin, cells were incubated with goat anti-mouse VE-cadherin Abs (2.5 μg/106 cells; R & D Systems) on ice for 20 min and then labeled with rabbit anti-goat Alexa 488 Abs (0.625 μg/106 cells; R & D Systems) on ice for 20 min. Staining control 0.5% goat serum was used, followed by the identical secondary antibody staining. Flow cytometry was performed using FACScan system and analyzed with Cell Quest software (BD Biosciences). Each analysis included at least 10,000 events.
BMAC function assays (tube formation, migration, and adhesion activity) in vitro.
In tube formation assay, BMACs in endothelial basal medium-2(EBM-2) plus 5% fetal bovine serum (FBS) were plated in a 48-well cell culture plate (5 × 104 cells/well) precoated with 150 μl of growth factor-reduced Matrigel-Matrix (BD Biosciences), as described previously (13, 29). After 24 h of incubation, images of tube morphology were taken by inverted microscope (Nikon) at ×40 magnification, and tube lengths were measured at five random fields per well. In adhesion assay, BMACs were plated in 96-well plates (2.5 × 104 cells/well) precoated with 2.5 g/ml vitronectin. After 1 h of incubation, nonadherent cells were washed away and adherent cells fixed with 2% paraformaldehyde. Nuclei were stained with Hoechst (5 μg/l; Sigma) for 20 min. The number of adherent cells was counted at ×100 magnification, and the mean value of four wells was determined for each sample (13). Migration assay was performed using a modified Boyden's chamber assay, as described previously, with slight modification (15). Around 5 × 104 BMACs were placed into upper Boyden's chamber with EBM-2 and 5% FBS. The lower chamber was loaded with EBM-2 and 5% FBS and vascular endothelial growth factor (VEGF; 50 ng/ml). BMACs were allowed to migrate for 24 h. The cells remaining in the upper chamber were mechanically removed. Cells on the lower side of the membrane were fixed and stained with Hoechst and counted at ×100 magnification. The mean value of five different fields was determined for each sample. The data of functional assays were calculated and expressed as fold changes vs. the control group in each experiment. In some of the experiments, cells were treated with the selective SHH inhibitor cyclopamine (5 μM final concentration; R & D Systems), recombinant mouse SHH (NH2 terminus, 20 μg/ml final concentration; R & D Systems), or recombinant mouse TSP-1 (2.2 nM final concentration; R & D Systems) for 24 h before measurements.
Western blot analysis.
Western blot analysis was performed by SDS-PAGE, as we described previously (10). Skin tissue was homogenized and lysed using Cell Lytic MT lysis buffer (Sigma) with Protease Inhibitor Cocktail (1:100 vol/vol; Sigma) for 20 min on ice. After centrifugation for 15 min at 12,000 g (4°C), the protein content of the samples was determined by bicinchoninic acid (BCA) assay (Bio-Rad, Hercules, CA). For secreted TSP-1 protein measurement, the culture medium was replaced with 2 ml of serum-free medium, and the cells were incubated for 4 h (15). The conditioned medium was collected and concentrated with a Centricon-10 (Amicon, Danvers, MA), and protein concentrations were determined with the BCA assay. Equal amounts of protein (30 μg) were loaded onto 7.5% SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad). Immunobloting was performed by using antibodies directed against TSP-1 (mouse monoclonal anti-TSP-1; 1:400 Abcam) and actin (mouse monoclonal anti-actin, 1:1,000; Santa Cruz Biotechnology). Secondary antibodies included IRDye 800-conjugated rat anti-mouse antibody (1:4,000; Rockland) and Alexa Fluor 680 goat anti-rabbit IgG antibody (1:2,500; Rockland). The blot was read with an Odyssey imager (Li-Cor), and molecular band intensity was determined with Odyssey 2.1 software (Li-Cor).
Flow cytometric analysis of CD36 and CD47 expression on BMACs.
BMACs from either type 1 diabetic mice or normal mice were cultured in vitro for 7 days, as described above. Adherent cells were gently detached using 5 mmol/l EDTA-PBS at 37°C, washed, and incubated for 1 h on ice in PBS-0.5% (wt/vol) BSA together with FITC-labeled mouse Abs against CD47 (2 μg/1 × 106 cells; Santa Cruz Biotechnology), PE-labeled mouse Abs against CD36 (2 μg/1 × 106 cells; Santa Cruz Biotechnology), or their corresponding isotype control Abs, respectively. Flow cytometry was performed using FACScan system and analyzed with Cell Quest software (BD Biosciences). Each analysis included at least 10,000 events.
