Abstract
Numerous studies have demonstrated that both the hypothalamic paraventricular nuclei (PVN) and ventromedial nuclei (VMN) regulate energy homeostasis through behavioral and metabolic mechanisms. Receptors for pituitary adenylate cyclase-activating polypeptide (PACAP) are abundantly expressed in these nuclei, suggesting PACAP may be critical for the regulation of feeding behavior and body weight. To characterize the unique behavioral and physiological responses attributed to select hypothalamic cell groups, PACAP was site-specifically injected into the PVN or VMN. Overall food intake was significantly reduced by PACAP at both sites; however, meal pattern analysis revealed that only injections into the PVN produced significant reductions in meal size, duration, and total time spent eating. PACAP-mediated hypophagia in both the PVN and VMN was abolished by PAC1R antagonism, whereas pretreatment with a VPACR antagonist had no effect. PACAP injections into the VMN produced unique changes in metabolic parameters, including significant increases in core body temperature and spontaneous locomotor activity that was PAC1R dependent whereas, PVN injections of PACAP had no effect. Finally, PACAP-containing afferents were identified using the neuronal tracer cholera toxin subunit B (CTB) injected unilaterally into the PVN or VMN. CTB signal from PVN injections was colocalized with PACAP mRNA in the medial anterior bed nucleus of the stria terminalis, VMN, and lateral parabrachial nucleus (LPB), whereas CTB signal from VMN injections was highly colocalized with PACAP mRNA in the medial amygdala and LPB. These brain regions are known to influence energy homeostasis perhaps, in part, through PACAP projections to the PVN and VMN.
Keywords: feeding, activity, temperature, hypothalamus, rat
pituitary adenylate cyclase-activating polypeptide (PACAP) is a key regulator of several hypothalamic systems, including stress (1), osmoregulation (17), thermoregulation (21), and body weight (27). PACAP was first discovered to influence energy homeostasis through inhibition of feeding in mice following a single intracerebroventricular (icv) injection of the peptide (39). These results combined with reports of diet-specific alterations of PACAP mRNA expression in the hypothalamic ventromedial nuclei (VMN) suggest that PACAP is responsive to nutritional status and directly tied to metabolic systems linked to energy expenditure (27, 40). In addition to reducing food intake, icv administration of PACAP modulates autonomic nerve activity (55) as well as hepatic glucose production (60). As a ligand, PACAP binds to three different G protein-coupled receptors, the PAC1 receptor (PAC1R), and the receptors originally discovered as targets of vasoactive intestinal polypeptide (VIP), VPAC1R and VPAC2R (10). Although PACAP stimulates all three receptor subtypes, it has the highest affinity for the PAC1R, whereas VIP is less likely to utilize the PAC1R due to its low binding affinity compared with the VPACRs (18, 34).
While PACAP regulation of body weight has been examined, previous studies have largely depended on either indiscriminant pharmacological icv injections of ligands or transgenic mouse lines that produce global gene knockouts that cannot isolate contributions of PACAP signaling to discrete brain regions. Moreover, PACAP and PAC1R knockout mice are often accompanied by complications such as lethality, low birth weight, and temperature sensitivity (20, 21, 47). Due to the limited availability of conditional PACAP transgenics, site-specific injections of PACAP and related pharmacological agents directly into discrete hypothalamic nuclei are necessary to selectively investigate the role of PACAP signaling within the hypothalamus.
Recently, we reported that site-specific injections of PACAP isolated to the VMN reduce food intake as well as increase core body temperature and locomotor activity (50). Despite abundant PACAP receptor expression in several different hypothalamic nuclei, little attention has been given to other hypothalamic cell groups with regard to PACAP's effects on food intake and body weight. In the few studies that have examined PACAP in the hypothalamus, the paraventricular nuclei (PVN) are reported to show PACAP terminal immunoreactivity (9, 35), and following direct administration of PACAP into the PVN changes in grooming behavior (44) and hepatic glucose production are observed (60). However, these studies did not report on PACAP-induced changes in feeding or body weight. Given that PVN lesion studies demonstrate an imperative role for this area of the hypothalamus in the regulation of energy balance (36), and both the PVN and PACAP have been linked to melanocortin signaling involved in control of body weight (4, 40), further examination of the relationship between PACAP signaling and PVN-mediated energy homeostasis is warranted.
To examine the effects of PACAP signaling in the hypothalamus on food intake and energy expenditure, we have executed site-specific PACAP injections in two hypothalamic nuclei, the PVN and the VMN. Despite the fact that loss of function in either of these nuclei produces obesity (7), the resulting physiology and behaviors suggest that the PVN and VMN are differentially responsive to PACAP, especially in terms of energy expenditure. Finally, using neuronal tracing, we examined PACAP-expressing circuits that could provide likely sources of PACAP release into these regions of the hypothalamus.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan, Madison, WI) weighing 225–250 g were individually housed in a climate-controlled room with a 12:12-h light-dark cycle. Animals had free access to Harlan standard diet (8604 formulation) and water. Food consumption was measured with a BioDAQ Food Intake Monitor (Research Diets, New Brunswick, NJ) or calculated by preweighing food in each bin and subtracting the weight of noningested and spilled food at the end of each measurement period. All procedures using animals were approved by the Marquette University Institutional Animal Care and Use Committee.
Surgery
Animals were anesthetized with a ketamine-xylazine-acepromazine (77:1.5:1.5 mg·ml−1·kg ip) cocktail and placed in a stereotaxic apparatus. Bilateral guide cannulae (26 gauge; Plastics One, Roanoke VA) were placed 3 mm dorsal to the target site in all animals and secured to the surface of the skull with an acrylic resin. The stereotaxic coordinates for the PVN injection site were anterior/posterior, −1.7 mm from bregma; medial/lateral, ± 0.5 mm from midline; dorsal/ventral, −4.9 mm, and for the VMN anterior/posterior, −2.5 mm from bregma; medial/lateral, ± 0.6 mm from midline; dorsal/ventral, −6.2 mm from surface of the skull based on The Rat Brain in Stereotaxic Coordinates, 6th Edition (48). Injectors extended 3 mm past the ventral tip of the cannulae reaching an injection site of −7.9 mm for PVN and −9.2 mm for VMN ventral from the surface of the skull. The upper incisor bar was positioned −3.3 mm below horizontal zero. A bilateral dummy stylet placed in the guide cannulae was used to maintain patency. The animals were given at least 5 days to recover after cannula installation before receiving drug or vehicle injections, during which time the animals were handled and dummy stylets were removed and replaced daily to acclimate the animals to the physical handling necessary during experiments. Correct cannula placements were confirmed at the conclusion of each experiment by microscopic examination of Nissl-stained sections, and only those with correct placement were included in the studies.
Microinjections and Injection Spread
In all experiments, PACAP (50 pmol/0.25 μl per side; PACAP38; California Peptide Research, Napa, CA), VIP (50 pmol/0.25 μl per side; California Peptide ResearchA), maxadilan (50 pmol/0.25 μl per side; a generous gift from Dr. Ethan Lerner), PACAP(6–38) (500 pmol/0.25 μl per side; Anaspec, Fremont, CA), VIP(6–28) (500 pmol/0.25 μl per side; Bachem, Torrance, CA), or saline vehicle was microinjected through bilateral guide cannulae over ∼2 min in awake animals while being gently restrained. Following each injection, an additional minute elapsed before removing injectors to minimize backflow of injected material. The optimal injection volume and subsequent spread within the VMN were determined previously (50), and the same procedure was used for PVN injection spread analysis. Briefly, biotinylated PACAP (50 pmol/0.25 μl per side; PACAP38-biotin, Anaspec) was injected into the PVN or VMN. One hour following biotinylated PACAP injections, animals were perfused with 0.9% NaCl and 4% paraformaldehyde in phosphate-buffered saline, and brains were removed. Brain sections containing the injection sites and surrounding areas were then immunohistochemically stained using a primary antibody against biotin. Immunohistochemical signal was then compared with the cresyl violet staining of adjacent sections to determine surgical accuracy and the spread of the biotinylated PACAP injection within the target injection site.
