Abstract
The impact of the GLP-1 receptor agonist lixisenatide on postprandial glucose disposition was examined in conscious dogs to identify mechanisms for its improvement of meal tolerance in humans and examine the tissue disposition of meal-derived carbohydrate. Catheterization for measurement of hepatic balance occurred ≈16 days before study. After being fasted overnight, dogs received a subcutaneous injection of 1.5 μg/kg lixisenatide or vehicle (saline, control; n = 6/group). Thirty minutes later, they received an oral meal feeding (93.4 kJ; 19% protein, 71% glucose polymers, and 10% lipid). Acetaminophen was included in the meal in four control and five lixisenatide dogs for assessment of gastric emptying. Observations continued for 510 min; absorption was incomplete in lixisenatide at that point. The plasma acetaminophen area under the curve (AUC) in lixisenatide was 65% of that in control (P < 0.05). Absorption of the meal began within 15 min in control but was delayed until ≈30–45 min in lixisenatide. Lixisenatide reduced (P < 0.05) the postprandial arterial glucose AUC ≈54% and insulin AUC ≈44%. Net hepatic glucose uptake did not differ significantly between groups. Nonhepatic glucose uptake tended to be reduced by lixisenatide (6,151 ± 4,321 and 10,541 ± 1,854 μmol·kg−1·510 min−1 in lixisenatide and control, respectively; P = 0.09), but adjusted (for glucose and insulin concentrations) values did not differ (18.9 ± 3.8 and 19.6 ± 7.9 l·kg−1·pmol−1·l−1, lixisenatide and control, respectively; P = 0.94). Thus, lixisenatide delays gastric emptying, allowing more efficient disposal of the carbohydrate in the feeding without increasing liver glucose disposal. Lixisenatide could prove to be a valuable adjunct in treatment of postprandial hyperglycemia in impaired glucose tolerance or type 2 diabetes.
Keywords: type 2 diabetes, hepatic glucose metabolism, mixed meal
the incretin hormone glucagon-like peptide-1 (GLP-1) has a number of beneficial direct and indirect effects on carbohydrate metabolism, including increasing insulin secretion in the presence of hyperglycemia, suppressing glucagon secretion, delaying gastric emptying, and stimulating glucose disposal by the liver and skeletal muscle (reviewed in Ref. 4). GLP-1 is released in response to meal ingestion, and therefore, it might be anticipated that it would have important therapeutic implications for patients with impaired glucose tolerance or type 2 diabetes. However, the biological half-life of GLP-1 is only ∼2 min due to its rapid degradation by dipeptidyl peptidase IV (DPP-IV), and this limits the practicality of treatment with GLP-1 itself (26). Lixisenatide, a novel once daily prandial GLP-1 receptor agonist developed to provide the benefits of GLP-1 in a longer lasting formulation (12, 43, 60), was granted marketing authorization by the European Medicines Agency in February 2013 (16). It was also approved for use in Mexico, Australia, and Japan. It is considered a prandial agent because of its substantial effect in lowering prandial glucose concentrations and improving Hb A1C levels (43, 48, 50). Its very high affinity for the GLP-1 receptor (≈4 times that of GLP-1) has led to its being developed for once a day administration despite its relatively short half-life (2–4 h) (5). In dose-ranging studies, once daily treatment was observed to provide a better ratio of efficacy to tolerability than twice-daily dosing (43).
Chronic (12 wk) treatment of obese prediabetic Zucker diabetic fatty (ZDF) rats with lixisenatide reduced basal glucose concentrations and improved oral glucose tolerance (61). In lean glucose-tolerant ZDF rats, on the other hand, lixisenatide treatment did not alter blood glucose (61). Acute treatment of conscious dogs with lixisenatide at doses of 0.15–5 μg/kg prior to an oral glucose tolerance test significantly reduced the maximum glucose response (49–73%) and the plasma insulin concentrations (60–75%) (60).
The effect of 12 wk of lixisenatide monotherapy on mixed-meal disposition has been reported in humans with type 2 diabetes (17). Lixisenatide treatment reduced the 2-h postprandial glucose concentrations seven- to eightfold compared with placebo and was associated with significant improvement in Hb A1c (doubling the number of subjects with Hb A1c <7%). Recent investigations have confirmed the benefit of lixisenatide in reducing postprandial glucose levels in individuals with type 2 diabetes following the consumption of a standard meal (1, 17, 39, 46, 47, 50, 56).
GLP-1 is an insulin secretagogue, and lixisenatide is fully active at the GLP-1 receptor. It has been shown to preserve β-cell mass and insulin mRNA expression in the db/db mouse and to preserve both the first- and second-phase insulin response in the ZDF rat (62). It also augmented the first-phase insulin response to an intravenous glucose challenge in nondiabetic humans (6) and improved β-cell function assessed by homeostasis model assessment-B in a 24-wk study of humans with type 2 diabetes (1). Nevertheless, the reduction in 2-h postprandial glucose concentrations with lixisenatide treatment in humans was associated with a reduction in 2-h postprandial insulin concentrations as well (13, 51). Thus, consistent with the results from humans treated with GLP-1 (28, 33), the effect of lixisenatide in improvement of glucose tolerance does not seem to be attributable simply to enhanced insulin secretion.
The liver plays an extremely important part in the disposal of carbohydrate from a glucose load or meal (11, 14), but it is difficult to quantify the role of the liver in glucose disposal in the human under physiological conditions because of the invasiveness of the catheterization required. Recent data indicate that lixisenatide delays gastric emptying (27), and Woerle et al. (64) reported that the splanchnic bed disposed of more of the carbohydrate from a mixed meal when gastric emptying in humans was delayed by pramlintide administration. Lixisenatide's impact on the relative roles of the liver and extrahepatic tissues in glucose disposal have not been examined under physiological conditions. For this reason, the current studies were carried out to examine the effect of lixisenatide on the disposition of a mixed meal in the conscious dog, a model in which it is possible to quantify hepatic balance precisely.
METHODS
Animals and experimental preparation.
The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee, and the animals were housed and cared for according to Association for Assessment and Accreditation of Laboratory Animal Care guidelines. The studies were carried out in conscious overnight-fasted male or female mongrel dogs (20.1–26.4 kg) that were fed once daily a diet of meat and chow providing 31% protein, 52% carbohydrate, 11% fat, and 6% fiber based on dry weight. Approximately 16 days before study, each dog underwent a laparotomy for placement of ultrasonic flow probes (Transonic Systems, Ithaca, NY) around the portal vein and the hepatic artery as well as insertion of silicone rubber catheters for sampling in a hepatic vein, the portal vein, and a femoral artery, as described in detail elsewhere (31). The animals were studied only if they met established criteria prior to study (31). On the morning of the study, catheters and flow probe leads were exteriorized from their subcutaneous pockets under local anesthesia (31). An angiocath (Deseret Medical, Sandy, UT) was inserted into a cephalic vein for infusion of indocyanine green dye.
Experimental design.