Data analysis.
All values were expressed as means ± SE. The statistical significance of differences between the two groups was determined with Student's two-tailed t-test. When more than two treatment groups were compared, one-way ANOVA followed by LSD post hoc testing was used (39). In all tests, P < 0.05 was considered statistically significant.
RESULTS
TSP1−/− BMACs possessed potent angiogenic ability and robust SHH pathway protein expressions.
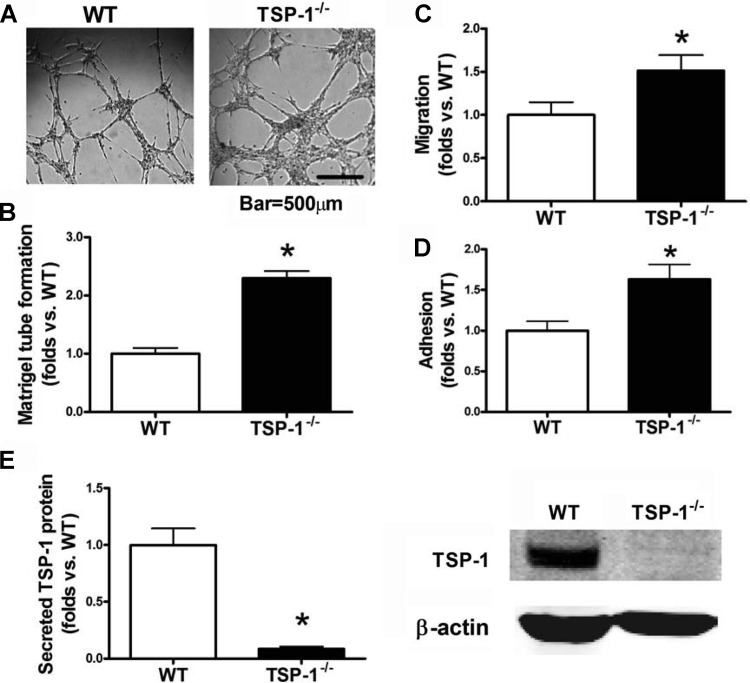
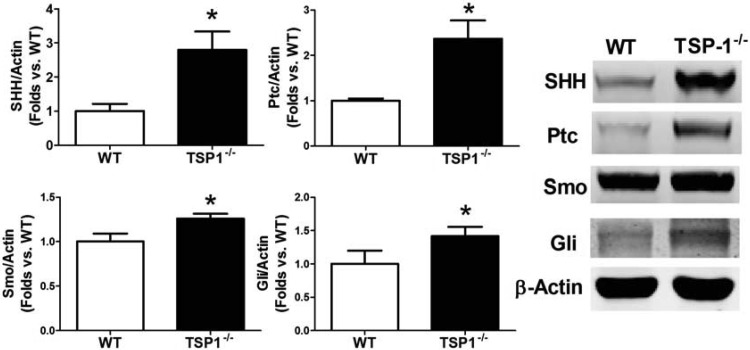
TSP-1−/− BMACs demonstrated significantly improved in vitro tube formation, adhesion, and migration ability (all P < 0.05 vs. WT; Fig. 1, A–D). The endogenous expression of TSP-1 protein was knockout in TSP-1−/− mice derived BMACs (Western blotting, P < 0.05 vs. WT; Fig. 1E). Meanwhile, to determine whether these protections were related to SHH signaling, SHH pathway proteins in BMACs were detected by Western blot. Compared with WT BMACs, TSP-1−/− BMACs had higher expression of SHH, Ptc, and Smo (Fig. 2). Notably, the WT BMACs expressed fairly low levels of SHH pathway proteins, particularly SHH and Ptc, suggesting that the SHH pathway may be comparatively silent in BMACs but that TSP-1 deletion may reactivate this signaling pathway.
Fig. 1.
Bone marrow-derived angiogenic cell (BMAC) function can be improved significantly by thrombospondin-1 (TSP-1) deletion. BMACs were isolated from TSP-1−/− mice and their wild-type (WT) controls. BMAC function was evaluated by Matrigel tube formation, migration, and adhesion. A: TSP-1−/− BMAC formed a significantly better tube network on Matrigel compared with WT controls. B: bar graph of accumulated tube length form by WT and TSP-1−/− BMACs; n = 6/group. C: bar graph of WT and TSP-1−/− BMAC adhesion; n = 6/group. D: bar graph of WT and TSP-1−/− BMAC adhesion; n = 6/group. E: Western blot analysis of TSP-1 protein expression in WT and TSP-1−/− mice; n = 6/group. Representative bands are shown at right. *P < 0.05 vs. WT.
