Abstract
Insulin resistance, a hallmark of metabolic disorders, is a risk factor for diabetes and cardiovascular disease. Impairment of insulin responsiveness in vascular endothelium contributes to insulin resistance. The reciprocal relationship between insulin resistance and endothelial dysfunction augments the pathophysiology of metabolism and cardiovascular functions. The most abundant green tea polyphenol, epigallocatechin-3-gallate (EGCG), has been shown to have vasodilator action in vessels by activation of endothelial nitric oxide synthase (eNOS). However, it is not known whether EGCG has a beneficial effect in high-fat diet (HFD)-induced endothelial dysfunction. Male C57BL/6J mice were fed either a normal chow diet (NCD) or HFD with or without EGCG supplement (50 mg·kg−1·day−1) for 10 wk. Mice fed a HFD with EGCG supplement gained less body weight and showed improved insulin sensitivity. In vehicle-treated HFD mice, endothelial function was impaired in response to insulin but not to acetylcholine, whereas the EGCG-treated HFD group showed improved insulin-stimulated vasodilation. Interestingly, EGCG intake reduced macrophage infiltration into aortic tissues in HFD mice. Treatment with EGCG restored the insulin-stimulated phosphorylation of eNOS, insulin receptor substrate-1 (IRS-1), and protein kinase B (Akt), which was inhibited by palmitate (200 μM, 5 h) in primary bovine aortic endothelial cells. From these results, we conclude that supplementation of EGCG improves glucose tolerance, insulin sensitivity, and endothelial function. The results suggest that EGCG may have beneficial health effects in glucose metabolism and endothelial function through modulating HFD-induced inflammatory response.
Keywords: green tea, epigallocatechin-3-gallate, high-fat diet, insulin resistance, endothelial dysfunction, vasodilation
the reciprocal relationship between insulin resistance and endothelial dysfunction plays an important role in pathophysiology of cardiometabolic syndrome (33, 44). Endothelial dysfunction is one of the characteristics of insulin resistance, and insulin resistance is a hallmark of endothelial dysfunction. Obesity is associated with metabolic and cardiovascular disorders, including insulin resistance, diabetes, atherosclerosis, and hypertension (33, 50, 62, 66). Hypertrophied adipocytes spill fatty acids to circulation, which is one of the mechanisms for activation of proinflammatory response through Toll-like receptors (TLR) (20, 23, 38, 65, 75, 79). This proinflammatory response releases various cytokines, including IL-6, IL-8, and TNFα, and increases infiltrated macrophages (15, 16, 45). Activation of TLRs stimulates c-Jun NH2-terminal kinase (JNK) and inhibitor of nuclear factor-κB kinase-β (IKKβ), which are the well-known negative regulators for insulin-signaling pathways (18, 31, 54, 82). Inflammatory cytokines inhibit the specific pathway for activating insulin receptor-mediated signaling, thereby inhibiting insulin action.
Endothelial dysfunction is characterized by a reduction of vasodilation. Vascular endothelium maintains vascular tone by secreting various vasodilators and vasoconstrictors, such as nitric oxide (NO) and endothelin-I (13). One of the vascular actions of insulin is stimulating production of NO to promote vascular function, which contributes to facilitation of glucose disposal in skeletal muscle (3, 62, 71).
Consumption of green tea is negatively associated with reduced mortality caused by cardiovascular events (37). Green tea contains polyphenolic compounds that have beneficial health effects in the prevention of the cardiometabolic syndrome (9, 28, 29, 58, 61). Epicatechin, catechins, epicatechin-3-gallate, and epigallocatechin-3-gallate (EGCG) are the major polyphenols found within green tea, and EGCG is the most abundant form found in green tea (28, 67, 77). In an epidemiological study, it has been suggested that EGCG has antidiabetic, antiobesity, and anti-inflammatory effects (56). EGCG improves endothelial function and insulin sensitivity while reducing blood pressure and protecting against ischemia reperfusion injury in spontaneously hypertensive rats (58). In a randomized triple-crossover controlled study, moderate and high doses of EGCG were given to patients with borderline hypertriglycerolemia, and the treatment reduced plasma triacylglycerol levels by 15.1 (moderate dose) and 28.7% (high dose) (70).
Despite many studies demonstrating that EGCG has beneficial health effects in various pathophysiological conditions, the effects of EGCG in obesity-induced endothelial dysfunction with regard to proinflammatory response are not reported. In this study, we hypothesize that the EGCG can ameliorate the impaired vasodilator actions of insulin in mice fed high-fat diet (HFD).
MATERIALS AND METHODS
Reagents.
Anti-phosphorylated (p)Akt and anti-Akt were obtained from Cell Signaling Technology (Beverly, MA). Anti-IRS-1 was obtained from Millipore (Billerica, MA), Anti-phosphorylated insulin receptor substrate-1 (p-IRS-1; Tyr612) was from BD Biosciences (San Jose, CA), and anti-β-actin was obtained from Sigma-Aldrich (St. Louis, MO). EGCG was provided by Dr. Hara Yukihiko (Tea Solutions, Hara Office, Tokyo, Japan).
