Abstract
In older adults, changes in skeletal muscle composition are associated with increased fibrosis, loss of mass, and decreased force, which can lead to dependency, morbidity, and mortality. Understanding the biological mechanisms responsible is essential to sustaining and improving their quality of life. Compared with young mice, aged mice take longer to recover from muscle injury; their tissue fibrosis is more extensive, and regenerated myofibers are smaller. Strong evidence indicates that cells called pericytes, embedded in the basement membrane of capillaries, contribute to the satellite-cell pool and muscle growth. In addition to their role in skeletal muscle repair, after tissue damage, they detach from capillaries and migrate to the interstitial space to participate in fibrosis formation. Here we distinguish two bona fide pericyte subtypes in the skeletal muscle interstitium, type-1 (Nestin-GFP−/NG2-DsRed+) and type-2 (Nestin-GFP+/NG2-DsRed+), and characterize their heretofore unknown specific roles in the aging environment. Our in vitro results show that type-1 and type-2 pericytes are either fibrogenic or myogenic, respectively. Transplantation studies in young animals indicate that type-2 pericytes are myogenic, while type-1 pericytes remain in the interstitial space. In older mice, however, the muscular regenerative capacity of type-2 pericytes is limited, and type-1 pericytes produce collagen, contributing to fibrous tissue deposition. We conclude that in injured muscles from aging mice, the pericytes involved in skeletal muscle repair differ from those associated with scar formation.
Keywords: pericytes, skeletal muscle, fibrous tissue, aging
most chronic diseases are characterized by excessive fibrous tissue deposition, altered tissue architecture, and organ dysfunction (49, 76). Typically, fibrosis increases in all organs with aging (27, 43). Following skeletal muscle injury, stem cells proliferate, differentiate, and fuse into myoblasts with injured myofibers during the repair phase (39). Connective tissue accumulation in the skeletal muscle has been reported when the injury involves the basal lamina (31). While the connective tissue contributes to cytoarchitecture stability during the healing process, excessive fibrogenic cells proliferation and fibrous tissue deposition may interfere with muscle regeneration, leading to incomplete functional recovery (69). Compared with young, aged mice take longer to recover from muscle injury; their regenerated myofibers are smaller, and tissue fibrosis is significantly increased (13).
The biological process underlying fibrous tissue deposition with aging is incompletely understood. Various cell types have been proposed to produce fibrous tissue in several organs including skeletal muscle (29), resident fibroblasts (3), bone marrow-derived circulating fibrocytes (14), epithelial cells (52), endothelial cells (79), and more recently, pericytes (28). Besides their role in initiating a fibrogenic response to pathology in various organs, pericytes act as multipotent stem cells in tissue repair (23). In the skeletal muscle, they contribute to the satellite cell pool and muscle growth (26).
Based on markers and morphology, pericytes have been identified as a heterogeneous cell population (11). However, their diverse differentiation potential was not explored until we demonstrated their heterogeneity in the skeletal muscle (7, 10). Whether a specific pericyte subtype contributes to skeletal muscle fibrosis with aging is unknown.
Here, by using a Nestin-GFP/NG2-DsRed transgenic mouse, we show the presence of two bona fide pericyte subtypes, type-1 (Nestin-GFP−/NG2-DsRed+) and type-2 (Nestin-GFP+/NG2-DsRed+), in the skeletal muscle. These cells express the pericyte markers platelet-derived growth factor receptor-β (PDGFR-β) and CD146 and are associated with microvessels. Our in vitro studies show that type-2 but not type-1 form muscle cells when exposed to myogenic differentiation medium. Additionally, we found that type-1 but not type-2 pericytes are fibrogenic when exposed to transforming growth factor-β (TGF-β) in culture. Transplantation studies indicate that type-2 pericytes contribute only to new muscle formation after injury but not to fibrogenesis tissue in the old mouse. Of relevance is the fact that type-2 pericyte regenerative myogenic capacity varies depending whether they are injected in either young or old animals, indicating that the host environment is crucial for efficient muscle regeneration. Moreover, type-1 pericytes do not form muscle in vivo but contribute to increased muscle fibrous tissue in older mice.
We conclude that type-1 pericytes contribute to muscle fibrous tissue formation with aging and envision the possibility of using type-2 and type-1 pericyte as a cellular target to improve skeletal muscle repair and reduce fibrosis in older mammals, respectively.
MATERIALS AND METHODS
Animals.
Nestin-GFP transgenic mice (our colony) were maintained homozygous for the transgene on the C57BL/6 genetic background (56). Aging Friend Virus B (FVB) mice (our colony) have long been used as a model of aging skeletal muscle in our laboratory (9, 66); young (3–5 mo), middle-aged (11–14 mo), and old (22–26 mo) mice were used. NG2-DsRed transgenic mice expressing DsRed-T1 under the control of the NG2 promoter (10) were purchased from the Jackson Laboratory (Bar Harbor, ME). β-Actin-DsRed transgenic mice expressing red fluorescent protein variant DsRed.MST under the control of the chicken β-actin promoter coupled with the cytomegalovirus (CMV) immediate-early enhancer (9) were purchased from the Jackson Laboratory. All tissues of β-actin-DsRed transgenic mice fluoresce red. Nestin-GFP mice were crossbred with NG2-DsRed and β-actin-DsRed mice to generate, respectively, Nestin-GFP/NG2-DsRed and Nestin-GFP/β-actin-DsRed double-transgenic mice. All mice colonies were housed in a pathogen-free facility of the Animal Research Program at Wake Forest School of Medicine (WFSM) under 12:12-h light/dark cycle and fed ad libitum. Both male and female homozygous mice were used, and their ages ranged from 3 to 5 mo. The WFSM Animal Care and Use Committee approved handling and procedures.
Primary antibodies.
Table 1 shows the antibodies, their dilution, and source.
Table 1.
Antibodies, concentration, and source
| Antibody | Dilution | Source | Location |
|---|---|---|---|
| Rabbit anti-PDGFR-β | 1:250 | Dr. W. Stallcup | Sanford-Burnham Medical Research Institute, CA |
| Rat anti-mouse CD146 | 1:250 | BioLegend (cat. no. 134702) | San Diego, CA |
| Rat anti-CD31 (PECAM-1) | 1:100 | BD Biosciences (cat. no. 558736) | San Jose, CA |
| Rabbit anti-type I collagen | 1:1,000 | AbD Serotec (cat. no. 2150–1410) | Raleigh, NC, |
| Mouse anti-MHC (MF 20) | 1:2,000 | Developmental Studies Hybridoma Bank, University of Iowa | Iowa City, IA |
| Rabbit anti-NG2 Chondroitin sulfate proteoglycan | 1:100 | Chemicon-Millipore (cat. no. AB5320) | Temecula, CA |
| Mouse anti-Pax7 | 1:100 | Developmental Studies Hybridoma Bank, University of Iowa | Iowa City, IA |
| Mouse anti-FSP1 | 1:100 | Novus Biologicals (cat. no. H00006275-M01) | Littleton, CO |
| Mouse anti-fast myosin skeletal heavy chain | 1:100 | Abcam (cat. no. ab51263) | Cambridge, MA |
PDGFR-β, platelet-derived growth factor receptor-β; PECAM-1, platelet endothelial cell adhesion molecule-1; MHC, myosin heavy chain; FSP1, fibroblast-specific protein 1; NG2, neuron-glial antigen 2.
Immunohistochemistry.
To detect DsRed and GFP fluorescence or DsRed fluorescence from 3-mo-old Nestin-GFP/NG2-DsRed mice or FVB mice of different ages injected with DsRed+ pericytes, respectively, extensor digitorum longus (EDL) and tibialis anterior (TA) muscles were dissected; fixed in 4% paraformaldehyde (PFA) overnight; immersed in 10, 20, and 30% sucrose solutions for 60, 45, and 30 min, respectively; embedded in OCT; and rapidly frozen in liquid nitrogen to prepare 10-μm thick cryosections. Muscle sections were fixed with 4% PFA for 30 min, then permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO), and blocked to saturate nonspecific antigen sites using 5% (vol/vol) goat serum/PBS (Jackson Immunoresearch Laboratories, West Grove, PA) overnight at 4°C. The next day, the sections were incubated with primary antibodies at room temperature for 4 h and visualized using appropriate species-specific secondary antibodies conjugated with Alexa Fluor 488, 568, or 680 at 1:1,000 dilution (Invitrogen, Carlsbad, CA). Muscle sections were counterstained with Hoechst 33342, mounted on slides using Fluorescent Mounting Medium (DakoCytomation, Carpinteria, CA), and examined under fluorescence microscopy.
Fluorescence-activated cell sorting.
