Abstract
Hypertonic saline (HS) inhalation therapy benefits cystic fibrosis (CF) patients [Donaldson SH, Bennet WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. N Engl J Med 354: 241–250, 2006; Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT; the National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. N Engl J Med 354: 229–240, 2006]. Surprisingly, these benefits are long-lasting and are diminished by the epithelial Na+ channel blocker amiloride (Donaldson SH, Bennet WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. N Engl J Med 354: 241–250, 2006). Our aim was to explain these effects. Human bronchial epithelial (hBE) cells from CF lungs were grown in inserts and were used in three experimental approaches: 1) Ussing chambers to measure amiloride-sensitive short-circuit currents (INa); 2) continuous perfusion Ussing chambers; and 3) near “thin-film” conditions in which the airway surface of the inserts was exposed to a small volume (30 μl) of isosmotic or HS solution as the inserts were kept in their incubation tray and were subsequently used to measure INa under isosmotic conditions (near thin-film experiments; Tarran R, Boucher RC. Methods Mol Med 70: 479–492, 2002). HS solutions (660 mosmol/kgH2O) were prepared by adding additional NaCl to the isosmotic buffer. The transepithelial short-circuit current (ISC), conductance (GT), and capacitance (CT) were measured by transepithelial impedance analysis (Danahay H, Atherton HC, Jackson AD, Kreindler JL, Poll CT, Bridges RJ. Am J Physiol Lung Cell Mol Physiol 290: L558–L569, 2006; Singh AK, Singh S, Devor DC, Frizzell RA, van Driessche W, Bridges RJ. Methods Mol Med 70: 129–142, 2002). Exposure to apical HS inhibited INa, GT, and CT. The INa inhibition required 60 min of reexposure to the isosmotic solution to recover 75%. The time of exposure to HS required to inhibit INa was <2.5 min. Under near thin-film conditions, apical exposure to HS inhibited INa, but as osmotically driven water moved to the apical surface, the aqueous apical volume increased, leading to an amiloride-insensitive decrease in its osmolality and to recovery of INa that lagged behind the osmotic recovery. Amiloride significantly accelerated the recovery of INa following exposure to HS. Our conclusions are that exposure to HS inhibits hBE INa and that amiloride diminishes this effect.
Keywords: hyperosmotic saline, cystic fibrosis, Na+ transport, amiloride, human bronchial epithelial cells
the importance of fluid and electrolyte transport in the maintenance of healthy airways is well established (5, 9). Adequate hydration of the airway surface layer (ASL) volume and mucus is essential for optimal mucociliary clearance (MCC) (31). Water fluxes are dictated primarily by net electrolyte transport, and in the airways, Na+, K+, Cl−, and HCO3− are the principal transported electrolytes. Elevated Na+ absorption and inadequate anion secretion of patients with cystic fibrosis (CF) led to a reduced ASL and thereby impaired MCC. Several clinical trials have shown a therapeutic benefit from maneuvers designed to increase ASL in patients with CF. These maneuvers consist of using either hyperosmotic saline (HS; 8, 11) or hyperosmotic mannitol (7, 18, 28, 32) aerosols and found profound improvement in several measures of lung function including forced expiratory volume in 1 s, MCC, frequency of exacerbations, days on antibiotics, and well-being. Because the benefit of HS was expected to be short-lived, one trial included a group of patients pretreated with amiloride (8), a blocker of the epithelial Na+ channel (ENaC). Amiloride was expected to block the absorption of Na+ and thereby prolong the duration of action of the HS. Surprisingly, amiloride had a negative impact on the benefit of HS. Donaldson et al. (8) suggested that amiloride blocks plasmalemmal water permeability, but this hypothesis remains controversial (21). HS aerosol treatment has quickly become a first-line therapy for CF patients (14, 40). However, several important observations remain to be explained about this therapy, including the apparent long duration of its beneficial effects and the paradoxical effect of amiloride.
The purpose of this work was to study the effect of apical HS exposure on amiloride-sensitive sodium transport (INa) in primary cultures of human bronchial epithelial (hBE) cells from CF donors. The results demonstrate that HS produces a prolonged inhibition in INa. Furthermore, the continuous presence of amiloride does not affect osmotic water fluxes in hBE cells but accelerates the recovery of INa following a HS exposure. These results should help in explaining and developing aerosol therapies for treating patients with pathologies resulting from dehydration of the airways such as CF and exercise-induced asthma.1
MATERIALS AND METHODS
Cell Culture
hBE cells were obtained under Institutional Review Board-approved protocols at the Universities of Pittsburgh and North Carolina. In brief, the CF-hBE cells were obtained from lungs explanted during transplantation. Cells were dissociated by enzymatic digestion, expanded in growth media, seeded as p3 cultures onto 6.5-mm PET Transwell inserts in a differentiation media (27) under air-liquid interface conditions, and studied when polarized (3–5 wk). Criteria for acceptance of the inserts was threefold: 1) appearance of a homogenous monolayer of cells without holes, 2) absence of liquid at the apical surface, and 3) a transepithelial resistance >200 Ω·cm2.
Short-Circuit Current Studies
CF-hBE cells grown on the Transwell inserts were mounted in either conventional Costar Ussing chambers or continuously perfused Ussing chambers (see below). The short-circuit currents (ISC) were measured under symmetrical isosmotic conditions at both sides of the epithelium or when the apical side was replaced with HS buffer. The transepithelial net Na+ flux via the apical ENaC (INa) was defined as the amiloride-sensitive ISC. This was determined by adding 10 μM amiloride to the apical side.
Transepithelial Impedance Analysis
To determine the changes in membrane surface area in response to changes in extracellular osmolality, the total transepithelial capacitance (CT) was measured. This was accomplished by transepithelial impedance analysis as previously described (6, 35). This technique also allowed measurement of ISC and transepithelial resistance (RT).
Exposure to HS
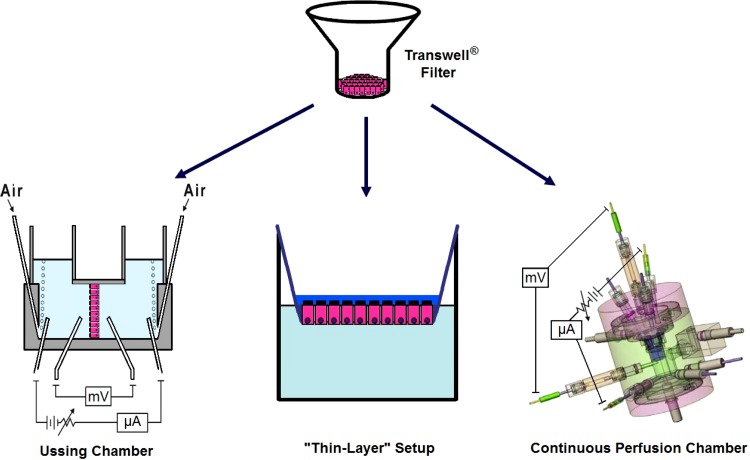
The following three experimental approaches were used to determine the effect of HS on INa (Fig. 1).
Fig. 1.