Fig. 2.
TSP-1−/− BMACs possess increased Sonic hedgehog (SHH) pathway proteins. Western blot analysis was performed to detect SHH pathway component proteins, including SHH ligand, the immediate binding protein patched (Ptc), the membrane receptor smoothened (Smo), and the transcriptional factor Gli, in BMACs from WT and TSP-1−/− mice; n = 6/group. *P < 0.05 vs. WT. Representative bands are shown at right.
Diabetic BMACs lack angiogenic response to SHH.
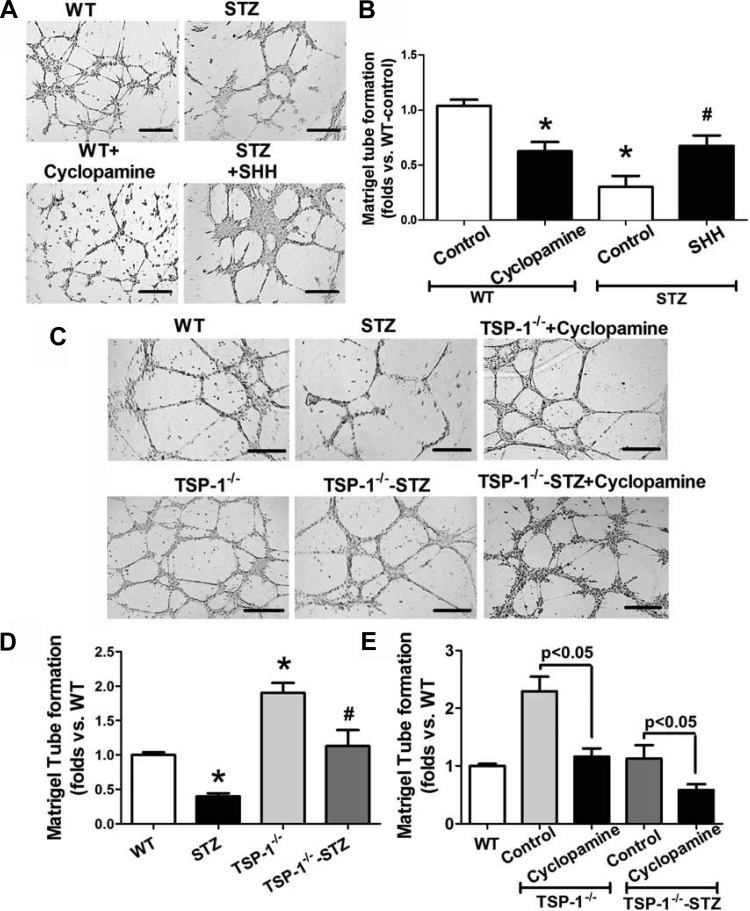
To determine whether or not the SHH pathway stimulates BMAC angiogenesis, we treated normal BMACs with selective SHH inhibitor cyclopamine (5 μM) for 24 h and tested their tube formation. The result demonstrated that normal BMAC angiogenesis was compromised by cyclopamine (P < 0.05 vs. WT-control; Fig. 3, A and B). Conversely, after treatment with recombinant mouse SHH NH2 terminus (20 μg/ml solution; R & D Systems) for 24 h, diabetic BMACs demonstrated significantly improved tube formation (P < 0.05 vs. STZ-control; Fig. 3, A and B).
Fig. 3.
Streptozotocin (STZ) BMACs have deficient responses to SHH-induced angiogenesis. TSP-1-knockout mice significantly rescue BMAC angiogenesis by increasing SHH. Selective SHH inhibitor cyclopamine (5 μM; R & D Systems) or recombinant mouse SHH (NH2 terminus, 20 μg/ml; R & D Systems) was used to treat cells for 24 h before measurements. A: representative pictures of Matrigel tube network form by BMACs from WT mice with or without cyclopamine and BMACs from STZ-induced type 1 diabetic mice with or without SHH. Bar, 500 μm. B: bar graph of accumulated tube length among WT + control, WT + cyclopamine, STZ + control, and STZ + SHH groups; n = 5/group, *P < 0.05 vs. WT + control; #P < 0.05 vs. STZ + control. C: representative pictures showing that TSP-1−/− BMACs formed better network on Matrigel than WT BMACs under normal physical and STZ-induced type 1 diabetic conditions, but cyclopamine-treated TSP-1−/− BMACs formed poor tubes. Bar, 500 μm. D: bar graph of accumulated tube length among WT, STZ, TSP-1−/−, and TSP-1−/− groups; n = 5/group. *P < 0.05 vs. WT; #P < 0.05 vs. STZ. E: bar graph showing that the improved tube formation in TSP-1−/− BMACs was impaired by treatment with cyclopamine; n = 5/group.
