Abstract
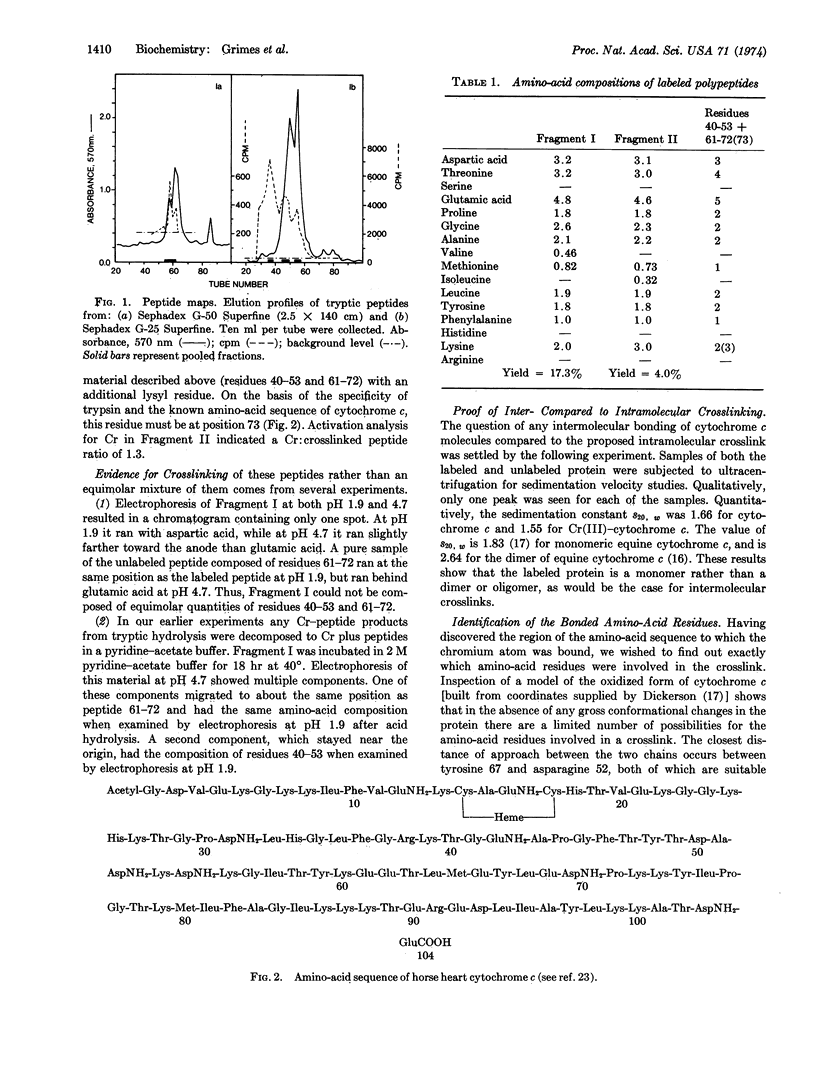
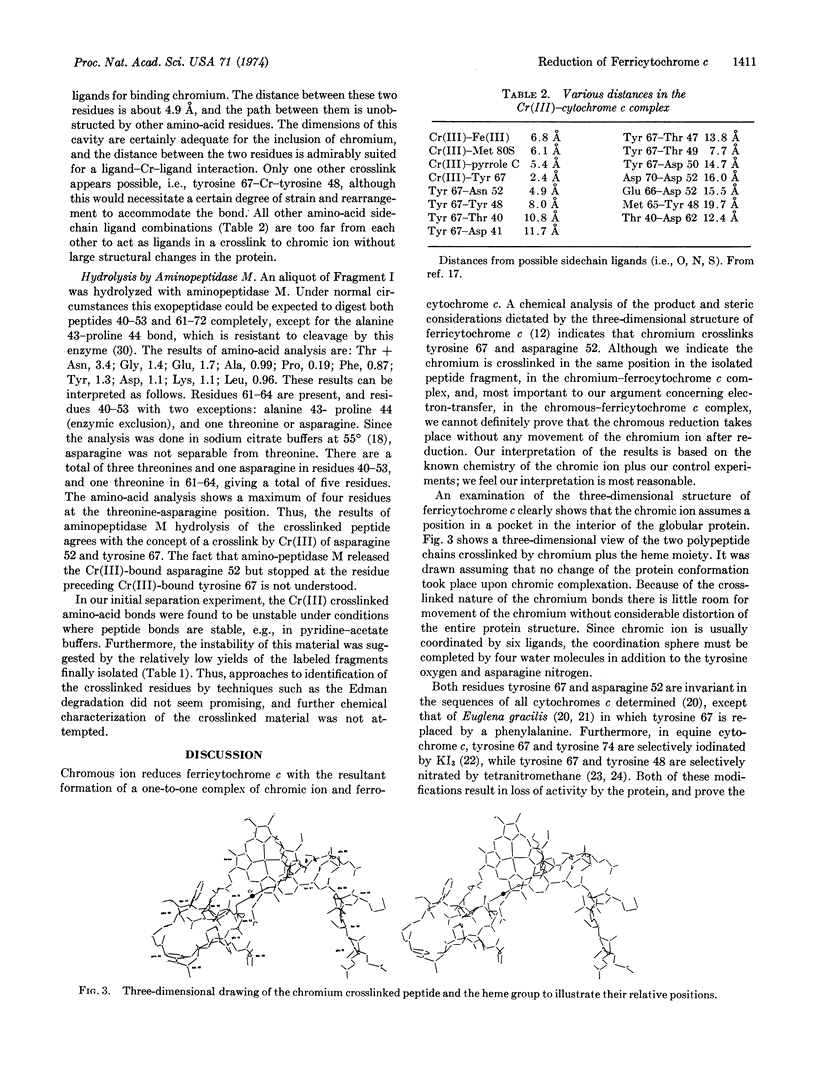
Chromous ion reacts with ferricytochrome c to yield a one-to-one Cr(III)-ferrocytochrome c complex. This material, when hydrolyzed by trypsin and subjected to chromatographic procedures, yielded two fragments containing chromium. The amino-acid compositions and chemical characteristics of each of these fragments indicated that the chromium had crosslinked two segments of polypeptide chain; these were residues 40-53-Cr(III)-residues 61-72 and residues 40-53-Cr(III)-residues 61-73. Examination of a model of the ferricytochrome c molecule indicated that only two residues of the crosslinked peptides were sufficiently close to allow crosslinking to take place. These residues were tyrosine 67 and asparagine 52. Enzymatic hydrolysis of one of those fragments by aminopeptidase M supported this identification. The position of the chromic ion implies what is the path of electron transfer from the chromous ion to the ferric ion in this chemical reduction of cytochrome c, and suggests a possible path of electron transfer in biological oxidation-reduction reactions.