Diagram depicting the three experimental setups used to measure the effect of hypertonic saline (HS) on cystic fibrosis-human bronchial epithelial (CF-hBE) cells grown at an air-liquid interface on Transwell (top) inserts. Left: the conventional Ussing chamber. Center: the close to “thin-layer” setup. It consisted of the deposit of a small volume (30 μl) of either isosmotic or hyperosmotic solution as the inserts were kept in their incubation tray. Subsequently, the inserts were mounted on an Ussing chamber to measure sodium transport (INa) under isosmotic conditions. Right: the continuous perfusion Ussing chamber that allowed continuous and rapid (<1 min) replacement of the bathing solutions.
Ussing chamber.
The Transwell inserts were directly mounted in the conventional Costar Ussing chamber under isosmotic conditions at both sides of the epithelium. The apical and basolateral chamber volumes were 5 and 3.5 ml, respectively. Removal of added amiloride or replacement of the apical solution was achieved by replacing this solution with 12 volumes of the desired solution.
Continuously perfused ussing chamber.
This setup permitted continuous replacement of the apical and basolateral volumes (2 ml) every minute. This setup allowed fast intermittent pulses of amiloride under isosmotic or hyperosmotic conditions and thereby allowed measurement of the time-dependent changes in INa.
Near “thin-film” conditions.
To test whether the results obtained with the Ussing chamber could be reproduced under conditions closer to a “thin-film” type experiment of the type pioneered by the University of North Carolina group (24, 37–39), near “thin-film” experiments were performed. The strategy consisted of adding 30 μl of either isosmotic or hyperosmotic buffers to the apical surface of the Transwell inserts while they remained in the incubation plates and measuring INa (see below). The 30 μl volume was selected because it allowed reliable sampling of aliquots (7 μl) to measure changes in apical osmolality at various incubation times. Subsequently, the time courses of changes in osmolality of the apical fluid and of INa were monitored. At the various incubation times, 7 μl aliquots of the apical solution were taken to quantify their osmolality by vapor pressure osmometry (Wescor, Logan, UT). It should be noted that although this aliquot volume was not replaced, net water flow from the basolateral reservoir (containing 700 μl of fluid) continued to move to the apical side until the osmolalities of both reservoirs (apical and basolateral) were equal (see results). The effect of apical HS on INa was measured by mounting the Transwell inserts in the Ussing chamber under isosmotic apical and basolateral solutions (see Fig. 1).
Buffer Solutions
Solutions contained (in mM) 120 NaCl, 1 glucose, 1.2 CaCl2, 1.2 MgCl2, 5 HEPES, and 4.2 KCl, The pH of all solutions was 7.3 at 36.5°C. pH was adjusted with Tris(hydroxymethyl)aminomethane hydrochloride (Tris·HCl). The osmolality of the isosmotic solution was 310 ± 10 mosmol/kgH2O and was adjusted with an appropriate mix of tris base [Tris(hydroxymethyl)aminomethane/Tris·HCl] to maintain the pH. The HS (660 ± 10 mosmol/kgH2O) was attained by adding additional NaCl to the isosmotic buffer.
Statistics
Each Transwell insert was considered an independent measurement. For comparison of two groups, Student's t-analysis was used; multiple-comparison procedures (<4 groups) were performed using the Bonferroni test. P < 0.05 was considered statistically significant.
RESULTS
Effect of Apical Na+ Hyperosmolarity on Transepithelial Short-Circuit Current, Conductance, and Capacitance in CF-hBE Cells
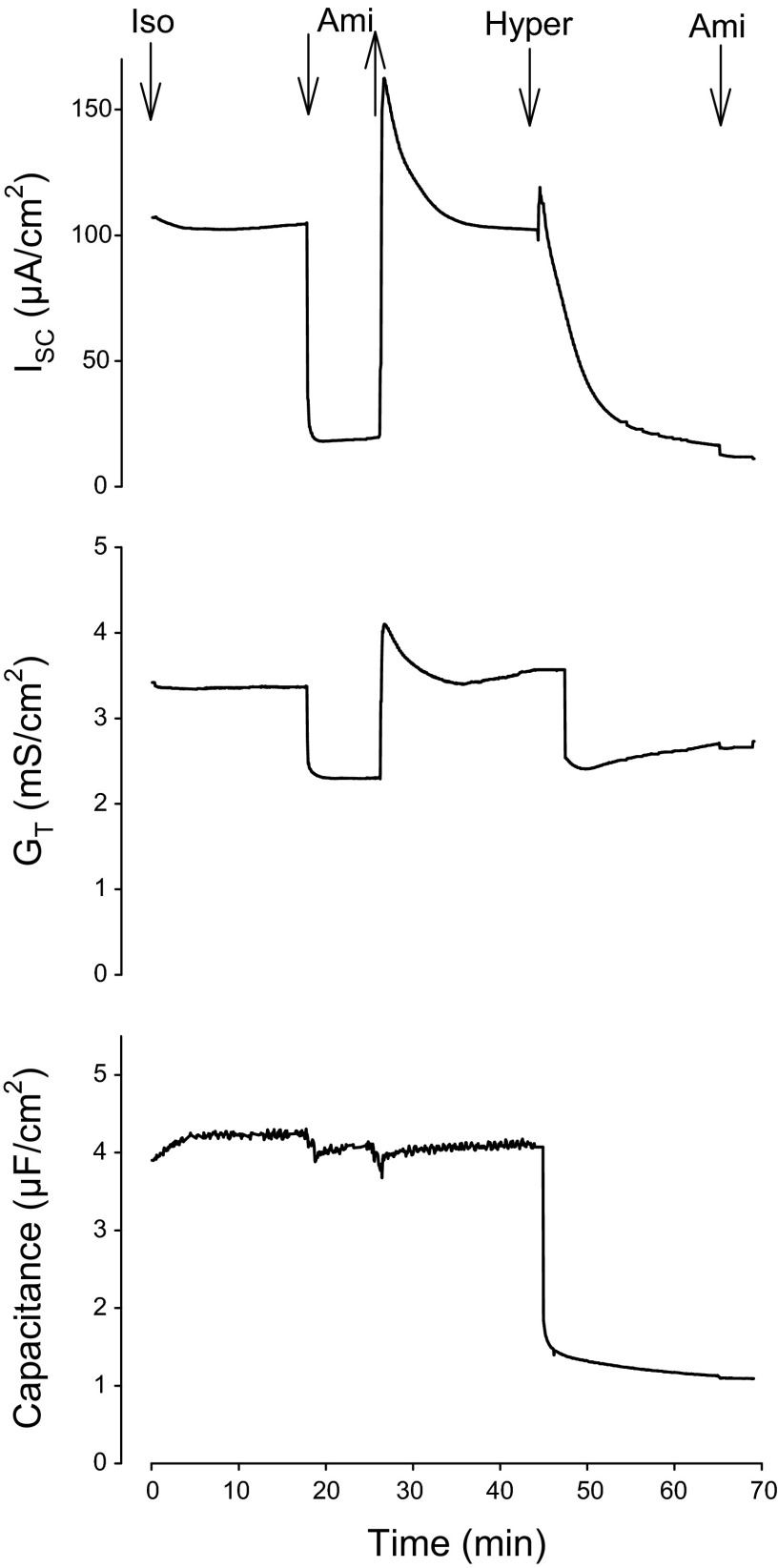