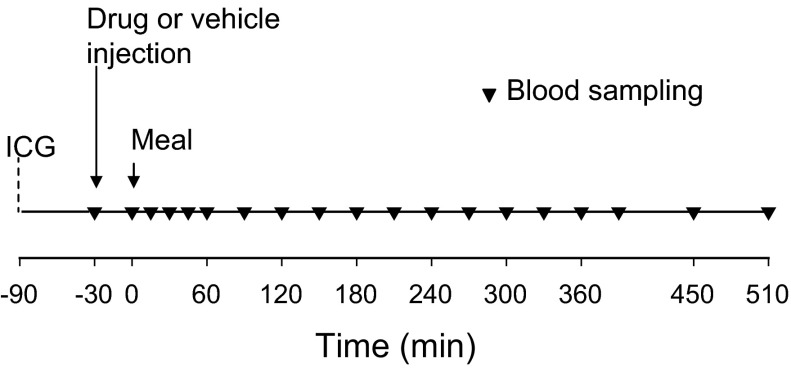
Each experiment consisted of a 60-min equilibration period (−90 to −30 min), a 30-min basal period (−30 to 0 min), and a 510-min experimental period (0–510 min). At −90 min, a continuous infusion of indocyanine green dye (0.08 mg/min; Sigma, St. Louis, MO) was begun in all dogs. At −30 min, seven dogs (lixisenatide group) received a subcutaneous injection of lixisenatide 1.5 μg/kg (Sanofi, Paris, France), and seven dogs (control group) received a subcutaneous vehicle (0.9% saline) injection (Fig. 1). At 0 min the study timer was stopped, and a liquid mixed meal [93.4 kJ/kg; energy supplied as 19% protein (Beneprotein, Novartis Medical Nutrition, Minneapolis, MN), 71% glucose polymers (Polycose; Abbott Nutrition, Columbus, OH), and 10% lipid (Microlipid; Nestlé Healthcare, Minnetonka, MN)] was drunk by the dogs as it was gradually injected into the mouth with a syringe. Five-hundred milligrams of acetaminophen (paracetamol; Sigma) was added to the meal for the last four of the control dogs and the last five of the lixisenatide dogs to assess gastric emptying. Ingestion of the meal took no longer than 3 min, and the timer was restarted as soon as meal intake was complete.
Fig. 1.
Timeline of procedures. A continuous peripheral venous infusion of indocyanine green (ICG) began at −90 min, followed by drug or vehicle (0.9% saline) injection at −30 min. The meal was given as soon as the 0-min baseline blood sampling was complete.
Samples were taken from the femoral artery, hepatic portal vein, and hepatic vein during the 30 min before the meal and every 15–30 min during the 510-min postprandial period to assess the gastrointestinal, hepatic, and nonhepatic disposition of the meal. One dog in each group vomited within 1 h after the meal was delivered. These dogs were deleted from the database, and thus the final groups contained six dogs each. At the end of study, the dogs were euthanized with an overdose of anesthetic, a laparotomy was performed, and the stomach was opened and drained for measurement and visual examination of the gastric fluid.
Hematocrit, blood lactate, alanine, and glycerol and plasma glucose, insulin, glucagon, and cortisol were measured as described previously (31). Plasma acetaminophen concentrations were measured by HPLC following the addition of 2-acetaminophenol as the internal standard and deproteinization of the plasma with Ba(OH)2 and ZnSO4 by a modification of the methods of Ameer et al. (3) and O'Connell and Zurzola (36). Plasma GLP-1 was measured with an ELISA method that specifically quantifies the active forms of GLP-1 (Linco Research, St. Charles, MO).
Calculations and data analysis.
Hepatic blood flow was measured using ultrasonic probes and indocyanine green extraction. The two methods yielded similar results, but the data reported here were calculated with the ultrasonic-determined flows because their measurement did not require an assumption regarding the relative contribution of arterial and portal flow to total hepatic blood flow. The rate of glucose delivery to the liver, or hepatic glucose load, was calculated as: loadin = (Fa × Sa) + (Fp × Sp), where Fa and Fp represent blood or plasma flow (as appropriate for the substrate under examination) in the artery and hepatic portal vein, respectively, and Sa and Sp represent the blood or plasma substrate concentrations in the two vessels. Net hepatic substrate balance (NHB) was calculated as loadout − loadin, where loadout = Fh × Sh, Fh is the total hepatic blood or plasma flow, and Sh is the substrate concentration in the hepatic vein. Net hepatic fractional substrate extraction was calculated as net hepatic substrate balance/loadin. Net gut glucose balance was calculated as (Sa − Sp) × Fp. Nonhepatic glucose uptake was net gut glucose output minus net hepatic glucose uptake (NHGU), which was adjusted for changes in the mass of the glucose pool. Glucose clearance was calculated as hepatic or nonhepatic glucose uptake, as appropriate, divided by the arterial glucose concentration. Net hepatic carbon retention, shown previously to be a close estimate of hepatic glycogen synthesis (53), was calculated as NHGU minus net hepatic lactate output (in glucose equivalents).
The area under the curve (AUC) was calculated for the postprandial period (0–510 min) using the trapezoidal rule, with the AUCs expressed as change from the basal concentrations or rates (ΔAUC), unless otherwise indicated.
SigmaStat (SPSS, Chicago, IL) was used for statistical analysis. Time course data were evaluated with repeated-measures ANOVA. Where significant differences between groups in their responses over time were found, Tukey's test was utilized for post hoc analysis to identify the time points where the groups differed. Unpaired Student's t-test was used for comparison of AUCs.
RESULTS
Acetaminophen concentrations.
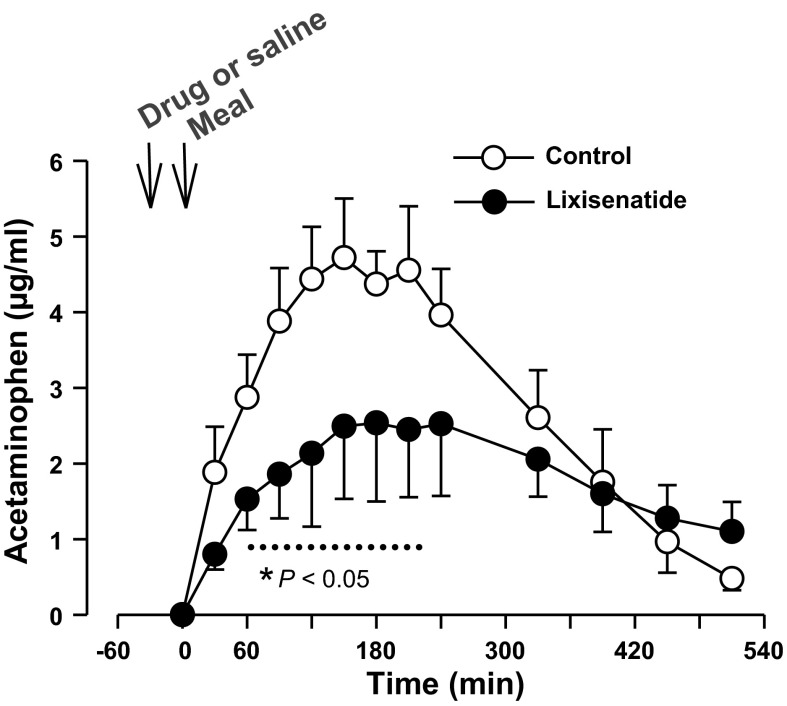
In the four control and five lixisenatide dogs that received acetaminophen to assess gastric emptying (Fig. 2), the peak plasma concentration in the lixisenatide group was only 53% of that in the control dogs (P < 0.05), whereas the overall ΔAUC in the lixisenatide dogs was 65% of that in the control group (924 ± 228 vs. 1,412 ± 240 ng·ml−1·510 min−1, P < 0.05).
Fig. 2.
Arterial plasma acetaminophen concentrations in the control (n = 4) and lixisenatide (n = 5) groups. Data are means ± SE. *P < 0.05 between groups (main effect significant; dotted line indicates data points that differed on post hoc analysis).