Experiments
Feeding behavior.
Animals were weighed daily and acclimated to the BioDAQ Food Intake Monitor for at least 7 days before the onset of the experiment. On the experiment day, ∼1 h prior to lights off, rats were injected bilaterally with vehicle or 50 pmol PACAP. Feeding measurements were collected for the next 24 h, as well as a final measurement of body weight at 24 h postinjection. For BioDAQ meal pattern analysis, the data were analyzed over the first 6 h postinjections to determine latency to meal onset, meal amount and duration, and eating rate. Meals were defined as food intake of 0.2 g or more with less than 15 min elapsing between feeding bouts (13, 14). For studies involving antagonism of PACAP receptors, rats were bilaterally pretreated with saline, PACAP(6–38) (500 pmol/0.25 μl per side, Anaspec) or VIP(6–28) (500 pmol/0.25 μl per side, Bachem). PACAP(6–38) is a widely used PAC1R antagonist; however, it also has antagonistic properties at the VPAC2R (19, 27, 40, 52), and VIP(6–28) is reported to be a potent nonselective VPAC receptor antagonist (16, 38, 54) shown to be effective in the hypothalamus (28). Five minutes later, rats received a second bilateral injection of either saline or 50 pmol PACAP followed by subsequent physiological and behavioral measurements.
Thermogenesis and spontaneous locomotor activity.
At the time of cannulation surgery, some animals were also implanted intraperitoneally with telemetry probes (Mini-Mitter, Sunriver, OR) to record core body temperature and spontaneous locomotor activity while the animals were in their home cage. For telemetry experiments, rats received bilateral injections of saline or 50 pmol PACAP at the onset of the light cycle and were placed back into their home cage, which was positioned over a receiver platform. Telemetric data for core body temperature and spontaneous locomotor activity were collected remotely every 5 min. Core body temperature data were averaged by the hour, and spontaneous locomotor activity data were summed to give cumulative activity over a specified amount of time.
Glucose, pancreatic hormone, and triglyceride measurements.
Blood was collected via tail vein before and after injections of saline or PACAP into the PVN or VMN in tubes containing EDTA and then immediately chilled on ice until centrifugation. Plasma was then collected and stored at −20°C until processing of samples. Glucose measurements were performed via a glucose oxidase colorimetric assay (Sigma-Aldrich, St. Louis, MO). Insulin and glucagon levels were measured by radioimmunoassay (Millipore, Billerica, MA). For triglyceride measurements, interscapular brown adipose tissue (BAT) was harvested 3 h postinjections into the VMN and snap-frozen in liquid nitrogen. BAT was homogenized in radioimmunoprecipitation assay buffer (Boston BioProducts, Ashland, MA) containing protease inhibitor cocktail (Roche, Indianapolis, IN). Triglyceride content of BAT was measured using a colorimetric assay (Sigma-Aldrich) and normalized to protein concentration using the DC protein assay (Bio-Rad, Hercules, CA).
In Situ Hybridization
Brains were sectioned coronally at 12 μm using a cryostat, thaw-mounted onto electrostatically clean slides, and stored at −80°C until postfixed. Prior to hybridization, sections were postfixed in 4% paraformaldehyde, rinsed in 0.1 M PBS (pH 7.4), equilibrated in 0.1 M triethanolamine (pH 8.0), and acetylated in triethanolamine containing 0.25% acetic anhydride. Standard in vitro transcription methods were used to generate both sense and antisense riboprobes recognizing VGLUT2 (Cullinan, Milwaukee, WI), PACAP, VPAC2R, and PAC1R transcripts (Choi, Milwaukee, WI), which were subsequently diluted in hybridization cocktail (Amresco, Solon, OH) with tRNA. Sections were hybridized overnight at 55°C with either digoxigenin (DIG) or fluorescein (FITC)-labeled riboprobes. After hybridization, slides were treated with RNase A and stringently washed in 0.1× SSC at 65°C (PACAP and VGLUT2) or 0.5× SSC at 65°C (VPAC2R and PAC1R) for 30 min. Slides were then incubated with an antibody against DIG or FITC conjugated to horseradish peroxidase (HRP; Roche) overnight at 4°C. Riboprobe signal was further enhanced using the TSA-Plus fluorophore system with either fluorescein or Cy3 (PerkinElmer; Waltham, MA). Image capture was performed using fluorescent microscopy (Axioskop-2; Zeiss, Thornwood, NY) and Axiovision image analysis software (Zeiss).
Tract Tracing and Immunohistochemistry
For PVN and VMN tract tracing experiments, 0.1 μl (PVN) or 0.2 μl (VMN) of biotinylated cholera toxin subunit B was stereotaxically microinjected over 10 min, and injectors were left in place for an additional 5 min before removal. Seven days later, animals were euthanized and brains were collected. Brains were sectioned by cryostat at 12 μm and prepared for PACAP or VGLUT2 fluorescent in situ hybridization as described above. Following in situ hybridization, slides were then probed with a primary antibody against biotin (goat anti-biotin; Vector Labs, Burlingame, CA) overnight at 4°C, and the antibody signal was visualized using a donkey anti-goat Alexa fluor 594-conjugated secondary antibody (Life Technologies, Grand Island, NY). Upon completion of fluorescent staining, representative sections from regions containing both PACAP signal from in situ hybridization and CTB immunolabeling were counted for total number of CTB-positive cells and the number of CTB-positive cells also expressing PACAP mRNA.
Biotinylated PACAP injection spread was visualized following standard free-floating immunohistochemical techniques. Floating coronal sections were incubated in primary antibody against biotin for 24 h at 4°C. After a washing in PBS, sections were incubated in biotinylated secondary antibody (Vector Labs) for 1 h at room temperature. Following a second wash, sections were incubated in a peroxidase-based avidin-biotin solution using the Vectastain Elite ABC kit (Vector Labs) for 1 h at room temperature. Immunohistochemical staining was visualized using a nickel-enhanced 3,3′-diaminobenzidine (DAB) chromogen solution.
Statistics
Data are presented as means ± SE and were analyzed statistically by analysis of variance (with repeated measures when appropriate). Fischer LSD analysis was used for all post hoc group comparisons. Statistical analyses were performed using Sigma Plot 11 software (Systat Software, San Jose, CA). P values < 0.05 were considered statistically significant.