The effect of apical HS on transepithelial amiloride-sensitive ISC, GT, and CT was studied in CF-hBE cells using transepithelial impedance analysis. Figure 2 illustrates typical results from CF-hBE cells (n = 6–18): transepithelial ISC (top), GT, (middle), and CT (bottom). Under isosmotic conditions, the average values of ISC, GT, and CT were 85.9 ± 7.9 μA/cm2, 2.8 ± 0.4 mS/cm2, and 3.4 ± 1.6 μF/cm2, respectively (n = 18). Figure 2 shows that, under normal isosmotic conditions, addition of 10 μM amiloride at the apical surface produced an almost complete (89 ± 8.8%; n = 12) inhibition in ISC. As expected, the reduction in ISC was accompanied by a decrease in GT (32 ± 7%; n = 12), indicating inhibition of an ionic conductive pathway (i.e., epithelial Na+ channels, ENaC). In some cells the addition of amiloride also produced a small reduction in CT, but the average change in CT of all experiments was not statistically significant (4.7 ± 4%; n = 12). Thereafter, amiloride was then removed by exchange of the mucosal bath with 12 volumes of control (isosmotic) buffer and the ISC was allowed to return to baseline. Interestingly, removal of amiloride produced an overshoot in ISC and GT, which appeared to be a function of the time of exposure to the ENaC blocker.2 Subsequently, in 6 inserts, mucosal bath was then exchanged with 12 volumes of the HS (660 mosmol/kgH2O). This manipulation was followed by a rapid transient increase in ISC (30 ± 14 μA/cm2) followed by a sustained decrease in ISC (91 ± 13%), GT (30 ± 3%), and CT (87 ± 23%). The initial brief increase in ISC may be due to an immediate and transitory rush of Na+ into the cell driven by its sudden increase in electrochemical gradient. The subsequent decrease in ISC, GT and CT following exposure to HS reached steady values below the control baselines. Amiloride was then added to obtain a second measure of ENaC-mediated Na+ flux. Under this condition, the amiloride-sensitive ISC was 95.4 ± 1.1% smaller as compared with the one observed under isosmotic conditions. Clearly, apical HS significantly inhibited Na+ transport in CF-hBE cells accompanied by the expected reduction in GT and a reduction in CT, which could be explained by cell shrinkage and/or endocytosis.
Fig. 2.
Representative time course (n = 6–18) of the effect of amiloride (Ami, 10 μM) and apical HS (660 mosmol/kgH2O) on short-circuit current (ISC, in μA/cm2; top), transepithelial conductance (GT, in mS/cm2; middle), and capacitance (in μF/cm2; bottom) on CF-hBE cells. The insert was mounted on an Ussing chamber that allowed transepithelial impedance analysis (see materials and methods). At 17 min of incubation, under isosmotic conditions (Iso), 10 μM amiloride was added to the apical bath. At 23 min, amiloride was washed out by exchange of the mucosal bath with 12 volumes of the isosmotic solution. At 45 min, the mucosal bath was exchanged with 60 ml of HS (Hyper, 660 mosmol/kgH2O). Finally, at 66 min, 10 μM amiloride was reapplied to the apical solution. See text for details.
The Hyperosmotic-Induced Inhibition in Na+ Transport Is Protracted
The reduced response to amiloride demonstrates that exposure to HS leads to pronounced inhibition in INa. The next step consisted of studying the reversibility of this inhibition. The protocol consisted of first measuring the amiloride-sensitive ISC under isosmotic conditions (ΔINa Iso1) and then exchanging the mucosal bath with HS (660 mosmol/kgH2O) and monitoring the ISC for 20–40 min until a steady ISC reading was attained. Amiloride was then added for a second time to get INa under hyperosmotic conditions (ΔHyper). The mucosal bath was then exchanged back to the isosmotic buffer and the ISC was monitored for an additional 20–120 min. Amiloride was then added for a third time to determine the amiloride-sensitive ISC recovery (ΔINa Iso2). The %INa recovery was calculated using the following equation:
| (1) |
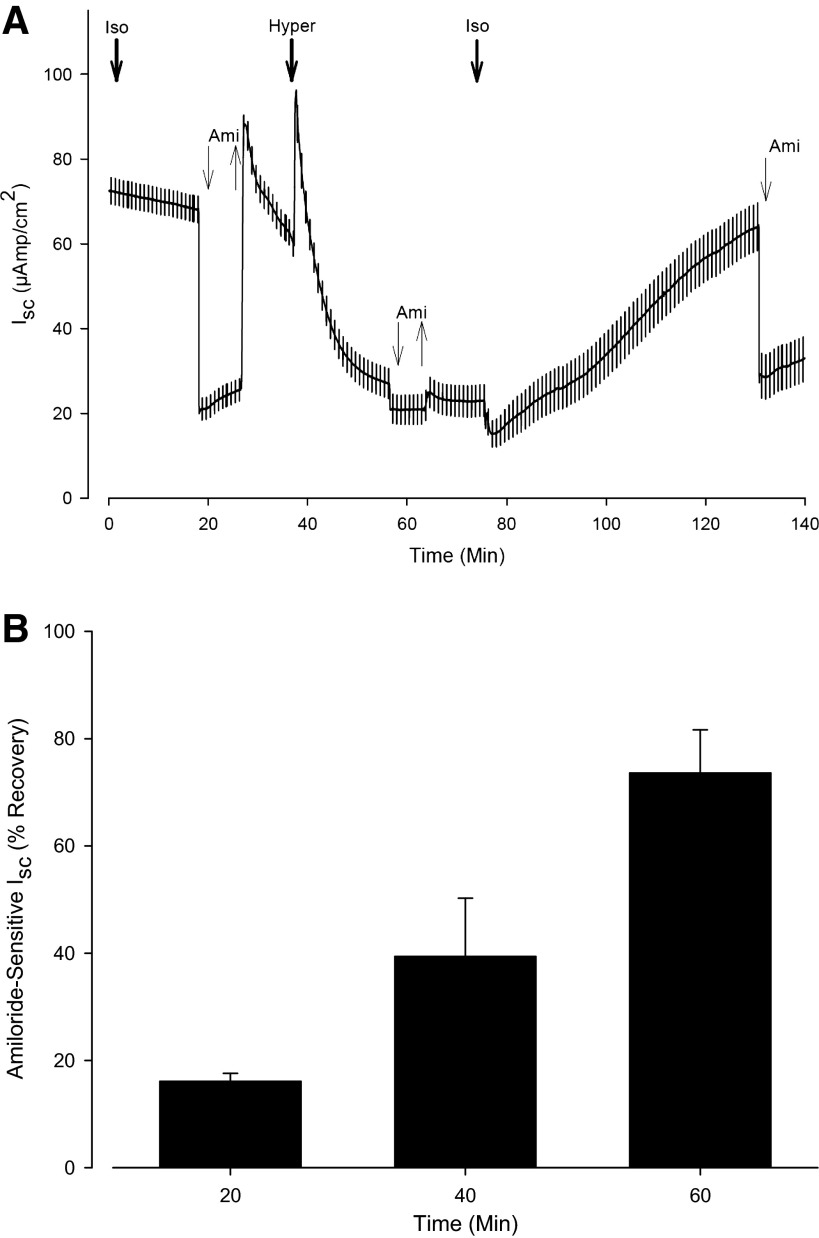
Figure 3A shows a representative experiment following the above protocol. Exposure to HS produced an 87% inhibition in INa. In this instance the CF-hBE cells were allowed to recover for 50 min following the HS exposure and the INa recovery was 69%. Control cells continuously exposed to isotonic solutions for the same length of time maintained 90 ± 8% of the INa.