Fluorescence-activated cell sorting (FACS) was carried out on a BD FACS (Aria Sorter, San José, CA) at 4°C and a pressure of 20 psi, using a laser at the 488-nm line, a 530/30 band-pass filter, a 100-μm sorting tip, and 34.2-kHz drive frequency. The sorting apparatus was sterilized with 10% bleach. This instrument allowed us to characterize cells by size as well as fluorescence. Data acquisition and analyses were performed using BD FACS Diva 5.0.3 software, gated for a high level of GFP, DsRed, or APC expression. The clear separation of GFP+ from GFP− cells (7) and DsRed+ from DsRed− cells as well as the low flow rate explains the ease and accuracy of sorting (10). Sorted cells were reanalyzed to confirm their fluorescence profile (7, 10).
Pericyte subtypes isolation from Nestin-GFP/NG2-DsRed mice skeletal muscle by FACS.
Cells were sorted immediately from a pool of hindlimb muscles after skeletal muscle dissection and dissociation. Hindlimb muscles from young-adult (3–5 mo) Nestin-GFP/NG2-DsRed transgenic mice were prepared as described before (10). Briefly, muscles were carefully dissected away from the surrounding connective tissue and minced, then digested by gentle agitation in 0.2% (wt/vol) type-2 collagenase in Krebs solution at 37°C for 2 h, and dissociated by trituration and resuspension in 0.25% trypsin/0.05% EDTA in PBS for 15 min at 37°C. After centrifuging at 1,500 rpm for 5 min, the supernatant was removed, and the pellet was resuspended in growth medium. Aggregates were removed by passing them through a 40-μm cell strainer before sorting. Cells were centrifuged at 1,500 rpm for 5 min. The supernatant was removed, and the pellet was resuspended in 1% FBS in PBS and analyzed for GFP and DsRed fluorescence to sort the different cell populations based on these two markers. The gate was set using cells isolated from C57BL6 wild-type mice. Isolated type-1 (Nestin-GFP−/NG2-DsRed+) and type-2 (Nestin-GFP+/NG2-DsRed+) pericytes were used to track the fate of those cells in vitro under myogenic or fibrogenic conditions.
Reverse transcription-polymerase chain reaction.
To detect the mRNA expression in cells, total RNA was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA), RNA was dissolved in sterile, RNase-free water (Invitrogen) and quantitated spectrophotometrically at 260 nm. Reverse transcription-polymerase chain reaction (RT-PCR) was performed in accordance with the manufacturer's instructions using the SuperScript III First-Strand synthesis system for RT-PCR system (Invitrogen). For each experiment, equivalent amounts of intact RNA (0.1–0.2 μg) were used. As negative controls, the RT reactions were performed in the absence of RNA (only water) or reverse transcriptase. The cDNA was amplified by PCR using the primers included in Table 2. PCR Master Mix was purchased from Promega (Madison, WI). Each PCR reaction contained 1× Promega PCR Master Mix, 1 μM of each primer, and the cDNA of the cells used in each case (Nestin-GFP−/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, and Nestin-GFP+/NG2-DsRed− cells). The volume of each reaction was brought up to 50 μl with water. DNA amplification was carried out as follows: denaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min. After 35 cycles, the reactions were incubated at 72°C for 7 min to increase the yield of amplification. PCR products were verified with DNA 2% agarose gel electrophoresis.
Table 2.
Genes, GenBank accession numbers, coding regions, and primers
| Gene | GenBank Accession Numbers | Coding Regions | Forward Primer and Positions | Reverse Primer and Positions |
|---|---|---|---|---|
| CD146 | NM_023061.2 | CDS: 33–1797 | AAGAGGAGAGCACCGATGAA (922–941) | TTACTTTCTGCCTCGCAGGT (1147–1128) |
| NG2 | NM_139001.2 | CDS: 87–7070 | GCACGATGACTCTGAGACCA (3020–3039) | AGCATCGCTGAAGGCTACAT (3242–3223) |
| PDGFR-β | NM_001146268.1 | CDS: 430–3729 | CCGGAACAAACACACCTTCT (2511–2530) | TATCCATGTAGCCACCGTCA (2656–2637) |
| Pax7 | NM_011039.2 | CDS: 58–1569 | CATCCTTAGCAACCCGAGTG (1215–1234) | AGTAGGCTTGTCCCGTTTCC (1567–1548) |
| Myf5 | NM_008656.5 | CDS: 204–971 | AGACGCCTGAAGAAGGTCAA (483–502) | TGGAGAGAGGGAAGCTGTGT (899–880) |
| Col1a1 | NM_007742.3 | CDS: 100–4461 | CACCCTCAAGAGCCTGAGTC (3765–3784) | GTTCGGGCTGATGTACCAGT (3998–4017) |
| S100a4 (FSP1) | NM_011311.2 | CDS: 53–358 | TTGTGTCCACCTTCCACAAA (87–106) | GCTGTCCAAGTTGCTCATCA (225–244) |
| Scx | NM_198885.3 | CDS: 165–788 | TGGCCTCCAGCTACATTTCT (529–548) | TGTCACGGTCTTTGCTCAAC (746–765) |
| GAPDH | NM_008084.2 | CDS: 51–1052 | GTGGCAAAGTGGAGATTGTTGCC (118–140) | GATGATGACCCTTTTGGCTCC (407–387) |
Myogenic induction in vitro.
Freshly isolated pericyte subtypes (2.5 × 103 cells/cm2) were cultured on laminin-precoated plates (Invitrogen) for 2 days in growth medium [DMEM-high glucose (Invitrogen), supplemented with 2% l-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin, and 10% (vol/vol) FBS (Invitrogen)], followed by 14 days in differentiation medium [DMEM (Invitrogen) containing 2-mM l-glutamine (Invitrogen) and 1% penicillin/streptomycin (Invitrogen), supplemented with 2% horse serum (Invitrogen)] in a 5% CO2 atmosphere (80, 81). Medium was changed every 3 days until elongated, multinucleated myotubes appeared. After day 14 in culture, cells were fixed in 4% PFA at room temperature, and myosin heavy chain (MHC) expression was analyzed and quantified.
Fibrogenic induction in vitro.
Fibrogenic differentiation was induced in fibrogenic medium for 5 days as described elsewhere (29). Briefly, freshly isolated pericyte subtypes were plated onto laminin-coated plates (Invitrogen) and cultured for 5 days in DMEM supplemented with 2% horse serum 10% (vol/vol) (Invitrogen), with 2% l-glutamine, 50 U/ml penicillin, and 50 mg/ml streptomycin, supplemented with 2.5 ng/ml of TGF-β1. Medium was changed twice. After day 5 in culture, cells were fixed in 4% PFA at room temperature, and type I collagen expression was analyzed and quantified.
Immunocytochemistry.
Cultured cells were fixed with 4% PFA for 30 min, then permeabilized in 0.5% Triton X-100 (Sigma), and blocked to saturate nonspecific antigen sites using 5% (vol/vol) goat serum/PBS (Jackson Immunoresearch Laboratories) overnight at 4°C. The next day, the cells were incubated with primary antibody at room temperature for 4 h and visualized using appropriate species-specific secondary antibody conjugated with Alexa Fluor 680 at 1:1,000 dilution (Invitrogen). They were counterstained with Hoechst 33342 reagent at 1:2,000 dilution (Invitrogen) to label the DNA and mounted on slides for fluorescent microscopy with Fluorescent Mounting Medium (DakoCytomation).
Isolation of type-1 and type-2 DsRed+ pericytes.
Hindlimb muscle cells were isolated from young adult (3–5-mo-old) Nestin-GFP/β-actin-DsRed mice as described above (9). After being counted, cells were centrifuged at 1,500 rpm for 5 min and resuspended in 100-μl 1% FBS in PBS/106 cells. First, an aliquot was collected for use as unlabeled control (labeled with only the secondary APC anti-rabbit, without the primary anti-NG2 antibody) to set the gate. The remaining cells were incubated with the primary APC anti-mouse NG2 antibody for 45 min and washed in 1% FBS in PBS. They were then incubated for 30 min with APC anti-rabbit secondary antibody, washed in PBS with 1% FBS, and run on a BD FACS flow cytometer (Aria Sorter). Sorting was done based on GFP and APC fluorescence. Isolated Nestin-GFP+/NG2-APC+/β-actin-DsRed+ and Nestin-GFP−/NG2-APC+/β-actin-DsRed+ cells were used in cell fate tracking experiments to evaluate muscle and fibrous tissue formation in vivo.
Muscle injury and cell transplantation.
Skeletal muscle regeneration was studied in TA muscle injured by intramuscular injection of barium chloride (BaCl2) as described previously (32). Specifically, young, middle-aged, and old FVB mice were anesthetized with isoflurane/O2 inhalation. TA muscles were injected with 50 μl of 1.2% BaCl2 dissolved in sterile PBS 1 day before cell transplantation. At 24 h postinjury, type-1 (Nestin-GFP−/NG2-APC+/β-actin-DsRed+) or type-2 (Nestin-GFP+/NG2-APC+/β-actin-DsRed+) pericytes were isolated from donor Nestin-GFP/β-actin-DsRed mice, resuspended in PBS (3 × 105 cells per TA), and slowly injected into the damaged muscle of the acceptor mice. As controls, injured TA muscles of all groups were injected with PBS. As our transplantation experiments were performed in immune-competent (FVB) mice, immunosuppression was induced in all mice to suppress immune rejection of the transplanted cells as described before (6). Briefly, all animals received daily subcutaneous injections of cyclosporine A (15 mg/kg sc; Novartis, East Hanover, NJ) begining 2 days before of transplantation and continuing for the duration of the experiment. Mice were killed 14 days postinjection, and TA muscles ere collected and processed for immunohistochemistry as described above. The area and number of newly formed DsRed+ myofibers were quantified in the muscle sections. Also, the area with collagen was quantified in old compared with young muscle sections. DsRed+ cells positive to type I collagen, which were localized at the interstitial muscle space were counted in muscle sections.