Glucose metabolism.
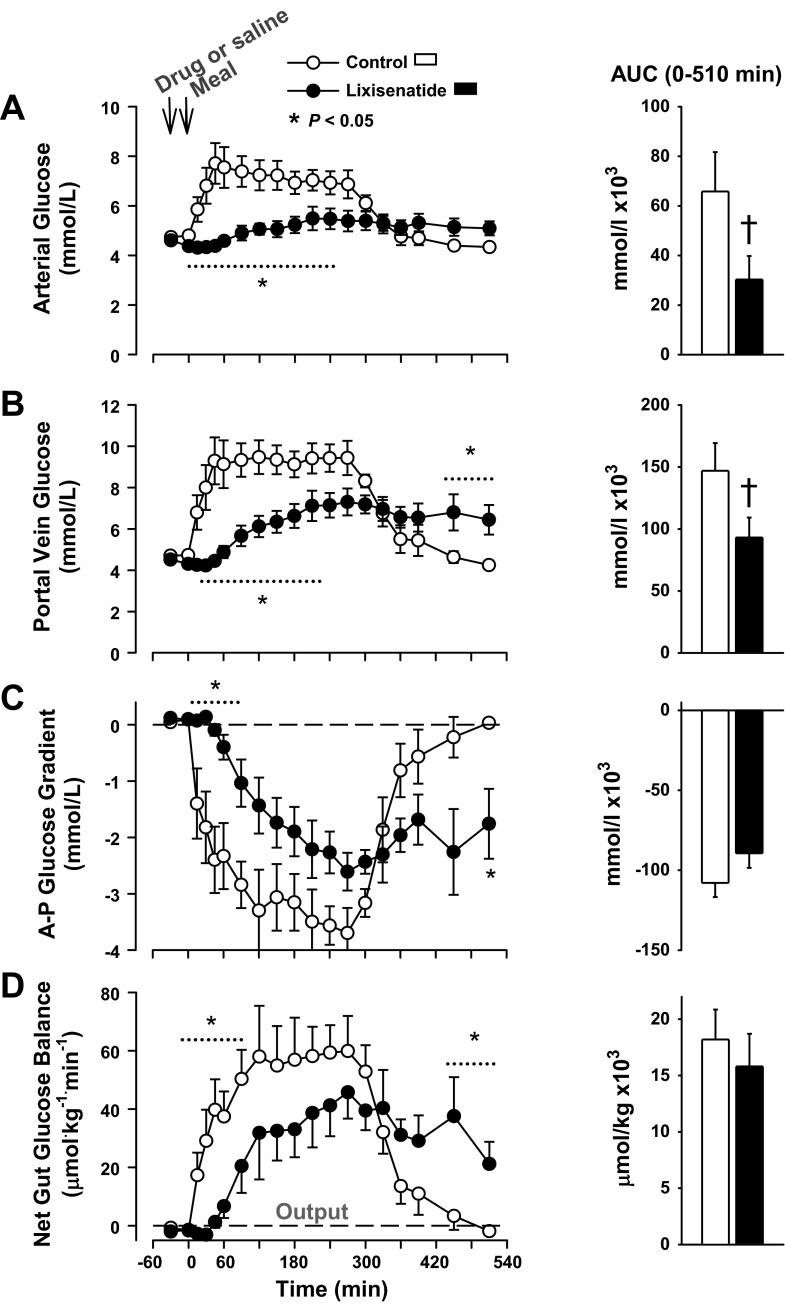
The basal arterial and portal blood glucose concentrations were ∼4.6 mmol/l in both groups (Fig. 3). In the control group, the arterial glucose concentrations peaked 45 min after the meal was administered and remained elevated above basal for >300 min after the meal. In the lixisenatide group, the rise in blood glucose was markedly blunted, and the peak was delayed until ∼4 h after the meal. The mean arterial blood glucose concentration remained slightly but significantly elevated above basal at the end of the study in lixisenatide group. The ΔAUC for arterial blood glucose in the lixisenatide group was ≈45% of that in the control group (P < 0.05; Fig. 3). The portal vein glucose concentration in the control group reached 9.5 ± 0.8 mmol/l and was elevated for >330 min, whereas the peak portal vein glucose in the lixisenatide group was 7.2 ± 0.7 mmol/l (P < 0.05 vs. control), and the concentration remained significantly above basal at the end of study. The ΔAUC for arterial blood glucose in the lixisenatide group was ∼63% of that in the control group (P < 0.05). The ΔAUC for portal blood glucose concentration was similarly suppressed in the lixisenatide group relative to control. A negative arterioportal (A-P) glucose gradient was present in the control group by 15 min, and it existed until after 450 min (Fig. 3). The development of a negative A-P gradient was delayed in the lixisenatide group until ≈45 min, but it persisted until after the final sampling point (P = 0.07 between AUCs in the 2 groups).
Fig. 3.
Arterial (A) and portal vein (B) blood glucose, the arterial-portal (A-P) glucose gradient (C), and net gut glucose balance (D) in the control and lixisenatide groups; n = 6/group. Data are means ± SE. There was a significant (P < 0.05) group × time interaction for all time course data; *data points that differed on post hoc analysis. The histograms show the areas under the curve (AUCs) for the postprandial period; †P < 0.05 between groups using an unpaired t-test.
Absorption of the meal was evident (the gut shifted from net uptake to output of glucose) within 15 min in the control group, but it was delayed >30 min in the group receiving lixisenatide (Fig. 3). The peak rates of net gut glucose output were 59.9 ± 12.0 and 45.8 ± 9.0 μmol·kg−1·min−1 in the control and lixisenatide groups, respectively (P < 0.05). Net absorption of the carbohydrate appeared to be complete within 510 min in the control group, but it continued until the end of the study in the lixisenatide group. As a result, the ΔAUC of gut glucose output did not differ significantly between groups (P = 0.2).