TSP-1 deletion rescues diabetic BMAC function by increasing SHH.
To further elucidate the impact of TSP-1 deletion on BMAC angiogenesis in diabetes, we tested tube formation of BMACs from STZ-induced diabetic TSP-1−/− mice (TSP-1−/−-STZ). Our results indicated that whereas STZ-BMACs had impaired tube formation (P < 0.05 vs. WT; Fig. 3, C and D), TSP-1−/−-STZ BMACs possessed significantly better tube formation compared with WT-STZ BMACs (P < 0.05; Fig. 3, C and D). These effects were blunted by cyclopamine (P < 0.05 vs. TSP-1−/−-STZ BMACs, P > 0.05 vs. WT-STZ BMACs; Fig. 3E).
CD36−/−BMACs but not CD47−/−BMACs had high SHH signaling.
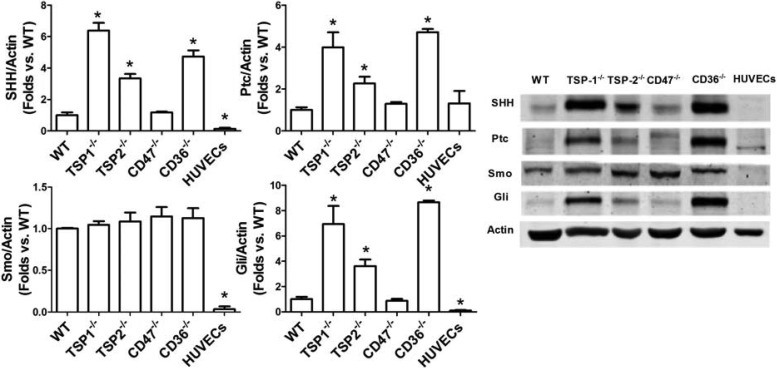
CD36 and CD47 are two major receptors of TSP-1. To determine which of the receptors, CD36 or CD47, mediates the effects of TSP-1 on the SHH pathway, BMACs from CD36−/− mice and CD47−/− mice were harvested for measurement of SHH pathway protein expressions. In addition, to compare the SHH pathway in the major members of thrombospodin family, BMACs from TSP-2−/− mice were also harvested for this detection. Human umbilical vein endothelial cells (HUVECs) at passages 5–8 served as mature endothelial cell controls. Our result showed that WT BMACs had higher expression of SHH, Smo, and Gli protein expressions than HUVECs did (P < 0.05; Fig. 4). Importantly, TSP-1−/−, TSP-2−/−, and CD36−/− BMACs, but not CD47−/− BMACs, possessed much higher SHH pathway protein expressions than WT BMACs did (all P < 0.05). Furthermore, TSP-1−/− BMACs had higher SHH pathway protein expression than TSP-2−/− BMACs did (P < 0.05).
Fig. 4.
SHH pathway proteins are increased in TSP-1, TSP-2, and CD36-knockout endothelial progenitor cells (EPCs) but not in CD47-knockout EPCs. Western blot analysis was performed to detect SHH pathway component proteins, including SHH ligand, the immediate binding protein Ptc, the membrane receptor Smo, and the transcriptional factor Gli, in BMACs from WT, TSP-1−/−, TSP-2−/−, CD47−/−, and CD36−/− mice. Human umbilical vein endothelial cells (HUVECs) were used as mature endothelial cell references; n = 4/group. *P < 0.05 vs. WT. Representative bands are shown at right.
