Abstract
Infections with enteric pathogens like enterotoxigenic Escherichia coli (ETEC) is a major health issue worldwide and while diarrhea is the major problem, prolonged, severe, and dual infections with multiple pathogens may also compromise the nutritional status of the infected individuals. There is almost nothing currently known about the effect of ETEC infection on intestinal absorptions of water-soluble vitamins including thiamin. We examined the effect of ETEC infection on intestinal uptake of the thiamin using as a model the human-derived intestinal epithelial Caco-2 cells. The results showed that infecting confluent Caco-2 monolayers with live ETEC (but not with boiled/killed ETEC or nonpathogenic E. coli) or treatment with bacterial culture supernatant led to a significant inhibition in thiamin uptake. This inhibition appears to be caused by a heat-labile and -secreted ETEC component and is mediated via activation of the epithelial adenylate cyclase system. The inhibition in thiamin uptake by ETEC was associated with a significant reduction in expression of human thiamin transporter-1 and -2 (hTHTR1 and hTHTR2) at the protein and mRNA levels as well as in the activity of the SLC19A2 and SLC19A3 promoters. Dual infection of Caco-2 cells with ETEC and EPEC (enteropathogenic E. coli) led to compounded inhibition in intestinal thiamin uptake. These results show for the first time that infection of human intestinal epithelial cells with ETEC causes a significant inhibition in intestinal thiamin uptake. This inhibition is mediated by a secreted heat-labile toxin and is associated with a decrease in the expression of intestinal thiamin transporters.
Keywords: hTHTR1, hTHTR2, transport, vitamin B1
thiamin (vitamin b1), a member of the water-soluble family of micronutrients, is essential for normal human health and well-being. The vitamin acts as a cofactor in a variety of cellular metabolic reactions related to energy metabolism and reduction of cellular oxidative stress and in maintaining mitochondrial health and function (3, 30). Thus it is not surprising that deficiency of this micronutrient is associated with negative consequences at the cellular (e.g., impairment in energy metabolism, oxidative stress, mitochondrial dysfunction, apoptosis; Refs. 4, 6) and systemic (neurological and cardiovascular disorders; Refs. 3, 31) levels.
Humans cannot synthesize thiamin endogenously and must obtain the vitamin from exogenous sources via intestinal absorption. The gut, therefore, plays an important role in regulating and maintaining overall thiamin body homeostasis. Previous studies from our laboratory and others have characterized many physiological/biological aspects of the human intestinal thiamin uptake process (10, 25, 27, 28, 29). These studies have established that the intestinal thiamin uptake process occurs via a specific carrier-mediated mechanism that involves both of the human thiamin transporter-1 and -2 (hTHTR-1 and hTHTR-2; products of the SLC19A2 and SLC19A3 genes, respectively). Expression of the hTHTR-1 protein has been shown to be at both the apical and basolateral membrane domains of the absorptive epithelia (28), while that of the hTHTR-2 protein is restricted to the apical membrane domain of these polarized cells (29).
The enterotoxigenic Escherichia coli (ETEC) represents a heterogeneous group of pathogenic bacteria that colonize the human small intestine (reviewed in Ref. 15) and is a major cause of diarrhea, especially in developing countries where over 400 million cases (mostly in children) occur annually (35). In addition to causing acute secretory diarrhea, repeated bouts of infection with ETEC and dual infection with other enteric pathogens affect the nutritional status of the infected children and delay their growth (12, 13, 16, 17, 18, 19, 23). In developed countries, infection with ETEC is also a major cause of traveler's diarrhea including military personal (8, 22).
Following their colonization of the small gut, ETEC strains produce and translocate the plasmid-encoded heat-labile (LT) and/or heat-stable (ST) toxins. The LT and ST toxins are responsible for causing the loss of electrolyte and fluid into the gut lumen and utilize different signaling mechanisms. The LT activates the enzyme adenylate cyclase, which leads to an increase in intracellular cAMP and activation of PKA, which then activates the cystic fibrosis transmembrane regulator (CFTR) chloride channel. The ST activates guanylyl cyclase C (GC-C) leading to an increase intracellular accumulation of cGMP, activation of cGMP-dependent protein kinase II, which then activates CFTR and also causes an inhibition in NaCl absorption (reviewed in Refs. 15 and 21).
The diarrheal disease caused by ETEC begins with a sudden onset of watery stool and may also be associated with vomiting, which exacerbate dehydration. While most ETEC cases are usually self-limited and last 3–4 days, some of these cases continue for a significantly longer period (5, 36). Repeated bouts of infection with ETEC and dual infection with other pathogenic bacteria can occur and may predispose infected children to malnutrition (16, 17, 18, 23). What effect ETEC has on intestinal absorption of water-soluble vitamins like thiamin is not known. Since thiamin plays an important role in maintaining normal health, growth, and development, and since the human body has a limited capability to store sufficient amounts of this vitamin, a prolonged and repeated infection with ETEC and dual infection with other pathogens may negatively impact the overall nutritional status of thiamin, leading to further aggravation of the health status of the infected children (many of whom are already nutritionally compromised). For this reason, we undertook the current investigations and have examined the effect of ETEC infection on intestinal thiamin uptake. We used human-derived intestinal epithelial Caco-2 cells as model, since previous investigations have shown the suitability of this cellular system in similar investigations (2, 14). The results showed that infection of intestinal epithelial cells with ETEC leads to a significant inhibition in thiamin uptake and that such an inhibition is mediated by alteration in physiological and molecular parameters of the involved hTHTR-1 and -2. Furthermore, the inhibition appears to be mediated by the LT toxin of ETEC.