Microscopy, cell imaging, and counting.
An inverted motorized fluorescent microscope (IX81; Olympus, Tokyo, Japan) with an Orca-R2 Hamamatsu CCD camera (Hamamatsu, Japan) was used for image acquisition. Camera drive and acquisition were controlled by a MetaMorph Imaging System (Olympus). Ten arbitrary microscopic fields were counted in each immunostained plate or tissue section, and values were pooled from parallel duplicates per time point and individual experiment.
Statistical analysis.
Results are expressed as the means ± SE. Statistical significance was assessed using Student's t-test or one-way ANOVA followed by Holm-Sidak or Tukey post hoc tests using SigmaPlot11 (Systat Software; San Jose, CA). P < 0.05 was considered significant.
RESULTS
Two bona fide pericyte subpopulations associated to microvessels are present in the skeletal muscle.
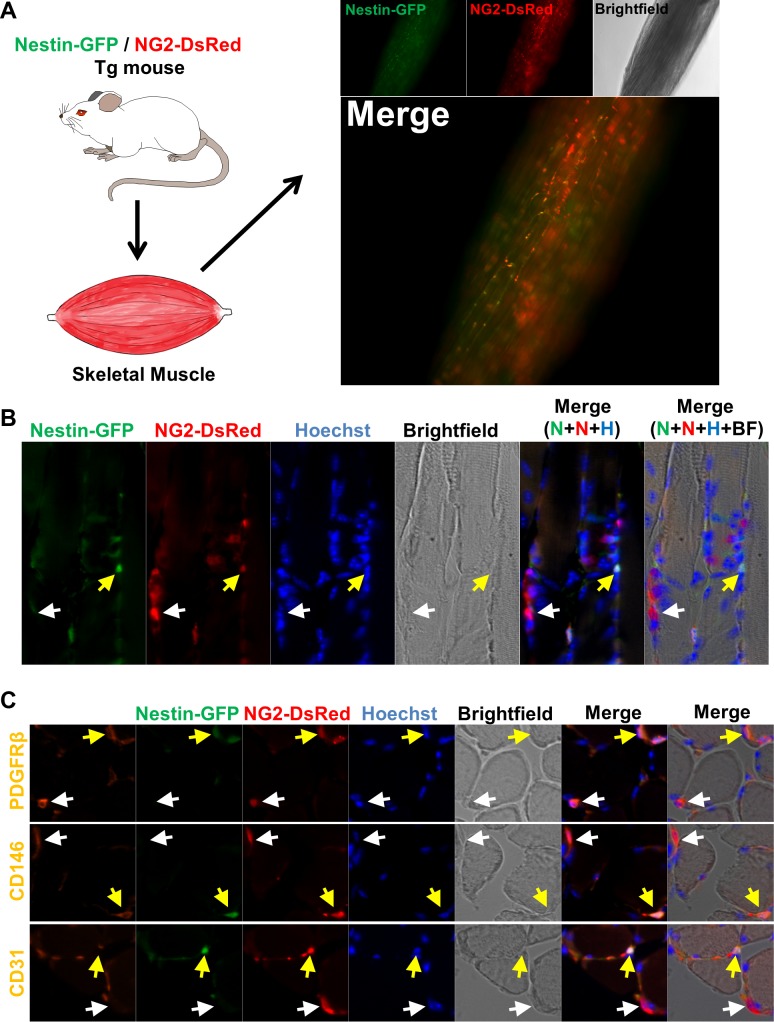
Pericytes are commonly recognized by their location and gene expression patterns rather than a precisely defined phenotype. Neuron-glial 2 chondroitin sulfate proteoglycan (NG2) has been used to detect them (62). We conducted a histological analysis of skeletal muscle from Nestin-GFP/NG2-DsRed mice using Nestin and NG2 regulatory elements to control GFP and DsRed expression, respectively. We discovered two types of pericytes; one expressed Nestin-GFP in the interstitium and the other did not. We termed them type 1 (Nestin-GFP−/NG2-DsRed+) and type 2 (Nestin-GFP+/NG2-DsRed+) (Fig. 1, A and B).
Fig. 1.
Two pericyte subtypes are associated with skeletal muscle microvessels. A: histological analysis of pericyte subtypes in the skeletal muscle of double transgenic Nestin-GFP/NG2-DsRed mice. Whole lumbricalis muscle examined immediately after dissection. NG2-DsRed, Nestin-GFP, brightfield, and merged images are shown. NG2-DsRed+ cells can be detected; some overlap with Nestin-GFP fluorescence. B: longitudinal section of EDL muscle from Nestin-GFP/NG2-DsRed mice illustrating the 2 pericyte subtypes, type-1 (Nestin-GFP−/NG2-DsREd+; white arrow) and type-2 (Nestin-GFP+/NG2-DsRed+; yellow arrow). A–C show the same muscle area for different channels [Nestin-GFP (N), NG2-DsRed (N), Hoechst (H), brightfield (BF), merged fluorescence, and merged fluorescence and brightfield images]. C: immunofluorescence on transverse sections from the same muscle shown in B, stained with anti- platelet-derived growth factor receptor-β (PDGFR-β), anti-CD146, and anti-CD31 antibodies, confirms the presence of 2 types of pericytes, both PDGFR-β+/CD146+ and associated with microvessels (CD31+). Panels show identical muscle areas from left to right: PDGFR-β (1st line), CD146 (2nd line), or CD31 (3rd line, orange), Nestin-GFP+ (green), NG2-DsRed (red), Hoechst (blue), brightfield, and merged images. White arrows indicate type-1 pericytes (Nestin-GFP−/NG2-DsRed+), and the yellow arrows indicate type-2 (Nestin-GFP+/NG2-DsRed+).
We confirmed that both types express two pericytic markers in addition to NG2 proteoglycan: a cell-surface tyrosine kinase receptor (the platelet-derived growth factor receptor PDGFR-β/CD140; Ref. 28) and a melanoma-specific cell-adhesion molecule, CD146, also known as MCAM, M-CAM, and MUC18 (23). While neither type expressed the endothelial cell marker platelet endothelial cell adhesion molecule-1 (PECAM-1), a transmembrane glycoprotein also known as CD31 (23) (Fig. 1C), they surrounded CD31+-labeled blood vessels (orange); pericytes typically surround small vessels with endothelial cells (23).
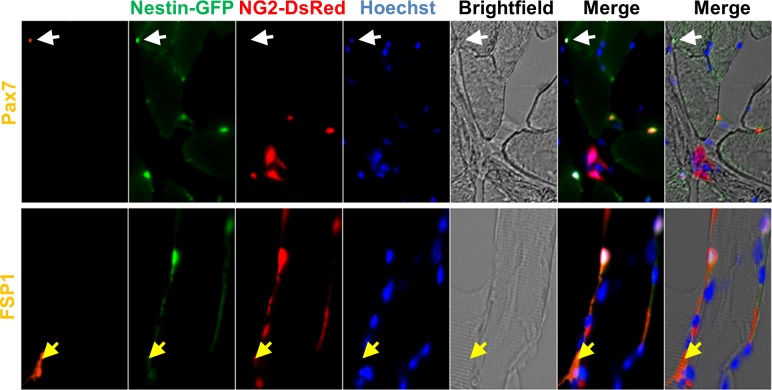
Like pericytes, satellite cells express GFP in Nestin-GFP transgenic mice (25) but not NG2-DsRed (Fig. 2) in Nestin-GFP/NG2-DsRed mice. As fibroblasts are also present in the skeletal muscle interstitium, we examined whether pericytes express fibroblast-specific protein 1 (FSP1; Ref. 42). Our results indicate that they do, but FSP1+ fibroblasts do not express NG2-DsRed (Fig. 2).
Fig. 2.
Satellite cells and fibroblasts do not express NG2-DsRed. Skeletal muscle from Nestin-GFP/NG2-DsRed transgenic mice shows Pax7 (satellite cells marker), fibroblast-specific protein 1 (FSP1; a fibroblast marker), Nestin-GFP, NG2-DsRed, and Hoechst staining in the same region. Brightfield and merged images are also shown. White arrow shows a satellite cell (Nestin-GFP+/Pax7+) that does not express NG2-DsRed; the yellow arrow indicates a Nestin-GFP−/FSP1+ but NG2-DsRed− fibroblast.