Fig. 3.
Recovery of INa following its inhibition by exposure to apical HS (660 mosmol/kgH2O). A: representative time course (n = 12) of the experimental protocol. After 17 min of exposure to the isosmotic solution (300 mosmol/kgH2O), 10 μM amiloride was added to the apical bath. At 27 min of incubation, amiloride was removed. At 37 min of incubation, the apical solution was replaced by the HS solution (660 mosmol/kgH2O). Once a steady value of ISC was attained (i.e., at 56 min of incubation), amiloride was added at the apical side for a second time. At 63 min of incubation, amiloride was removed. When a steady value of ISC was measured (i.e., at 75 min), the apical solution was replaced by the isosmotic solution. This manipulation produced a transient decrease in ISC attributed to a reduction in the driving force for Na+ entry into the cell. After an allowance of 55 min of recovery (i.e., at 130 min of incubation), amiloride was added for a third and final time. The recovery of INa following reexposure to the isosmotic solution was calculated using Eq. 1. B: summary of 16 experiments (n = 4 at each recovery time measured) in which the %INa recovery following exposure to HS (660 mosmol/kgH2O) was measured at various recovery times.
Figure 3B shows the summary of 12 experiments in which the %INa recovery following the exposure to HS was measured following various recovery times. The results show that, ensuing inhibition of Na+ transport, it takes about an hour of recovery time for ISC to regain values comparable to those originally measured under isosmotic conditions. These results suggest that the inhibition in INa extends beyond the period of exposure to a hypertonic solution and suggest that the benefits from HS extend beyond the initial period of exposure and perhaps help explain the remarkable positive outcome of the clinical trials (7, 18, 28, 32) and the successful clinical use of HS for CF patients (14, 40).
The Onset of HS-Induced Inhibition of INa Is Rapid
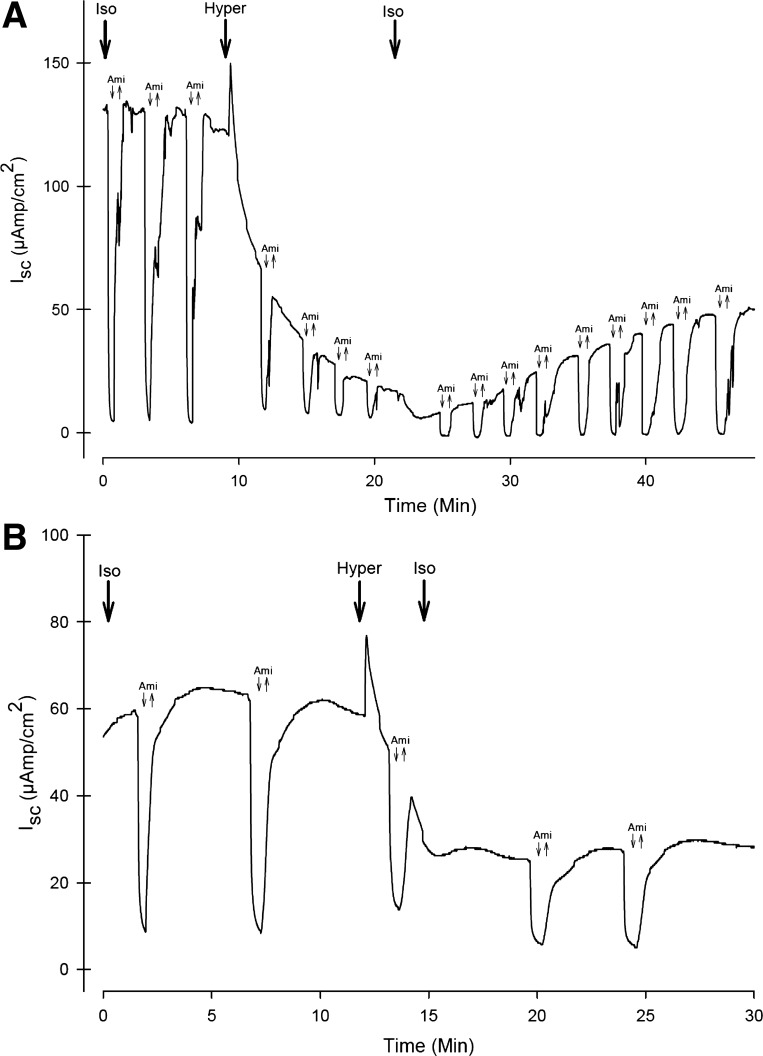
To establish how long cells must be exposed to HS to initiate the inhibition in INa, the continuous perfusion Ussing chamber setup was used. Figure 4A shows a representative example of an ISC trace from a series of six CF-hBE inserts in response to repetitive (every 3 min) exposure to amiloride under isosmotic and HS (660 mosmol/kgH2O) conditions. The figure shows that, under isosmotic conditions (300 mosmol/kgH2O), repetitive application of amiloride (downward facing arrows) produced reductions in ISC of similar magnitude. Once a steady ISC was established, exposure to 660 mosmol/kgH2O HS for 15 min inhibited INa by 85 ± 9.6%. The figure also shows that, as expected, the inhibition in INa outlived the actual time of exposure to the HS when the isosmotic conditions were reinstated. In this instance the recovery in ISC after 30 min of reexposure to the isosmotic solution only produced a recovery of 30%. To further study how long the HS exposure should be to produce the inhibition in ISC, similar experiments were performed, but in this instance the exposure to the HS was for 2 min. Figure 4B shows a representative example from a series of four inserts showing that exposure to 660 mosmol/kgH2O HS for only 2 min inhibited INa by 60 ± 7% and that the inhibition in INa outlived the actual time of exposure to HS when the isosmotic conditions were reinstated.
Fig. 4.
Time course of the inhibition and recovery of INa in response to exposure and removal of an apical HS (660 mosmol/kgH2O) in CF-hBE inserts mounted in the continuously perfused Ussing chamber (see materials and methods). A: representative example (n = 6) of the INa inhibition and recovery in response to exposure (for 12 min) and removal (for 30 min) to apical HS. Apical amiloride was applied and removed every 3 min. After 10 min of incubation in the isosmotic solution, the HS was applied to the apical bath. At 22 min of incubation, the isosmotic solution was reestablished at the apical bath. B: time course of the effect of apical HS (660 mosmol/kgH2O) application for 2 min. After 12 min of exposure to the isosmotic solution, the HS solution was applied at the apical side. After 2 min of this exposure (14 min of incubation), the apical solution was reestablished. See text for further details.