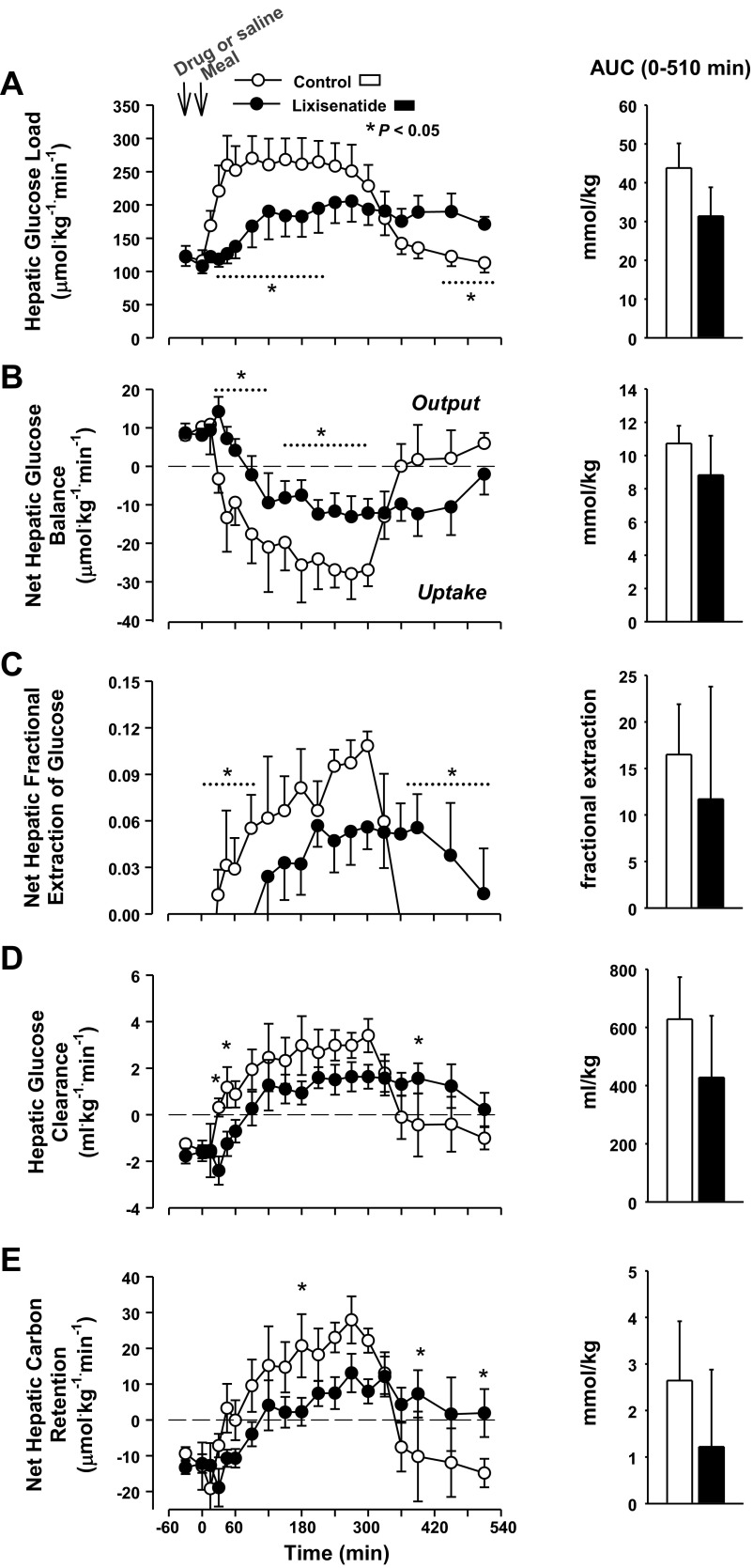
Because of the overall reduction in glycemia, the hepatic glucose load was lower in the lixisenatide group than in the control group for >5 h after the meal (P < 0.05; Fig. 4). The hepatic load had returned to basal in the control group by the end of study, whereas it remained significantly elevated in the lixisenatide group. Because of this, the ΔAUC for hepatic glucose load in the lixisenatide group was only 28% smaller than in the control group (P = 0.1). The control group switched from net hepatic glucose output to NHGU within 30 min after the meal, but the shift to NHGU was delayed until 90 min in the lixisenatide group, and the rate remained significantly lower in lixisenatide through 300 min (Fig. 4). NHGU ceased by ≈360 min in the control group but was ongoing at the end of study in the lixisenatide group, so the ΔAUC of NHGU was not different in lixisenatide or control (P = 0.24). Net hepatic fractional extraction of glucose and hepatic glucose clearance followed a pattern similar to that of NHGU. The AUC of net hepatic carbon retention did not differ between groups (P = 0.24; Fig. 4).
Fig. 4.
Hepatic glucose load (A), net hepatic glucose uptake (B), net hepatic fractional glucose extraction (C), hepatic glucose clearance (D), and net hepatic carbon retention (E) in the control and lixisenatide groups. There was a significant (P < 0.05) group × time interaction for all parameters. *Data points that differed on post hoc analysis. There were no significant differences in postprandial AUCs between groups (histograms); n = 6/group. Data are means ± SE.
The assimilation of the meal was incomplete at the end of study in the lixisenatide group, as can be seen from the acetaminophen concentrations and net gut glucose balance. The acetaminophen concentrations provide an independent tool for estimating the time remaining for meal assimilation. If the rate of change of acetaminophen concentrations over the last hour of study is projected forward until the concentrations fall to zero in the lixisenatide group, it can be estimated that gastric release of the acetaminophen (and presumably the meal) would have continued for ≈4 h past the end of the study. If the mean rate of NHGU observed between groups over the last hour of study had continued for an additional 4 h, then the AUC of NHGU in the lixisenatide group would total 10.3 ± 3.3 mmol/kg, remarkably similar to the AUC of 10.7 ± 1.1 mmol/kg observed in the control group.
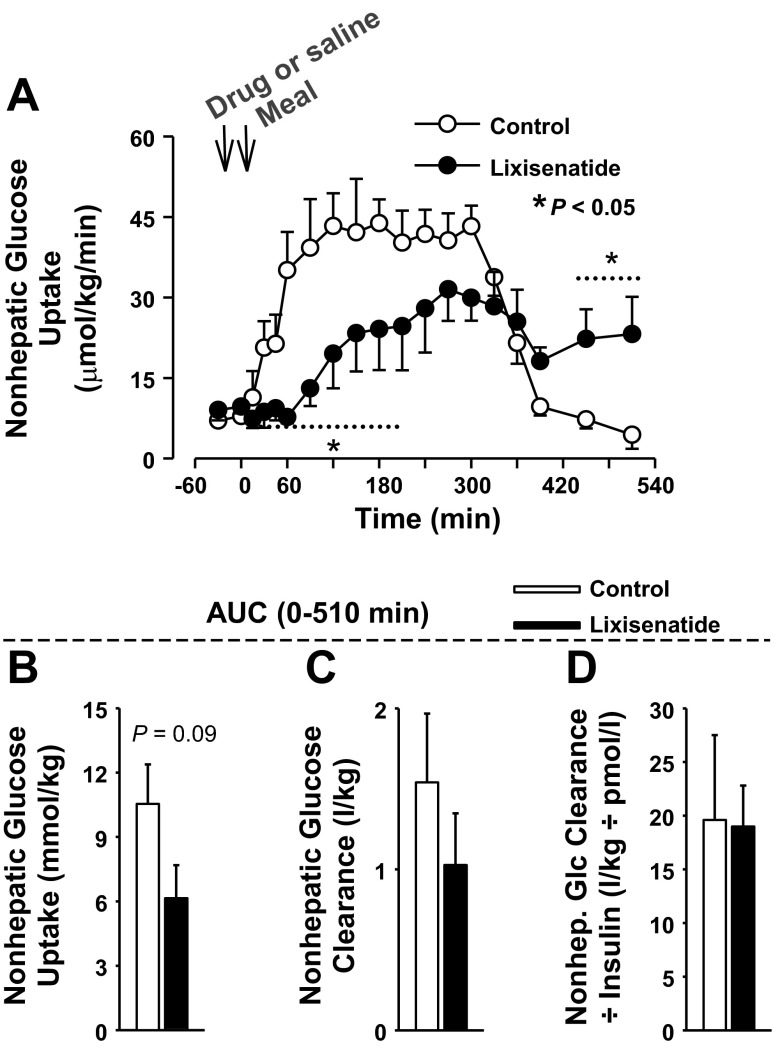
Nonhepatic glucose uptake was significantly greater in the control group than in the lixisenatide group for >3 h after the meal (Fig. 5). The ΔAUC of nonhepatic glucose uptake for the postprandial period was reduced >40% in lixisenatide (10,541 ± 1,854 and 6,151 ± 4,321 μmol·kg−1·510 min−1, P = 0.09). Since the glycemic concentrations differed, nonhepatic glucose clearance was calculated (Fig. 5). Although there was a significant group × time effect of lixisenatide on nonhepatic glucose clearance, the ΔAUC did not differ between groups (P = 0.3). When nonhepatic glucose clearance was divided by the insulin concentrations, neither the individual time points nor the AUCs differed (P = 0.9 for comparison of AUCs; Fig. 5).