Pericytes have been reported to have fibrogenic (28, 29) and myogenic (23, 26) potential, although myogenic potential has not been reported for fibrogenic progenitors (29). Whether distinct pericyte subtypes contribute to either muscle repair or the excessive fibrogenesis that occurs in older adults is unknown. Here, we propose that the muscle vasculature from adult and aged mammals contains two types of pericytes, one responsible for muscle tissue repair, the other for fibrous tissue accumulation with aging (28).
Type-1 pericytes are fibrogenic, while type-2 pericytes are myogenic in vitro.
To determine whether fibrogenic or myogenic potential arises from the distinct, lineage-committed pericyte subtypes described above (10), we isolated type-1 and type-2 pericytes from mononucleated cells dissociated from Nestin-GFP/NG2-DsRed mouse skeletal muscle by cell sorting (Fig. 3) and cultured them separately in myogenic (see Fig. 5, A–C) or fibrogenic (see Fig. 5, D–G) induction medium.
Fig. 3.
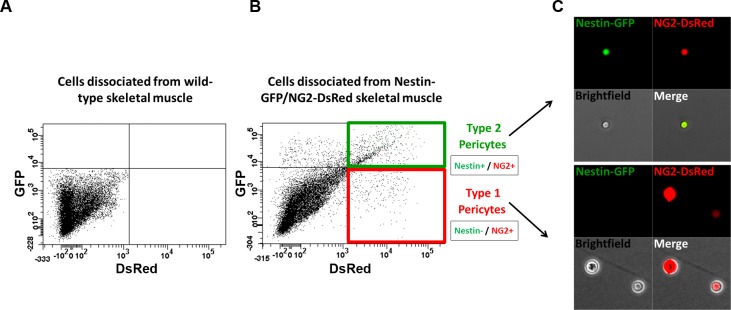
Isolation of skeletal muscle pericyte subtypes from Nestin-GFP/NG2-DsRed mice by sorting. Representative flow cytometry dot plot showing GFP vs. DsRed fluorescence with the gate set using cells isolated from skeletal muscle of wild-type mice (A). B: Nestin-GFP/NG2-DsRed skeletal muscle-derived cells were divided into 4 populations: Nestin-GFP+/NG2-DsRed-, Nestin-GFP−/NG2-DsRed+ (type-1 pericytes), Nestin-GFP+/NG2-DsRed+ (type-2 pericytes), and Nestin-GFP−/NG2-DsRed- cells. C: single cells in culture dishes immediately after sorting. Localization of Nestin-GFP+ (green) and NG2-DsRed+ (red) cells in freshly sorted fractions. All cells in the fraction of type-1 pericytes (Nestin−/NG2+) were Nestin-GFP negative and NG2-DsRed positive and Nestin-GFP and NG2-DsRed positive in the fraction of type-2 pericytes (Nestin+/NG2+).
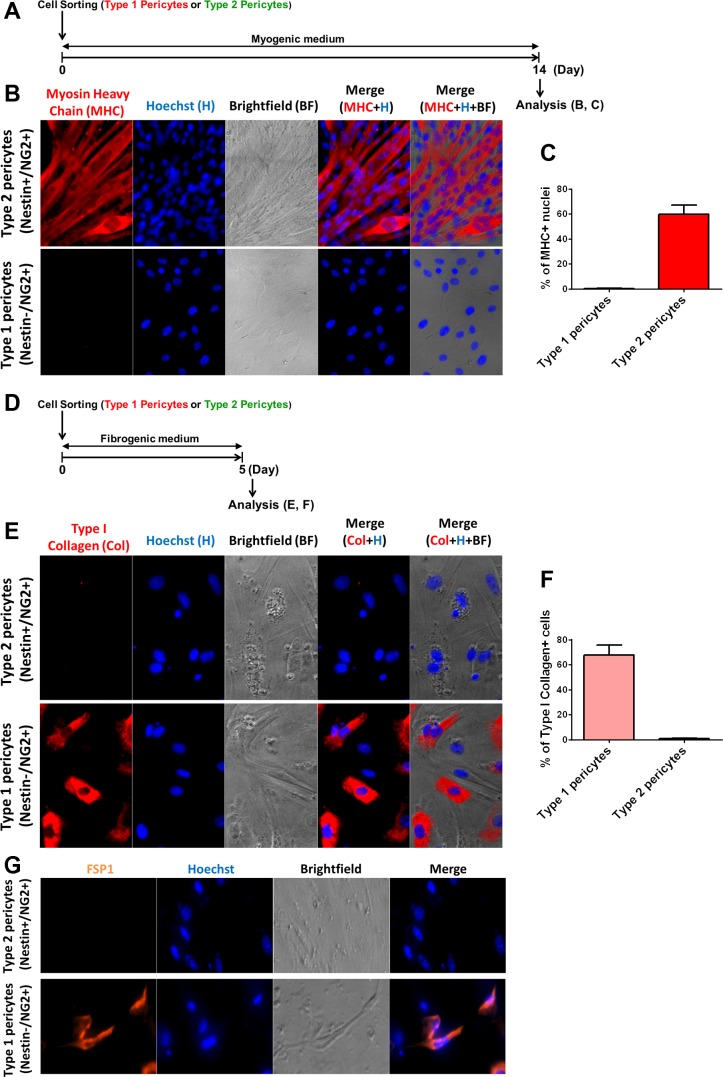
Fig. 5.
Type-1 pericytes produce type I collagen, while type-2 pericytes differentiate into myotubes in vitro. Myogenic and fibrogenic induction of freshly isolated pericyte subtypes from Nestin-GFP/NG2-DsRed mouse muscle. A: time frame of myogenic differentiation in vitro: freshly isolated pericytes (Fig. 3) were cultured for 2 wk in myogenic differentiation medium. B: after 14 days in differentiation medium, Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells were stained with anti-myosin heavy chain (MHC) antibody. C: percentage of MHC+ nuclei derived from each pericyte subtype was counted and normalized to the total number of nuclei (n = 3). Data are means ± SE. D: time frame for fibrogenic in vitro differentiation: freshly isolated pericytes (Fig. 3) were cultured for 5 days in fibrogenic medium containing TGF-β. E: after 5 days in fibrogenic medium, Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells were stained with anti-type I collagen antibody. F: percentage of type I collagen+ cells derived from each pericyte subpopulation was counted and normalized to the total cell number (n = 3 preparations). G: Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells were stained with FSP1 antibody at day 5 in fibrogenic conditions.
Our previous work show that, in contrast to type-2 pericytes, satellite cells do not express NG2 proteoglycan and are located beneath the basal lamina (53, 77). To confirm the purity of our pericyte subpopulations, we examined the expression of specific markers in our isolated sorted cells by RT-PCR (Fig. 4, A and B). All pericytes expressed CD146, NG2 and PDGFR-β mRNA but not the satellite cell markers Pax7 (48, 61, 65, 68) or Myf5 (5). Type I collagen mRNA was expressed in satellite cells (Nestin-GFP+/NG2-DsRed−) as reported (71), and type-1 pericytes (Nestin-GFP−/NG2-DsRed+), but absent in type-2 pericytes (Nestin-GFP+/NG2-DsRed+). Additionally, pericytes did not express the fibroblast markers FSP1(42) and Scleraxis (22, 55).
Fig. 4.
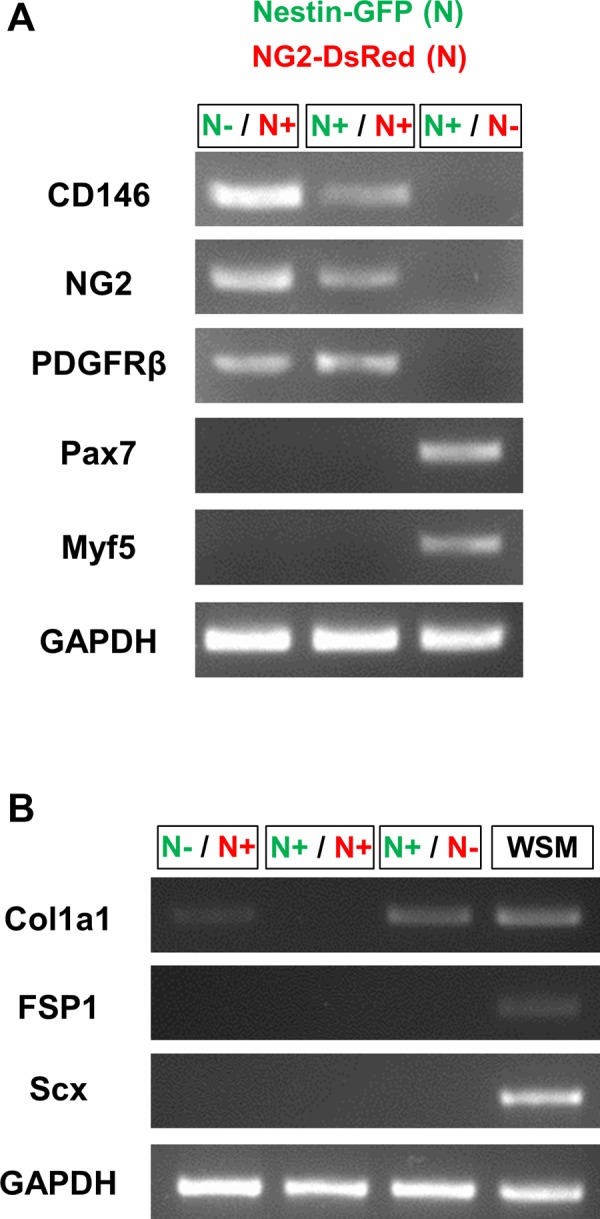
Nestin-GFP and NG2-DsRed cells gene expression. A: representative RT-PCR agarose gel from 3 experiments showing CD146, NG2, PDGFR-β, Pax7, Myf5 expression, and control GAPDH. The pericyte markers CD146, NG2, and PDGFR-β were present in Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells but absent in Nestin-GFP+/NG2-DsRed- cells. The satellite cell markers Pax7 and Myf5 were detected only in Nestin-GFP+/NG2-DsRed- cells. B: RT-PCR agarose gel representative of 3 experiments, showing type I collagen (Col1a1), FSP1, Scleraxis (Scx) expression, and control GAPDH. Type I collagen gene was expressed in Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed- cells, but absent in Nestin-GFP+/NG2-DsRed+ cells. The fibroblast markers were not present in Nestin-GFP−/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, or Nestin-GFP+/NG2-DsRed− cells. cDNA from whole skeletal muscle dissociated cells presorting (WSM) was used as a positive control.