Animals.
All animal procedures were performed in accordance with the guidelines and with the approval of the Animal Use and Care Committee at The University of Alabama at Birmingham. All animals were maintained in a temperature-controlled facility with a 12:12-h light-dark cycle. Twenty male C57BL/6J mice, (6 wk of age) were obtained from The Jackson Laboratory (Bar Harbor, ME) and equally divided into four groups. Mice were fed normal chow (10% kcal from fat; Research Diet D12450B) or HFD (60% kcal from fat; Research Diet D12492) without and with EGCG supplement (50 mg·kg−1·day−1) for 10 wk. All animal groups were given access to water and food ad libitum. A 0.1% concentration of EGCG was prepared by dissolving in sterile water and administered via oral gavage each day. Amount given in milliliters was based on the body weight measured weekly. Tissue samples were stored in RNAlater solution until needed. The remaining tissue samples were flash-frozen in liquid nitrogen. For plasma collection, the blood was centrifuged after a blood clot was formed, and supernatant was collected for further analysis conducted by the University of Alabama at Birmingham's (UAB) Nutrition Science Core facility. Plasma insulin and mouse leptin were measured using a Millipore RIA kit.
Cell culture.
Bovine aortic endothelial cells (BAECs) were maintained in F-12K media containing 5% fetal bovine serum (FBS), endothelial cell growth supplement (30 mg/l; BD Biosciences), heparin sulfate (50 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). All experiments conducted on BAECs were before their sixth passage.
Measurement of fasting glucose and insulin in serum.
Mice were fasted overnight, and the blood was collected before euthanization. Glucose was measured with a hand-held glucometer (Alphatrak; Abbott, Abbott Park, IL). The serum was collected after centrifugation (2,200 g for 10 min at 4°C) of clotted blood. Radioimmunoassay was performed to determine the serum insulin levels by using a Millipore (Billerica, MA) RIA kit. These analyses were conducted by the UAB core facility. Quantitative insulin sensitivity check index (QUICKI) was calculated as 1/{log [fasting insulin (μU/ml)] + log [fasting glucose (mg/dl)]} (8, 40, 60).
Functional assessment for isolated mesentery arterioles.
Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Mesenteric arteries were excised from the animal and placed in a cooled (4°C) chamber containing dissection buffer [145 mM NaCl, 4.7 mM KCl, 2 mM CaCl, 1.2 mM MgSO4, 1 mM NaH2PO4, 5 mM glucose, 3 M 3-(N-morpholino) propansulfonic acid buffer, 2 mM pyruvate, 0.02 mM EDTA, and 1% BSA, pH 7.4]. The isolated arteries were then cannulated with glass micropipettes with monofilament suture and mounted in a custom-designed tissue chamber (Living System Instrumentation, Burlington, VT). The arteries were pressurized to 45 mmHg intraluminally with the same buffer without flow and superfused with buffer but without albumin. The vessel preparations were positioned on the stage of an inverted microscope. The vessel segments were gradually warmed to 37°C during a 30-min equilibration period. After baseline diameter was established, arteries were exposed to phenylephrine (1 μM) until a maximal contraction was achieved (5 min). The vessels were subsequently stimulated with various stimulators (10−11 to 10−5 M, 3 min/concentration), including insulin, acetylcholine, or sodium nitroprusside. The dilator responses to insulin were observed and recorded. Measurement of vessel diameter (in μm) was performed with an electronic video caliper (Living Systems Instumentation, St. Albans, VT), and recorded by using Lab Chart software (AD Instruments, Colorado Springs, CO).
The data are expressed as means ± SE. The vasodilator responses to insulin were calculated as percent relaxation from the phenylephrine constriction according to the following equation:
IDtreat is the diameter that was obtained when the vessel was treated with insulin, IDPE is the diameter that was obtained when the vessel was constricted with phenylephrine, and IDw/oCa2+ is the maximal passive diameter that was observed when the vessel was fully dilated in buffer containing 2 mM EGTA and 100 μM adenosine without Ca2+.
Preparation of cell lysates and immunoblotting.
Before lysis, cells were washed briefly with ice-cold PBS. Cells were then scraped in lysis buffer containing 50 mM Tris (pH 7.2), 125 mM NaCl, 1% Triton X-100, 0.5% NP-40, 1 mM EDTA, 1 mM Na3VO4, 20 mM NaF, 1 mM Na pyrophosphate, and complete protease inhibitor cocktail (Roche Applied Science). Cell debris was pelleted by centrifugation of samples at 17,000 g for 10 min at 4°C. Supernatants were then boiled with Laemmli sample buffer for 5 min, and proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with specific antibodies, as described in the figure legends using standard methods. Immunoblots were visualized and quantified by Image Analyzer (Vision Work) and UVP.
RT-PCR.