RESULTS
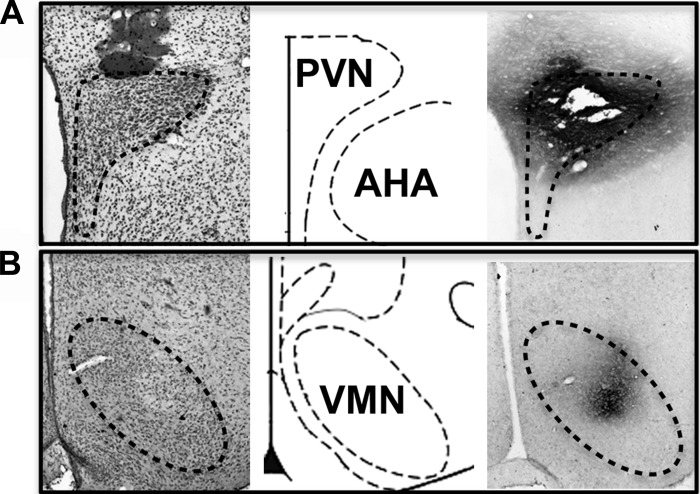
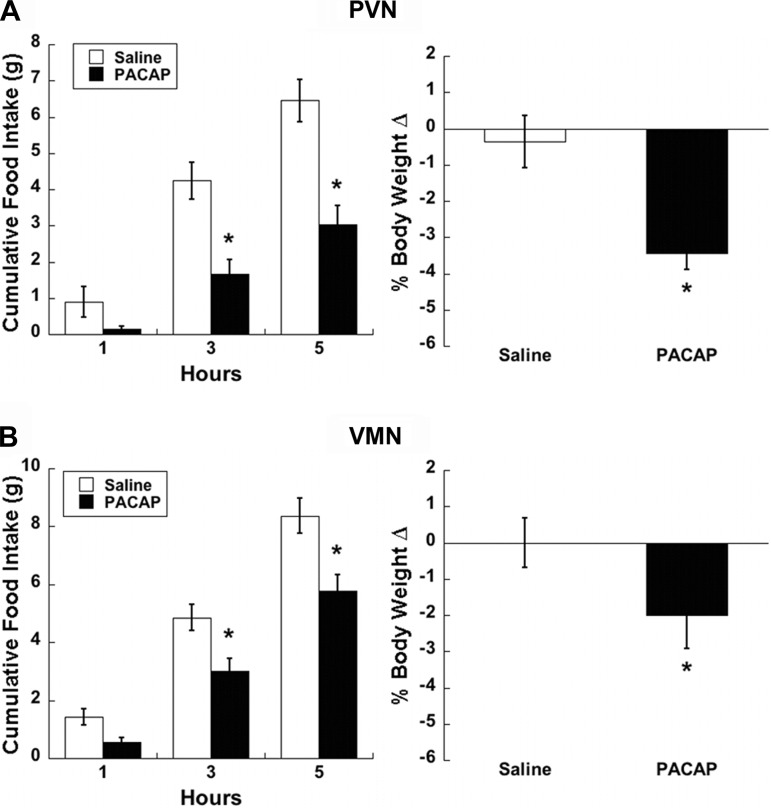
To assess diffusion of PACAP microinjections into our target nuclei, we injected a biotinylated PACAP peptide to simulate behavioral experiments followed by histological analysis of injection sites. Following immunohistochemical detection of the biotinylated PACAP (Fig. 1), we confirmed that peptide injection volumes were appropriate and did not diffuse outside of the PVN and VMN. Our prior studies had indicated that bilateral PACAP injections of 50 pmol into the VMN significantly decreased food intake (50), but the effects of PACAP injections into the PVN on food intake have yet to be reported. We performed feeding behavior experiments under free-feeding conditions starting at ∼1 h prior to lights off following saline or PACAP injections into the PVN and compared subsequent responses to PACAP-induced hypophagia in the VMN. Feeding behavior in both PVN and VMN animals treated with PACAP showed significant main effects by two-factor repeated-measures ANOVA (treatment P < 0.01, time P < 0.001, interaction P < 0.05). Multiple comparison analysis performed at 1, 3, and 5 h postinjection resulted in significant differences at 3 (PVN, P < 0.001, VMN, P < 0.01) and 5 h (PVN, P < 0.001, VMN, P < 0.001) compared with controls (Fig. 2, A and C). Body weight following the injection day was also significantly decreased, with PVN PACAP-treated animals losing 3% (Fig. 2B, P < 0.001) and VMN PACAP treated animals losing 2% (Fig. 2D, P < 0.05) of their preinjection body weight.
Fig. 1.
Injection spread of biotin-labeled pituitary adenylate cyclase-activating polypeptide (PACAP). Staining for biotin-labeled PACAP (far right) accompanied by adjacent cresyl violet staining (far left) show that spread of injectate is contained within the boundaries of the PVN (A; Bregma −1.8 mm) or VMN (B; Bregma −3.0 mm). Schematic image corresponding to the level of injection site is shown in the middle, adapted from The Rat Brain in Stereotaxic Coordinates 6th Edition (48). PVN, paraventricuar nuclei; AHA, anterior hypothalamic area; VMN, ventromedial nuclei.
Fig. 2.
PACAP injections into the PVN or VMN both decrease feeding behavior and body weight. Bilateral 50 pmol PACAP injections (0.25 μl/side) into the PVN (A) or VMN (B) result in significantly reduced cumulative food intake 3 and 5 h postinjection (left), accompanied by reduction in body weight 24 h later (right). Data are expressed as means ± SE. *P < 0.05 vs. saline.
To better characterize the hypophagia produced by hypothalamic PACAP signaling, we evaluated meal patterns from the experiment shown in Fig. 2 (Table 1). PVN injections of PACAP significantly decreased meal size (1st meal P < 0.05, 2nd meal P < 0.01), meal duration (1st meal P < 0.01, 2nd meal P < 0.01), average meal size (P < 0.01), time spent eating (total time, P < 0.05, %time, P < 0.05), and rate of eating (P < 0.01), while also increasing the time it took to initiate the first meal (1st meal P < 0.05, 2nd meal P = 0.375). In contrast, PACAP administration into the VMN produced a decrease only in the rate of eating (P < 0.05) and increased the latency to initiate the first meal (1st meal P < 0.05, 2nd meal P = 0.863). Although PACAP-treated animals ate less, neither PVN- nor VMN-injected animals showed significantly altered 24-h intake.
Table 1.
Meal pattern analysis following PACAP treatment
| PVN |
VMN |
|||
|---|---|---|---|---|
| Saline | PACAP | Saline | PACAP | |
| Latency to 1st meal, min | 47.9 ± 12.8 | 105.9 ± 25.1* | 29.6 ± 6.8 | 89.3 ± 27.5* |
| Latency to 2nd meal, min | 93.9 ± 13.1 | 75.6 ± 15.6 | 62.8 ± 10.1 | 60.2 ± 11.6 |
| Duration of 1st meal, min | 42.6 ± 7.6 | 14.5 ± 4.0* | 37.4 ± 5.8 | 48.8 ± 7.1 |
| Duration of 2nd meal, min | 31.9 ± 4.8 | 15.4 ± 1.8* | 28.8 ± 4.5 | 22.9 ± 3.6 |
| Amount of 1st meal, g | 2.8 ± 0.7 | 1.0 ± 0.2* | 2.8 ± 0.4 | 2.1 ± 0.3 |
| Amount of 2nd meal, g | 3.0 ± 0.4 | 1.4 ± 0.3* | 3.1 ± 0.5 | 2.5 ± 0.5 |
| Average meal size, g | 3.1 ± 0.3 | 1.5 ± 0.2* | 2.8 ± 0.2 | 2.3 ± 0.3 |
| Meal frequency | 2.6 ± 0.2 | 2.5 ± 0.4 | 3.7 ± 0.3 | 3.3 ± 0.3 |
| Total time spent eating, min | 91.2 ± 8.4 | 56.3 ± 14.5* | 107.2 ± 6.9 | 104.6 ± 10.6 |
| %Total time spent eating | 25.3 ± 2.3 | 15.6 ± 4.0* | 30.5 ± 1.7 | 29.1 ± 3.0 |
| Rate of eating, mg/min | 22.0 ± 1.8 | 13.1 ± 2.7* | 27.6 ± 1.1 | 21.0 ± 2.2* |
| 24-h Intake, g | 19.4 ± 1.2 | 16.9 ± 1.0 | 21.7 ± 0.8 | 19.3 ± 1.1 |
Data are means ± SE. Meal patterns were analyzed for 6 h postinjection. PVN, paraventricular nuclei; VMN, ventromedial nuclei; PACAP, pituitary adenylate cyclase-activating polypeptide.