MATERIALS AND METHODS
Materials
[3H]thiamin (specific activity 19 Ci/mmol; radiochemical purity >97%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). All other cell culture reagents and other chemicals used in this study were of molecular biology grade and were obtained from commercial sources.
Methods
Cell culture.
The human-derived intestinal epithelial Caco-2 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA) and were grown in MEM containing 4.5 g/l glucose, 10% FBS, and streptomycin (10 mg/l) under standard conditions at 37°C. Confluent Caco-2 monolayers (at 5–6 days postconfluence) were used for examining the effect of ETEC on thiamin uptake. Cells were maintained overnight in antibiotic-free MEM media supplemented with 0.5% FBS before bacterial infection.
Bacterial culture and infection of Caco-2 cells.
The ETEC strains used in this study were the wild-type ETEC strain H10407 (ATCC), Δ eltA H10407 (jf570; a kind gift from Dr. J. M Fleckenstein of the University of Washington University, St. Louis, MO; Ref. 9), and the nonpathogenic isolate HS4. Overnight bacterial cultures, grown in Luria-Bertani broth, were harvested by centrifugation. The bacterial pellet was then washed once with PBS and diluted into MEM media (without any serum or antibiotics) to the desired multiplicity of infection (MOI). Confluent Caco-2 monolayers, maintained overnight under serum deprived condition, were infected with ETEC at a MOI of 200 for 3 h (unless otherwise stated). Following infection, cell monolayers were treated with freshly prepared gentamicin solution (50 μg/ml) for 30 min to remove adherent ETEC from the cell surface. After the gentamicin treatment, cells were washed once with PBS and incubated for 6 h (unless otherwise stated) at 37°C in a 5% CO2 water-jacketed incubator. The cells were used for further studies. To examine whether dual infection of ETEC with enteropathogenic EPEC will lead to an additive inhibition in thiamin uptake, we infected confluent Caco-2 monolayers simultaneously with both bacteria (at 50 MOI for both) for 2 h. Following gentamycin treatment for 30 min, Caco-2 cells were incubated for an additional 6 h then used for uptake study.
Bacterial supernatants.
The overnight culture supernatant was collected by centrifuging the bacterial culture and then sterilized by passing through a 0.22-μm sterile syringe filter. To examine the effect of the heat treatment, the same culture supernatant was boiled in a water bath (15 min) and used for incubation.
Thiamin uptake.
Thiamin uptake by confluent Caco-2 monolayers treated with ETEC was performed as described by us previously (2). In brief, cells were incubated with Krebs-Ringer (K-R) buffer (pH 7.4) at 37°C for 7 min (initial rate of uptake; Ref. 25). [3H]thiamin was added to the incubation medium at the onset of incubation, and the uptake reaction was terminated by the addition of 2 ml of ice-cold K-R buffer followed by immediate aspiration. Cells were then rinsed twice with ice-cold buffer, lysed with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then analyzed for radioactivity in a liquid scintillation counter. Total protein content of Caco-2 cell digests was then measured using a Bio-Rad kit (Bio-Rad, Richmond, VA) following manufacturer's protocol.
Real-time PCR analysis.
Total RNA was isolated from confluent Caco-2 cells; those treated with ETEC and those treated with nonpathogenic E. coli using TRIzol (Life Technologies). To avoid amplification from contaminating DNA the total RNA was treated with DNase and transcribed to cDNA using i-script kit (Bio-Rad). The cDNA thus prepared was used for quantitative real-time PCR using gene specific primers in a CFX96 real-time i-cycler (Bio-Rad) to determine relative expression. For the SLC19A2, the primers used were forward, 5′-GCCAGACCGTCTCCTTGTA-3′; and reverse, 5′-TAGAGAGGGCCCACCACAC-3′. For the SLC19A3, the primers used were forward, 5′-TTCCTGGATTTACCCCACTG-3′; and reverse, 5′-GTATGTCCAAACGGGGAAGA-3′. For β-actin the primers were forward, 5′-CATCCTGCGTCTGGACCT-3′; and reverse, 5′- TAATGTCACGCACGATTTCC-3′, and relative expression was quantified by normalizing Ct values with corresponding β-actin.
Western blot analysis.
For Western blot analysis, cells were disrupted using RIPA buffer (Sigma) containing complete protease inhibitor cocktail (Roche, Nutley, NJ). The soluble fraction was isolated by centrifugation at 8,000 g for 5 min, and total protein content was measured using a Bio-Rad kit. Equal amounts of protein were loaded in a 10% mini gel (Invitrogen) for Western blot analysis. The protein was transferred to PVDF membrane, blocked overnight, and probed simultaneously either with anti-hTHTR-1 (cat. no: SC27656; Santa Cruz Biotechnology, Santa Cruz, CA) or hTHTR-2 (cat. no: AB103950; Abcam, Cambridge, MA) antibodies (raised in rabbit) and mouse monoclonal β-actin antibody (raised in mouse; cat. no: SC-47778; Santa Cruz Biotechnology). The blot was then incubated with anti-rabbit IR 800 dye and anti-mouse IR 680 dye (LI-COR Biosciences, Lincoln, NE) secondary antibodies (1:25,000) for 1 h at room temperature. Relative expression was quantified by comparing the fluorescent intensities in an Odyssey Infrared imaging system (LI-COR) using Odyssey application software (version 3.0) with respect to corresponding β-actin.