Effect of HS on INa Under Near “Thin-Film” Conditions
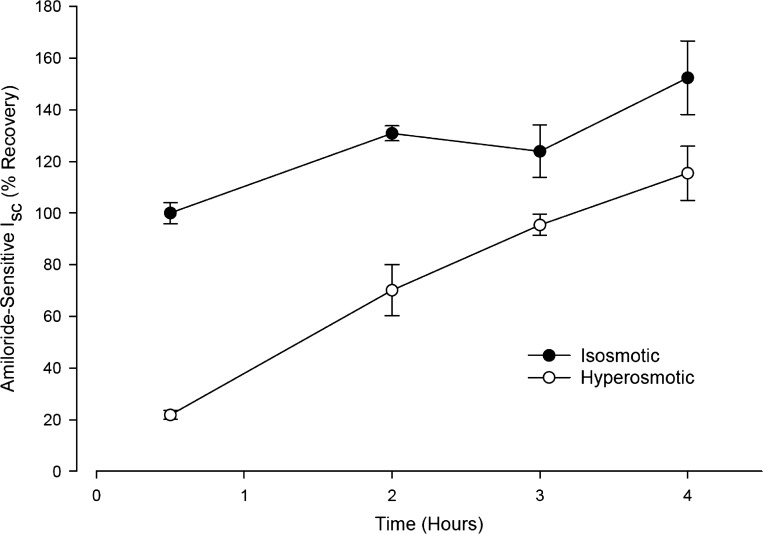
To test whether the HS-induced reduction in INa could be reproduced under conditions closer to physiological conditions, near “thin-film” (24, 37–39) experiments were performed. To minimize the volume of the buffer at which the apical side of the epithelium was exposed, 30 μl of either isosmotic or hypertonic buffers were added to the apical side of the Transwell inserts while they remained in the incubation plates. Subsequently, the time courses of the changes in osmolality of the hypertonic apical solution and of the changes in INa were determined. Figure 5 shows that in CF-hBE cells, addition of the isosmotic apical buffer (closed circles, n = 6) produced a time-dependent increase in INa in relation to the initial INa measured after 30 min of incubation (normalized to 100%). The rate of this increase in ISC was 16% per hour. Since all of the Transwell inserts were treated with the same care for washing and mounting on the Ussing chambers at the various incubation times, this increase in ISC was unlikely to be due to mechanical stimulation of INa. On the other hand, increases in INa have been reported in cultured hBE cells in response to dilution of the native ASL with 20 μl of isosmotic Ringer (39). This effect has been attributed to dilution of apical soluble serine protease inhibitors (CAP inhibitors), which would otherwise inhibit membrane-tethered serine proteases (CAP1) that activate ENaC (25, 26). Figure 5 also shows that exposure to HS (open circles, 660 mosmol/kgH2O) produced an 80 ± 3% inhibition in INa at 30 min of incubation compared with when the inserts were exposed to the isosmotic solution. The 80% inhibition in INa under these “thin-film” conditions is similar to the 91 ± 13% inhibition observed in the Ussing chamber studies (Fig. 2) and the 85 ± 9.6% inhibition in the continuous perfusion studies (Fig. 4A). Under the continuous presence of apical HS, the INa recovered with time with a slope of 28.6% per hour. This rate is 1.8 times faster than the increase in INa observed under isosmotic conditions but slower than the recovery observed in the Ussing chamber experiments upon reexposure to isosmotic conditions, where it reached 73 ± 7.5% in 1 h (Fig. 3B). After 4 h of incubation, the INa values under HS conditions reached similar INa values as those measured under isosmotic conditions at 30 min of incubation. Concomitant with the recovery of INa there was an increase in buffer volume at the apical surface. Although this increase in buffer volume was not directly measured, it was observed visually and was quantified indirectly by a reduction in the osmolality of the apical buffer (see below). The recuperation of ISC observed following exposure to HS is explained by two factors: 1) dilution in the apical osmolality as osmotically driven water moves from the epithelium and/or serosal side to the apical side and dilutes the hypertonic buffer (see below), and 2) recovery of Na+ transport following exposure to the HS.
Fig. 5.
Time course of the changes in INa measured on inserts of CF-hBE cells under near “thin-film” conditions. Inserts were left in the incubation tray and were subjected to exposure to 30 μl of either isosmotic (closed circles, n = 6) or HS (open circles, 660 mosmol/kgH2O, n = 6). At 30 min and 2, 3, and 4 h of incubation, the inserts were removed from the incubation tray, washed with isosmotic buffer, and mounted in Ussing chambers to measure INa under isosmotic conditions. The values were normalized (100%) to the INa measured following exposure to isosmotic conditions after 30 min of incubation (see text for further details).
Lag in Recovery of ISC Compared With Apical Osmolality Following Exposure to Hyperosmolality Under Near “Thin-Film” Conditions
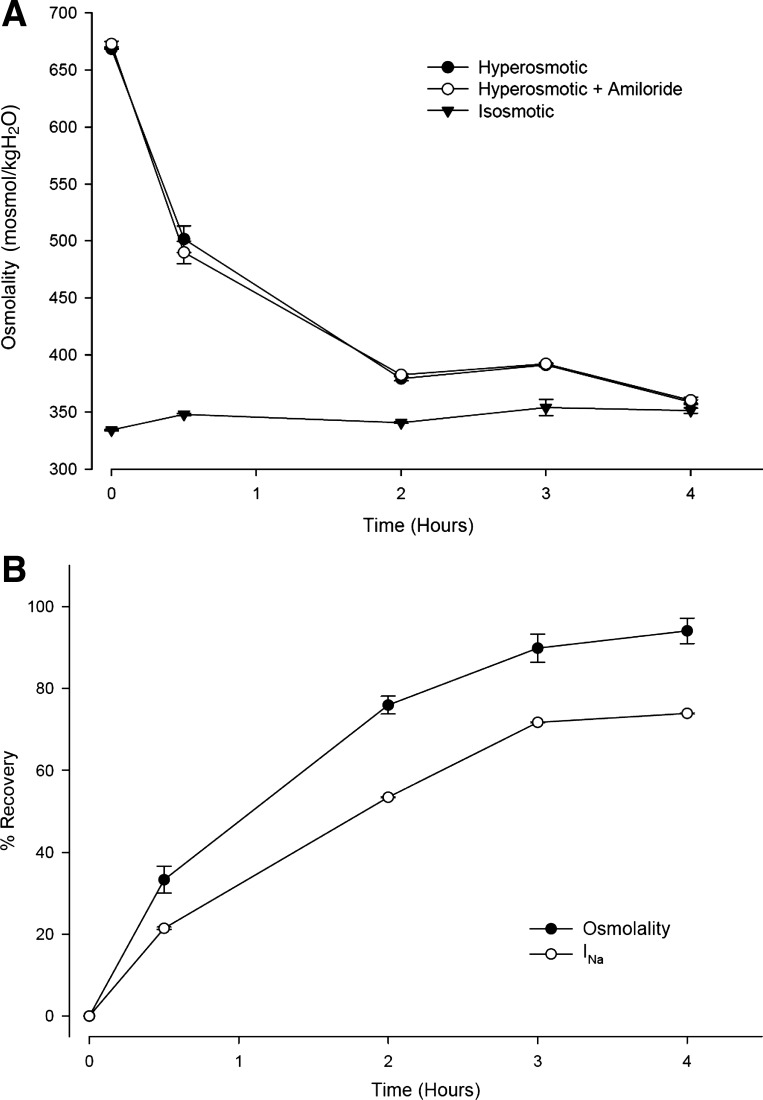
Figure 6A shows time courses (n = 6) of apical osmolalities in response to exposure to either isosmotic (inverted triangles) or HS (660 mosmol/kgH2O, circles) in the absence (closed symbols) or presence of 10 μM amiloride (open circles). The experimental protocol consisted of adding 30 μl of isosmotic or HS solution in the absence or presence of amiloride to the apical surface of inserts left in the incubation tray according to the near “thin-film” preparation (see materials and methods). At the indicated times, the osmolality of aliquots of the apical volume was measured. The graph shows that under isosmotic conditions the osmolality did not change for up to 4 h. The presence of amiloride had no effect on the osmolality (data not shown for clarity). In contrast, under HS conditions, there was decay in the apical solution osmolality, reaching the isosmotic values after 4 h of incubation. The presence of amiloride had no effect on this recovery. As explained above, this reduction in osmolality was due to dilution of the apical buffer as osmotically driven water moved from the basolateral side and the cytoplasm to the apical side. Since the original HS osmolality of the apical buffer was 660 mosmol/kgH2O, a reduction in osmolality to 360 mosmol/kgH2O at 4 h of incubation (Fig. 6A) indicates that there was a 1.83-fold dilution of the apical buffer resulting from an osmotically driven net flow of 240 μl of H2O from the basolateral solution, bringing the apical solution to a final volume of 540 μl. The fact that amiloride produced no effect on the apical osmolality under isosmotic and HS conditions argues against the proposal that amiloride inhibits water channels (8).