The cells were treated as described in the figure legends. Total RNA was prepared by using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. One microgram of total RNA was used for cDNA synthesis by using the Omniscript RT Kit (Qiagen, Valencia, CA). Then the cDNA was subjected to semiquantitative PCR analysis by using the Hat Star Taq Master Mix Kit (Qiagen). PCR product was visualized with fluorescent dye (Envirosafe DNA/RNA Stain; Helixx Technologies), and the image was analyzed and quantified by Image Analyzer (Vision Works) and UVP. Black and white colors were inverted by using the software. The primers for each gene areas follows: β-actin forward, TGTTACCAACTGGGACGACA; β-actin reverse, GGGGTGTTGAAGGTCTCAAA; F4/80 forward, TGCCACAACACTCTCGGAAGCTAT; F4/80 reverse, TCCTGGAGCACTCATCCACATCTT.
RESULTS
EGCG reduced HFD-induced weight gain without altered food intake.
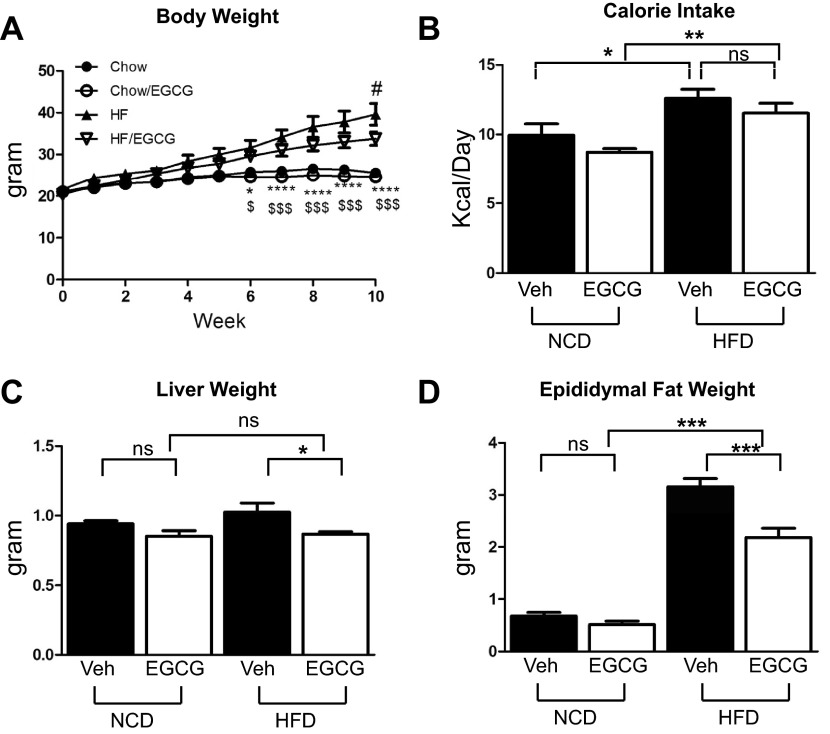
To examine whether supplementation of EGCG has an effect on HFD-induced weight gain, we measured body weight once/wk. Mice fed HFD gained more weight compared with the mice fed normal chow diet (NCD) after 6 wk of feeding. Mice on a HFD supplemented with EGCG gained less weight compared with mice on a HFD without EGCG supplementation on the 10th wk (Fig. 1A). Food intake was higher in HFD groups, but there was no difference in food intake whether EGCG was supplemented or not in both NCD and HFD groups (Fig. 1B). We also measured weights of liver and epididymal adipose tissue. HFD increased weights of both liver and adipose tissue, and EGCG supplementation reduced the weights of liver and adipose tissue only in the HFD mice (Fig. 1, C and D).
Fig. 1.
Epigallocatechin-3-gallate (EGCG) prevented high-fat diet (HFD)-induced weight gain. Six-week-old C57BL/6J mice were fed normal chow diet (NCD) or HFD with and without EGCG for 10 wk. We measured body weight (A), caloric intake (B), liver weight (C), and epididymal fat mass (D). Mice fed HFD and EGCG gained less weight than the mice fed HFD only. Data are presented as means ± SE (n = 5). The data were analyzed by 1-way ANOVA combined with Newman-Keuls post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 for NCD vs. HFD; $P < 0.05 and $$$P < 0.001, NCD and EGCG vs. HFD and EGCG; #P < 0.05 for HFD vs. HFD/EGCG. HF, high fat; ns, not significant; Veh, vehicle.
EGCG ameliorated HFD-induced insulin resistance.