Promoter activity: transfection and reporter gene assay.
To study the promoter activity, full-length SLC19A2 and SLC19A3 promoter-luciferase reporter constructs, generated previously in our laboratory (20, 24) were used. For transfection intestinal epithelial Caco-2 cells were cotransfected with test construct (3 μg of each) and the control plasmid Renilla luciferase-thymidine kinase (pRL-TK; 100 ng; Promega, Madison, WI). Following 24 h of transfection, cells were infected with ETEC as described before. Following infection cells were washed with PBS, lysed with passive lysis buffer and luciferase activity was measured by the Dual Luciferase Assay system (Promega).
Statistical analysis.
All uptake data presented in the study are means ± SE of multiple separate determinations and are expressed in terms of femtomoles per milligram protein per 7 min. Thiamin uptake by the carrier-mediated process was determine by subtracting uptake of a physiological concentration of [3H]thiamin in the presence of a high pharmacological concentration of unlabeled thiamin (1 mM) from uptake in its absence. Statistical significance was determined from Student's t-test or one-way ANOVA with statistical significance set at <0.05. All results presented here are means ± SE of at least three experiments. Promoter activity data (in arbitrary units) are expressed as fold over pGL3-Basic.
RESULTS
Effect of ETEC infection on thiamin uptake by human intestinal epithelia Caco-2 cells.
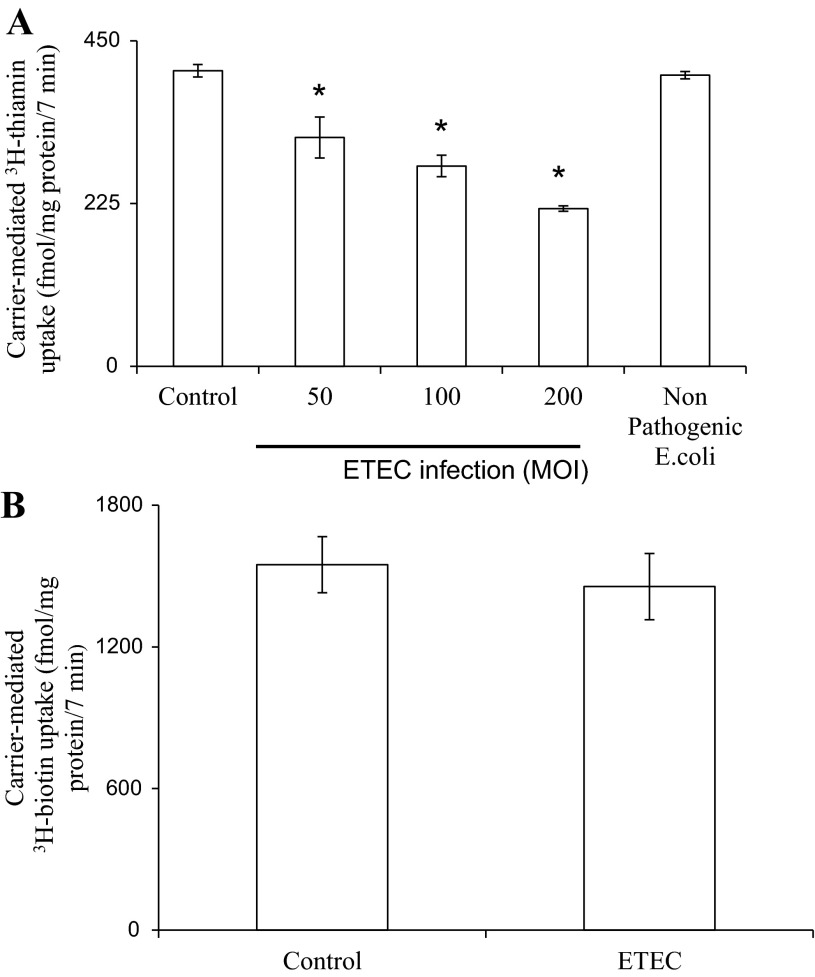
We examined the effect of infecting confluent Caco-2 monolayers for 3 h with different MOI of ETEC (50, 100, and 200 MOI) or with a nonpathogenic E. coli (200 MOI) on the initial rate (7 min; as mentioned before; Ref. 25) of thiamin (15 nM) uptake. Uptake studies were performed 6 h posttreatment with ETEC as described in Methods. The results showed that ETEC inhibit thiamin uptake and that the inhibition is bacterial load dependent; ∼40% inhibition was observed with 200 MOI (Fig. 1A). The nonpathogenic E. coli, on the other hand, did not affect thiamin uptake by Caco-2 cells. We also examined the effect of ETEC infection (200 MOI) on uptake of the unrelated water-soluble vitamin B7 (biotin) by confluent Caco-2 monolayers but found no effect. The latter finding suggests that ETEC effect on thiamin uptake by intestinal epithelial cells is not a generalized phenomenon (Fig. 1B).