Fig. 6.
Effect of apical isosmotic and HS (660 mosmol/kgH2O) solutions on INa and apical osmolality in CF-hBE inserts under near “thin-film” conditions. A: time course of the changes in apical osmolality of inserts left in the incubation trays and subjected to the addition of 30 μl of either isosmotic (▼, n = 6) or HS (660 mosmol/kgH2O, ● and ○) in the absence (▼ and ●, n = 6) or presence of 10 μl amiloride (○, n = 6). At the indicated times, the osmolality of aliquots of the apical solutions was measured. B: time course of the recovery of the apical osmolality (●) and of INa (○) from a group of six CF-hBE cells. The INa recovery lagged behind the osmolality recovery (see text for further details).
Figure 6B shows a comparison of the time courses of recovery of the apical osmolality (closed circles) and of INa (open circles) from a group of six CF-hBE filters. The figure shows that the recovery in the amiloride-sensitive ISC followed a similar time course as the recovery in apical osmolality but that it was smaller in magnitude. The INa recovery lagged behind the osmolality recovery by 12, 22, 18, and 20% at 0.5, 2, 3, and 4 h of incubation, respectively. The fact that the recovery of INa consistently lagged behind the recovery of apical osmolality indicates again that Na+ transport inhibition by exposure to HS is protracted.
Amiloride Partially Blunts the Na+ Transport Inhibitory Response to the HS
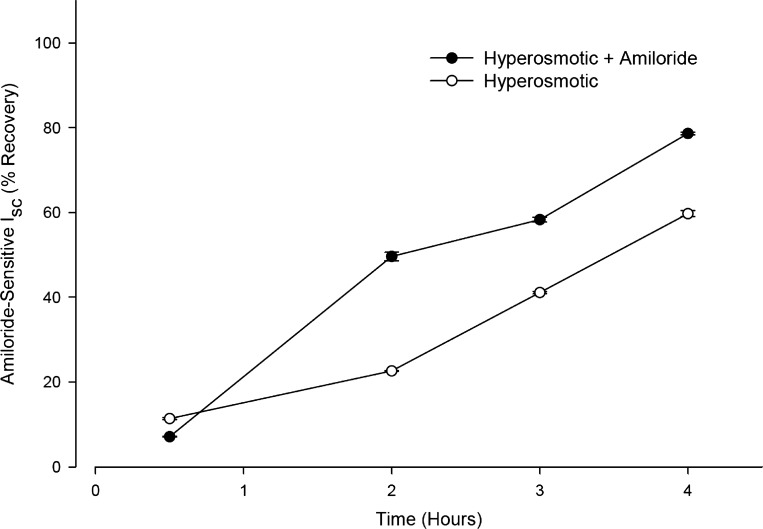
On the basis of these observations we reasoned that the paradoxical effect of amiloride on HS inhalation in CF patients may be due to a protective effect of amiloride on the HS-induced inhibition of Na+ transport. To test his hypothesis, the effect on the rate of recovery of INa following exposure to HS (660 mosmol/kgH2O) under near “thin-film” conditions was compared in the presence or absence of amiloride in CF-hBE cells (n = 6). The protocol consisted of exposing the apical surface of the inserts to 30 μl of either isosmotic or HS in the absence or presence of 10 μM amiloride and comparing the INa at various incubation times. Figure 7 depicts a comparison of the relative INa of inserts exposed to either HS (open circles) or an identical HS but containing amiloride (closed circles). The figure shows that following the first 30 min of incubation and up to 4 h of incubation, cells exposed to HS plus amiloride exhibited a significantly larger INa than cells exposed to HS alone. This indicates that exposure to amiloride accelerated the recovery of INa following the HS-induced inhibition of INa. This result suggests that amiloride, despite being an inhibitor of ENaC, can behave as a “protector” of HS-induced Na+ transport inhibition.
Fig. 7.
Time course of the effect of 10 μM amiloride on the recovery of INa following exposure to HS (660 mosmol/kgH2O) under near “thin-film” conditions in CF-hBE cells. The inserts were exposed to 30 μl HS (660 mosmol/kgH2O) in either the absence (○, n = 6) or presence (●, n = 6) of amiloride. At the various indicated times the inserts were washed with isosmotic buffer and mounted on Ussing chambers to measure INa under isosmotic conditions. SEs are shown where values extend beyond symbols (see text for further details).
DISCUSSION
Justification of the Use of the Hyperosmotic Challenges
In healthy individuals the airway epithelium may not be normally subjected to osmotic stress. Thus, until recently there has been little reason to investigate the regulatory responses of the airway epithelium in response to a hyperosmotic challenge. However, two lines of clinical research suggest that there is much to be gained from the further investigation of the responses of the airway epithelium to HS: 1) it has been proposed that exercise-induced bronchoconstriction in asthmatic and nonasthmatic subjects may result from an increase in osmolarity of the ASL (2); 2) inhalation of HS aerosols produces profound improvement in several measures of lung function in CF patients (8, 11). The results here presented demonstrate that even a brief (i.e., 2 min, Fig. 4B) apical exposure to 660 mosmol/kgH2O (obtained by adding additional NaCl to the isosmotic buffer) produced a protracted inhibition of INa. The fact that this inhibition outlives the time of exposure to the HS (Figs. 3–7) indicates that this manipulation produces a protracted change in the processes that mediate the amiloride-sensitive currents (i.e., ENaC and/or Na-K-ATPase and/or basolateral K+ channels) in CF-hBE cells.