P < 0.05 vs. appropriate anatomic control.
Both the PVN and VMN are hypothalamic regions known to regulate peripheral glucose as well as feeding behavior (29, 56). Similarly, central PACAP has been implicated in glucose homeostasis (60). The effect of PACAP infusion into the PVN or VMN on plasma glucose was assessed in animals fasted overnight (Table 2). PACAP administration in both the PVN and VMN produced significant increases in plasma glucose concentrations (PVN P < 0.05, VMN P < 0.05). Next, in nonfasted animals, we analyzed insulin and glucagon levels to assess the effects of PACAP on pancreatic hormone secretion. Neither PVN nor VMN injections of PACAP significantly altered pancreatic hormone levels, although insulin did show a trend to decrease with PACAP injections into the VMN (P = 0.11).
Table 2.
Change in levels of glucose and pancreatic hormones 60 min following PACAP treatment
| PVN |
VMN |
|||
|---|---|---|---|---|
| Saline | PACAP | Saline | PACAP | |
| Glucose Δ fasted, mg/dl | 19.1 ± 2.2 | 29.8 ± 4.5* | 9.2 ± 2.5 | 17.5 ± 2.0* |
| Insulin Δ, ng/ml | 0.03 ± 0.18 | −0.06 ± 0.13 | −0.01 ± 0.21 | −0.55 ± 0.18 |
| Glucagon Δ, pg/ml | −6.6 ± 6.2 | 5.5 ± 6.5 | −2.9 ± 4.2 | −13.2 ± 13.1 |
Data are means ± SE.
P < 0.05 vs. appropriate anatomic control.
The diversity of effects that PACAP produces physiologically and behaviorally may be due to its multiple receptor subtypes, and the anatomic distribution of specific receptor expression. Fluorescent in situ hybridization revealed that the VPAC2R and PAC1R in the hypothalamus each have distinctive expression patterns (Fig. 3). VPAC2R mRNA expression was abundant in the central dorsomedial nuclei (DMN) and the medial PVN, whereas VPAC2R expression in the VMN and arcuate (ARC) was not detected. On the other hand, PAC1R mRNA expression was much more widely distributed, with abundant expression found throughout the hypothalamic nuclei, including PVN, DMN, VMN, and ARC nuclei. Furthermore, the wide distribution of PAC1R mRNA expression may represent expression in both glial and neuronal cell types (15). Sense probes did not show any signal for either receptor probe (data not shown).
Fig. 3.
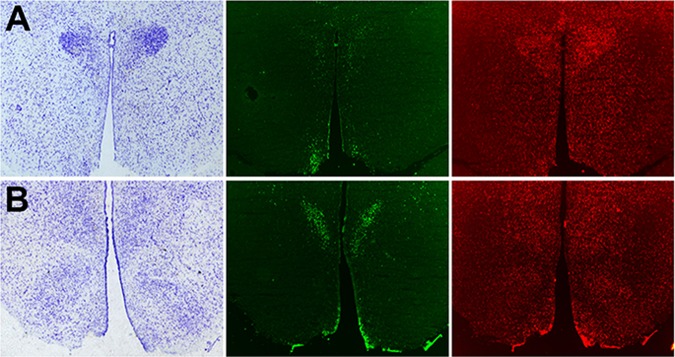
In situ hybridization of hypothalamic PACAP receptor mRNA. mRNA expression of the VPAC2R (middle) and PAC1R (far right) in the hypothalamic PVN (A; Bregma −1.8 mm), and VMN (B; Bregma −3.0 mm) accompanied by adjacent sections stained with cresyl violet depicting the corresponding hypothalamic anatomy in far left.
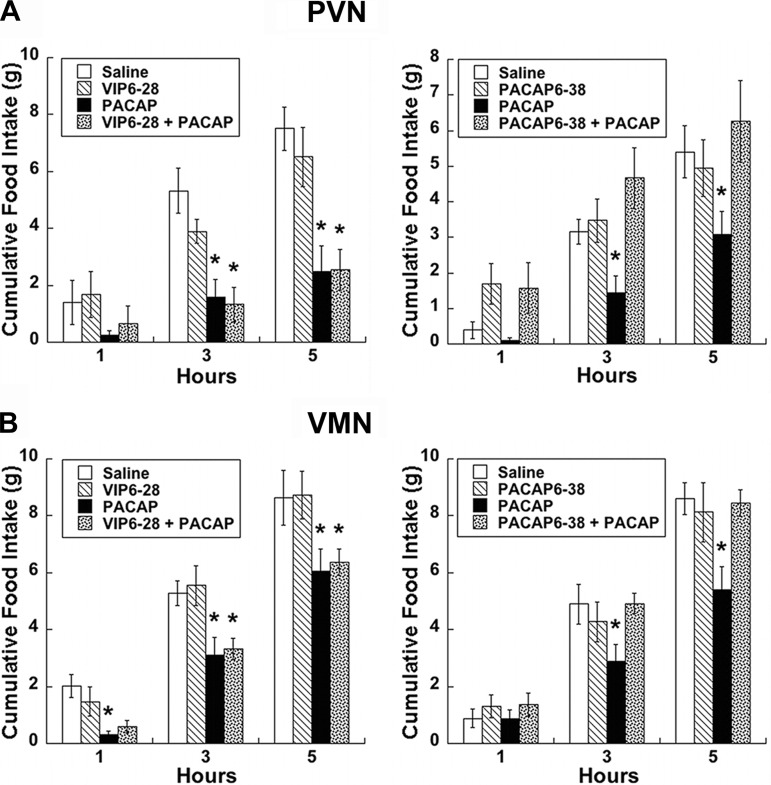
Due to the expression of multiple PACAP receptors in the hypothalamus, we assessed the involvement of PAC1 and VPAC receptors in the regulation of feeding behavior by administering receptor antagonists prior to PACAP injections. Pretreatment with VIP(6–28) did not alter the suppression of feeding behavior produced by PACAP in either the PVN or the VMN compared with controls (Fig. 4, A and C). However, administration of PACAP(6–38) completely reversed the effects of PACAP administration into the PVN at both 3- (P < 0.05) and 5-h (P < 0.01) time points (Fig. 4B). Similarly, pretreatment with PACAP(6–38) blocked the effects of PACAP administration into the VMN at both 3 (P < 0.05) and 5 h (P < 0.001) postinjection (fig. 4D).
Fig. 4.
PACAP effects on feeding are mediated by PAC1R. In both PVN (A) and VMN (B), PACAP-induced hypophagia (50 pmol/side) is prevented by PAC1R antagonist PACAP(6–38) (500 pmol/side; right), but not the VPACR antagonist vasoactive intestinal polypeptide (VIP)(6–28) (500 pmol/side; left). Data are expressed as means ± SE. *P < 0.05 vs. saline.