Fig. 1.
Enterotoxigenic Escherichia coli (ETEC) infection inhibits thiamin uptake by confluent Caco-2 monolayers. A: Caco-2 cells at 3–4 days after confluency were serum starved (overnight) then infected with wild-type ETEC at different multiplicity of infection (MOI; 50, 100, and 200) or with nonpathogenic E. coli (HS4) at MOI of 200 for 3 h. Uptake of thiamin was subsequently measured as described in Methods. *P < 0.01, compared with control. B: effect of ETEC (at 200 MOI, 3 h) on [3H]biotin uptake by Caco-2 cells.
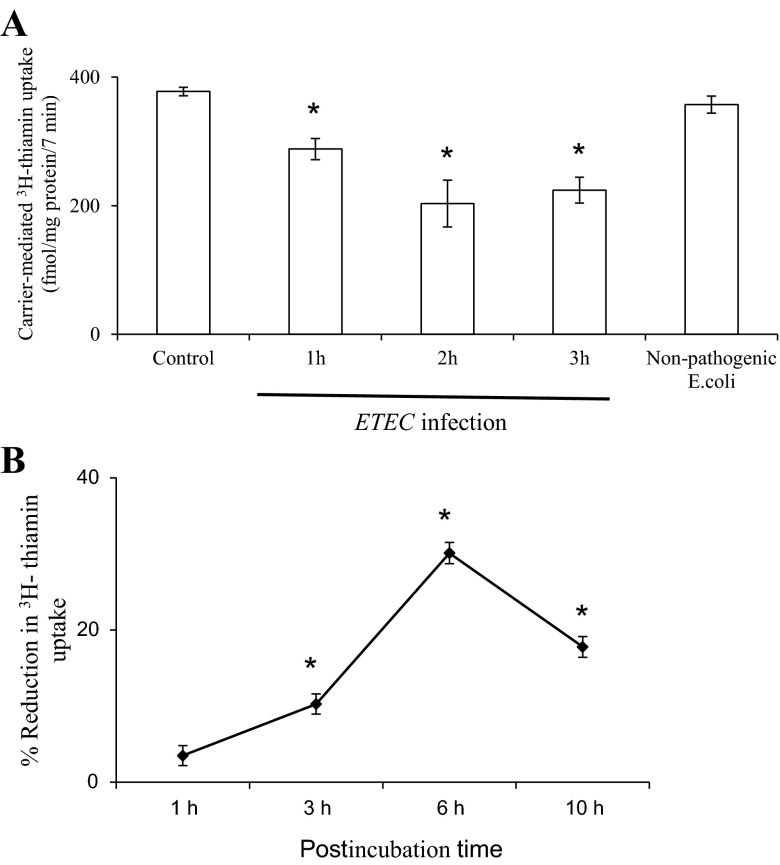
In another study, we examined the effect of exposure time (1, 2, and 3 h) to ETEC (200 MOI) on initial rate of thiamin uptake by confluent Caco-2 monolayers. The results (Fig. 2A) showed an increase in the degree of inhibition in thiamin uptake with increasing treatment time from 1 to 2 h; no further increase in the degree of inhibition in thiamin uptake was observed upon further increase in the exposure time (Fig. 2A). We also investigated the effect of different postinfection times (1, 3, 6, and 10 h) of confluent Caco-2 monolayers following treatment with ETEC (200 MOI for 3 h) on initial rate of thiamin uptake. The results showed a progressive increase in the degree of inhibition for up to 6 h posttreatment, but the degree of inhibition decreased thereafter (Fig. 2B).
Fig. 2.
A: time course of ETEC effect on thiamin uptake by confluent Caco-2 monolayers. Caco-2 cells were infected with ETEC for 1, 2, and 3 h, followed by postinfection incubation of 6 h. *P < 0.01, compared with control. B: effects of postinfection time on thiamin uptake by Caco-2 cells. Caco-2 monolayers were infected with ETEC for 3 h and postincubated for 1, 3, 6, and 10 h. Thiamin uptake was measured as described in Methods. Values are means ± SE of 3 separate uptake determinations. *P < 0.01, compared with control.