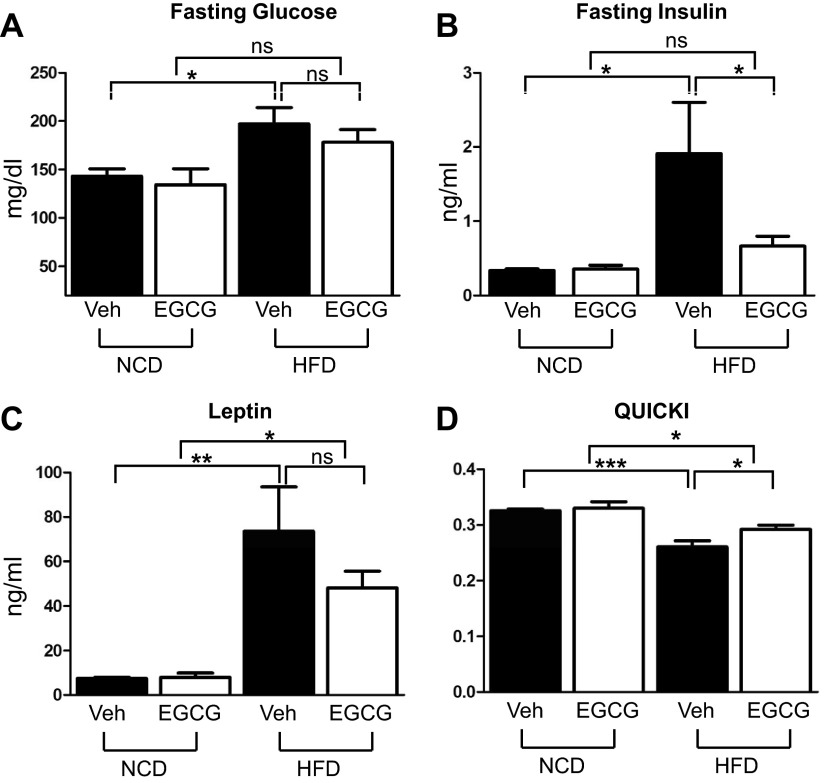
We measured fasting glucose and insulin levels to evaluate whether EGCG supplementation prevents HFD-induced insulin resistance. The fasting glucose level was elevated by HFD, and EGCG intake has a trend of reducing fasting glucose level, but the values were not statistically significant [high fat (HF): 38.8 ± 2.01 g; HF/EGCG: 34.4 ± 1.72 g; P < 0.05]. The fasting glucose level in the EGCG-treated HFD group is not different from the NCD groups (Fig. 2A). Fasting insulin level was greatly increased by HFD, and supplementation of EGCG reduced the fasting insulin level significantly (Fig. 2B). Serum leptin level was increased by HFD, and EGCG intake suppressed leptin level, but the values were not statistically significant (Fig. 2C). The insulin sensitivity surrogate index QUICKI indicates that the HFD mice were more insulin resistant compared with NCD mice, and EGCG supplementation ameliorated HFD-induced insulin resistance (Fig. 2D).
Fig. 2.
EGCG ameliorated HFD-induced insulin resistance. Six-week-old C57BL/6J mice were fed NCD or HFD with or without EGCG for 10 wk. Food was removed the night before euthanization. Serum was collected, and fasting blood glucose (A), insulin (B), and leptin (C) levels were determined as described in materials and methods. D: EGCG intake ameliorated HFD-induced insulin resistance. Insulin sensitivity was determined by quantitative insulin sensitivity check index (QUICKI). Data are presented as means ± SE (n = 5). The data were analyzed by 1-way ANOVA combined with Newman-Keuls post hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001, NCD vs. HFD.
EGCG effect on insulin-stimulated vasodilation in mesentery arteries.
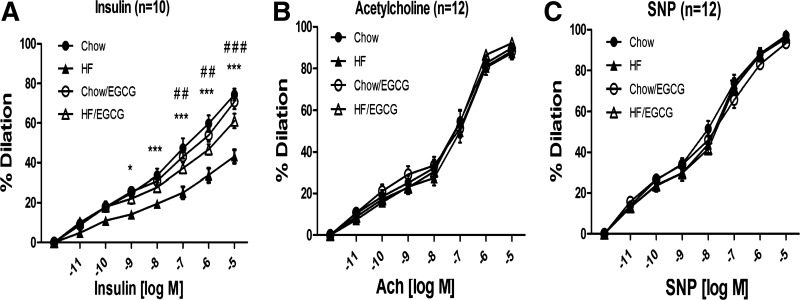
To examine whether EGCG has an ability to ameliorate HFD-induced endothelial dysfunction, the effects of EGCG on vasodilator actions of various reagents in mesentery arteries were examined (Fig. 3). The vessels dilated in response to insulin in a dose-dependent fashion, and there was no significant difference between the vessels from NCD and NCD/EGCG. Vessels isolated from HFD mice showed significantly impaired insulin-stimulated vasodilation compared with the vessels isolated from NCD mice. However, vessels from EGCG-treated HFD mice showed significant improvement of vasodilation compared with the vessels isolated from HFD mice (Fig. 3A). Acetylcholine (ACh) is a well-known cholinergic agonist that stimulates endothelial NO synthase (eNOS) mainly through a Ca2+-mediated mechanism. Interestingly, neither HFD nor EGCG has an effect on ACh-mediated vasodilation (Fig. 3C). Then, we examined whether NOS-independent vasodilation is altered by HFD or EGCG. We treated sodium nitroprusside (SNP), which generates NO independent of NO synthase (NOS) activity to dilate vessels. There was no difference in the SNP-stimulated vasodilation between groups. These results suggest that HFD causes impairment of vasodilator actions of insulin but not ACh- or SNP-stimulated vasodilation, and supplementation of EGCG for 10 wk was able to prevent it.
Fig. 3.