To investigate myogenesis in vitro, we examined the expression of MHC, a marker of myogenic lineage (63), in cells exposed to myogenic differentiation medium for 14 days to allow myotube formation. We found that type-2 (Nestin-GFP+/NG2-DsRed+) but not type-1 (Nestin-GFP−/NG2-DsRed+) pericytes differentiated into myotubes (Fig. 5B). The percentage of nuclei per MHC+ myotubes normalized to total nuclei in type-1 and type-2 pericyte cultures was 0.34 ± 0.10 (n = 3 replicates, 500 nuclei counted) and 60 ± 13% (n = 3 replicates, 500 nuclei counted), respectively (Fig. 5C).
To examine the fibrogenic potential of the two skeletal muscle pericyte subpopulations, we exposed them to fibrogenic medium containing TGF-β1, a profibrotic cytokine of well-established potency that is overexpressed in most fibrotic tissues (19). Type-1, but not type-2 pericytes, differentiated into FSP1+ fibroblast-like cells (Fig. 5G) and expressed type I collagen (Fig. 5E). The percentage of type-1 and type-2 pericyte-derived type I collagen+ cells per total nuclei was 68 ± 8.1 (n = 3 replicates, 200 cells counted) and 0.9 ± 0.3% (n = 3 replicates, 200 cells counted), respectively (Fig. 5F).
Type-2 pericytes are myogenic in vivo.
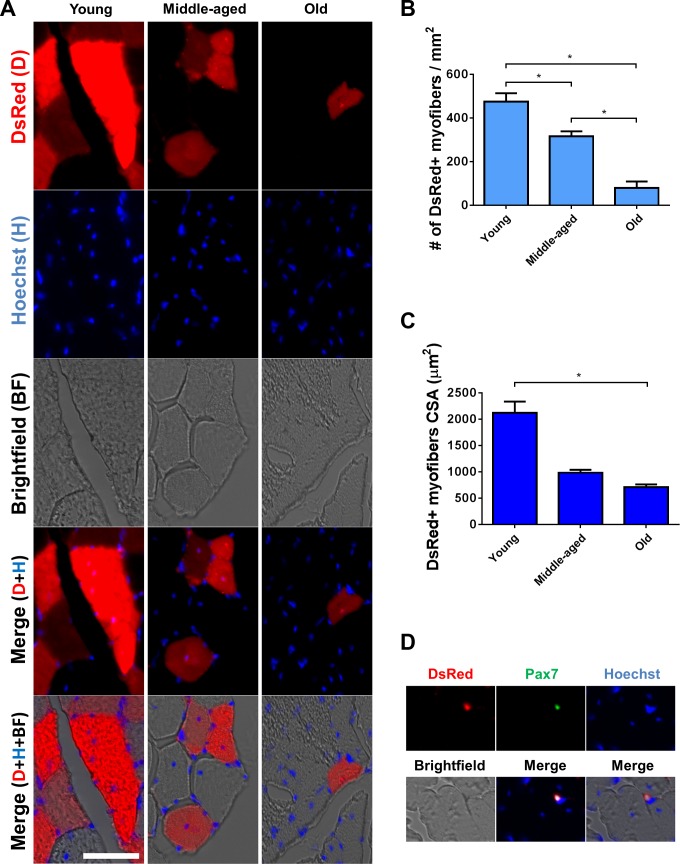
As purified type-2 pericytes demonstrated myogenic differentiation in vitro, we next examined their myogenic potential in skeletal muscle. Immediately after cell sorting, we transplanted the pericyte subtypes freshly isolated from the muscles of Nestin-GFP/β-actin-DsRed transgenic (Tg) mice into the TA muscles of wild-type mice of different ages (young, middle-aged, and old) (Fig. 6B) using BaCl2 injection (Fig. 6) to induce well-confined muscle degeneration without damaging the basal membrane (16). DsRed fluorescence (9) allowed us to identify donor fibers in the host muscle (Fig. 6A). The purity of these cell populations was confirmed by flow cytometric reanalysis as reported (10). After 2 wk, very few interstitial DsRed+ cells were observed in the muscles from young mice injected with type-1 pericytes, and no DsRed+ myofibers were detected (Fig. 6C). In contrast, transplanted muscles from animals injected with type-2 pericytes showed many newly formed DsRed+ myofibers (Figs. 6D and 7A).
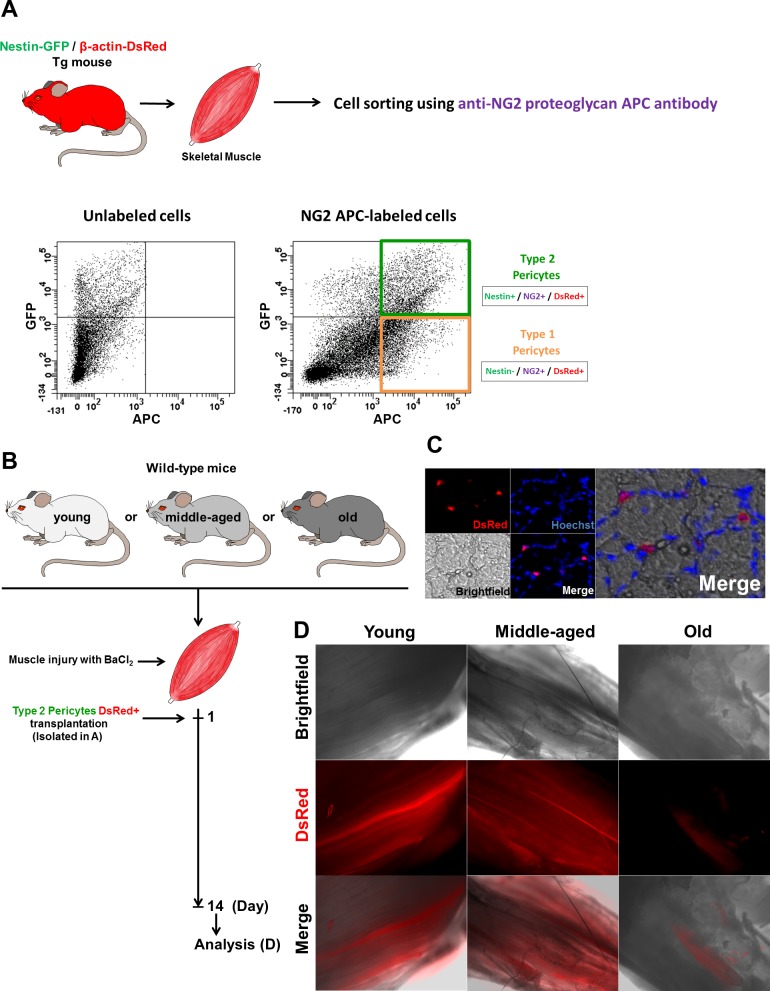
Fig. 6.
Skeletal muscle type-2 pericyte fate in vivo. A: pericytes subtypes from Nestin-GFP/β-actin-DsRed double-transgenic mice were isolated and sorted using an anti-NG2 proteoglycan APC antibody. All cells are DsRed+ and can be tracked in vivo. Representative dot plots showing GFP vs. APC fluorescence with the gate set using unlabeled cells before and after labeling with NG2 APC antibody. B: transplantation of isolated type-2 DsRed+ pericytes into injured skeletal muscles. C: representative tibialis anterior (TA) muscle section from a young mouse showing transplanted type-1 pericytes (DsRed+) remaining in the interstital space. Panels show identical muscle areas. Nuclei were stained with Hoechst 33342. D: whole TA muscle visualized immediately after dissection from young, middle-aged, and old mice 2 wk after type-2 DsRed+ pericytes transplantation. Brightfield, DsRed fluorescence, and merged images are shown. Notice the scarcity of DsRed+ cells in the muscle from old animals.