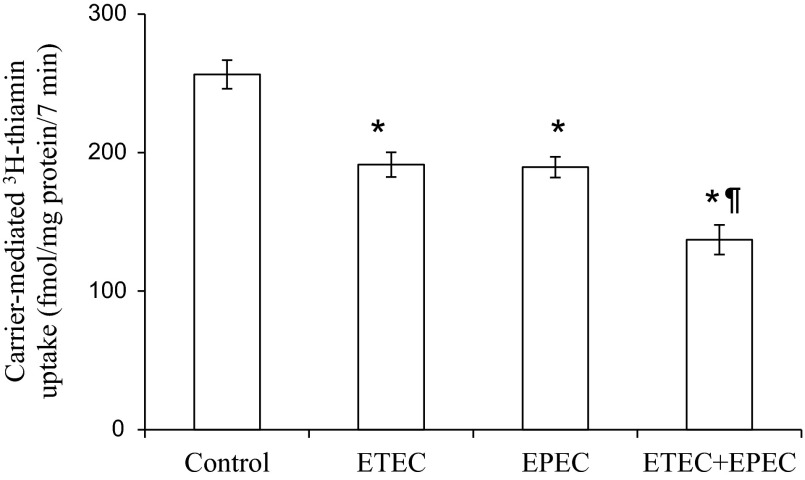
As mentioned earlier, dual infection of ETEC with other pathogens like EPEC is not uncommon (1, 11, 32, 34) and it is believed that such a dual infection could increase the virulence of the pathogens (7). Thus we also examined the effect of dual infection of confluent monolayers of Caco-2 cells with ETEC and EPEC (both at 50 MOI) on carrier-mediated thiamin uptake. Infection with both pathogens was done simultaneously for 2 h, and thiamin uptake was measured 6 h postinfection. The results showed a compounded inhibition in thiamin uptake over what was seen with the single pathogen (Fig. 3).
Fig. 3.
Effect of dual infection of confluent Caco-2 monolayers with ETEC and EPEC on thiamin uptake. Caco-2 monolayers were infected for 2 h with wild-type ETEC and EPEC (both at MOI of 50) together or separately followed by postincubation for 6 h as mentioned earlier. Initial rate of thiamin uptake was then measured as described in Methods. *P < 0.01, relative to control and *¶P < 0.01, relative to infection by individual bacteria alone.
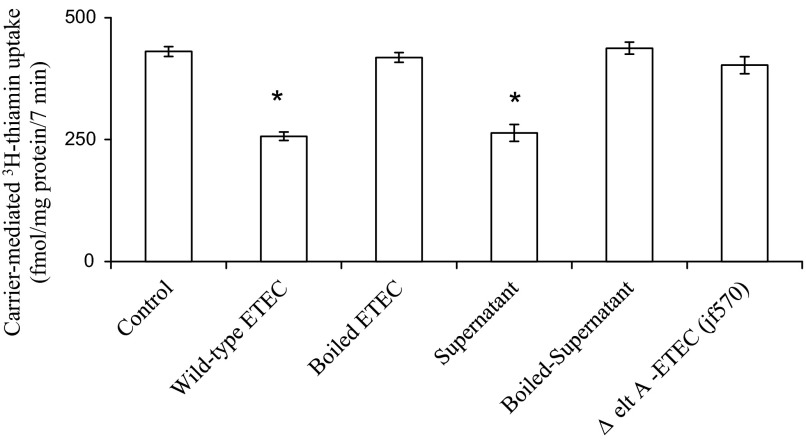
Direct contact of live ETEC is not necessary for the inhibition in thiamin uptake by Caco-2 cells.
In these studies, we examined and compared the effect of treating confluent Caco-2 monolayers with live ETEC, heat-killed (boiled) ETEC, or ETEC culture supernatant for 3 h on initial rate of thiamin (15 nM) uptake. The results showed that while live bacteria and culture supernatant inhibit thiamin uptake, boiled bacteria was without an effect (Fig. 4). We also examined the effect of boiled ETEC culture supernatant as well as that of an ETEC mutant that lacks the ability to produce LT toxins (jf570; Ref. 9) on thiamin uptake by confluent Caco-2 monolayers. In both cases the results showed a complete disappearance of the inhibitory effect of ETEC on thiamin uptake (Fig. 4). The above results indicate that a LT-secreted component is the cause of ETEC-induced inhibition in thiamin uptake by intestinal epithelial cells.
Fig. 4.
Direct contact of live ETEC is not necessary for the inhibition of thiamin uptake by Caco-2 cells. Confluent monolayers of Caco-2 were treated (for 3 h) with wild-type ETEC, boiled (killed) ETEC, culture supernatant of ETEC, or mutant (Δ eltA; jf570) ETEC followed by measurement of the initial rate of carrier-mediated thiamin uptake. Results represent means ± SE of at least 3 separate experiments. *P < 0.01, compared with control.
Effect of ETEC infection on expression of hTHTR-1 and hTHTR-2.
A recent study from our laboratory has established that both hTHTR-1 and hTHTR-2 are involved in the thiamin uptake by intestinal epithelial cells (25). Thus we examined the effect of ETEC infection of confluent Caco-2 monolayers on expression of hTHTR-1 and hTHTR-2 at the protein and mRNA levels. The former was performed by means of Western blot analysis (using total proteins extracted from ETEC-infected and -coinfected Caco-2 cells and specific anti-hTHTR-1 and anti-hTHTR-2 polyclonal antibodies), while the latter was done by mean of quantitative PCR (using total RNA samples). The results showed a significant (P < 0.05 for both) decrease in the level of expression of the hTHTR-1 and hTHTR-2 proteins (Fig. 5, A and B, respectively) and mRNA in cells infected with ETEC compared with control cells and those that were treated with nonpathogenic E. coli (Fig. 5, C and D, respectively). Changes in mRNA level could occur via different mechanisms, one of which is changes in transcription rate of the particular gene. To test this possibility, we examined the effect of ETEC infection on the activity of the full-length SLC19A2 and SLC19A3 promoters (fused to the luciferase reporter gene) transfected into confluent Caco-2 monolayers (see Methods). The results showed significantly (P < 0.05 for both) lower activity for both the SLC19A2 and SLC19A3 promoters in ETEC-infected Caco-2 cells compared with control (untreated) cells (Fig. 5, E and F, respectively).