EGCG improved insulin-stimulated vasodilation. C57BL/6J mice were fed NCD or HFD without or with EGCG for 10 wk. Mesentery arteries were isolated from mice. The arteries were canulated onto glass micropipettes and pressurized to 45 mmHg using a Pressure Servo System and warmed to 37°C for 30 min of stabilization. Arterioles were preconstricted with phenylephrine (1 μM) and then exposed to increasing concentrations of insulin (A), acetylcholine (ACh; B), or sodium nitroprusside (SNP; C). The vasodilatory activity in response to each stimulator on the arteries was observed and recorded using a data acquisition system. Endothelial function in response to insulin was improved in the arteries isolated from EGCG-treated HFD mice compared with the arteries isolated from HFD mice. The vasodilatory responses to ACh and SNP were not different between groups. Data are presented as means ± SE (n = 5). The data were analyzed by repeated measurements of 2-way ANOVA combined with Bonferroni post hoc test. Values are significantly different (*P < 0.05 and ***P < 0.001, NCD vs. HFD; ##P < 0.01 and ###P < 0.001, HFD vs. HFD/EGCG) from the values for the indicated groups.
EGCG prevents HFD-induced inflammation by inhibition of macrophage infiltration.
Macrophage infiltration plays an important role in both atherosclerosis and insulin resistance (42, 52, 75, 79). We examined the expression levels of F4/80, a cell surface receptor and a marker for macrophage, by RT-PCR analysis whether EGCG can prevent macrophage infiltration in HFD mice. More macrophages were infiltrated into HFD mice aorta, which was prevented by treatment with EGCG (Fig. 4, A and B). Interestingly, macrophage infiltration in adipose tissue was not observed in the HFD mice (Fig. 4, C and D). The result suggests that EGCG prevents the HFD-induced macrophage infiltration in aorta.
Fig. 4.
EGCG reduced HFD-induced macrophage infiltration in vascular tissues but not in adipose tissue. Aorta (A and B) and epididymal adipose tissue (C and D) were collected from NCD or HFD mice. Total RNA was isolated from tissues and subjected to RT-PCR analysis. Macrophage infiltration was examined by F4/80 mRNA (A–D). Macrophage infiltration was increased in HFD aorta but not in HFD adipose tissue compared with NCD. EGCG prevented macrophage infiltration in HFD mice aorta. Data are presented as means ± SE (n = 5). The data were analyzed by 1-way ANOVA combined with Newman-Keuls post hoc test. *P < 0.05; **P < 0.01.
Treatment with EGCG prevents the saturated fatty acid-mediated impairment of insulin-signaling pathways in primary vascular endothelial cells.
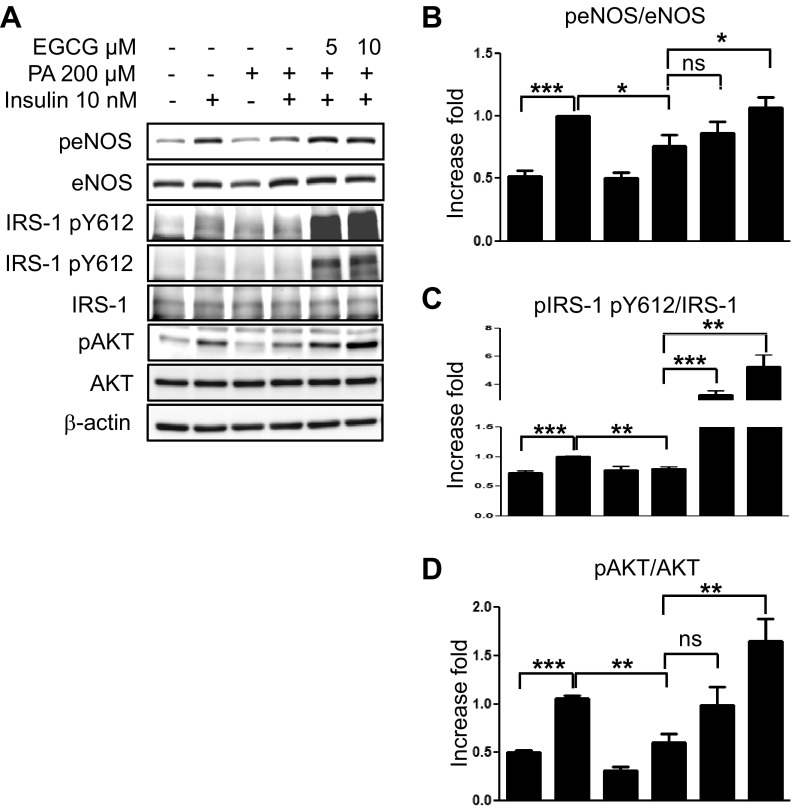
Insulin-signaling pathways in vascular endothelial cells contribute to vasodilator actions of insulin. The insulin receptor stimulating the phosphatidylinositol (PI) 3-kinase pathway to activate Akt plays an especially important role in the stimulation of eNOS and NO production that culminates in vasodilation (46, 47). Saturated fatty acid (SFA)-mediated inhibition of insulin-stimulated Akt leads to impairment of vascular actions of insulin (24, 25, 30). Thus, we examined whether EGCG has an effect to prevent the SFA-mediated impairment of insulin-signaling pathways in bovine aortic primary endothelial cells. We treated BAECs with BSA or palmitate (200 μM, 5 h) and then treated the cells with vehicle, insulin alone, or insulin plus EGCG for the times indicated in the figure legends. Treatment with palmitate decreased insulin-stimulated phosphorylation of eNOS (Ser1179), IRS-1 (Tyr612), and Akt (Ser473). Treatment with EGCG restored the insulin-stimulated phosphorylation of eNOS, IRS-1, and Akt (Fig. 5). The results suggest that EGCG prevents SFA-induced impairment of insulin signaling in vascular endothelial cells, which may be the mechanism for the protective effects of EGCG in HFD-induced endothelial dysfunction.
