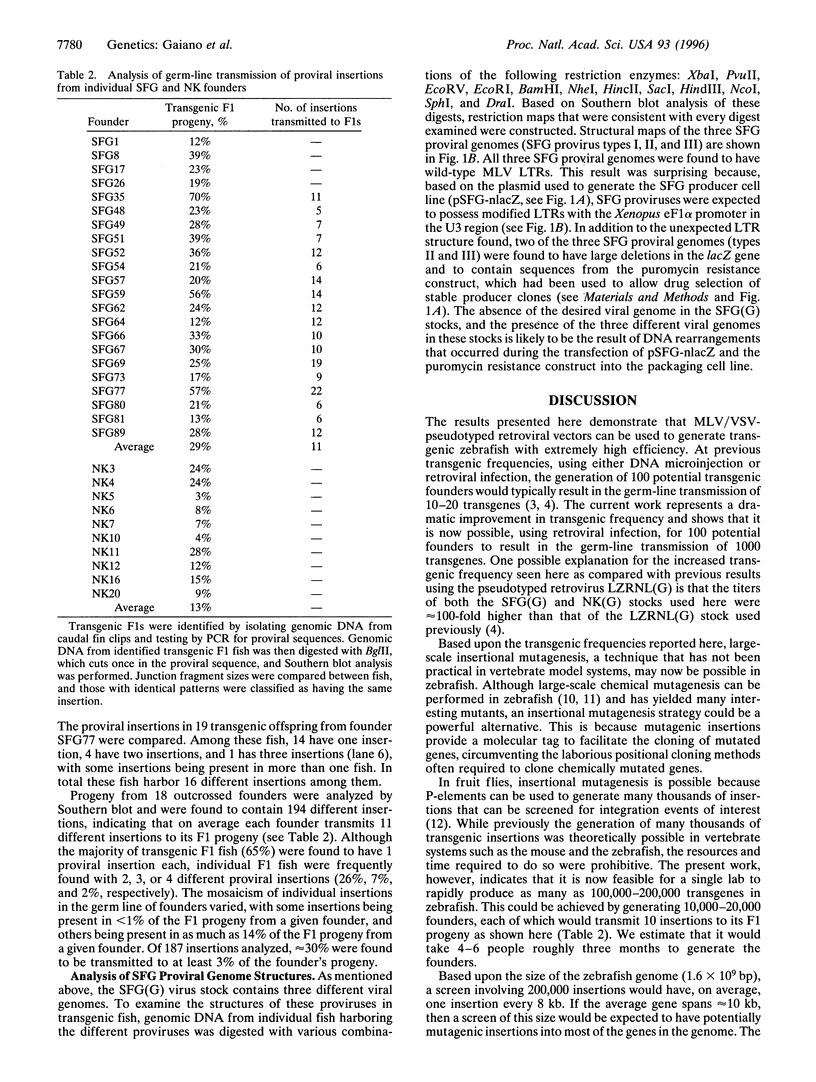
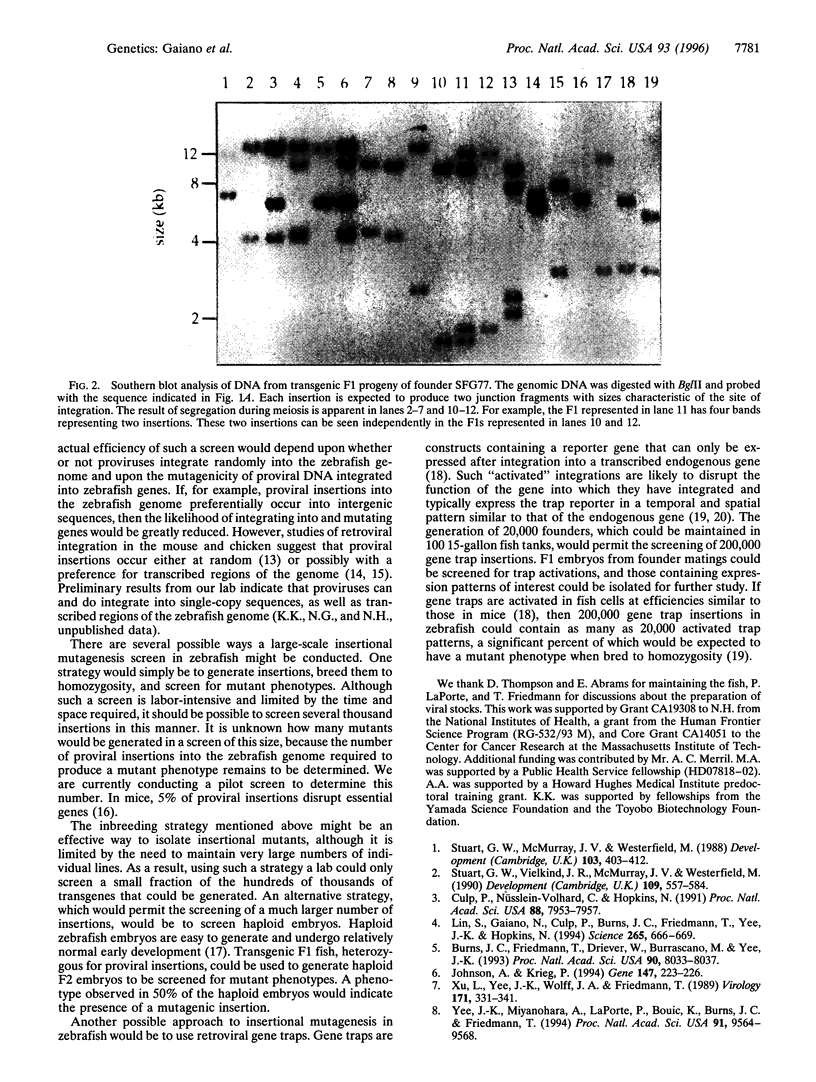
Abstract
An important technology in model organisms is the ability to make transgenic animals. In the past, transgenic technology in zebrafish has been limited by the relatively low efficiency with which transgenes could be generated using either DNA microinjection or retroviral infection. Previous efforts to generate transgenic zebrafish with retroviral vectors used a pseudotyped virus with a genome based on the Moloney murine leukemia virus and the envelope protein of the vesicular stomatitis virus. This virus was injected into blastula-stage zebrafish, and 16% of the injected embryos transmitted proviral insertions to their offspring, with most founders transmitting a single insertion to approximately 2% of their progeny. In an effort to improve this transgenic frequency, we have generated pseudotyped viral stocks of two new Moloney-based genomes. These viral stocks have titers up to two orders of magnitude higher than that used previously. Injection of these viruses resulted in a dramatic increase in transgenic efficiency; over three different experiments, 83% (110/133) of the injected embryos transmitted proviral insertions to 24% of their offspring. Furthermore, founders made with one of the viruses transmitted an average of 11 different insertions through their germ line. These results represent a 50- to 100-fold improvement in the efficiency of generating transgenic zebrafish, making it now feasible for a single lab to rapidly generate tens to hundreds of thousands of transgenes. Consequently, large-scale insertional mutagenesis strategies, previously limited to invertebrates, may now be possible in a vertebrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Culp P., Nüsslein-Volhard C., Hopkins N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7953–7957. doi: 10.1073/pnas.88.18.