Fig. 7.
Type-2 pericytes participate in the formation of fewer and smaller myofibers in injured old mice. A: 2 wk after transplantation, DsRed+ myofibers (red) are present in the muscle of mice injected with type-2 pericytes. Panels show identical muscle areas from top to bottom: DsRed fluorescence (first line), Hoechst (blue), brightfield, merged fluorescence, and merged fluorescence and brightfield images. B and C: quantification of the data illustrated in A. B: number of DsRed+/type I collagen+ cells normalized to the cross-sectional area at day 14 of cell transplantation into injured skeletal muscle from old mice (n = 3 muscles). For data analysis, we used one-way ANOVA followed by Holm-Sidak test. C: cross-sectional area of DsRed+ myofibers in young, middle-aged, and old mice (n = 3 muscles). For data analysis, we used one-way ANOVA followed by Tukey test. D: representative TA muscle section from a transplanted mouse (as in A), showing a rare type-2 pericyte (DsRed+) expressing the satellite cell marker Pax7. Panels show identical muscle areas. Nuclei were stained with Hoechst 33342. *P < 0.05.
Regenerative capacity of type-2 pericytes decreased when transplanted into aged skeletal muscle.
Aged microenvironments or niches inhibit the regenerative capacity of tissue stem cells (74). Whether they influence the differentiation potential of pericyte subpopulations is unknown. To address this question, we injected young, middle-aged, and old mice with type-2 pericytes and found that their myogenic regenerative capacity decreased in the aged microenvironment; the same number of cells formed fewer myofibers in old mice compared with their younger littermates. Quantitative analysis revealed that Nestin-GFP+/NG2+/β-actin-DsRed+ cells participated in the formation of 474 ± 39 (n = 3 muscles), 316 ± 23 (n = 3 muscles), and 79 ± 30 (n = 3 muscles) DsRed+ myofibers per mm2 in young, middle-aged, and old animals, respectively (Fig. 7, A and B). Compared with young mice, middle-aged, and old mice showed a 33 and 83% decrease in DsRed+ cells, respectively. Additionally, the cross-sectional area of the newly formed fibers was smaller in old than young and middle-aged mice. The area of DsRed+ myofibers was 2,123 ± 211, 984 ± 54, and 707 ± 54 μm2 in young, middle-aged, and old animals, respectively (Fig. 7, A and C). Compared with young mice, middle-aged and old mice showed a 53 and 66% decrease in the DsRed+ area, respectively.
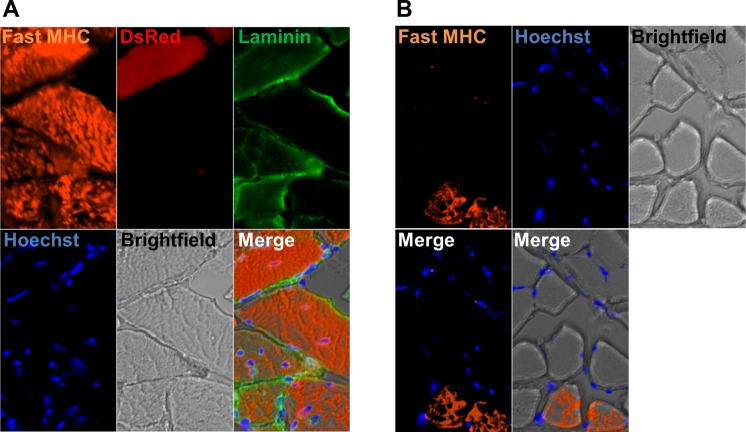
Skeletal muscle exhibits marked plasticity; myofiber type readily converts in response to distinct stimuli (12, 34, 38, 40). During muscle formation, fast myosin is expressed before slow myosin appears (41). To determine DsRed+ myofiber type, we examined their MHC isoform expression. Our results show that type-2 pericytes fused to fast (type II) myofibers (Fig. 8A). As the adult TA muscle is composed of only fast fibers (36), while soleus muscles are a mixture of fast and slow fibers (30), we used the soleus muscle as a control for our antibody (Fig. 8B).
Fig. 8.
DsRed+ muscle fibers in muscles transplanted with DsRed+ type-2 pericytes are fast (type II). A: all fibers express fast myosin heavy chain in TA muscle injected with DsRed+ type-2 pericytes. B: soleus muscle, used as a staining control, also shows some fibers expressing fast myosin heavy chain. A and B show the same area for different channels: Fast MHC staining (orange), DsRed (red), Laminin (green), Hoechst (blue), brightfield, merged fluorescence images, and fluorescence and brightfield merge image; n = 3.
In addition to fusing to developing myofibers, skeletal muscle pericytes have been reported to enter the satellite-cell compartment and express satellite-cell markers (26). Since satellite cells do not express NG2 proteoglycan, they did not contaminate the pool of injected type-2 pericytes, and any DsRed+ satellite cells detected after transplantation had to originate from the pericyte subtype. Indeed, in examining the expression of the satellite-cell marker Pax7 in DsRed+ cells derived from the transplanted type-2 pericytes, we did find DsRed+/Pax7+ cells in TA muscles from young animals, although very rarely (0.92 ± 0.92 DsRed+/Pax7+ cells per mm2), indicating that besides myofibers, type-2 pericytes may generate satellite cells (Fig. 7D).
Type-1 but not type-2 pericytes contribute to fibrous tissue formation in skeletal muscle from old mice.
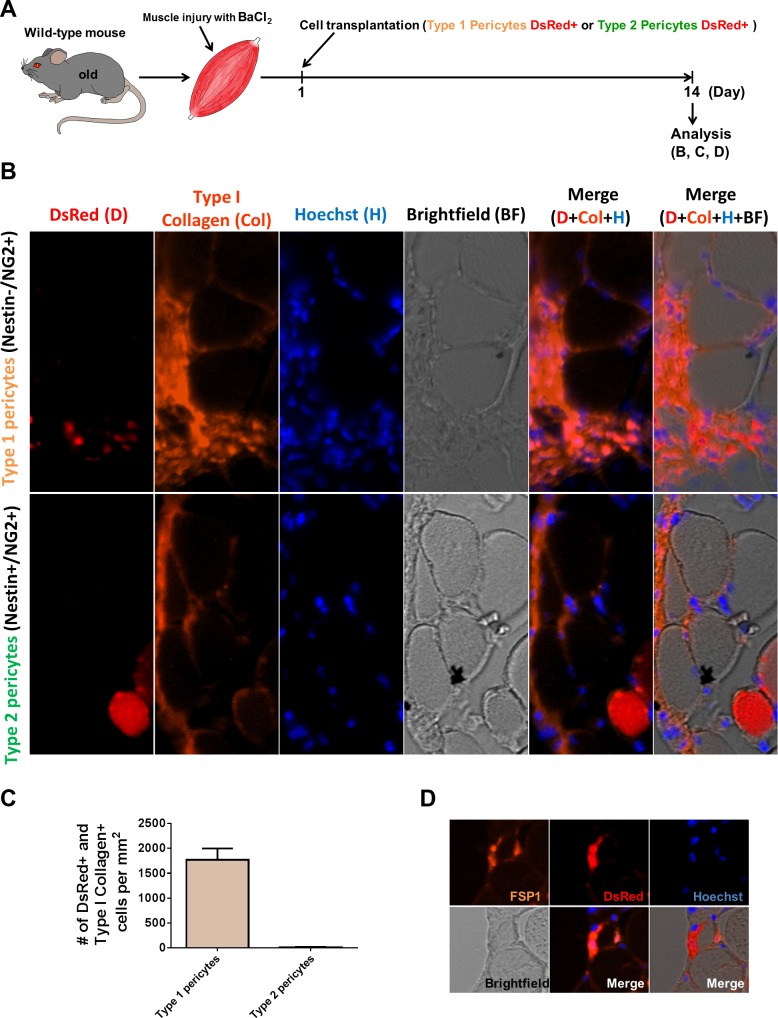
Because type-1 pericytes showed fibrogenic differentiation potential in vitro, we next evaluated their in vivo capacity to form fibrous tissue in skeletal muscle. Fibrous tissue accumulation is observed only when myogenic regeneration fails due to aging or disease (29) and not in response to injury in young healthy mice. Therefore, we transplanted type-1 and type-2 pericytes into the injured skeletal muscle of old mice to analyze their fibrotic potential (54). Previous works have measured collagen accumulation in intact aging skeletal muscle using biochemical and histochemical techniques (1, 33, 37, 47, 57).
We sorted the pericyte subtypes from β-actin-DsRed/Nestin-GFP mice using a rabbit anti-NG2 proteoglycan and APC anti-rabbit secondary antibodies. Since every cell expresses DsRed (10), even when transdifferentiated into another cell type, this technique allowed us to track isolated type-1 and type-2 pericytes after injection (Fig. 9A).