Fig. 5.
A: there was a significant (P < 0.05) group × time interaction for nonhepatic glucose uptake. *Data points that differed on post hoc analysis. The histograms show the AUC0–510 min for nonhepatic glucose uptake (B), nonhepatic glucose clearance (C), and nonhepatic glucose clearance adjusted for arterial plasma insulin concentrations (D). There were no significant differences in the AUCs of the 2 groups, although the AUC of nonhepatic glucose uptake tended to be larger in the control group; n = 6/group. Data are means ± SE.
Hormonal response.
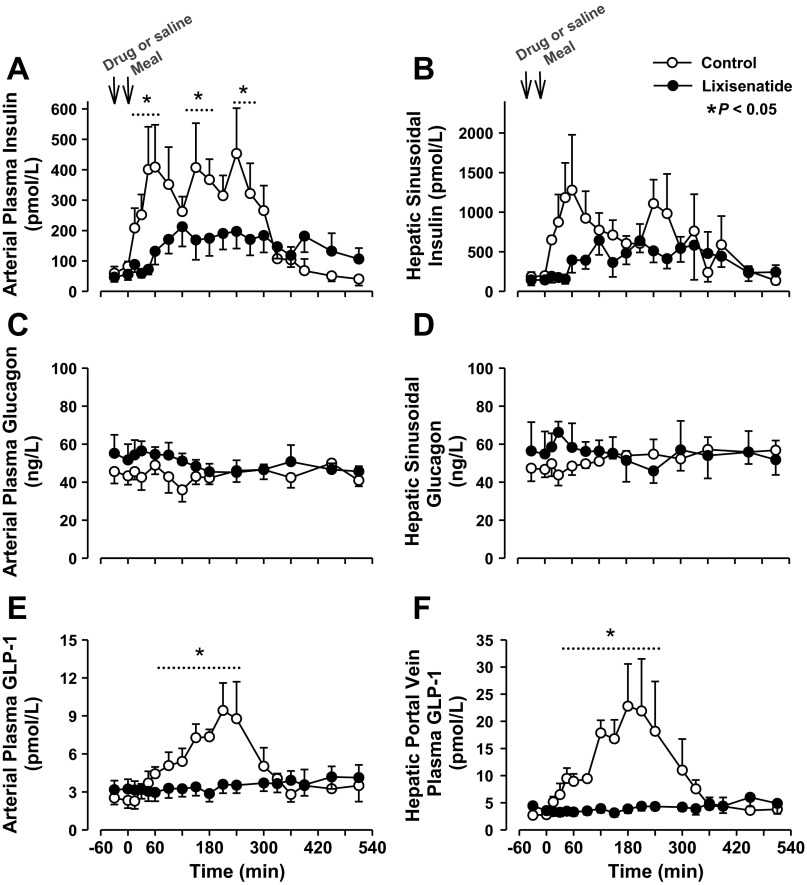
The arterial plasma insulin response in the lixisenatide group was 44% smaller than in the control group (ΔAUC 51,813 ± 9,735 vs. 87,088 ± 18,875 pmol·l−1·510 min−1, P < 0.05), and the hepatic sinusoidal insulin response also tended to be blunted (37% less in lixisenatide than control), as shown in Fig. 6. Arterial plasma glucagon concentrations did not differ in the two groups and remained nearly basal throughout (Fig. 6). Arterial and portal vein GLP-1 concentrations demonstrated a robust increase after the meal in the control group, but there was little or no change from basal in the lixisenatide group (Fig. 6). Arterial plasma cortisol concentrations did not change significantly from basal in either group during the experiments (data not shown).
Fig. 6.
Arterial (A) and hepatic sinusoidal (B) insulin, arterial (C) and hepatic sinusoidal (D) glucagon, and arterial (E) and portal vein (F) glucagon-like peptide-1 (GLP-1) in the control and lixisenatide groups; n = 6/group. Data are means ± SE. *P < 0.05 between groups (main effect significant; dotted line indicates data points that differed on post hoc analysis).
Metabolite concentrations and balance.
During the first half-hour of the postprandial period, the arterial concentrations of nonesterified fatty acids (NEFA) were significantly higher in the lixisenatide vs. the control group (Table 1). Although the NEFA concentrations in the lixisenatide group remained numerically higher for ≥300 min postprandially, post hoc testing revealed no significant differences after 30 min. Arterial blood glycerol concentrations in the basal state were lower in the lixisenatide group than in the control group, but the early postprandial concentrations did not differ between groups (Table 1). As a result, the decline in arterial glycerol concentrations between the basal period and the 30-min postprandial sample was three times as large in the control group as in the lixisenatide group (P < 0.05). During the last 150 min of study, glycerol concentrations in both groups began to return toward basal. During this time, the concentrations in the control group again were significantly greater than those in the lixisenatide group. Net hepatic glycerol uptake did not differ between groups (P = 0.3).
Table 1.
NEFA, glycerol, lactate, and alanine concentrations and balances
| Postprandial Period, min |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group and Parameter | Basal Period | 30 | 60 | 120 | 180 | 240 | 300 | 360 | 450 | 510 |
| Arterial plasma NEFA, μmol/l* | ||||||||||
| Control | 813 ± 129 | 283 ± 44 | 150 ± 35 | 110 ± 30 | 94 ± 20 | 73 ± 7 | 77 ± 9 | 289 ± 130 | ||
| Lixisenatide | 673 ± 111 | 558 ± 153† | 443 ± 156 | 263 ± 101 | 199 ± 58 | 146 ± 37 | 125 ± 27 | 154 ± 38 | ||
| Arterial blood glycerol, μmol/l* | ||||||||||
| Control | 92 ± 15 | 39 ± 7 | 26 ± 6 | 21 ± 5 | 23 ± 4 | 21 ± 4 | 26 ± 5 | 62 ± 14 | 54 ± 9 | 78 ± 15 |
| Lixisenatide | 69 ± 12† | 51 ± 13 | 40 ± 10 | 30 ± 10 | 25 ± 6 | 26 ± 5 | 20 ± 3 | 34 ± 5† | 45 ± 17 | 47 ± 16† |
| Net hepatic glycerol uptake, μmol·kg−1·min−1 | ||||||||||
| Control | 1.0 ± 0.5 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.2 | 1.5 ± 0.7 | 0.6 ± 0.4 | 0.7 ± 0.4 |
| Lixisenatide | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.9 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.4 | 0.5 ± 1.1 |
| Arterial blood lactate, μmol/l* | ||||||||||
| Control | 645 ± 118 | 784 ± 178 | 1,122 ± 124 | 875 ± 104 | 742 ± 79 | 736 ± 94 | 745 ± 103 | 719 ± 50 | 999 ± 142 | 1,104 ± 195 |
| Lixisenatide | 654 ± 109 | 663 ± 158† | 612 ± 121† | 607 ± 80† | 568 ± 45 | 533 ± 46 | 543 ± 66 | 612 ± 90 | 788 ± 70 | 837 ± 162 |
| Net hepatic lactate output, μmol·kg−1·min−1 | ||||||||||
| Control | 3.9 ± 6.6 | 16.5 ± 3.8 | 18.6 ± 5.0 | 10.8 ± 4.8 | 9.7 ± 3.8 | 7.6 ± 2.8 | 8.0 ± 3.4 | 17.5 ± 9.0 | 16.6 ± 3.5 | 18.6 ± 5.5 |
| Lixisenatide | 4.3 ± 2.8 | 9.9 ± 5.8 | 10.6 ± 5.2 | 10.4 ± 3.6 | 8.2 ± 1.5 | 7.4 ± 1.7 | 6.6 ± 1.6 | 11.0 ± 2.6 | 14.9 ± 4.6 | 13.6 ± 6.7 |
| Arterial blood alanine, μmol/l* | ||||||||||
| Control | 381 ± 45 | 417 ± 45 | 428 ± 46 | 386 ± 24 | 351 ± 19 | 335 ± 12 | 363 ± 19 | 405 ± 18 | 512 ± 34 | 541 ± 51 |
| Lixisenatide | 379 ± 44 | 356 ± 49 | 342 ± 46† | 311 ± 28† | 294 ± 17 | 294 ± 19 | 294 ± 15† | 311 ± 17 | 353 ± 17 | 386 ± 37 |
| Net gut alanine output, μmol·kg−1·min−1 | ||||||||||
| Control | 0.9 ± 0.2 | 1.7 ± 0.5 | 2.1 ± 0.7 | 2.6 ± 0.7 | 2.9 ± 0.7 | 3.6 ± 0.9 | 3.2 ± 0.8 | 2.2 ± 0.6 | 2.1 ± 0.4 | 1.2 ± 0.2 |
| Lixisenatide | 0.8 ± 0.1 | 0.7 ± 0.2 | 1.2 ± 0.3 | 2.0 ± 0.8 | 1.8 ± 0.5 | 2.4 ± 0.6 | 2.3 ± 0.6 | 2.2 ± 0.3 | 1.6 ± 0.5 | 1.8 ± 0.3 |
| Net hepatic alanine uptake, μmol·kg−1·min−1 | ||||||||||
| Control | 2.6 ± 0.4 | 3.5 ± 0.3 | 3.9 ± 0.8 | 4.7 ± 0.6 | 5.0 ± 0.8 | 4.7 ± 0.9 | 4.4 ± 0.8 | 2.8 ± 0.7 | 2.3 ± 0.8 | 2.7 ± 1.2 |
| Lixisenatide | 2.5 ± 0.4 | 2.7 ± 0.5 | 3.0 ± 0.6 | 4.1 ± 1.8 | 3.6 ± 0.7 | 4.4 ± 0.8 | 3.7 ± 0.7 | 3.2 ± 0.6 | 3.2 ± 0.7 | 3.6 ± 0.7 |
Data are means ± SE. NEFA, nonesterified fatty acid.
Significant differences between groups in their responses over time identified by ANOVA (P < 0.05);
time points where post hoc testing indicated differences between groups.