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Krieg P. A. pXeX, a vector for efficient expression of cloned sequences in Xenopus embryos. Gene. 1994 Sep 30;147(2):223–226. doi: 10.1016/0378-1119(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Lin S., Gaiano N., Culp P., Burns J. C., Friedmann T., Yee J. K., Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994 Jul 29;265(5172):666–669. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Haffter P., Nüsslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994 Mar 1;4(3):189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherdin U., Rhodes K., Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990 Feb;64(2):907–912. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L., Schier A. F., Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994 Apr;136(4):1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., McMurray J. V., Westerfield M. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development. 1988 Jun;103(2):403–412. doi: 10.1242/dev.103.2.403. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Vielkind J. R., McMurray J. V., Westerfield M. Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development. 1990 Jul;109(3):577–584. doi: 10.1242/dev.109.3.577. [DOI] [PubMed] [Google Scholar]
- Withers-Ward E. S., Kitamura Y., Barnes J. P., Coffin J. M. Distribution of targets for avian retrovirus DNA integration in vivo. Genes Dev. 1994 Jun 15;8(12):1473–1487. doi: 10.1101/gad.8.12.1473. [DOI] [PubMed] [Google Scholar]
- Wurst W., Rossant J., Prideaux V., Kownacka M., Joyner A., Hill D. P., Guillemot F., Gasca S., Cado D., Auerbach A. A large-scale gene-trap screen for insertional mutations in developmentally regulated genes in mice. Genetics. 1995 Feb;139(2):889–899. doi: 10.1093/genetics/139.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- Yee J. K., Miyanohara A., LaPorte P., Bouic K., Burns J. C., Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]