Fig. 9.
Type-1 pericytes are fibrogenic in vivo in old mouse skeletal muscle. A: time frame for type-1 or type-2 DsRed+ pericyte isolation and injection into injured skeletal muscles from old wild-type mice. B: TA muscle cross sections from old mice injected with type-1 or type-2 DsRed pericytes 2 wk after transplantation. Panels show identical muscle areas from left to right: DsRed, type I collagen, Hoechst, brightfield, merged fluorescence, and merged fluorescence and brightfield images. Anti-type I collagen staining confirms that type-1 pericytes stay in the interstitium and do not differentiate into muscle cells; type-2 pericytes do not express type I collagen and fuse in DsRed+ myofibers. C: quantification of the data illustrated in B. Number of DsRed+/type I collagen+ cells normalized to the cross-sectional area at day 14 of cell transplantation into injured skeletal muscle from old mice (n = 3 muscles). D: TA muscle cross-sections from old mice injected with type-1 DsRed+ pericytes 2 wk after transplantation. All panels show the same muscle area for different channels (FSP1 staining, DsRed, Hoechst, brightfield, merged fluorescence, and fluorescence and brightfield merged images). Type-1 pericytes are located in the interstitium and express the fibroblast marker FSP1.
Two weeks after cell transplantation, muscles were harvested and stained for type I collagen (Fig. 9B). We observed few DsRed+ mononucleated cells in the interstitium of muscles injected with type-2 pericytes; almost no DsRed+ cells expressing type I collagen (9.4 ± 9.4 DsRed+/type I collagen+ cells/mm2; n = 3 muscles; Fig. 9, B and C); and newly formed DsRed+ myofibers (Fig. 9B). In contrast, the interstitial connective tissue of muscles transplanted with type-1 pericytes was rife with DsRed+ cells expressing type I collagen (1,772 ± 228 DsRed+/type I collagen+ cells per mm2: n = 3 muscles) and FSP1 (Fig. 9D) and had no regenerated DsRed+ myofibers, indicating that type-1 pericytes are fibrogenic in vivo (Fig. 9, B and D).
DISCUSSION
Pericytes are found in vascularized tissues and recognized by their histological location rather than a precisely defined phenotype. However, some antigens, such as neuron-glial 2 chondroitin sulphate proteoglycan (NG2; Ref. 23), PDGFR-β (28), and a melanoma-specific cell-adhesion molecule CD146 (23) have been identified as pericytic molecular markers. We distinguished two bona fide subpopulations of pericytes in skeletal muscle, types 1 and 2. They express these markers and surround the endothelial cells in the microvasculature. Although pericytic differentiation in several tissues supports their multipotency (23), whether pericyte subpopulations are differentially committed to specific lineages is not known.
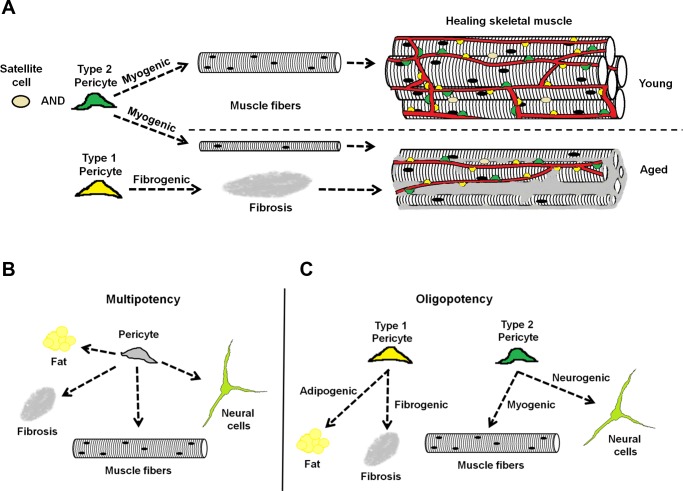
We found that both in vitro and in vivo, type-2 (Nestin-GFP+/NG2-DsRed+) pericytes are myogenic, while type-1 are fibrogenic. Future studies targeting their specific capacities could establish a therapeutic role, either to enhance myogenesis or to decrease fibrous tissue accumulation, in skeletal muscle from aging mammals (Fig. 10A).
Fig. 10.
Diagram of the role of pericyte subtypes in healing young and old skeletal muscle. Two subpopulations of pericytes are associated with blood vessels in the skeletal muscle: type-1 (yellow) and type-2 (green). A: we propose that during muscle repair, type-2 pericytes together with satellite cells contribute to myogenesis, producing larger myofibers in young than in old mice, while type-1 pericytes participate in the fibrous tissue accumulation observed in aged mice. B: classical model of the pericyte as a multipotent stem cell able to differentiate into fat, fibrous tissue, muscle, or neural cells. C: our model proposes that pericytes are heterogeneous and multipotent, but their subtypes are oligopotent and their ability to differentiate more restricted.
Type-1 pericytes as cellular targets for antifibrotic therapy in skeletal muscle.
Pericytes have been found to contribute to fibrous tissue production in several organs, including muscle (28). Once activated, they escape from the microvessels into the interstitium and participate in the formation of scar tissue, progression of fibrosis, and deterioration of organ function (28). However, they have also been associated with regenerative functions. In skeletal muscle, for example, they participate in vasculogenesis and contribute to the satellite-cell pool and muscle growth (23, 24, 26). Thus removing all pericytes to prevent or reduce fibrosis would impede tissue repair after injury.
Here, we adduce data indicating that a distinct type of pericyte directs each of these separate outcomes. Type 1 contributes to fibrosis in aging skeletal muscle, while only type 2 is myogenic. Instead of the whole pericyte population, future studies should target type 1 for antifibbrotic therapy in skeletal muscle and, further, determine whether specific pericyte subpopulations vary in their function and regenerative capacity in other organs.
Age-dependent mechanisms may activate different pericyte subtypes.
Fibrous tissue accumulates in the skeletal muscle with aging (75), but the mechanisms remain poorly understood (21). Some studies suggest that the decrease in, and reduced function of, stem cells play an essential role (58); others that significant changes to the skeletal muscle pericyte microenvironment impair regeneration (35). Some pericytes may not express a specific receptor that mediates the signaling pathway required for their differentiation, resulting in the emergence of a subpopulation with poor sensitivity to a specific agonist. Reduced expression of the notch-ligand delta affects notch signaling to impair muscle regeneration (21). The TGF-β, Wnt, and IGF pathways have also been associated with age-dependent impairment of muscle regeneration (4, 18).
The constitutively active PDGFR-α-receptor knockin mouse exhibits fibrosis both systemically and in the skeletal muscle (60). Consequently, it could be used to determine whether aging-impaired signaling through PDGFRs affects different pericyte subtypes (2) and how extrinsic and intrinsic pericyte changes impair muscle regeneration. Cell-intrinsic changes may be reversible or irreversible but in any case represent an additional source of heterogeneity. One pericyte subtype may be more prone to senescence or apoptosis, and the aged environment may select for a subtype with distinct regenerative potential (20). Pericyte subtype characterization also suggests further investigation of reported heterogenous stem cells (72) and their role in tissue regeneration in various organs. Future studies should determine whether the fate of the pericyte changes when they are exposed to such ligands as IGF-I, PDGF-AA, Wnt, and delta and whether their differentiation potential remains unchanged after exposure to PDGFR-α-, TGF-βR-, IGFR-, frizzled-, or notch-Fc chimeric receptors, which compete with pericyte receptors for ligands in vitro.
Host microenvironment is critical for type-2 pericyte myogenicity.
Total muscle size decreases over the lifespan (51), and old muscle regenerative capacity is severely impaired (21), which can lead to disability, particularly in patients with other diseases or organ impairments. Note that when exposed to a younger microenvironment or experimental activation of specific signaling, old muscle can regain its regenerative capacity (21).
Our data show that the myogenic regenerative capacity of type-2 pericytes decreases when injected in older host animals: the same number generated fewer and smaller myofibers. We suggest that the deleterious age-muscle microenvironment could be modified to improve type-2 pericyte-dependent muscle regeneration.
Total muscle decrease with aging is associated with fewer fibers (51), but many studies also report myofiber atrophy with a substantial decrease in fiber size (73). Reduced regenerative potential of muscle stem cells (15) is one of several mechanisms proposed to explain age-related muscle loss. Here, we show that the regenerative capacity of type-2 pericytes is impaired in the old. To what extent this impairment leads to fiber loss or atrophy compared with the effects of other myogenic stem cells in skeletal muscle has not been established.
In general, pericytes are multipotent, but their subpopulations exhibit lineage restriction.
Strong evidence indicates that pericytes are multipotent stem cells, able to form various tissues. They have been shown to improve heart function following myocardial infarction in animal models (45) and to form myotubes with high efficiency (23, 26), and their osteogenic differentiation and chondrogenic and adipogenic potential are also confirmed (23). They accelerate wound healing (78) and contribute to fibrous tissue formation (28, 29). Their role in forming and stabilizing engineered blood vessels (24) suggests a use in vasculogenic therapy, and they can be induced to develop into neural cells (44).