Fig. 5.
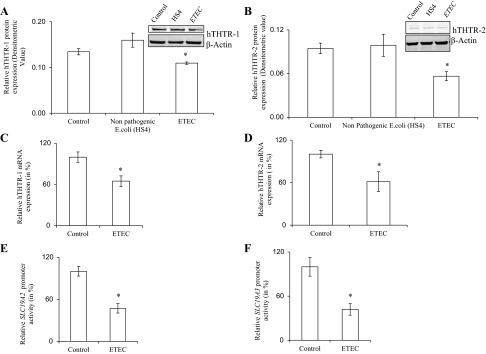
Effect of ETEC infection on expression of human thiamin transporter-1 and -2 (hTHTR-1 and -2) at the protein (A and B), mRNA (C and D), and promoter (E and F) levels. Confluent monolayers of Caco-2 cells were infected with ETEC (200 MOI for 3 h along with 6 h postincubation) and total protein was isolated by treating with RIPA buffer. Western blot analysis was performed using specific anti-hTHTR-1 (A) and anti-hTHTR-2 (B) polyclonal antibodies. Representative gel image is shown. *P < 0.05, compared with control. Quantitative real-time PCR analysis was performed using total RNA from Caco-2 cells treated with ETEC or nonpathogenic E. coli and hTHTR-1 (C)- and hTHTR-2 (D)-specific primers as described in Methods. *P < 0.05, compared with control. Effect of ETEC infection on activity of the SLC19A2 (E) and SLC19A3 (F) promoters. Caco-2 cells were transfected with SLC19A2 and SLC19A3-luciferase reporter plasmid constructs or a control pGL3-basic vector by use of Lipofectamine 2000. Cells were then infected (3 h) with ETEC or nonpathogenic E. coli (200 MOI) followed 6 h postinfection incubation. Luciferase assay was performed as described in Methods. Firefly luciferase activity was normalized relative to the activity of simultaneously expressed Renilla luciferase. All experiments were run on at least 3 separate occasions. Results of a representative experiment are shown. Values are means ± SE. *P < 0.05, compared with control.
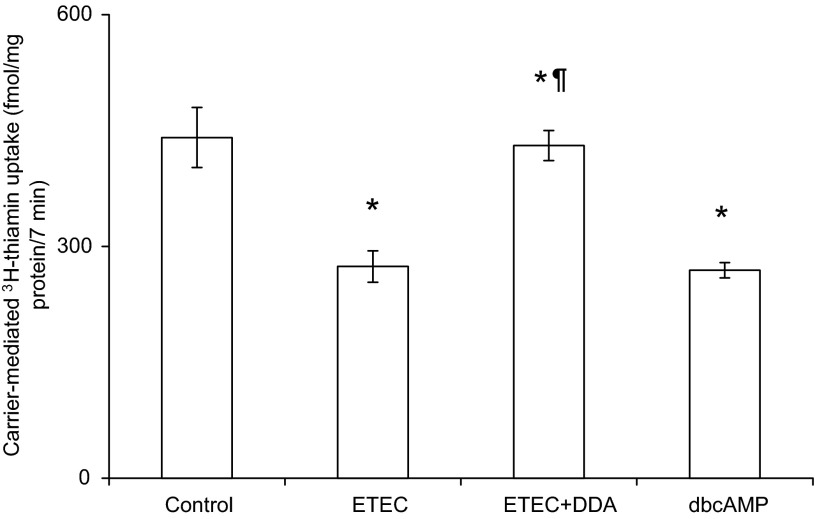
Role of adenylate cyclase in mediating the inhibitory effect of ETEC on intestinal thiamin uptake.
As mentioned earlier, LT activates adenylate cyclase leading to an increase in intracellular cAMP level. Since LT appears to be involved in mediating the inhibitory effect of ETEC on thiamin uptake by Caco-2 cells, we examined the effect of treating ETEC-infected cells with a cell-permeable inhibitor of adenylate cyclase, 2′,5′-dideoxyadenosine, on the initial rate of thiamin uptake. The results indeed showed a complete prevention of the inhibitory effect of ETEC in thiamin uptake (Fig. 6). To confirm that the process itself is orchestrated by cAMP, we tested the effect of treating confluent Caco-2 cells monolayers with dibutyryl-cAMP (1 mM) under identical conditions to those used in ETEC treatment (i.e., for a total of 10-h incubation with dibutyryl-cAMP) on initial rate of thiamin uptake and indeed observed a significant (P < 0.01) inhibition (Fig. 6). These findings suggest that the inhibition in thiamin uptake by ETEC is mediated via induction in activity of adenyl cyclase and the subsequent increase in intracellular cAMP level.
Fig. 6.
Effect of inhibiting adenylate cyclase activity of Caco-2 cells on the inhibitory effect of ETEC on thiamine uptake. Confluent Caco-2 cells monolayers were infected with ETEC as in Fig. 4 in the presence and absence of the adenylate cyclase inhibitor [2′,5′-dideoxyadenosine (DDA); 100 μM] or with dibutyryl-cAMP (dbcAMP; 1mM) in absence of ETEC. Data are means ± SE of at least 3 different experiments. *P < 0.01, relative to control and *¶P < 0.01, relative to ETEC infection in absence of adenylate cyclase inhibitor.