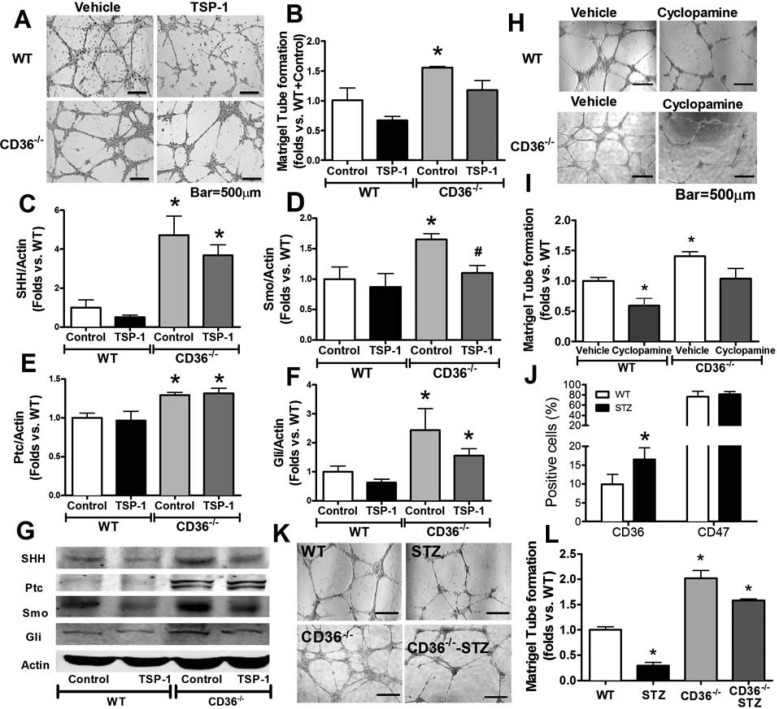
CD36-mediated detrimental effects of TSP-1 on BMAC function and SHH in diabetes.
To investigate whether or not CD36 mediates the detrimental effects of TSP-1 on BMAC function and SHH signaling, CD36−/− BMACs as well as WT BMACs were treated with recombinant TSP-1 protein (2.2 nM; R & D Systems) for 24 h. The concentration TSP-1 was known to induce endothelial cell apoptosis in vitro. The in vitro angiogenic ability of BMACs after treatment was evaluated by assessing tube formation. Our data demonstrated that the impairment induced by STP-1 was absent in CD36−/− BMACs (P < 0.05 vs. CD36−/−, BMACs + vehicle; Fig. 5, A and B). Second, we detected SHH pathway proteins in CD36−/− BMACs and WT BMACs with or without TSP-1 stimulation. Our results demonstrated that the increased expressions of SHH, Ptc, and Gli in CD36−/− BMACs (P < 0.05 vs. WT + vehicle) were not suppressed by TSP-1 (P < 0.05 vs. WT + TSP-1, P > 0.05 vs. CD36−/− + vehicle; Fig. 5, C, E, F, and G). Smo was increased in CD36−/− BMACs but decreased by TSP-1 (P < 0.05 vs. WT + vehicle, P < 0.05 vs. CD36−/− + vehicle; Fig. 5D). CD36−/− BMACs displayed potent in vitro angiogenic ability, as measured by Matrigel tube formation, which was impaired by inhibition of the SHH pathway using cyclopamine (P < 0.05 vs. CD36−/− + vehicle; Fig. 5, H and I). Furthermore, to understand the role of CD36 in TSP-1-induced BMAC dysfunction in diabetes, we detected CD36 and CD47 expression in normal and diabetic BMACs using flow cytometry. As demonstrated in Fig. 5J, CD36 expression was significantly upregulated in diabetic BMACs (P < 0.05 vs. WT), whereas CD47 remained unchanged. Matrigel tube formation indicated that under both normal physical and diabetic conditions, CD36−/− BMACs had improved in vitro angiogenic ability compared with WT BMACs (P < 0.05; Fig. 5K).
Fig. 5.
TSP-1 impairs EPC function and SHH pathway via CD36 in diabetes. Recombinant mouse TSP-1 (2.2 nM; R & D Systems) was used to treat cells for 24 h before measurement of tube formation. A: representative pictures of Matrigel tube network formation by WT BMACs with or without TSP-1 and CD36−/− BMACs with or without TSP-1. B: bar graph of accumulated tube length among WT + control, WT + TSP-1, CD36−/− + control, and CD36−/− + TSP-1 groups; n = 5/group. Western blot analysis was performed to detect SHH pathway component proteins, including SHH ligand (C), the membrane receptor Smo (D), the immediate binding protein Ptc (E), and the transcriptional factor Gli (F), in BMACs from WT and TSP-1−/− mice; n = 6/group. G: representative bands of Western blot analysis. Selective SHH inhibitor cyclopamine (5 μM; R & D Systems) were used to treat cells for 24 h before measurement of tube formation. H: representative pictures of Matrigel tube network form by WT BMACs with or without cyclopamine and CD36−/− BMACs with or without cyclopamine. I: bar graph of accumulated tube length among WT + vehicle, WT + cyclopamine, CD36−/− + vehicle, and CD36−/− + cyclopamine groups; n = 5/group. J: flow cytometric analysis of CD36 and CD47 expression in BMACs from WT and STZ-induced type 1 diabetic mice; n = 5/group. *P < 0.05 vs. WT. K: representative pictures showing that CD36−/− BMACs formed a better network on Matrigel than WT BMACs under normal physical and STZ-induced type 1 diabetic conditions. L: bar graph of accumulated tube length among found groups; n = 5/ group. *P < 0.05 vs. WT + control; #P < 0.05 vs. STZ + control.