Arterial blood lactate and alanine concentrations (Table 1) were significantly greater in the control vs. lixisenatide group during the postprandial period (P < 0.05), but net hepatic lactate output and alanine uptake, as well as net gut alanine output, did not differ significantly between groups (P = 0.2, 0.4, and 0.2, respectively). Neither the concentrations nor the net hepatic balances of glutamine and glutamate differed significantly between groups (data not shown).
Gastrointestinal tolerance.
In the control dogs, only a small amount (5–10 ml) of clear fluid was visible in the stomach at necropsy, with none of the liquid formula diet apparent. In the lixisenatide group, all stomachs contained bile-stained fluid with what appeared to be curdled formula. The formula volume was ≈10–15% of that administered.
DISCUSSION
Lixisenatide significantly reduced the postprandial glycemic excursion in the conscious dog despite the fact that postprandial insulin concentrations were also reduced in the treatment group. Although lixisenatide was associated with a reduction in the peak arterial glucose concentration and in the maximal rate of gut glucose output, its action prolonged the glycemic elevation and the period of absorption so that there was no difference between groups in the AUC for key parameters, including arterial blood glucose, net gut glucose output, net hepatic glucose uptake, or nonhepatic glucose uptake. The rate of NHGU was significantly lower in the lixisenatide group for the first 5 h of the postprandial period, but NHGU continued through the end of study in the treatment group, unlike the control group. As a result, there were no significant differences between groups in the AUC0–510 for NHGU or net hepatic fractional extraction of glucose, which takes into account the differences between groups in hepatic glucose load, a major determinant of the rate of NHGU (11). Moreover, there was ongoing absorption of glucose in our lixisenatide group at the end of the study, and it is possible that NHGU would have continued for some time after the final sampling point such that the total NHGU in the lixisenatide group eventually equaled or exceeded that in the control group. If the rate of change in acetaminophen concentrations is projected forward and NHGU is adjusted for the continued gastric emptying of acetaminophen, as described in results, then it is likely that the AUC of NHGU would have been the same in the two groups. Unfortunately, it was not possible to prolong these acute experiments sufficiently to allow us to determine when NHGU ceased in the lixisenatide group. Our findings contrast with those of Woerle et al. (64), who reported that the splanchnic bed disposed of more of the carbohydrate from a mixed meal when gastric emptying in humans was delayed by pramlintide administration. However, exogenous glucose Ra in the human study was still elevated well above basal at the conclusion of their experiments, particularly in the pramlintide-treated group, and thus it is impossible to determine exactly what contribution the splanchnic bed made to glucose disposal.
The acetaminophen (paracetamol) concentrations indicate that slowing the rate of gastric emptying likely explains at least part of the effect. Acetaminophen is absorbed in the small intestine but not the stomach, and its appearance in the circulation is dependent largely on the rate of gastric emptying (22). Plasma acetaminophen concentrations in normal humans ingesting a liquid meal correlate remarkably well with results from scintography, considered the “gold standard” for evaluation of gastric emptying (18). The AUC0–180 of acetaminophen concentrations was reduced >50% in dogs given lixisenatide vs. placebo prior to an oral glucose tolerance test (OGTT) (60). The longer duration of observation post-acetaminophen ingestion in the current study likely explains why the AUC0–510 was reduced only 35% in the presence of lixisenatide. Net gut output of glucose in our animals was dependent not just upon the rate of gastric emptying but also upon the rates of hydrolysis of the glucose polymers in the meal and absorption in the small intestine. Nevertheless, the markedly lower net gut glucose output in the lixisenatide group early in the postprandial period is in general agreement with the acetaminophen data. The tendency toward blunting of the net gut output of alanine and the significant reduction in the arterial concentrations of alanine, as well as the presence of some of the meal in the stomach at the end of study, are also consistent with a delay in nutrient assimilation in the lixisenatide group. Indeed, although measurement of the stomach contents can provide only a rough estimate of the amount of the diet in the stomach at the end of study, the post-study volume of intragastric formula in the lixisenatide-treated dogs was approximately equivalent to the amount that remained unabsorbed based on the net gut glucose output data. The reduction in the rate of gastric emptying with lixisenatide treatment is consistent with results from [13C]octanoic acid breath tests in humans with type 2 diabetes. After 4 wk of daily treatment, the gastric emptying rate for a labeled breakfast meal decreased 85% compared with the pretreatment measurement in the lixisenatide-treated group, whereas a placebo-treated group exhibited no decrease in gastric emptying (27). GLP-1 may alter gastric emptying by both reducing antral activity and stimulating pyloric contraction (54, 63). The delay of gastric emptying by GLP-1, GLP-1 receptor agonists, or DPP-IV inhibitors apparently involves central neural mediation (15, 24, 40, 52), although whether or not the vagal afferent innervation is required for the response is unclear (24, 32).