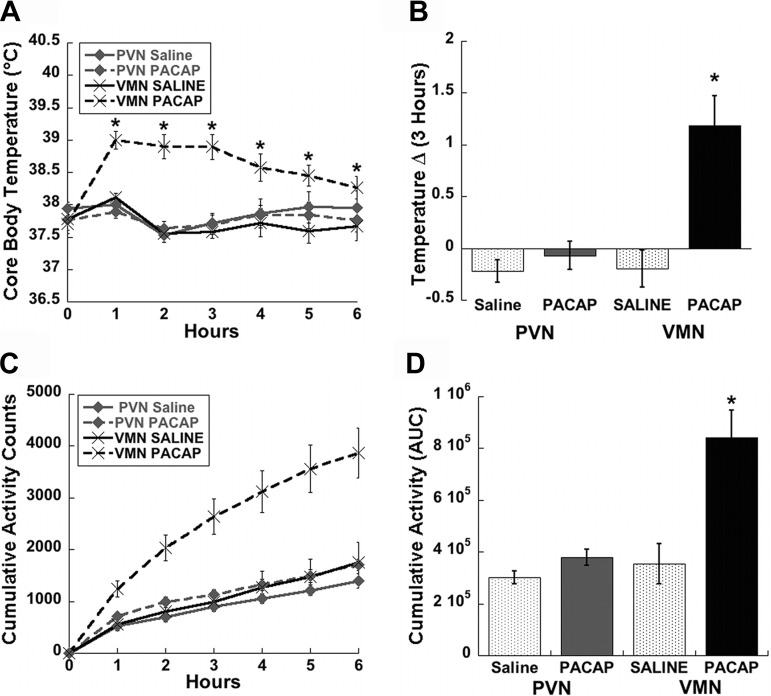
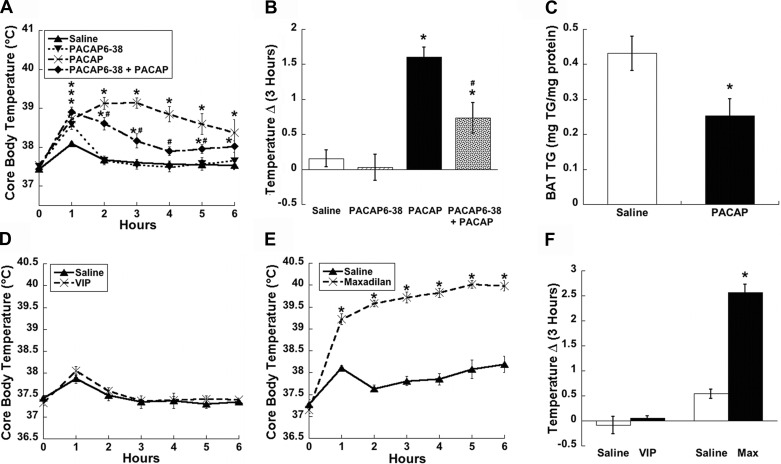
To gain further insight as to how central PACAP signaling impacts body weight regulation, we examined its effects on behaviors relevant to energy expenditure, namely thermogenesis and spontaneous locomotor activity. These telemetric experiments were similar to the feeding studies except they were performed at the onset of the light phase, when core body temperature is at its circadian trough. Surprisingly, PACAP infusion into the PVN had no effect on either core body temperature (Fig. 5, A and B) or activity (Fig. 5, C and D) compared with saline-treated controls. By contrast, animals that were injected with PACAP into the VMN displayed marked and long-lasting increases in both temperature (Fig. 5, A and B, P < 0.05) and activity (Fig. 5, C and D, P < 0.01) compared with saline-treated controls.
Fig. 5.
VMN but not PVN injections of PACAP increase thermogenesis and activity. Time course of core body temperature (A) and 3 h temperature change (Δ; B) show a significant increase following PACAP injections (50 pmol) into the VMN; however, injections into the PVN do not affect temperature. Time course for cumulative activity (C) and area under the curve (AUC; D) analysis depict a similar significant increase only in animals receiving injections of PACAP into the VMN. Data are expressed as means ± SE. *P < 0.05 vs. saline.
In the VMN, PACAP(6–38) significantly attenuated the increased thermogenesis produced by PACAP administration alone from 2–5 h postinjection (Fig. 6, A and B, P < 0.05). Additionally, interscapular BAT (iBAT) triglyceride content per milligram of protein at 3 h postinjection into the VMN was significantly decreased (Fig. 6C, P < 0.05). Although PACAP(6–38) only attenuated the effects of PACAP-induced thermogenesis, we used agonists for the VPAC and PAC1 receptors, VIP and maxadilan, respectively, to confirm the specific involvement of PAC1R receptors. As predicted, VIP injections into the VMN did not affect body temperature (Fig. 6, D and F), whereas the PAC1R-specific agonist, maxadilan, produced a large and long-lasting increase in core body temperature (Fig. 6, E and F, P < 0.001) similar to PACAP-induced thermogenesis.
Fig. 6.
PACAP-mediated increases in temperature by the VMN are PAC1R dependent. A and B: PAC1R antagonist PACAP(6–38) (500 pmol/side) attenuates the time course and 3-h temperature change (Δ) of PACAP-induced thermogenesis (50 pmol/side). C: interscapular brown adipose tissue (iBAT) triglyceride (TG) content was significantly decreased 3 h following injections of PACAP into the VMN. D: VMN injections of VPACR agonist VIP (50 pmol/side) did not alter the time course of core body temperature, whereas PAC1R-specific agonist maxadilan (50 pmol/side) caused significant increases in the time course (E) and 3 h temperature change (Δ; F) in core body temperature similar to PACAP injections. Data are expressed as means ± SE. *P < 0.05 vs. saline group; #P < 0.05 vs. PACAP group.
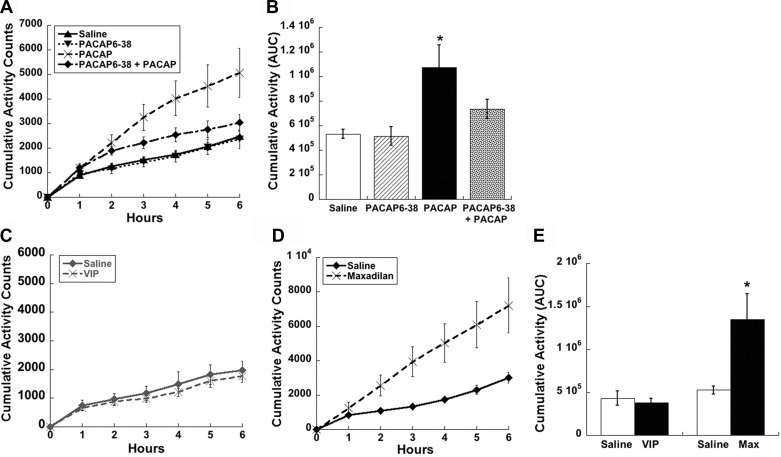
The PAC1 receptor also mediates the increased activity following PACAP infusion into the VMN. PACAP(6–38) blocked the PACAP-induced increase in spontaneous locomotor activity (Fig. 7, A and B, P < 0.05), unlike the effect on temperature, which was only partially inhibited. Further investigation with VIP and maxadilan confirmed that the increase in locomotor activity induced by PACAP is dependent on the PAC1R. VIP did not alter activity compared with controls (Fig. 7, C and E), whereas maxadilan produced large increases in cumulative activity compared with saline treated controls (Fig. 7, D and E, P < 0.05).
Fig. 7.
PACAP-mediated increases in activity by the VMN are PAC1R dependent. A: time course of PACAP-induced spontaneous locomotor activity (50 pmol/side) is blocked by PAC1R antagonist PACAP(6–38) (500 pmol/side), shown by area AUC analysis (B). C: VMN injections of VPACR agonist VIP (50 pmol/side) did not alter the time course of activity, whereas PAC1R-specific agonist maxadilan (50 pmol/side) caused significant increases in the time course of locomotor activity shown by AUC analysis similar to PACAP injections (D and E). Data are expressed as means ± SE. *P < 0.05 vs. saline group.