DISCUSSION
SHH plays a crucial role in adult blood vessel formation. To date, the endogenous regulation of SHH is largely unknown. We provide the first evidence that the TSP-1/CD36 pathway contributes to SHH signaling defects in diabetes, resulting in dysfunction of angiogenic cells. Our work generated novel mechanistic insights into the upstream regulation of SHH in angiogenesis, which may help to establish a SHH-based therapeutic regimen as a novel approach to improve tissue perfusion.
The microcircumstances under which angiogenesis occurs are composed of a careful orchestra of various vasoactive molecules that regulate cellular behavior via direct autocrine and paracrine activities and indirect activities that are mediated by proteins and growth factors. In the present study, we found that BMACs isolated from TSP-1-null mice displayed significant active angiogenic ability under both normal physical and diabetic conditions, suggesting possible involvement of TSP-1 in impaired angiogenesis in diabetes. Meanwhile, we found that TSP-1−/− BMACs possess significantly active SHH signaling proteins, including SHH ligand, the membrane receptors Ptc and Smo, and the transcriptional factor Gli, in parallel with their active angiogenesis in vitro. The reactivation of the SHH signaling pathway has an important regulatory role in injury-induced angiogenesis, as inhibition of SHH function results in impaired upregulation of various angiogenic molecules, decreased muscle blood flow, and reduced capillary density. The expression of SHH signaling proteins was weak in WT BMACs according to Western blot results. However, in TSP-1−/− BAMCs, all of the component proteins in SHH pathways were elevated, especially for SHH ligand, suggesting that one of the mechanisms underlying the detrimental effects of TSP-1 on angiogenesis is promoting SHH expression.
Furthermore, our data demonstrate that normal BMAC tube formation was abolished by inhibiting the SHH pathway and that exogenous supplement of SHH to diabetic BMACs did not induce an improvement in tube formation. These data indicate that the response of BMACs to SHH is impaired in diabetes, which may be a contributing mechanism to impaired angiogenesis in diabetes. TSP-1 deletion significantly improves BMAC tube formation, which was blunted by simultaneously inhibiting SHH. The canonical SHH pathway is that the binding of SHH to Ptc releases Smo, which transmits its signal to the cytosolic transcription factor Gli, leading to the activation of downstream genes. However, recent reports show that SHH-induced protective effects may also be mediated by various Gli-independent pathways such as phosphoinositide 3-kinase/Akt, Rho kinase, and GTPase RhoA, etc. (7, 36, 46, 48). These pathways are suggested to mediate SHH effects on EC activities. In the present study, we did not look further into other pathways that may be involved. However, our data suggest that TSP-1 suppresses the canonical transduction pathway of SHH, resulting in impaired BMAC function in diabetes.
TSPs bind to various cell surface structures to exert their antiangiogenic effects. CD36 and CD47 are two major receptors for TSPs. We compared the SHH pathways in BMACs isolated from TSP-1−/−, TSP-2−/−, CD36−/−, and CD47−/− mice to determine which of the molecules has a greater impact on SHH pathways. Our data indicated a strong elevation of SHH, Ptc, and Gli in CD36−/− BMACs compared with WT mice and CD47−/− mice. Furthermore, the tube formation of CD36−/− BAMCs was not impaired by in vitro stimulation of recombinant TSP-1 proteins, suggesting that CD36 is an important receptor mediating TSP-1 activities in BMACs. Upon TSP-1 stimulation, CD36 deletion blocked the downregulation of Ptc and Gli but failed to block the downregulation of SHH and Smo. In CD36−/− BMACs treated with SHH inhibitor cyclopamine, tube formation was impaired. These data suggest an essential role of the SHH pathway in CD36's regulation of angiogenesis. The signaling pathways downstream of CD36 involve ligand-dependent recruitment and nonreceptor tyrosine kinases and specific mitogen-activated protein kinases, which actively participate in immunity, metabolism, and angiogenesis (6, 24, 41). However, this is the first evidence to show that CD36 suppresses SHH to impair angiogenesis. There are other potential mechanisms that contribute to TSP-1/CD36-induced deficiency of the SHH pathway, but extensive future work is required given that the endogenous regulation of the SHH pathway is unclear.