Keywords: Cr(III)-ferrocytochrome c complex, crosslinkage
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson J. W., Gray H. B., Holwerda R. A., Westhead E. W. Kinetics of the reduction of metalloproteins by chromous ion (laccase-cytochrome c-plastocyanins-temperature-rate constants). Proc Natl Acad Sci U S A. 1972 Jan;69(1):30–33. doi: 10.1073/pnas.69.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Dickerson R. E. The structure and history of an ancient protein. Sci Am. 1972 Apr;226(4):58–passim. doi: 10.1038/scientificamerican0472-58. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Kowalsky A. A study of the mechanism of electron transfer in cytochrome c. Chromium as a probe. J Biol Chem. 1969 Dec 25;244(24):6619–6625. [PubMed] [Google Scholar]
- Landon M., Melamed M. D., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. I. Isolation of tryptic peptides from the carboxymethylated protein. J Biol Chem. 1971 Apr 25;246(8):2360–2373. [PubMed] [Google Scholar]
- Landon M., Piszkiewicz D., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. II. Sequences of tryptic peptides. J Biol Chem. 1971 Apr 25;246(8):2374–2399. [PubMed] [Google Scholar]
- Lin D. M., Niece R. L., Fitch W. M. The properties and amino-acid sequence of cytochrome c from Euglena gracilis. Nature. 1973 Feb 23;241(5391):533–535. doi: 10.1038/241533a0. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., LUSTGARTEN J. Interconversion of horse heart cytochrome C monomer and polymers. J Biol Chem. 1962 Nov;237:3397–3405. [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- McDowall M. A., Smith E. L. Amino acid sequence of dog heart cytochrome c. J Biol Chem. 1965 Dec;240(12):4635–4647. [PubMed] [Google Scholar]
- McGowan E. B., Stellwagen E. Reactivity of individual tyrosyl residues of horse heart ferricytochrome c toward iodination. Biochemistry. 1970 Jul 21;9(15):3047–3053. doi: 10.1021/bi00817a017. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The amino-acid sequence of cytochrome c from Euglena gracilis. Nature. 1973 Feb 23;241(5391):531–533. doi: 10.1038/241531a0. [DOI] [PubMed] [Google Scholar]
- Schejter A., Sokolovsky M. Nitration of horse heart ferricytochrome C with tetranitromethane. FEBS Lett. 1969 Aug;4(4):269–272. doi: 10.1016/0014-5793(69)80252-8. [DOI] [PubMed] [Google Scholar]
- Skov K., Hofmann T., Williams G. R. The nitration of cytochrome c. Can J Biochem. 1969 Jul;47(7):750–752. doi: 10.1139/o69-114. [DOI] [PubMed] [Google Scholar]
- Smith L., Davies H. C., Reichlin M., Margoliash E. Separate oxidase and reductase reaction sites on cytochrome c demonstrated with purified site-specific antibodies. J Biol Chem. 1973 Jan 10;248(1):237–243. [PubMed] [Google Scholar]
- Takano T., Swanson R., Kallai O. B., Dickerson R. E. Conformational changes upon reduction of cytochrome c. Cold Spring Harb Symp Quant Biol. 1972;36:397–404. doi: 10.1101/sqb.1972.036.01.051. [DOI] [PubMed] [Google Scholar]
- Winfield M. E. Electron transfer within and between haemoprotein molecules. J Mol Biol. 1965 Jul;12(3):600–611. doi: 10.1016/s0022-2836(65)80314-x. [DOI] [PubMed] [Google Scholar]
- Yandell J. K., Fay D. P., Sutin N. Mechanisms of the reactions of cytochrome c. II. The rate of reduction of horse-heart ferricytochrome c by chromium(II). J Am Chem Soc. 1973 Feb 21;95(4):1131–1137. doi: 10.1021/ja00785a022. [DOI] [PubMed] [Google Scholar]