It is important to recognize that the osmolality of the HS solution used in our experiments may appear higher than would be expected to encounter under normal and even pathophysiological conditions in the airways. However, we estimate these osmolalities to be in the range of osmolalities at which airway epithelial cells of CF patients undergoing HS therapy may be exposed. Considering that these patients receive 5 ml of 7% (1.2 M) NaCl solution (2.4 osM) for 30 min and that 70% of the inhalation is swallowed, then 1.5 ml of solution reaches the airways. Therefore, the inhaled volume of 1.5 ml with an osmolality of 2,400 mosmol/kgH2O would be dissolved in a maximal volume of 5 to 8 ml (9) if homogeneously distributed across the entire ASL, and would yield a final osmolality of ≃700–930 mosmol/kgH2O. Thus, the chosen osmolality of 660 mosmol/kgH2O used in the in vitro studies reported here is within the range of those expected to be reached in the airways of patients undergoing HS therapy. It is pertinent to consider that other in vitro studies have used osmolalities much larger than the ones utilized in these studies (e.g., calculated 2,720–27,200 mosmol/kgH2O; Ref. 8).
Ussing Chamber Versus Near “Thin-Layer” Experiments
The Ussing chamber experiments here described (Figs. 2–4) were performed with a 5 ml mucosal bath volume, which, compared with the cell volume, can be considered infinite. The ASL of in vitro airway cells has been reported to have a height of only 7 μm (37, 39). Thus it was important to assess whether the results obtained employing the Ussing chamber conditions could be reproduced in a preparation closer to an in vivo condition. Our approach was to use a preparation closer to a “thin film” preparation (Figs. 5–8). We chose to add a volume of 30 μl to the apical surface of the inserts because this volume allowed us to reliably monitor the changes in apical osmolality. Admittedly, this volume is still very large compared with what the ASL normally is in situ in human lungs. However, the fact that our “thick” (i.e., Ussing chamber) and closer to “thin-film” preparations yielded similar outcomes argues in favor of the physiological validity of the observed inhibition of INa by HS (Figs. 2–5).
Protracted Inhibition of INa From Exposure to HC-NaCl Solutions
Previous observations indicate that exposure to luminal HS conditions inhibits Na+ transport across human airway epithelia under physiological conditions. For example, physical exercise with increased ventilation leads to airway dehydration which in turn reduces the amiloride-sensitive inhibition in nasal epithelia potential difference in normal and CF patients (15). Likewise, exposure to luminal HS inhibits the amiloride-sensitive potential difference in respiratory epithelium measured in controls and CF patients (16). However, to the best of our knowledge, there are no reports available assessing the effect of HS on the amiloride-sensitive ISC in hBE cells. The results here presented not only document a HS-induced inhibition of INa but also show that this inhibition outlasts the duration of the exposure to the HS. Figure 3B shows that hBE cells exposed to 20 min of HS slowly recovered their INa following reexposure to the isosmotic conditions. This recovery of INa corresponds to a slow reestablishment of the pathways responsible for the transepithelial transport of Na+. Thus, the HS-induced inhibition of INa is protracted.
Protective Effect of Amiloride on HC-NaCl-Induced Inhibition of INa
The success achieved with HS in the clinical trials was unexpected because the airways epithelium has a high water permeability and cannot sustain an osmotic gradient (24). Thus water was expected to move quickly into the airways and then back out as Na+ and Cl− were absorbed. In an effort to prolong the duration of action of hypertonic saline, Donaldson et al. (8) included a treatment group with HS plus amiloride. However, instead of improving the effect of HS, the inclusion of amiloride attenuated the treatment benefit of HS. In an effort to explain this apparent paradoxical effect of amiloride the authors performed some in vitro experiments and concluded from their results that amiloride blocks water channels. This conclusion, however, is controversial since Verkman and coworkers (21) have shown that amiloride does not block the water channels found in airway cells (aquaporins 1, 3, 4, and 5) and did not observe an inhibitory effect of amiloride on the water permeability in primary cultures of hBE cells. In our hands, the fact that the presence of amiloride did not affect the recovery of osmolality at the apical surface of CF-hBE cells exposed to HC-NaCl in the close to “thin-film” preparation (Fig. 6A) also argues against a possible blockage of amiloride on water permeability.
The inclusion of amiloride during the HS resulted in an accelerated recovery of INa during reexposure to isosmotic conditions (Fig. 7). This suggests that amiloride partially protects the epithelium from the inhibitory effects of the HS. Intracellular Na+ concentration is determined by cell volume and the entry and exit rates of Na+. Hyperosmotic solutions will shrink the cell, causing intracellular Na+ concentration ([Na+]i) to increase. Until ENaC activity is downregulated, Na+ entry resulting from exposure to HS solutions will exacerbate the rise in [Na+]i caused by cell shrinkage. Amiloride by blocking Na+ entry would tend to attenuate the rise in [Na+]i and thereby blunt the downregulation of ENaC activity. We speculate that this effect of amiloride may help explain why amiloride had a negative impact in the clinical trials with HS (8).
Possible Mechanisms Responsible for the HS-Induced Inhibition of INa
The inhibitory effect of HS on INa could be due to inhibition of Na+ transport at either the apical surface (i.e., via ENaC) and/or basolateral membranes (e.g., via Na-K-ATPase and/or K+ channels). Furthermore, these effects could result from either the increases in the extra and/or intracellular Na+ concentrations and/or the concomitant changes in cell volume. The phylogenetic relationship of ENaC to mechanosensitive ENaC/degenerin gene family (22) suggests that ENaC might be mechanosensitive as well (12). While the possibility that HS could affect the Na-K-ATPase remains, there is considerable evidence supporting the notion that Na+ and cell shrinkage affect ENaC activity. The latter, for example, has been reported to activate ENaC in rat hepatocytes (4) and rat epithelial ENaC expressed in Xenopus oocytes (19), but to inhibit this channel in mouse trachea (33). This is further complicated by the fact that, at least in rat hepatocytes, it has been shown that subunits α, β, and γ of ENaC are related to hypertonicity-induced cation channels (30). In any event, there is strong consensus that either exposure to hyperosmolality or shear stress alters ENaC activity. This has led to the proposal that these channels may act as stretch sensors (10) playing a critical role in epithelial cell volume regulation (19).
Regarding the possible role of Na+ in regulating ENaC there are two main mechanisms documented: 1) a fast inhibition by extracellular Na+ (self-inhibition) (13, 29) and 2) a slower and longer lasting inhibition due to increases in [Na+]i (feedback inhibition) (1, 3, 34). The fact that in the experiments here reported the HS-induced inhibition of INa was protracted and that amiloride accelerated the recovery of this inhibition (Figs. 3, 4, and 7) suggests that a likely mechanism involved for this effect is feedback-inhibition. Three possibilities that could be involved in this process are 1) a possible enhancement of ENaC endocytosis via the ubiquitin ligase NEDD-4–2 that binds to ENaC, ubiquitinates the channel leading to its endocytosis, and degradation (23, 36); 2) an intracellular Na+-dependent regulation of ENaC degradation by altering the accessibility of ENaC cleavage sites to proteases (20); and 3) a Na+-dependent slowing down of insertion of active ENaC. While this would be a slow process compared with the observed “rapid” rate of INa inhibition by HS, a slowdown of insertion of channels could explain the slow rate of recovery. The first two mechanisms have ENaC leaving the membrane, but the possibility that ENaC remains in the membrane in a nonconducting state cannot be ruled out at present.