In CD47−/− BMACs only Smo was significantly elevated, whereas Gli was not changed compared with WT BMACs, suggesting that CD47 may act on Smo to induce Gli-independent effects in BAMCs. We actually stimulated CD47−/− BMACs with TSP-1 but observed similar reduction in tube formation compared with WT BMACs treated with TSP-1 (data not shown). To compare the situation in diabetes, we detected CD36 and CD47 expression in diabetic BMACs and their normal controls. Our results elucidate that CD36 is the major receptor mediating the adverse effects of TSP-1 on BMAC function under diabetic conditions because CD36 is significantly upregulated, whereas CD47 stays unchanged. The fact that CD36−/− diabetic BMACs still showed strong tube formation ability supports this view. It has been discovered that CD47 mediates TSP-1's detrimental effects in a number of settings, the majority of which are related to elevation of nitric oxide (NO), cyclic GMP activities, and NADPH oxidase (8, 20, 37). We reported that SHH increases NO in human umbilical vein endothelial cells (1). SHH improves tissue repair through a variety of molecules in a Gli-independent manner (26, 36). Alhough further investigations are needed to confirm the relationship between the CD47 and SHH pathways, our observation sheds light on the role of CD47 in the regulation of angiogenesis.
It should be pointed out that we focused on the TSP-1/CD36/SHH pathway in regulating BMAC function under diabetic conditions in this study. However, our study does not exclude the possibility that other potential molecules are involved in the regulation of TSP-1 on angiogenesis. It was reported recently that TSP-1 disruption abrogated age-associated capillary rarefaction in type 2 diabetic (db/db) mice mice, attenuating myocardial upregulation of angiopoietin-2, a mediator that induces vascular regression. In vitro, TSP-1 stimulation increased macrophage but not endothelial cell angiopoietin-2 synthesis (12). It is expected that multiple pathways are involved based on previous findings (27). Future studies are needed to elucidate potential mechanisms that mediate the impact of TSP-1 in angiogenesis.
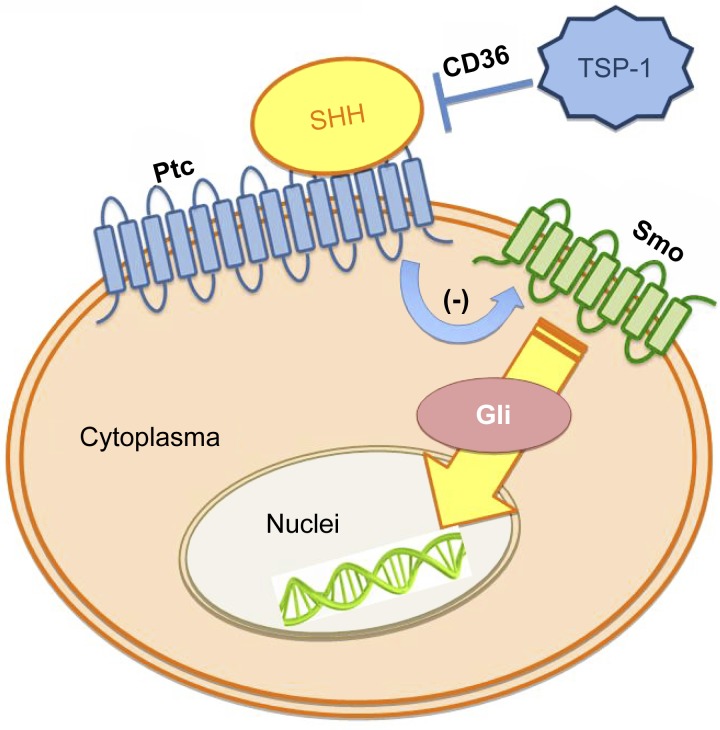
In conclusion, the importance of the SHH pathway in the regulation of vascular development and postnatal neovasculogenesis has long been recognized. However, information regarding the endogenous regulation of this pathway is very limited. We discovered that TSP-1 contributes critically to SHH deficiency in diabetes via its receptor CD36 (Fig. 6). This study yields novel insights into SHH regulation in diabetes, providing useful information regarding potential target molecules in future angiogenesis-based therapies (12).