DISCUSSION
Our aim in this study was to examine the effect of infection of human intestinal epithelial cells with ETEC on the ability of these cells to take up thiamin and to delineate the mechanism involved in the observed effect. We used the human-derived intestinal epithelial Caco-2 cells as model for these investigations. As mentioned earlier, pathogenic E. coli is classified (based on their pathogenesis) into different variants with ETEC and EPEC representing common pathogens infecting humans, especially infants and children, in the developing world (reviewed in Ref. 21). For ETEC for example, over 400 million episodes are reported annually in children worldwide (reviewed in Ref. 21), with significant mortality among children of <5 yr of age (estimated at 380,000 deaths per year; Ref. 33). While malnourishment (which is caused mainly by the low socioeconomic status of the affected children and the multiple diarrhea episodes) prolongs enteric infection and increases its severity (12, 13, 16, 17, 19), the reverse could also be true in that prolonged infection with these pathogens could negatively impact the nutritional status of the infected subjects (12, 13, 16, 17, 18, 19).
Our results in this study showed that ETEC, but not the nonpathogenic E. coli (HS4), causes a significant and MOI-dependent inhibition in carrier-mediated uptake of physiological concentration of thiamin. This inhibition did not appear to be generalized in nature, as uptake of the unrelated vitamin biotin was not affected by ETEC infection. Also, the degree of ETEC inhibition in thiamin uptake was found to increase as a function of increasing the exposure time of Caco-2 cells to ETEC and as a function of time postinfection (after removal of the bacteria). The latter demonstrates the persistent nature of the effect postexposure to the bacteria. The decrease in the inhibition in thiamin uptake after 10-h postinfection is most probably due to attenuation of the signal that cause the effect.
The inhibitory effect of ETEC on intestinal thiamin uptake did not appear to require direct contact of live bacteria with the epithelial cells, as indicated by the similar degree of inhibition in the vitamin uptake by Caco-2 cells treated with live bacteria and those treated with filtered bacterial culture supernatant. The latter finding also suggests that the factor that causes the inhibition in thiamin uptake is a secreted factor. Moreover, our data show that the secreted factor is LT as indicated by the findings that boiling the supernatant (or the bacteria) leads to disappearance of the inhibitory effect on thiamin uptake. This was further confirmed by the findings that the mutant ETEC, which is defective in producing LT toxin (9), did not affect thiamin uptake.
Previous studies have shown that dual infection of humans with ETEC and EPEC is not uncommon (1, 11, 32, 34) and that such a dual infection leads to an enhancement in the virulence of these bacteria (7). For this reason we also examined the effect of the dual infection of intestinal Caco-2 cells with ETEC and EPEC on thiamin uptake. The results showed that the inhibition is additive and, thus, more severe during coinfection compared with infection by one bacteria strain alone.
The ETEC-inducted inhibition in thiamin uptake by Caco-2 cells was associated with a significant reduction in the level of expression of the hTHTR-1 and -2 at the protein and mRNA levels. The latter finding of changes in message level of the thiamin transporters raised the possibility that the effect may, at least in part, be mediated at the level of transcription of the SLC19A2 and SLC19A3 genes. To test this possibility we examined the effect of ETEC infection on the activity of SLC19A2 and SLC19A3 promoters (transfected into Caco-2 cells) and indeed observed a significant reduction in the activity of these promoters as a result of infection of Caco-2 cells with ETEC. The inhibition in the transcription of the two thiamin uptake systems also suggests that the ETEC inhibitory effect also has a prolonged phase following the encounter of the absorptive cells with the organisms.
ETEC produces both LT and ST toxins and our data in the present study showed that it is the LT component that is responsible for the inhibitory effect of ETEC on intestinal thiamin uptake. Since the ETEC LT toxin is known to exert its effect through activation of adenylate cyclase (reviewed in Ref. 21), we examined the effect of inhibiting the adenylate cyclase system on the ability of ETEC to inhibit thiamin uptake. Such treatment has indeed shown complete prevention of the inhibitory effect of ETEC on thiamin uptake. We also observed that prolonged treatment of Caco-2 cells with dibutyryl-cAMP inhibits intestinal thiamin uptake. Thus it is reasonable to conclude that the ETEC inhibitory effect on intestinal thiamin uptake is mediated via an effect on the intestinal epithelial adenylate cyclase system and intracellular cAMP level.
In summary, our studies demonstrate for the first time that ETEC infection of human intestinal epithelial cells leads to a significant inhibition in thiamin uptake and that the inhibitory effect is mediated by a secreted ETEC LT toxin. Furthermore, this inhibition is associated with a decrease in the expression of hTHTR-1 and hTHTR-2 and in the activity of the SLC19A2 and SLC19A3 promoters. These data, when considered with the flushing effect of the ETEC-induced diarrhea of the “good” large intestinal micro biota (which generate considerable amount of thiamin that is absorbed in the colon; Ref. 26), demonstrate the negative effect of such an infection on the overall bioavailability of the essential micronutrient thiamin to the host.