In sum, the results presented here document that apical exposure to a HS solution (660 mosmol/kgH2O) attained by adding additional NaCl to the normal buffer produces a protracted inhibition of the amiloride-sensitive ISC (i.e., INa) in CF-hBE cells. This effect is observed in Ussing chambers and in a near to “thin-film” preparation. The rapid and protracted inhibition in INa likely results from cell shrinkage/endocytosis together with a likely increase in the intracellular Na+ concentration ([Na+]i). Amiloride, by presumably reducing the likely increases in [Na+]i, ameliorates the HS-induced inhibition of INa. These results may help explain the long-lasting beneficial effects of HS inhalers in CF patients and the antagonistic effect of combined HS with amiloride.
GRANTS
This work was supported in part by the Cystic Fibrosis Foundation (to H. Rasgado-Flores, R. J. Bridges, and S. H. Randell), the Cystic Fibrosis Foundation Research Development Program (to J. M. Pilewski), and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-072506 (to J. M. Pilewski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.R.F., W.V.D., J.M.P., S.H.R., and R.J.B. conception and design of research; H.R.F., V.K.M., and H.S. performed experiments; H.R.F., V.K.M., H.S., and R.J.B. analyzed data; H.R.F., J.M.P., S.H.R., and R.J.B. interpreted results of experiments; H.R.F., V.K.M., and H.S. prepared figures; H.R.F. and R.J.B. drafted manuscript; H.R.F., W.V.D., J.M.P., S.H.R., and R.J.B. edited and revised manuscript; H.R.F., W.V.D., J.M.P., S.H.R., and R.J.B. approved final version of manuscript.
Footnotes
This article is the topic of an Editorial Focus by Carole M. Liedtke (21a).
This phenomenon has been carefully studied using blocker-induced noise analysis of ENaC of frog skin by Helman and Baxendale (17). They found that blockage of ENaC leads to an increase in the density of apical ENaC from sources different from closed states of the channel, i.e., from dormant channels within apical membranes and/or from channel-containing vesicles inserted into apical membranes from stored sites within the cytoplasm.
REFERENCES
- 1. Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol 574: 333–347, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson SD. Exercise-induced bronchoconstriction in the 21st century. J Am Osteopath Assoc 111, Suppl 7: S3–S10, 2011 [PubMed] [Google Scholar]
- 3. Awayda MS. Regulation of the epithelial Na+ channel by intracellular Na+. Am J Physiol Cell Physiol 277: C216–C224, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Böhmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A, Lin JT, Lang F, Wehner F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem 10: 187–194, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Danahay H, Atherton HC, Jackson AD, Kreindler JL, Poll CT, Bridges RJ. Membrane capacitance and conductance changes parallel mucin secretion in the human airway epithelium. Am J Physiol Lung Cell Mol Physiol 290: L558–L569, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Daviskas E, Robinson M, Anderson SD, Bye PT. Osmotic stimuli increase clearance of mucus in patients with mucociliary dysfunction. J Aerosol Med 15: 331–341, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Donaldson SH, Bennet WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354: 241–250, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Donaldson SH, Boucher RC. Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulm Med 9: 486–491, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT; the National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354: 229–240, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fronius M, Clauss W. Mechano-sensitivity of ENaC: may the (shear) force be with you. Pflügers Arch 455: 775–785, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Goss CH, Ratjen F. Update in cystic fibrosis 2012. Am J Respir Crit Care Med 187: 915–919, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit A. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med 164: 443–446, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hebestreit A, Kersting U, Hebestreit H. Hypertonic saline inhibits luminal sodium channels in respiratory epithelium. Eur J Appl Physiol 100: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Helman SI, Baxendale LM. Blocker-related changes of channel density. J Gen Physiol 95: 647–678, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, Robertson CF, Bye PT, LeSouef PN, Shalbolt B, Anderson SD, Charlton B. Inhaled mannitol improves lung function in cystic fibrosis. Chest 133: 1388–1396, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Ji HL, Fuller CM, Benos DJ. Osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes. Am J Physiol Cell Physiol 275: C1182–C1190, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Knight KK, Wentzlaff DM, Snyder PM. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J Biol Chem 283: 27477–27482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levin MH, Sullivan S, Nielson D, Yang B, Finkbeiner WE, Verkman AS. Hypertonic saline therapy in cystic fibrosis: evidence against the proposed mechanism involving aquaporins. J Biol Chem 281: 25803–25812, 2006 [DOI] [PubMed] [Google Scholar]
- 21a. Liedtke CM. Understanding the cellular mechanism for inhaled hyperosmotic saline therapy for patients with cystic fibrosis. Focus on “Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors.” Am J Physiol Cell Physiol (August 28, 2013). 10.1152/ajpcell.00250.2013 [DOI] [PubMed] [Google Scholar]
- 22. Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel: a new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 318: 95–99, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway. Am J Physiol Renal Physiol 290: F1285–F1294, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest 105: 1419–1427, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 281: 27942–27949, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Myerburg MM, McKenna EE, Luke CJ, Frizzell RA, Kleyman TR, Pilewski JM. Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L932–L941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neuberger T, Burton B, Clark H, Van Goor F. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol 741: 39–54, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Nilsson H, Dragomir A, Ahlander A, Johannesson M, Roomans G. Effects of hyperosmotic stress on cultured airway epithelial cells. Cell Tissue Res 330: 257–269, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Palmer LG, Sackin H, Frindt G. Regulation of Na+ channels by luminal Na+ in rat cortical collecting tubule. J Physiol 509: 151–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plettenberg S, Weiss E, Lemor R, Wehner F. Subunits α, β and γ of the epithelial Na+ channel (ENaC) are functionally related to the hypertonicity-induced cation channel (HICC) in rat hepatocytes. Pflügers Arch 455: 1089–1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Randell SH, Boucher RC; University of North Carolina Virtual Lung Group Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35: 20–28, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, Bye PT. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J 14: 678–685, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Schreiber R, König J, Sun J, Markovich D, Kunzelmann K. Effects of purinergic stimulation, CFTR and osmotic stress on amiloride-sensitive Na+ transport in epithelia and Xenopus oocytes. J Membr Biol 192: 101–110, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through”. Am J Physiol Renal Fluid Electrolyte Physiol 241: F579–F590, 1981 [DOI] [PubMed] [Google Scholar]
- 35. Singh AK, Singh S, Devor DC, Frizzell RA, van Driessche W, Bridges RJ. Transepithelial impedance analysis of chloride secretion. Methods Mol Med 70: 129–142, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380, 1996 [PMC free article] [PubMed] [Google Scholar]
- 37. Tarran R, Boucher RC. Thin-film measurements of airway surface liquid volume composition and mucus transport rates in vitro. Methods Mol Med 70: 479–492, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol 68: 543–561, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2: 1–41, 2010 [DOI] [PubMed] [Google Scholar]