Fig. 5.
EGCG restored insulin-stimulated phosphorylation of endothelial nitric oxide synthase (eNOS), insulin receptor substrate-1 (IRS-1), and Akt, which was inhibited by palmitate. Bovine aortic endothelial cells were incubated overnight with medium containing 0.1% horse serum and serum-starved for 2 h. Next, the cells were incubated with palmitate (200 μM, 5 h) and then treated with insulin (10 nM, 20 min). Cell lysate was collected and subjected to Western blotting using the indicated antibodies. Experiments were repeated at least 3 times. Data are presented as means ± SE (n = 4 for B, and n = 3 for C and D). Data were analyzed by 1-way ANOVA combined with Newman-Keuls post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
In the present study, we demonstrate that EGCG has protective effects on HFD-induced insulin resistance and endothelial dysfunction. The potential mechanism underlying the beneficial effects of EGCG may be through reduction of macrophage infiltration and augmentation of insulin-signaling pathways, leading to enhanced vasodilation. This is the first report showing that EGCG has a beneficial vascular effect in the HFD-induced insulin-resistant mouse model.
Reduction of body weight and improvement of insulin sensitivity.
We demonstrate that EGCG supplementation partially prevents HFD-induced weight gain without significant difference in calorie intake (Fig. 1, A and B). Although the food intake was not significantly reduced, there was a trend of slightly less food intake in EGCG-treated groups. The reduced food intake may contribute to the reduction of body weight over the longer period of feeding. As a corollary, liver and epididymal adipose tissue weights are reduced by EGCG treatment in the HFD groups, whereas there were no differences in the NCD groups (Fig. 1, C and D). However, there may be other mechanisms involved in EGCG-induced reduction of body weight. The effects of green tea extract (GTE) and EGCG on prevention of obesity have been shown in both genetic and diet-induced animal models (5, 51, 55, 68, 69). The underlying mechanisms may be a combination of reduced lipid absorption (5, 14) and increased lipolysis and fatty acid oxidation, etc. (21, 39, 76). We observed that EGCG treatment reduces fasting glucose and insulin levels in the HFD groups (Fig. 2, A and B). Because fasting glucose level is contributed mainly by hepatic gluconeogenesis, it is possible that EGCG may have an effect on reduced gluconeogenesis. In fact, it has been shown that EGCG reduces hepatic glucose production through activation of the CaMKKβ/AMPK pathway and/or inhibition of tyrosine phosphatase, which in turn, reduces the expression of gluconeogenic enzymes (9, 73, 81). The similar effects of EGCG and GTE are reported in both fructose- and HFD-induced insulin-resistant models (53, 78). These studies show that the expression levels of GLUT4 in the skeletal muscle and adipose tissue are increased by GTE or EGCG, which may be the mechanism for the anti-insulin resistance effect of green tea polyphenols. Nonetheless, green tea polyphenols may have an effect on enhancing energy metabolism, which leads to improved insulin sensitivity and prevention of weight gain.
Link between insulin resistance and endothelial dysfunction.
Insulin resistance is characterized by endothelial dysfunction, and endothelial dysfunction is a hallmark of insulin resistance (2, 33). The reciprocal relationship between endothelial function and insulin sensitivity is the physiological contributor for the link between metabolism and cardiovascular functions (28, 31, 33, 50). Previously, we showed that EGCG ameliorates insulin resistance and endothelial dysfunction in spontaneously hypertensive rats and that EGCG has a direct effect on vasodilation through the activation of the Fyn/PI 3-kinase/Akt/eNOS signaling pathway (29, 58). In the present study, we demonstrate that insulin-stimulated vasodilation is impaired in the mesenteric arteries isolated from the HFD group and that chronic treatment of EGCG treatment restored the insulin-stimulated vasodilation (Fig. 3). This is consistent with the previous studies showing that endothelial dysfunction causes impairment of nutritive capillary recruitment in the skeletal muscle that is associated with impaired glucose tolerance and insulin resistance (1, 2, 33, 36, 44, 59, 72). In contrast, HFD did not affect ACh-stimulated vasodilation (Fig. 3B). This result is consistent with the previous studies (22, 35, 57, 64), but other studies show that HFD leads to the impairment of vascular relaxation to ACh (4, 10, 63). ACh stimulates G protein-coupled receptor and the Ca2+/calmodulin-signaling pathway to activate eNOS, which is distinct from the insulin signaling to activate eNOS (46, 47). Thus, HFD may differentially affect insulin signaling and ACh-mediated signaling pathways. Endothelial-derived constricting factor or reactive oxygen species may also differentially contribute to the HFD-induced endothelial dysfunction (49). The discrepancy in results from different laboratories may be due to the genetic background of animals, diet, husbandry, severity of insulin resistance and vessels, or other factors. The future studies on the detailed mechanisms for the effects of HFD on insulin- and ACh-induced eNOS activation will reveal the discrepancies. Nonetheless, HFD causes the impairment of insulin-stimulated vasorelaxation that precedes the impairment of ACh responsiveness. This suggests that mild insulin resistance may not reveal the obvious impairment of ACh-stimulated vasodilation.