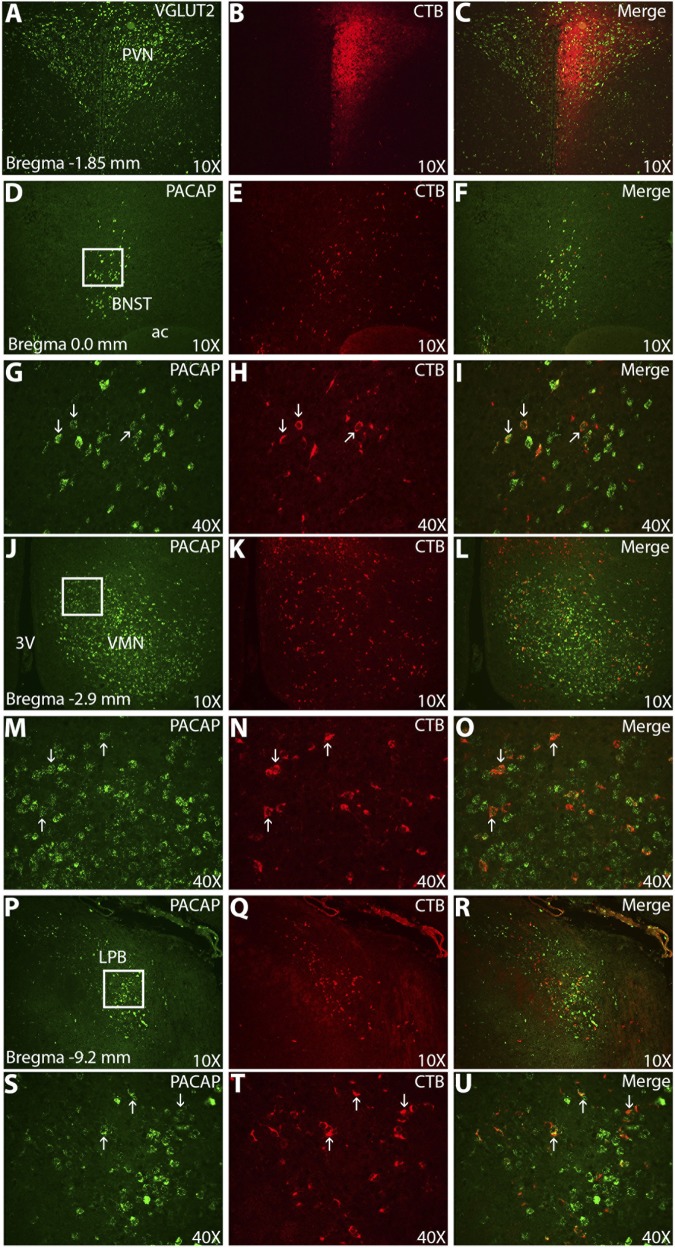
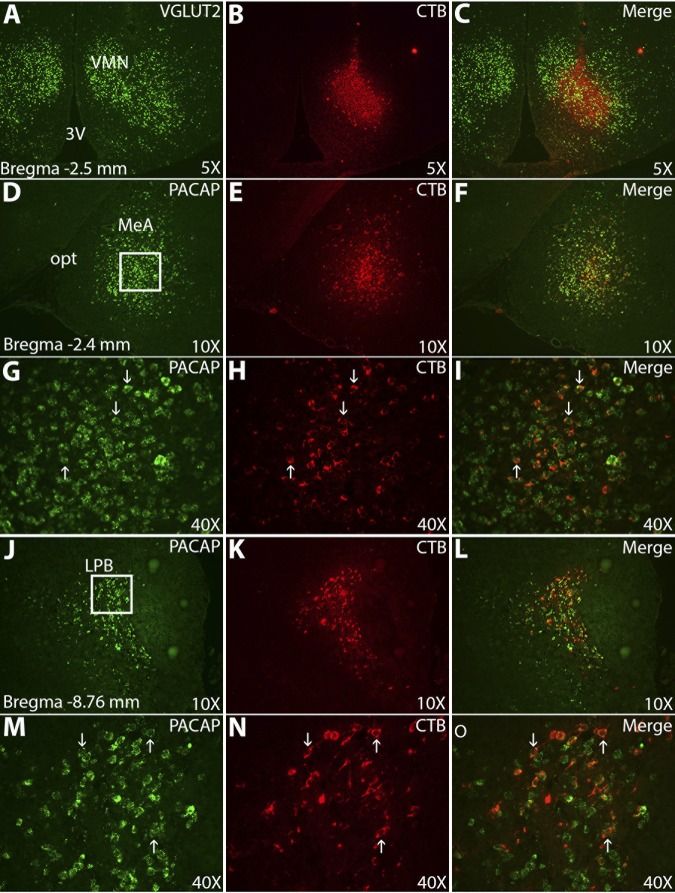
PACAP signaling in the hypothalamus drives several behaviors leading to reduced body weight; however, PACAP-expressing afferent circuits of the PVN and VMN are not well defined. We performed tract tracing with the neuronal tracer cholera toxin subunit B (CTB), coupled with fluorescent in situ hybridization for PACAP and VGLUT2 mRNA (Figs. 8 and 9). VGLUT2 fluorescent in situ hybridization was used as an anatomic marker for both the PVN and VMN. One week following unilateral stereotaxic injections of CTB into the PVN and VMN, we examined the distribution of both the neuronal tracer and PACAP mRNA. CTB retrograde signal from the PVN (Fig. 8) was found to be colocalized, with PACAP mRNA most notably in the anterior medial bed nucleus of the stria terminalis (BNST), VMN, and lateral parabrachial nucleus (LPB). Few dual-labeled cells were also found in the medial amygdala (MeA). With the exception of the BNST, which had 56% of CTB-positive cells coexpressing PACAP mRNA, the vast majority of CTB cells in the VMN (95%) and LPB (92%) coexpressed PACAP mRNA. Retrograde CTB tracing from the VMN (Fig. 9) was abundantly colocalized with PACAP mRNA in the MeA (85%) ipsilateral to the injection site, as well as in the LPB (90%).
Fig. 8.
PACAP-containing afferents of the PVN include the anterior medial bed nucleus of the stria terminalis (BNST), VMN, and lateral parabrachial nucleus (LPB). Left: fluorescent in situ hybridization for VGLUT2 or PACAP identifying anatomic borders of nuclei and cell bodies. Middle: immunofluorescence for cholera toxin subunit B (CTB). Right: merged images. The majority of cells (∼90%) in the VMN and LPB projecting to the PVN were positive for PACAP, whereas ∼50% of cells projecting from the BNST were positive for PACAP. PVN injection site at ×10 (A–C); anterior medial BNST at ×10 (D–F) and ×40 (G–I); VMN at ×10 (J–L) and ×40 (M–O); LPB at ×10 (P–R) and ×40 (S–U). Boxes at left: area of subsequent high-magnification pictures; arrows, representative dual-labeled cells. 3V, 3rd ventricle; ac, anterior commissure.
Fig. 9.
PACAP-containing afferents of the VMN include the medial amygdala (MeA), and LPB. Left: fluorescent in situ hybridization for VGLUT2 or PACAP identifying anatomic borders of nuclei and cell bodies. Middle: immunofluorescence for CTB. Right: merged images. The majority of cells projecting to the VMN from the MeA and LPB were positive for PACAP (∼85–90%). VMN injection site at ×5 (A–C); MeA at ×10 (D–F) and ×40 (G–I); LPB at ×10 (J–L) and ×40 (M–O). Boxes at left: area of subsequent high-magnification pictures; arrows, representative dual-labeled cells. opt, optic tract.