GRANTS
Supported was provided by National Institutes of Health and the Department of Veterans Affairs Grants DK-56061, DK-58057, and AA-18071. N. S Chatterjee was supported by Fulbright Nehru Senior Research Fellowship 2011–12 (No: 1542/FN-SR/2011).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.G., N.S.C., and H.M.S. conception and design of research; A.G., N.S.C., and T.C. performed experiments; A.G., N.S.C., T.C., and H.M.S. analyzed data; A.G., N.S.C., and H.M.S. interpreted results of experiments; A.G. and T.C. prepared figures; A.G., T.C., and H.M.S. drafted manuscript; A.G., N.S.C., T.C., and H.M.S. edited and revised manuscript; A.G., N.S.C., T.C., and H.M.S. approved final version of manuscript.
REFERENCES
- 1. Adhikari M, Coovadi Y, Hewitt J. Enteropathogenic Escherichia coli (EPEC) and enterotoxigenic (ETEC) related diarrhoeal disease in a neonatal unit. Ann Trop Paediatr 5: 19–22, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Balasubramaniem A, Kumar J, Gail AH, Said HS. Enteropathogenic Escherichia coli inhibits intestinal vitamin B1 (thiamin) uptake: studies with human-derived intestinal epithelial Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 297: G825–G833, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berdanier CD. Advanced Nutrition-Micronutrients. New York: CRC, 1998, p. 183–219 [Google Scholar]
- 4. Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bouckenooghe AR, Jiang ZD, de la Cabada FJ, Ericsson CD, Dupont HL. Enterotoxigenic Escherichia coli as cause of diarrhea among Mexican adults and US travelers in Mexico. J Travel Med 9: 137–40, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58: 946–958, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Crane JK, Choudhari SS, Naeher TM, Duffey ME. Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli. Infect Immun 74: 1505–1515, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalton CB, Mintz ED, Wells JG, Bopp CA, Tauxe RV. Outbreaks of enterotoxigenic Escherichia coli infection in American adults: a clinical and epidemiologic profile. Epidemiol Infect 123: 9–16, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorsey FC, Fischer JF, Fleckenstein JM. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell Microbiol 8: 1516–1527, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamin uptake by human jejunal brush-border membrane vesicles. Am J Physiol Cell Physiol 281: C786–C792, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Eshel G, More A, Gluskin E, Karpuch J, Azizi E, Mundel G, Aladeim M. Acute gastoenteritis due to double infection with enteropathogenic Escherichia coli or Salmonella and another pathogen. Isr J Med Sci 26: 316–318, 1990 [PubMed] [Google Scholar]
- 12. Fagundes U, Andrade J. Acute diarrhea and malnutrition: lethality risk in hospitalized infants. J Am Coll Nutr 18: 303–308, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Fagundes U, Scaletsky IC. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. Sao Paulo Med J 118: 21–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleckenstein JM, Roy K, Fischer JF, Burkitt M. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect Immun 74: 2245–2258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect 12: 89–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg 47: 28–35, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66: 487–505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mata L. Diarrheal disease as a cause of malnutrition. Am J Trop Med Hyg 47: 16–27, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA., Jr Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 54: 185–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Nataro J, Kaper J. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11: 142–201, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18: 465–483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 75: 3961–3968, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta 1561: 180–187, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Said HM, Ortiz A, Kumar C, Chatterjee N, Dudeja P, Rubin S. Transport of thiamine in the human intestine: mechanism and regulation in an intestinal epithelial cell model Caco-2. Am J Physiol Cell Physiol 277: C645–C651, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am J Physiol Gastrointest Liver Physiol 281: G144–G150, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol 286: G491–G498, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamin transporter-1 (hTHTR1): intracellular trafficking and membrane targeting. J Biol Chem 278: 3976–3984, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamin transporter-2 (hTHTR2) in epithelial cells. J Biol Chem 281: 5233–5245, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Tanphaichitr V. Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M. New York: Lea and Febiger, 1994, p. 359–375 [Google Scholar]
- 31. Todd K, Butterworth RF. Mechanisms of selective neuronal cell death due to thiamine deficiency. Ann NY Acad Sci 893: 404–411, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Wada Y, Nakaoka Y, Kondo H, Nakazawa M, Jubo M. Dual infection with attaching and effacing Escherichia coli and enterotoxigenic Escherichia coli in post-weaning pigs. J Comp Pathol 114: 93–99, 1996 [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization Strategic Plan 1998–2001. Global Programme for Vaccines and Immunization, WHO/GPV/98.04. Geneva: World Health Organization, 1998, p.70–71 [Google Scholar]
- 34.World Health Organization New frontiers in the development of vaccines against enterotoxigenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Wkly Epidemiol Rec 74: 98–101, 1999 [PubMed] [Google Scholar]
- 35.World Health Organization Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec 81: 97–104, 2006 [PubMed] [Google Scholar]
- 36. Yoder JS, Cesario S, Plotkin V, Ma X, Kelly-Shannon K, Dworkin MS. Outbreak of enterotoxigenic Escherichia coli infection with an unusually long duration of illness. Clin Infect Dis 42: 1513–1517, 2006 [DOI] [PubMed] [Google Scholar]