Similar to NHGU, nonhepatic glucose uptake was significantly greater in the control group than in the lixisenatide group for more than 3 h after meal ingestion, but nonhepatic glucose uptake continued at a substantial rate in the lixisenatide group at the end of the experiments. Although the AUC of nonhepatic glucose uptake tended to be reduced in the lixisenatide group, when the rate was adjusted for the differences in glycemia (i.e., nonhepatic glucose clearance), the result was more similar between groups. When nonhepatic glucose clearance was further adjusted for the prevailing insulin concentrations, no differences were evident between groups. Thus, there was no evidence for either a specific hepatic or a nonhepatic effect of lixisenatide. Glycemic levels during the first half of the postmeal period in the current studies were significantly blunted in the dogs receiving lixisenatide. This is particularly relevant, given that the 2-h postload glucose concentration during an OGTT has been found to be predictive of both the progression of prediabetic individuals to diabetes and the risk of cardiovascular disease in type 2 diabetes (8, 10, 20, 35). Hb A1c is heavily influenced by postprandial glycemic levels (21). In obese prediabetic ZDF rats, 12 wk of treatment with lixisenatide vs placebo reduced Hb A1c significantly (1.7%) (61). Consistent with this, 12 wk of lixisenatide monotherapy in adults with type 2 diabetes significantly decreased 2-h postprandial plasma glucose concentrations and excursions (change from basal concentrations) after a standardized breakfast meal, reduced fasting plasma glucose, and doubled the number of subjects with Hb A1c concentrations <7% compared with placebo treatment (17). Subsequent randomized clinical investigations in adults with type 2 diabetes inadequately controlled with metformin (1), basal insulin with or without metformin (46, 47), basal insulin with or without a sulfonylurea (56), or pioglitazone (39) demonstrated that the addition of lixisenatide once daily for periods ranging from 13 to 24 wk was effective in reducing postprandial plasma glucose after a standardized test meal and lowering Hb A1c relative to placebo (43). Given the relationship between elevations in Hb A1c and cardiovascular disease and other complications of diabetes (7, 57), lixisenatide appears to hold promise for improving the care of selected individuals with type 2 diabetes.
The data from the current study and a previous study in dogs undergoing an OGTT (60) are generally consistent with those in humans given exogenous GLP-1 infusions or short-term treatment with a DPP-IV inhibitor in that insulin concentrations were not increased in the lixisenatide group during most of the postprandial period. Continuous intravenous GLP-1 slowed gastric emptying and brought about a dose-dependent reduction in insulin concentrations after a meal in both normal volunteers and those with type 2 diabetes (28, 33). Only with the use of erythromycin as a prokinetic agent was it possible to observe an insulinotropic effect of GLP-1 following meal feeding in normal subjects (30). Moreover, despite the increase in insulin secretion during combined treatment with erythromycin and GLP-1, the glucose-lowering effect of GLP-1 was markedly reduced by inhibition of its ability to delay gastric emptying (30). On the other hand, in subjects with type 2 diabetes, there was no effect of 10 days of treatment with the DPP-IV inhibitor vildagliptin on either the rate of gastric emptying or insulin concentrations following a mixed meal (59). However, after longer periods (6–52 wk) of vildagliptin therapy, β-cell effectiveness (insulin secretion in relation to glycemia) in individuals with prediabetes or type 2 diabetes was improved significantly relative to placebo (2, 49, 58). Thus it is possible that higher insulin levels might have been observed in dogs if lixisenatide treatment had continued chronically. Nevertheless, lixisenatide reduced both 2-h postprandial insulin concentrations as well as the 0- to 4-h postprandial insulin AUC in a dose-dependent manner in adults with type 2 diabetes inadequately controlled with metformin (44) while also reducing postprandial glucose concentrations dose dependently (43). Exenatide, another short-acting GLP-1 receptor agonist, exhibits a similar postprandial insulin-lowering effect (25). The insulin concentrations in the control group showed substantial fluctuations. This was likely due to the pulsatile nature of insulin secretion in the dog, as in the human (19, 41, 42). With 15- to 30-min sampling intervals, the samples may capture concentrations anywhere on the curve from peak to trough. Additionally, the absorption of a meal is not a smooth process but instead occurs in a rhythmic pattern induced by the migrating myoelectric complex (9, 45). This has been observed to entrain insulin secretion (45). Overall, our data from the lixisenatide group agree with those of Lorenz et al. (27) obtained in the human in that postprandial insulin secretion appeared to be appropriate to the glucose stimulation. Lixisenatide appears to improve glucose disposal (glucose clearance) even in the absence of obvious enhancement of insulin secretion.
Using the specific GLP-1 receptor antagonist exendin (9–39), Shirra et al. (55) determined that GLP-1's reduction of glucagon secretion can play a significant role in reducing fasting and postprandial glycemia. However, in the current acute study, there was only a small effect, if any, of lixisenatide to lower postprandial glucagon concentrations (Fig. 5).
Lixisenatide treatment slowed the suppression of NEFA concentrations and reduced the fall in glycerol concentrations in the postprandial period. These effects were likely related to the reduced insulin concentrations in the lixisenatide group. The lixisenatide group also had lower blood lactate concentrations in the early postprandial period, apparently due to a reduction in hepatic glycolysis related to the lower rates of NHGU in the lixisenatide group.
In conclusion, the reduction in the postprandial glycemic response in the current report, combined with the promising data from clinical trials (1, 17, 39, 46, 47, 50, 56), indicates that lixisenatide could be a useful adjunct in treatment of postprandial hyperglycemia in individuals with impaired glucose tolerance or type 2 diabetes. As suggested in humans (23, 34), our data demonstrate a delay in gastric emptying following administration of lixisenatide that allows more efficient disposal of the carbohydrate in the meal. Data from mice and humans suggest that this may be accompanied by improved postprandial triglyceride and lipoprotein metabolism (29, 37, 38, 65). There appears to be no specific effect of lixisenatide to increase liver glucose uptake.
GRANTS
These studies were supported by a grant from Sanofi. Lixisenatide (formerly described also as AVE0010 or ZP10) was licensed to Sanofi by Zealand Pharma (Denmark). These studies were facilitated by the Metabolic Physiology Shared Resource Core and the Hormone Assay & Analytical Services Core, Vanderbilt Diabetes Research and Training Center, supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-20593. A. D. Cherrington is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
DISCLOSURES
U. Werner is an employee of Sanofi.
AUTHOR CONTRIBUTIONS
M.C.M., M.S.S., and T.D.F. performed the experiments; M.C.M., M.S.S., and T.D.F. analyzed the data; M.C.M. and A.D.C. interpreted the results of the experiments; M.C.M. prepared the figures; M.C.M. drafted the manuscript; M.C.M., U.W., and A.D.C. edited and revised the manuscript; M.C.M., U.W., M.S.S., T.D.F., and A.D.C. approved the final version of the manuscript; U.W. and A.D.C. contrbiuted to the conception and design of the research.