DISCUSSION
The present study demonstrates that site-specific PACAP injections into either the VMN or the PVN of the hypothalamus both produce long-lasting reductions in food intake as well as changes in meal patterns, altered glucose homeostasis, and significant body weight loss 24 h postinjection. Importantly, site-specific hypothalamic injections do not cause sedation or aversive effects (50) that can sometimes arise following exogenous peptide administration. Although there are multiple PACAP receptor subtypes expressed in the hypothalamus, the reduction in feeding behavior appears to be mediated primarily by the PAC1R subtype, which is in agreement with prior feeding studies (27, 40, 50). Specifically, in both the PVN and VMN, the effects of PACAP could be blocked by the VPAC2R/PAC1R antagonist PACAP(6–38) but not by the VPAC1R/VPAC2R antagonist VIP(6–28). Interestingly, only PACAP injections into the VMN produced elevated locomotor activity and core body temperature, which were also dependent on PAC1R signaling.
To specifically address the impact of PACAP signaling on feeding behavior, we placed site-specific microinjections of PACAP into both the PVN and VMN, which produced significant reductions in feeding. However, there were differences in PACAP-mediated meal pattern changes in the PVN vs. the VMN. PACAP injected into the PVN produced several alterations in meal patterns, including increased latency to meal initiation, decreased meal size, decreased meal duration, decreased time spent eating, and decreased rate of eating. In contrast, PACAP injected into the VMN produced an increase only in the latency to meal initiation, and a decrease in the rate of eating. Taken together with the cumulative food intake data, PACAP's functional role in the PVN may be more specific to the regulation of feeding behavior than its actions in the VMN. These results are consistent with loss-of-function studies involving the PVN and VMN where there is a pronounced hyperphagia in PVN-lesioned animals, while loss of function following VMN lesions produces a more modest hyperphagia often restricted to the light period (7). In light of the current data, combined with anatomic and biochemical reports involving PACAP, stress, and the hypothalamus (1, 8, 12, 23), it is plausible that central stress pathways may play a substantial role in the anorectic response caused by PACAP within these hypothalamic nuclei. Further investigation of stress and satiety mechanisms utilizing PACAP signaling will be important to the understanding of the role of this pleiotropic neuropeptide.
Due to the heterogeneous PACAP receptor population in the hypothalamus, and in particular the PVN, it was necessary to determine which PACAP receptor subtype mediated the effects on food intake. The most abundant and widely distributed PACAP receptor in the hypothalamus is PAC1R, with high mRNA expression in all hypothalamic mediobasal cell groups. There is little evidence for any appreciable expression of VPAC1R mRNA in the hypothalamus; however, the VPAC2R is expressed in several hypothalamic areas, including the suprachiasmatic nuclei (SCN), PVN, and dorsomedial nuclei (DMN) (58). Expression of multiple PACAP receptor subtypes in the hypothalamus suggests that PACAP is a multifunctional neuropeptide signal that contributes to several behavioral and physiological systems. The feasibility of this concept is supported by reports linking PACAP to stress (51), anxiety (22), feeding (27, 40, 50), and glucose mobilization (60), all systems that may work synergistically and converge on PACAP-enriched anatomic areas such as the hypothalamus and amygdala (25).
We examined both the VPAC2R/PAC1R antagonist PACAP(6–38) and the VPAC1R/VPAC2R antagonist VIP(6–28) (16) on their ability to inhibit the effects of PACAP on feeding. Even though there are no data to support VPAC1R expression in the hypothalamus, we used VIP(6–28) to antagonize both the VPAC1R and VPAC2R subtypes to fully eliminate the participation of the VPAC receptors (58). Furthermore, because PACAP(6–38) has antagonistic activity at the VPAC2 receptor, VIP(6–28) was utilized to rule out possible contributions of VPAC2R antagonism by PACAP(6–38). Our findings were clear that in both the PVN and VMN pretreatment with PACAP(6–38) prior to PACAP injection completely abolished the effects of PACAP on feeding and significantly attenuated PACAP's effects on thermogenesis and locomotor activity. While we had reported previously that the PAC1R was important for the feeding phenotype observed following PACAP injection into the VMN (50), it is worthy to note that VIP(6–28) did not alter the effects of PACAP in the PVN on feeding behavior despite recent reports demonstrating the importance of PVN VPAC2Rs for the regulation of PACAP-induced hepatic glucose production (60). Taken together, there may be anatomic and receptor-specific roles for PACAP in the PVN, with PAC1R governing the feeding behavior response while VPAC2R may govern the sympathetic-driven glycemic response to PACAP.
Reduction in body weight is consistently found following central PACAP injections (12, 27, 50); however, pair-feeding studies suggest that the body weight loss is not entirely due to decreased feeding, but rather to an increased metabolic rate (27). Here, we find that PACAP signaling in the VMN augments core body temperature and spontaneous locomotor activity, both of which require PAC1R signaling. Furthermore, injections of the specific PAC1R agonist maxadilan in the VMN produced the same pattern of behaviors demonstrated by PACAP administration, whereas VIP injections had no effect on either temperature or activity. By contrast, stimulation of the PVN by PACAP did not significantly alter these measures of energy expenditure despite evidence of PVN regulation of sympathetic outflow to BAT (3, 46) and its control over the sympathetically driven PACAP-induced glycemic response (60). However, these results are in agreement with reports that PVN neurons inhibit sympathetic activity to BAT (33, 37).
VMN-injected animals exhibiting increased thermogenesis and activity also display increased iBAT UCP1 mRNA necessary for nonshivering thermogenesis 3 h postinjection of PACAP (50), as well as decreased BAT triglyceride stores, which is consistent with increased sympathetic nerve activation and lipolysis (6, 45). Whereas transynaptic retrograde tracing studies from the iBAT often fail to detect VMN neurons (5, 46), there is ample evidence supporting VMN activation of BAT thermogenesis, including both VMN-specific microinjection and genetic studies (2, 30, 49, 50). It is feasible that the VMN exerts its influences over hypothalamic structures that are labeled by transynaptic tracers from BAT such as the medial preoptic area, dorsomedial nuclei, or lateral hypothalamus (46, 61), allowing for indirect and downstream effects on BAT thermogenesis that are not observed in more proximal polysynaptic tracing experiments. Future studies are needed to reconcile the discrepancy between the anatomical and functional studies regarding VMN-mediated BAT thermogenesis.
Hypothalamic PACAP mRNA is predominantly expressed in the VMN (24, 27), suggesting that a prominent source of PACAP release in the PVN may originate from the VMN. In fact, much work has been done to characterize PACAP-containing afferents of the PVN, with evidence for PACAP immunolabeling of synaptic terminals (35) and expression of PACAP receptor subtypes (Fig. 4) (26, 53, 58). Furthermore, retrograde tracing from the PVN demonstrates colabeling of neurons with PACAP immunoreactivity (9); however, results using PACAP immunohistochemistry are often difficult to interpret, since available PACAP antibodies typically have low sensitivity and require the use of colchicine and antigen retrieval methods to obtain even low numbers of immunoreactive labeling of neuronal cell bodies (9). The current study has demonstrated that a number of known PVN afferent pathways utilize PACAP signaling including an input from the VMN. Taking into consideration the similar feeding behavior responses following stimulation of the VMN and PVN by PACAP injections, the established circuitry of glutamatergic VMN neurons projecting to the PVN (57), and the extremely high PACAP mRNA expression found in the VMN (24), it is not surprising that the VMN may contribute functionally important PACAP-expressing afferent inputs to the PVN. However, other significant sources of PACAP innervation to the PVN were found in the BNST, LPB, and sparse dual-labeling in the MeA. Although PACAP-containing cells from the LPB and MeA appear to project more extensively to the VMN, the BNST is well characterized to be a critical regulator of stress and anxiety with known inputs to the PVN and could have a potential role in PACAP-mediated hypophagia during times of stress (11, 22, 23).