Fig. 6.
Schema of our hypothesis. TSP-1 contributes to the SHH signaling defect, resulting in BMAC dysfunction in diabetes. This effect is at least partly via its receptor CD36.
GRANTS
This work was supported in part by National Institute of General Medical Sciences Grant R01-GM-077352 (to A. F. Chen), Veterans Affairs Rehabilitation Research & Development Merit Awards I01RX000244 and 1I01RX000652 (to A. F. Chen), American Diabetes Association Research Award 7-11-BS-23 (to A. F. Chen), National Science Foundation of China (NSFC) Key Research Project no. 81130004 (to A. F. Chen), NSFC for Distinguished Young Scholars of China Award no. 81300240 (to J. M. Wang), and American Heart Association Scientist Development Grant 13SDG16930098 (to J. M. Wang).
DISCLOSURES
J. S. Isenberg is Chair of the Scientific Advisory Boards and has an equity interest in Vasculox (St. Louis, MO) and Radiation Control Technologies, (New York, NY).
AUTHOR CONTRIBUTIONS
J.-M.W., T.R.B., and A.F.C. contributed to the conception and design of the research; J.-M.W. performed the experiments; J.-M.W. and J.S.I. analyzed the data; J.-M.W., J.S.I., T.R.B., and A.F.C. interpreted the results of the experiments; J.-M.W. prepared the figures; J.-M.W. drafted the manuscript; A.F.C. edited and revised the manuscript; A.F.C. approved the final version of the manuscript.
REFERENCES
- 1.Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martinez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J 21: 2735–2741, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113: 2413–2424, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res 104: 1095–1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayraktar M, Dundar S, Kirazli S, Teletar F. Platelet factor 4, beta-thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res 22: 90–94, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem 283: 5699–5707, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bujold K, Rhainds D, Jossart C, Febbraio M, Marleau S, Ong H. CD36-mediated cholesterol efflux is associated with PPARgamma activation via a MAPK-dependent COX-2 pathway in macrophages. Cardiovasc Res 83: 457–464, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 9: 570–579, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32: 2966–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 138: 707–717, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274: 19055–19062, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde AV, Frangogiannis NG. Thrombospondin-1 Induction in the Diabetic Myocardium Stabilizes the Cardiac Matrix, While Promoting Vascular Rarefaction Through Angiopoietin-2 Upregulation. Circ Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood 87: 2020–2028, 1996 [PubMed] [Google Scholar]
- 15.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res 98: 697–704, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer 9: 182–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol 173: 1100–1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg 247: 860–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide 21: 52–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 361: 1545–1551, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6: 41–48, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kazerounian S, Duquette M, Reyes MA, Lawler JT, Song K, Perruzzi C, Primo L, Khosravi-Far R, Bussolino F, Rabinovitz I, Lawler J. Priming of the vascular endothelial growth factor signaling pathway by thrombospondin-1, CD36, and spleen tyrosine kinase. Blood 117: 4658–4666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci 65: 700–712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med 11: 1197–1204, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6: 1–12, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Luo JD, Hu TP, Wang L, Chen MS, Liu SM, Chen AF. Sonic hedgehog improves delayed wound healing via enhancing cutaneous nitric oxide function in diabetes. Am J Physiol Endocrinol Metab 297: E525–E531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110: 2484–2493, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 120: 4207–4219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53: 1–114, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 58: 95–101, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Primo L, Ferrandi C, Roca C, Marchio S, di Blasio L, Alessio M, Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J 19: 1713–1715, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 107: 69–82, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, Qin G, Kishore R, Losordo DW. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol 49: 490–498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol 31: 162–169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest 104: 855–864, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res 105: 537–544, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Sheridan C. Genentech obtains proof of concept for hedgehog inhibition. Nat Biotechnol 27: 968–969, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2: re3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 107: 3209–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Tie L, Li XJ, Wang X, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. Am J Physiol Endocrinol Metab 296: E1423–E1429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am J Physiol Endocrinol Metab 300: E1135–E1145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia YP, Dai RL, Li YN, Mao L, Xue YM, He QW, Huang M, Huang Y, Mei YW, Hu B. The protective effect of sonic hedgehog is mediated by the phosphoinositide [corrected] 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience 209: 1–11, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology 112: 279–286, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res 71: 7061–7070, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab 299: E110–E116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]