REFERENCES
- 1.Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 38: 2543–2550, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahren B, Pacini G, Foley JE, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care 28: 1936–1940, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ameer B, Greenblatt DJ, Divoll M, Abernethy DR, Shargel L. High-performance liquid chromatographic determination of acetaminophen in plasma: single-dose pharmacokinetic studies. J Chromatogr 226: 224–230, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barnett AH. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid 6: 67–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker RH, Stechl J, Kapitza C, Msihid J. Augmentation of 1st-phase insulin release with lixisenatide in non-diabetic subjects (Abstract). Diabetes 61: A296, 2012 [Google Scholar]
- 7.Beisswenger PJ. Glycation and biomarkers of vascular complications of diabetes. Amino Acids 42: 1171–1183, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia 44: 2107–2114, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bueno L, Fioramonti J, Ruckebusch Y. Rate of flow of digesta and electrical activity of the small intestine in dogs and sheep. J Physiol 249: 69–85, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceriello A, Bortolotti N, Motz E, Pieri C, Marra M, Tonutti L, Lizzio S, Feletto F, Catone B, Taboga C. Meal-induced oxidative stress and low-density lipoprotein oxidation in diabetes: the possible role of hyperglycemia. Metabolism 48: 1503–1508, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 48: 1198–1214, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Christensen M, Knop FK, Holst JJ, Vilsboll T. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs 12: 503–513, 2009 [PubMed] [Google Scholar]
- 13.Christensen M, Knop FK, Vilsboll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs 20: 549–557, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr 141: 1643–1651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado-Aros S, Vella A, Camilleri M, Low PA, Burton DD, Thomforde GM, Stephens D. Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardio-vagal dysfunction. Neurogastroenterol Motil 15: 435–443, 2003 [DOI] [PubMed] [Google Scholar]
- 16.European Union Community Register of Medicinal Products for Human Use, 2013 (Online). http://ec.europa.eu/health/documents/community-register/html/h811.htm [10 April 2013].
- 17.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 35: 1225–1231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glerup H, Bluhme H, Villadsen GE, Rasmussen K, Ejskjaer N, Dahlerup JF. Gastric emptying: a comparison of three methods. Scand J Gastroenterol 42: 1182–1186, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Grubert JM, Lautz M, Lacy DB, Moore MC, Farmer B, Penaloza A, Cherrington AD, McGuinness OP. Impact of continuous and pulsatile insulin delivery on net hepatic glucose uptake. Am J Physiol Endocrinol Metab 289: E232–E240, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 25: 10–16, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hanefeld M, Temelkova-Kurktschiev T. Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis 12: 98–107, 2002 [PubMed] [Google Scholar]
- 22.Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol 47: 415–421, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz M, Rayner CK, Jones KL. Mechanisms and clinical efficacy of lixisenatide for the management of type 2 diabetes. Adv Ther 30: 81–101, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol 273: G920–G927, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88: 3082–3089, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Larsen J, Hylleberg B, Ng K, Damsbo P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 24: 1416–1421, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lorenz M, Pfeiffer C, Steinsträßer A, Becker RH, Rütten H, Ruus P, Horowitz M. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes - Relationship to postprandial glycemia. Regul Pept 185: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 88: 2719–2725, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Meier JJ, Gethmann A, Gotze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 49: 452–458, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes 54: 2212–2218, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Moore MC, Satake S, Lautz M, Soleimanpour SA, Neal DW, Smith M, Cherrington AD. Nonesterified fatty acids and hepatic glucose metabolism in the conscious dog. Diabetes 53: 32–40, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Nagell CF, Wettergren A, Ørskov C, Holst JJ. Inhibitory effect of GLP-1 on gastric motility persists after vagal deafferentation in pigs. Scand J Gastroenterol 41: 667–672, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Endocrinol Metab 273: E981–E988, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Nicolaus M, Brodl J, Linke R, Woerle HJ, Goke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab 96: 229–236, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Nijpels G, Popp-Snijders C, Kostense PJ, Bouter LM, Heine RJ. Fasting proinsulin and 2-h post-load glucose levels predict the conversion to NIDDM in subjects with impaired glucose tolerance: the Hoorn Study. Diabetologia 39: 113–118, 1996 [DOI] [PubMed] [Google Scholar]
- 36.O'Connell SE, Zurzola FJ. A rapid quantitative determination of acetaminophen in plasma. J Pharm Sci 71: 1291–1294, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 299: E318–E324, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Parlevliet ET, Schroder-van der Elst JP, Corssmit EP, Picha K, O'Neil K, Stojanovic-Susulic V, Ort T, Havekes LM, Romijn JA, Pijl H. CNTO736, a novel glucagon-like peptide-1 receptor agonist, ameliorates insulin resistance and inhibits very low-density lipoprotein production in high-fat-fed mice. J Pharmacol Exp Ther 328: 240–248, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab 15: 1000–1007, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Vilsbøll T, Knop FK, Holst JJ. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol 304: G1117–G1127, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Porksen N, Grofte T, Greisen J, Mengel A, Juhl C, Veldhuis JD, Schmitz O, Rossle M, Vilstrup H. Human insulin release processes measured by intraportal sampling. Am J Physiol Endocrinol Metab 282: E695–E702, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Porksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes 45: 1317–1323, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Ratner RE, Rosenstock J, Boka G; DRI6012 Study Investigators Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 27: 1024–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratner RE, Rosenstock J, Boka G, Silvestre L. Post-meal pharmacodynamic profile of AVE0010, a once-daily GLP-1 receptor agonist, in patients with type 2 diabetes inadequately controlled on metformin (Abstract). Diabetologia 52: S60, 2010 [Google Scholar]
- 45.Rayner DV. The relationships between glucose absorption and insulin secretion and the migrating myoelectric complex in the pig. Exp Physiol 76: 67–76, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, Ping L, Ye J, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 36: 2489–2496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, Ping L, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 36: 2497–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenstock J, Bergenstal RM, Blevins TC, Morrow LA, Prince MJ, Qu Y, Sinha VP, Howey DC, Jacober SJ. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care 36: 522–528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenstock J, Foley JE, Rendell M, Landin-Olsson M, Holst JJ, Deacon CF, Rochotte E, Baron MA. Effects of the dipeptidyl peptidase-IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care 31: 30–35, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and Safety of Lixisenatide Once Daily Versus Exenatide Twice Daily in Type 2 Diabetes Inadequately Controlled on Metformin: A 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 36: 2945–2951, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenstock J, Ratner RE, Boka G, Silvestre L. Post-meal effects of AVE0010, a once-daily GLP-1 receptor agonist, in type 2 diabetes inadequately controlled on metformin (Abstract). Diabetes 58: A151, 2009 [Google Scholar]
- 52.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 51: 1663–1671, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55: 243–251, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Göke B. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil 21: 609–618, e21–e22, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 14: 910–917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.No authors listed Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 58.Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM, Watson CE, Ligueros-Saylan MA, Foley JE, Holst JJ, Deacon CF, Kahn SE. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care 31: 108–113, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, Dunning BE, Foley JE, Rizza RA, Camilleri M. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 56: 1475–1480, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Werner U, Gerlach M, Hofmann M, Herling AW. The GLP-1 receptor agonist AVE0010 abolishes OGTT-induced blood glucose excursion in healthy, normoglycemic dog without risk of hypoglycemia (Abstract). Diabetes 56, Suppl 1: A129, 2007 [Google Scholar]
- 61.Werner U, Gerlach M, Hofmann M, Herling AW. Treatment of obese Zucker diabetic fatty rats with novel GLP-1 receptor agonist AVE0010 improves oral glucose tolerance and glycaemic control without risk of hypoglycaemia (Abstract). Diabetologia 49, Suppl 1: 398, 2006. 16374627 [Google Scholar]
- 62.Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 164: 58–64, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Wettergren A, Wojdemann M, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (7–36)amide on antral motility is antagonized by its N-terminally truncated primary metabolite GLP-1 (9–36)amide. Peptides 19: 877–882, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Gerich J, Göke B, Schirra J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 294: E103–E109, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 32: 1513–1519, 2012 [DOI] [PubMed] [Google Scholar]