In contrast to the PVN, the circuitry of PACAP-containing afferents to the VMN are far less studied, with no prior published reports to date. While the possibility of contralateral and intrinsic PACAP signaling within the VMN still exists (32, 43), CTB retrograde tracing following injections into the VMN robustly colabeled with fluorescent in situ hybridization signal for PACAP mRNA in two distinct regions, the MeA and LPB. Both brain regions have been strongly implicated in energy balance, so the MeA and LPB may be two distinct central pathways that converge on the PVN and VMN to manage both feeding behavior and metabolic systems. Lesions to the MeA produce hyperphagia and obesity, especially in females (31). Moreover, the MeA is also a glucose-sensing area of the brain that may respond to states of hypoglycemia, further strengthening a connection with the well-characterized glucostatic role of the VMN (62). The LPB also contributes to body weight regulation as demonstrated by the loss of GABAergic signaling to the parabrachial nuclei from agouti-related polypeptide (AgRP) neurons of the ARC resulting in starvation (59). Given their effect on feeding, the PVN and VMN may be significant efferent targets of the LPB whereby LPB disinhibition could lead to increased excitation of these areas and anorexia. Furthermore, the LPB are critical structures in the thermoregulation pathway (41, 42), thereby positioning the VMN as potential downstream targets of LPB signaling that, in turn, could modulate thermogenic responses.
Perspectives and Significance
The differential response to PACAP signaling within the PVN and VMN regarding feeding, temperature, and locomotor activity may reflect an anatomic divergence segregating the PVN as the predominant sight of action for PACAP-mediated hypophagia, while PACAP stimulation of the VMN may serve primarily to stimulate energy expenditure. The distinct and widespread population of PACAP receptors in the hypothalamus enables functionally diverse signaling by PACAP. Thus, it would be intriguing to determine whether multiple subtypes of PACAP receptors are expressed on the same cell or, more importantly, at the same synapse.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-074734.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.R. and S.C. conception and design of research; J.M.R., B.M., A.K.G., S.K.M., K.A.P., and S.C. performed experiments; J.M.R., B.M., A.K.G., S.K.M., K.A.P., and S.C. analyzed data; J.M.R., B.M., A.K.G., S.K.M., K.A.P., and S.C. interpreted results of experiments; J.M.R. and S.C. prepared figures; J.M.R. and S.C. drafted manuscript; J.M.R. and S.C. edited and revised manuscript; J.M.R. and S.C. approved final version of manuscript.
REFERENCES
- 1.Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138: 45–57, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir S. Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat. Brain Res 511: 341–344, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Amir S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res 508: 152–155, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology 140: 4081–4088, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Choi S, Horsley C, Aguila S, Dallman MF. The hypothalamic ventromedial nuclei couple activity in the hypothalamo-pituitary-adrenal axis to the morning fed or fasted state. J Neurosci 16: 8170–8180, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol 500: 761–776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther 121: 294–316, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494: 142–178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38: 2160–2169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29: 7015–7022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci 20: 3596–3605, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishbein VA, Coy DH, Hocart SJ, Jiang NY, Mrozinski JE, Jr, Mantey SA, Jensen RT. A chimeric VIP-PACAP analogue but not VIP pseudopeptides function as VIP receptor antagonists. Peptides 15: 95–100, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gillard ER, Leon-Olea M, Mucio-Ramirez S, Coburn CG, Sanchez-Islas E, de Leon A, Mussenden H, Bauce LG, Pittman QJ, Curras-Collazo MC. A novel role for endogenous pituitary adenylate cyclase activating polypeptide in the magnocellular neuroendocrine system. Endocrinology 147: 791–803, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology 127: 272–277, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Gourlet P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, Robberecht P. Fragments of pituitary adenylate cyclase activating polypeptide discriminate between type I and II recombinant receptors. Eur J Pharmacol 287: 7–11, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Gray SL, Cummings KJ, Jirik FR, Sherwood NM. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol 15: 1739–1747, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 143: 3946–3954, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci 42: 327–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453: 389–417, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hannibal J, Mikkelsen JD, Clausen H, Holst JJ, Wulff BS, Fahrenkrug J. Gene expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat hypothalamus. Regul Pept 55: 133–148, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 371: 567–577, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29: 14828–14835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermes ML, Kolaj M, Doroshenko P, Coderre E, Renaud LP. Effects of VPAC2 receptor activation on membrane excitability and GABAergic transmission in subparaventricular zone neurons targeted by suprachiasmatic nucleus. J Neurophysiol 102: 1834–1842, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24: 7604–7613, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci USA 108: 10673–10678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King BM. Amygdaloid lesion-induced obesity: relation to sexual behavior, olfaction, and the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 291: R1201–R1214, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Kiss J, Csaki A, Halasz B. Location of glutamatergic/aspartatergic neurons projecting to the hypothalamic ventromedial nucleus studied by autoradiography of retrogradely transported [(3)H]d-aspartate. Neuroscience 176: 210–224, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151: 645–657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam HC, Takahashi K, Ghatei MA, Kanse SM, Polak JM, Bloom SR. Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. Eur J Biochem 193: 725–729, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett 246: 145–148, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav 27: 1031–1040, 1981 [DOI] [PubMed] [Google Scholar]
- 37.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohney RP, Zigmond RE. Vasoactive intestinal peptide enhances its own expression in sympathetic neurons after injury. J Neurosci 18: 5285–5293, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 13: 1133–1135, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jegou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 34: 424–435, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107: 8848–8853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishizuka M, Pfaff DW. Intrinsic synapses in the ventromedial nucleus of the hypothalamus: an ultrastructural study. J Comp Neurol 286: 260–268, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regul Pept 128: 33–41, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamatsu-Ogura Y, Kitao N, Kimura K, Saito M. Brown fat UCP1 is not involved in the febrile and thermogenic responses to IL-1β in mice. Am J Physiol Endocrinol Metab 292: E1135–E1139, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Otto C, Hein L, Brede M, Jahns R, Engelhardt S, Grone HJ, Schutz G. Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation 110: 3245–3251, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2007, p. 456 [Google Scholar]
- 49.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 289: 401–402, 1981 [DOI] [PubMed] [Google Scholar]
- 50.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol 301: R1625–R1634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470: 492–497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem 207: 239–246, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience 67: 409–418, 1995 [DOI] [PubMed] [Google Scholar]
- 54.Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, Sasa M. Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res 809: 127–136, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Tanida M, Shintani N, Hashimoto H. The melanocortin system is involved in regulating autonomic nerve activity through central pituitary adenylate cyclase-activating polypeptide. Neurosci Res 70: 55–61, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5: 383–393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol 519: 1301–1319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology 135: 2662–2680, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137: 1225–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi CX, Sun N, Ackermans MT, Alkemade A, Foppen E, Shi J, Serlie MJ, Buijs RM, Fliers E, Kalsbeek A. Pituitary adenylate cyclase-activating polypeptide stimulates glucose production via the hepatic sympathetic innervation in rats. Diabetes 59: 1591–1600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 31: 1873–1884, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Podolsky N, Sang Z, Ding Y, Fan X, Tong Q, Levin BE, McCrimmon RJ. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes 59: 2646–2652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]