EGCG stimulates AMPK (9, 27, 61), which is another upstream kinase to phosphorylate eNOS at the same serine site that Akt phosphorylates (7). Thus, AMPK may mediate the actions of EGCG to improve insulin sensitivity and endothelial function. Because AMPK facilitates β-oxidation and autophagy to increase fatty acid oxidation, EGCG can contribute indirectly to the prevention of endothelial dysfunction and insulin resistance by facilitation of lipid metabolism. Thus, the physiological actions of EGCG may be through both direct and indirect mechanisms to augment insulin actions.
Anti-inflammatory effect of EGCG in HFD-induced obesity.
HFD increases circulating free fatty acids, which causes inflammation and lipotoxicity (26, 31, 32). The HFD-induced proinflammatory responses, including activation of IKKβ and JNK, production of cytokines, and cell adhesion molecules, are implicated as a mechanism for insulin resistance and endothelial dysfunction (18, 20, 25, 82). Moreover, obesity-induced macrophage infiltration plays the major role in insulin resistance (75, 79) and endothelial dysfunction (11, 74). The underlying mechanisms, such as increased serine phosphorylation of IRS-1 and ER stress, have been proposed (12, 19, 34, 41, 48, 54). Thus, EGCG may improve insulin signaling through inhibition of proinflammatory responses. We observed that macrophage infiltration occurs in the vasculature but not in adipose tissue (Fig. 4). This is consistent with the previous study showing that the endothelial dysfunction precedes metabolic insulin resistance (21). HFD causes stimulation of TLR, which leads to production of proinflammatory cytokines and endoplasmic reticulum stress (20, 23, 25, 31, 43). Considering the anti-inflammatory role of EGCG as a specific inhibitor for TLR2 and TLR4 (5, 6, 17, 80), it is conceivable that EGCG may have beneficial effects through the inhibition of TLRs in vascular endothelium and that it preserves insulin signaling even in the presence of SFAs.
In summary, the present study demonstrates that supplementation of EGCG prevents HFD-induced body weight gain, insulin resistance, and endothelial dysfunction. These beneficial health effects of EGCG may be due to the reduction of macrophage infiltration in the vasculature and improvement of insulin-signaling pathways. Thus, drinking green tea may prevent obesity-induced health problems. This suggests that the lifestyle adjustment by drinking tea may have therapeutic potential to improve public health and can be used to lower medical costs.
GRANTS
This study was supported by the American Diabetes Association (1-09-JF-33 and 1-12-BS-99 to J. Kim), the American Heart Association (13GRNT17220057 to J. Kim), and the UAB Diabetes Research Training Center-sponsored pilot and feasibility program supported by National Institutes of Health (P60-DK-079626).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.-J.J. and S.D.R. performed the experiments; H.-J.J., S.D.R., and J.-a.K. analyzed the data; H.-J.J., S.D.R., and J.-a.K. interpreted the results of the experiments; H.-J.J., S.D.R., and J.-a.K. prepared the figures; H.-J.J., S.D.R., and J.-a.K. edited and revised the manuscript; H.-J.J., S.D.R., and J.-a.K. approved the final version of the manuscript; S.D.R. and J.-a.K. drafted the manuscript; J.-a.K. contributed to the conception and design of the research.
ACKNOWLEDGMENTS
We thank Dr. Yukihiko Hara (Tea Solutions) for providing EGCG.