Pericytes are heterogeneous in location, origin, and morphology (82), and here we show that their molecular marker expression along the skeletal muscle vasculature also varies. However, in contrast to the current concept of skeletal muscle pericytes as multipotent stem cells with several differentiation potentials (Fig. 10B), the two oligopotent capillary pericytes we identified differ. Type-1 is responsible for ectopic adipocyte deposition (8) and fibrous tissue accumulation with aging, while type-2 accounts for regenerative capacity and, under special conditions, may be induced to differentiate into neural lineage (10). This new knowledge will advance tissue repair and regenerative medicine.
Limitations of this study.
Our transplantation studies are the first to show that pericytes participate differentially in myogenesis or fibrosis in vivo. However, their contribution relative to other myogenic and fibrogenic cells in skeletal muscle, namely, satellite cells and tissue-resident fibroblasts, respectively, remains to be studied. Although lack of muscle regeneration has been reported after satellite cell ablation (50, 54, 67), their interaction with fibroblasts is required for efficient muscle regeneration (59), and cross talk with other cell types, including pericytes, cannot be ruled out. Skeletal muscle fibroblasts do not participate directly, contributing myonuclei, to muscle regeneration. However, since their ablation alters the expansion of satellite cells and impairs muscle regeneration (59), research is needed to determine whether ablating type-2 pericytes, which do contribute myonuclei by fusion, can affect muscle regeneration.
Endogenous pericytes contribute to growth of a significant percentage of muscle fibers (26). Additionally, conditions that trigger skeletal muscle regeneration, such as muscle injury, dramatically increase pericyte contribution to skeletal myogenesis (26). Reports showing that selectively ablating satellite cells permanently hampers subsequent attempts to regenerate skeletal muscles (50, 59, 67) do not contradict our results because satellite cells may be required to induce other cell types to adopt a myogenic fate, as suggested by Sambasivan et al. (67). The relative contribution of pericytes to muscle repair is unknown since they fuse with myofibers and may generate satellite cells (26).
Multinucleated myofibers arise from the fusion of many cells with myogenic capacity in developing and regenerating muscle (17, 46, 64, 70). A recent study describes the ability of pericytes resident in postnatal skeletal muscle to fuse with myofibers and enter the satellite cell compartment (26). Here, we show that myofibers become DsRed+ after injection with type-2, but not type-1, pericytes because these pericytes fuse to the myofibers during tissue repair. Whether one or many type-2 pericytes fuse with individual damaged myofibers is unknown. We also show that, in vitro, only type-2 pericytes form myotubes.
Quantifying the contribution of endogenous pericyte subtypes to myogenesis and fibrous tissue formation with aging is enabled by recombination-based lineage tracing, and ablating a pericyte subpopulation would show whether satellite cell myogenic capacity is affected by its absence. The only marker we found differentially expressed in pericyte subpopulations is Nestin-GFP, which is also expressed in satellite cells (25), so tracking pericyte fate or ablating a subtype in vivo will require the discovery of new markers expressed in a pericyte subpopulation but not other cells. Future work characterizing the expression profiles of type-1 and type-2 pericytes will define these novel markers.
GRANTS
The present study was supported by a Wake Forest Pepper Center Pilot Project and PUSH grant from the Wake Forest Comprehensive Cancer Center (to O. Delbono and A. Mintz), National Institute on Aging Grants AG-13934 and AG-15820 (to O. Delbono), Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332), and a Glenn/AFAR Scholarship for Research in the Biology of Aging (to A. Birbrair).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B., A.M., and O.D. conception and design of research; A.B., T.Z., Z.-M.W., and M.L.M. performed experiments; A.B. and M.L.M. analyzed data; A.B., A.M., and O.D. interpreted results of experiments; A.B. and T.Z. prepared figures; A.B. and O.D. drafted manuscript; A.B., A.M., and O.D. edited and revised manuscript; O.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. G. N. Enikolopov from Cold Spring Harbor Laboratory (Cold Spring Harbor, NY) and Dr. W. Stallcup from the Sanford-Burnham Medical Research Institute (La Jolla, CA) for sharing with us the Nestin-GFP mouse and the rabbit anti-PDGFR-β antibody, respectively. Dr. James Wood, from the WFSM Comprehensive Cancer Center, contributed expertise on Flow Cytometry to our project.
REFERENCES
- 1. Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139: 677–689, 1984 [PMC free article] [PubMed] [Google Scholar]
- 2. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes JL, Glass WF., 2nd Renal interstitial fibrosis: a critical evaluation of the origin of myofibroblasts. Contrib Nephrol 169: 73–93, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 27: 9545–9559, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One 6: e16816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22: 2298–2314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319: 45–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10: 67–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20: 1703–1705, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev 71: 541–585, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994 [PMC free article] [PubMed] [Google Scholar]
- 15. Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev 9: 369–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol 16: 225–238, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Capers CR. Multinucleation of skeletal muscle in vitro. J Biophys Biochem Cytol 7: 559–566, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell 8: 676–689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest 100: 2697–2713, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25: 885–894, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36: 642–654, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 125: 87–99, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304: 246–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Duffield JS. The elusive source of myofibroblasts: problem solved? Nat Med 18: 1178–1180, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol 8: 241–276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18: 1262–1270, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Farnbach GC, Brown MJ, Barchi RL. A maturational defect in passive membrane properties of dystrophic mouse muscle. Exp Neurol 62: 539–554, 1978 [DOI] [PubMed] [Google Scholar]
- 31. Fechner G, Bajanowski T, Brinkmann B. Immunohistochemical alterations after muscle trauma. Int J Legal Med 105: 203–207, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol 192: 69–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4: 183–191, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Gollnick PD, Matoba H. Identification of fiber types in rat skeletal muscle based on the sensitivity of myofibrillar actomyosin ATPase to copper. Histochemistry 81: 379–383, 1984 [DOI] [PubMed] [Google Scholar]
- 35. Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7: 590–598, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Hamalainen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. Histochem Soc 41: 733–743, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90: 1137–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc 24: 197–205, 1992 [PubMed] [Google Scholar]
- 40. Jarvis JC, Mokrusch T, Kwende MM, Sutherland H, Salmons S. Fast-to-slow transformation in stimulated rat muscle. Muscle Nerve 19: 1469–1475, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Jerkovic R, Argentini C, Serrano-Sanchez A, Cordonnier C, Schiaffino S. Early myosin switching induced by nerve activity in regenerating slow skeletal muscle. Cell Struct Funct 22: 147–153, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kapetanaki MG, Mora AL, Rojas M. Influence of age on wound healing and fibrosis. J Pathol 229: 310–322, 2013 [DOI] [PubMed] [Google Scholar]
- 44. Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 11: 471–476, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109: 894–906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Konigsberg IR, McElvain N, Tootle M, Herrmann H. The dissociability of deoxyribonucleic acid synthesis from the development of multinuclearity of muscle cells in culture. J Biophys Biochem Cytol 8: 333–343, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21: 749–757, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172: 103–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leask A. Signaling in fibrosis: targeting the TGF beta, endothelin-1 and CCN2 axis in scleroderma. Front Biosci 1: 115–122, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat (Basel) 156: 173–186, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 45: 55–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 469: 311–324, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Mohan S, Radha E. Age-related changes in rat muscle collagen. Gerontology 26: 61–67, 1980 [DOI] [PubMed] [Google Scholar]
- 58. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16: 303–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23: 3430–3439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001 [DOI] [PubMed] [Google Scholar]
- 63. Parlakian A, Gomaa I, Solly S, Arandel L, Mahale A, Born G, Marazzi G, Sassoon D. Skeletal muscle phenotypically converts and selectively inhibits metastatic cells in mice. PLoS One 5: e9299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pietsch P. Differentiation in regeneration. I. The development of muscle and cartilage following deplantation of regenerating limb blastemata of Amblystoma larvae. Dev Biol 3: 255–264, 1961 [DOI] [PubMed] [Google Scholar]
- 65. Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172: 91–102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem 273: 28845–28851, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000 [DOI] [PubMed] [Google Scholar]
- 69. Stauber WT. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol 14: 61–70, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Stockdale FE, Holtzer H. DNA synthesis and myogenesis. Exp Cell Res 24: 508–520, 1961 [DOI] [PubMed] [Google Scholar]
- 71. Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4: 1964, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193, 2011 [DOI] [PubMed] [Google Scholar]
- 73. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis 6: 197–203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Zhang T, Birbrair A, Delbono O. Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis. Cytoskeleton (Hoboken) 70: 134–147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang T, Birbrair A, Wang ZM, Taylor J, Messi ML, Delbono O. Troponin T nuclear localization and its role in aging skeletal muscle. Age (Dordr) 35: 353–370, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zimmermann KW. Der feinere bau der blutkapillaren. Z Anat Entwicklungsgesch 68: 29–109, 1923 [Google Scholar]