REFERENCES
- 1.Baron AD. Insulin and the vasculature—old actors, new roles. J Investig Med 44: 406–412, 1996 [PubMed] [Google Scholar]
- 2.Baron AD. Insulin resistance and vascular function. J Diabetes Complications 16: 92–102, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49: 768–774, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Blaedel M, Raun K, Boonen HC, Sheykhzade M, Sams A. Early onset inflammation in pre-insulin-resistant diet-induced obese rats does not affect the vasoreactivity of isolated small mesenteric arteries. Pharmacology 90: 125–132, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 138: 1677–1683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun EH, Omura T, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett 585: 814–820, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 54: 1914–1925, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282: 30143–30149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa RR, Villela NR, Souza M, Boa BC, Cyrino FZ, Silva SV, Lisboa PC, Moura EG, Barja-Fidalgo TC, Bouskela E. High fat diet induces central obesity, insulin resistance and microvascular dysfunction in hamsters. Microvasc Res 82: 416–422, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest 102: 145–152, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 55: 2392–2397, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Endemann DH, Schiffrin EL. Nitric oxide, oxidative excess, and vascular complications of diabetes mellitus. Curr Hypertens Rep 6: 85–89, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes 36: 735–743, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Garcia C, Feve B, Ferre P, Halimi S, Baizri H, Bordier L, Guiu G, Dupuy O, Bauduceau B, Mayaudon H. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab 36: 327–338, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb 17: 332–341, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hong Byun E, Fujimura Y, Yamada K, Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol 185: 33–45, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54, Suppl 2: S73–S78, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Jang HJ, Kim HS, Hwang DH, Quon MJ, Kim JA. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am J Physiol Endocrinol Metab 304: E1077–E1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res 50: 188–210, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 288: H854–H860, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 25: 989–994, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 27: 321–333, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Montana V, Jang HJ, Parpura V, Kim JA. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation. J Biol Chem 288: 22693–22705, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JA. Mechanisms underlying beneficial health effects of tea catechins to improve insulin resistance and endothelial dysfunction. Endocr Metab Immune Disord Drug Targets 8: 82–88, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282: 13736–13745, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 302: E201–E208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JA, Koh KK, Quon MJ. The union of vascular and metabolic actions of insulin in sickness and in health. Arterioscler Thromb Vasc Biol 25: 889–891, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kim JA, Montagnani M, Chandrasekran S, Quon MJ. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin 8: 589–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension 44: 956–962, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA: 1255–1265, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lee MS, Kim CT, Kim Y. Green tea (−)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab 54: 151–157, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Liu YF, Herschkovitz A, Boura-Halfon S, Ronen D, Paz K, Leroith D, Zick Y. Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol Cell Biol 24: 9668–9681, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS One 3: e3204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol 29: 1370–1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 123: 1003–1004, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McVeigh GE, Cohn JN. Endothelial dysfunction and the metabolic syndrome. Curr Diab Rep 3: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276: 30392–30398, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16: 1931–1942, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, Minotti R, Barton M. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res 73: 368–375, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord 26: 1459–1464, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Nishiumi S, Bessyo H, Kubo M, Aoki Y, Tanaka A, Yoshida K, Ashida H. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice. J Agric Food Chem 58: 12916–12923, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O'Connor M, Manautou JE, Bruno RS. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem 22: 393–400, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Polychronopoulos E, Zeimbekis A, Kastorini CM, Papairakleous N, Vlachou I, Bountziouka V, Panagiotakos DB. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study). Eur J Nutr 47: 10–16, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292: E1378–E1387, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Quon MJ. Insulin signaling and the link to endothelial dysfunction. Endocr Pract 9, Suppl 2: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Quon MJ. QUICKI is a useful and accurate index of insulin sensitivity. J Clin Endocrinol Metab 87: 949–951, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology 151: 103–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M, Bhatnagar A, Hill BG. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ Res 111: 1176–1189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato J, O'Brien T, Katusic ZS, Fu A, Nygren J, Singh R, Nair KS. Dietary antioxidants preserve endothelium dependent vasorelaxation in overfed rats. Atherosclerosis 161: 327–333, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Schulman IH, Zhou MS, Jaimes EA, Raij L. Dissociation between metabolic and vascular insulin resistance in aging. Am J Physiol Heart Circ Physiol 293: H853–H859, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome—a review. Phytochemistry 70: 11–24, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Tokimitsu I. Effects of tea catechins on lipid metabolism and body fat accumulation. Biofactors 22: 141–143, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Unno K, Yamamoto H, Maeda K, Takabayashi F, Yoshida H, Kikunaga N, Takamori N, Asahina S, Iguchi K, Sayama K, Hoshino M. Protection of brain and pancreas from high-fat diet: effects of catechin and caffeine. Physiol Behav 96: 262–269, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Unno T, Tago M, Suzuki Y, Nozawa A, Sagesaka YM, Kakuda T, Egawa K, Kondo K. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br J Nutr 93: 543–547, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 3: 279–288, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277: 34933–34940, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Luo W, Wang J, Guo C, Wang X, Wolffe SL, Bodary PF, Eitzman DT. Obesity-induced endothelial dysfunction is prevented by deficiency of P-selectin glycoprotein ligand-1. Diabetes 61: 3219–3227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Siren J, Hamsten A, Fisher RM, Yki-Jarvinen H. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes 56: 2759–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr 26: 373S–388S, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr 43: 116–124, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr 128: 2334–2340, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Yasui K, Tanabe H, Miyoshi N, Suzuki T, Goto S, Taguchi K, Ishigami Y, Paeng N, Fukutomi R, Imai S, Isemura M. Effects of (−)-epigallocatechin-3-O-gallate on expression of gluconeogenesis-related genes in the mouse duodenum. Biomed Res 32: 313–320, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]